Abstract
Objective This study aimed to analyze, evaluate, and characterize novel cement-based carboxymethyl chitosan/amorphous calcium phosphate (CMC/ACP).
Materials and Methods The three cement groups studied were gypsum (Gyp), and CMC/ACP—gypsum cement-based 5% (5% CAG) and 10% (10% CAG). The groups were characterized using Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), setting time, and scanning electron microscopy (SEM) data. The characterization results were analyzed qualitatively, but the data for setting time were analyzed using SPSS ( p < 0.05).
Statistical Analysis Data were statistically analyzed. One-way analysis of variance was used to compare numerical (parametric) data between more than two separate groups followed by post hoc Tukey.
Results FTIR showed phosphate groups indicate the presence of calcium phosphate in the form of amorphous (ACP) in the CMC/ACP, CMC/ACP post-milled powder, and CMC/ACP cement-based (5% CAG and 10% CAG). XRD showed no difference in the diffraction spectra among the Gyp, 5% CAG, and 10% CAG groups. SEM images revealed that the CMC/ACP cement-based groups (5% CAG and 10% CAG) showed CMC/ACP cluster filled with hollow spaces between the gypsum crystals and aggregations surrounding the gypsum crystals. The CMC/ACP showed envelopes and attached to the crystalline structures of the gypsum. Setting times of 5% CAG and 10% CAG showed significant differences compared with Gyp ( p < 0.05).
Conclusion The result of our study showed that CMC/ACP cement-based (5% CAG and 10% CAG) demonstrated amorphous characteristic, which can stabilize calcium ions and phosphate group (ACP). In addition, the modification of gypsum using CMC/ACP as cement-based extended the time of setting.
Keywords: gypsum, CMC/ACP, carboxymethyl chitosan, amorphous calcium phosphate, dentin remineralization, Fourier-transform infrared spectroscopy, X-ray diffraction, scanning electron microscopy, setting time
Introduction
The concept of minimal invasive is done by removing infected dentin and maintaining affected dentin. Infected dentin is the outermost layer of caries containing bacteria and their byproducts, demineralized inorganic components, denaturation of collagen fibrils, and loss of vital odontoblast. 1 2 3 Meanwhile, on the affected dentin layer, denaturation has occurred, but it is bacteria free, has intact collagen, and still allows remineralization. 1 2 4 In the natural remineralization of dentin, there are two interrelated components, namely, collagen, which acts as a scaffold, and noncollagen protein, namely, dentin matrix protein 1 (DMP1), which plays an important role as a regulator and stabilizer of the nucleation of apatite minerals. 5 6 DMP1 has a high affinity for calcium ions and can bind to collagen through electrostatic interactions. DMP1 triggers the formation of nanocluster amorphous calcium phosphate (ACP) precursors. Then, DMP1 and ACP will form the DMP1/ACP nanocomplex. DMP1 works to stabilize ACP so that it does not aggregate and remains in nano size. Furthermore, the DMP1/ACP nanocomplex will penetrate the gap zone between collagens and form hydroxyapatite crystals so that intrafibrillar remineralization occurs. 7 In the caries process, some DMP1s will experience degradation of functions so that remineralization does not occur optimally on the demineralized dentin surface. 5
In the dentine remineralization process with a biomimetic strategy, the material used is expected to replace the function of degraded components such as DMP1. Several alternative materials can replace the function of DMP1 as a noncollagen protein that have been studied and used to stabilize ACP, namely, polyacrylic acid and polyaspartic acid, phosphorylated chitosan, and carboxymethyl chitosan (CMC). 5 Several past studies had looked for natural materials that may have an effect on the prevention of dentin demineralization. 5 8 9 One of the compounds obtained from natural materials is CMC, a derivative of chitosan and it is easily obtained from the crustacean shell. 10 CMC is rich in carboxyl groups, so it has a high calcium affinity in the CMC/ACP complex, which plays a role in the remineralization process. 4 Recent research by Chen et al and Annisa et al confirms that CMC/ACP in scaffold preparation can replace the role of DMP1 in the remineralization process in the formation of hydroxyapatite both extrafibrillar and intrafibrillar. 5 11
CMC/ACP preparations in scaffold form have been characterized. Several studies have shown that the material triggers guided tissue remineralization in both dentin and enamel, but the scaffold form was lacking good handling properties and is not practical in dentistry due to the short duration of use, complex storage, and complicated processing. 5 11 12 13 According to Yamin et al and Maharti et al, a mixture of gypsum and CMC/ACP can be one of the solutions to overcome this problem, and their study showed that evidently this mixture yields positive effects in dentin remineralization. 14 15 To date, none of the research explains how the preparation of CMC/ACP in the form of powder can be mixed as a cement material suitable for the field of dentistry as dentin remineralization. The current study aims to characterize the CMC/ACP material as a cement-based mixture.
Materials and Methods
Preparation of CMC/ACP Powder Materials
Preparation for CMC/ACP powder: 0.555 g of CaCl 2 was added to 10 mL deionized water under stirring for 5 minutes, and then 0.498 g of K 2 HPO 4 was added to this solution under stirring at 500 rpm. Then 2.5 g of CMC (PUI Kitosan and Material Maju, Universitas Sumatra Utara, Medan, Indonesia) was added to 20 mL of deionized water under stirring at 1,000 rpm until the CMC powder completely dissolved. Next, the CMC solution was added dropwise to the mixed solution (CaCl 2 + K 2 HPO 4 ) to form a CMC/ACP gel. The gel was immediately frozen at −80°C for 2 hours and then lyophilized in a vacuum freeze dryer intermittent time (at 12, 4, and 8 hours) to form a dry material of CMC/ACP that could be crushed into a powder using a mortar.
Preparation of CMC/ACP Post-Milled Powder
The powder resulting from lyophilization was subjected to a ball milling process using a high-energy shaker mill (Nano Center Indonesia, Serpong, Indonesia). The ball milling process was performed on the CMC/ACP powder for 30 minutes at 700 rpm with balls made of ceramic to obtain fine particle in the material. The above-mentioned milling time was chosen after a pilot study revealed that it yields the smallest particle smaller particles.
Preparation of Cement-Based CMC/ACP
Cement-based CMC/ACP was prepared according to previous report with some modification. 5 14 15 16 17 CMC/ACP cement was prepared with gypsum (calcium sulfate hemihydrate/CaSO 4 ∙½H 2 O—Sigma Aldrich), CMC/ACP post-milled powder (CAPM), and water at a ratio of 2.5 g:0.25 g (10% ratio) and/or 0.125 g (5% ratio):1.9 mL, respectively. There were three experimental cement-based groups containing gypsum—calcium sulfate hemihydrate (Gyp), CMC/ACP gypsum cement 5% (5% CAG), and CMC/ACP gypsum cement 10% (10% CAG).
Characterization of CMC/ACP, CAPM 5% CAG and 10% CAG
Fourier-Transform Infrared Spectroscopy Analysis
The functional groups present in the CMC, CMC/ACP, and CAPM, 5% CAG and 10% CAG were analyzed in the spectral range of 4,000 to 510 cm −1 using Nicolet iS50 Fourier-Transform Infrared Spectrometer (Thermo Fisher Scientific, United States).
X-ray Diffraction Analysis
The X-ray diffraction (XRD) pattern of CMC, CAPM, 5% CAG and 10% CAG were recorded using an X-ray diffractometer (D8 DIS- COVER, Bruker, United States) with Cu K α radiation (k = 1.54 degrees) and scanning rate of 1 step/s with step size of 0.1 degree/step. XRD test was performed to evaluate the crystallization phase of the material.
Morphological Analysis
Morphological analysis was used to observe crystalline structures of all groups (Gyp, 5% CAG, and 10% CAG) by field emission scanning electron microscopy (SEM, FEI Quanta 400, The Netherlands).
Setting Time
Samples of dimension 6 mm × 3 mm were prepared in three groups ( n = 6) consisting of Gyp, 5% CAG, and 10% CAG. A Vicat needle with 3N in weight and a flat-ended cylindrical needle tip of 1 mm in diameter were applied to the surface of the sample and indentations interval were 60 seconds. Setting time was recorded from the start of mixing to 60 seconds when the needle failed to penetrate a depth of more than 1 mm on the surface of the sample.
Statistical Analysis
Statistical testing was performed using Windows, version 23 of IBM SPSS software. The degree of significance was set to p- value < 0.05.
Results
Characterization of CMC/ACP, CAPM, 5% CAG and 10% CAG
Fourier-transform infrared spectroscopy (FTIR) spectra of CMC and CMC/ACP powder are shown in Fig. 1a, b . A band at 1,419.61 cm −1 of the -CH2COOH and peaks of the -NH2 at 1,599.99 for CMC indicate the presence of CMC in the form of carboxymethyl (-COO-1) and amine (-NH2). Phosphate groups (PO4 3− ) were detected in the CMC/ACP material at 1,005.90 and 1,085.43 cm −1 indicating interaction between Ca 2+ and CMC. Phosphate groups indicating calcium phosphate in the form of amorphous (ACP) were present in CMC/ACP. The milling process did not alter the functional groups of the CAPM compared with CMC/ACP ( Fig. 1c ). FTIR spectra of sulfate were evident in Gyp (1,121.28 cm −1 ), 5% CAG (1,124.38 cm −1 ), and 10% CAG (1,122.87 cm −1 ), and carboxyl functional groups were detected at 1,681.3 to 3,549.31 cm −1 (5% CAG) and 1,679.62 to 3,609.15 cm −1 (10% CAG); 5% CAG and 10% CAG showed two peaks shifted to 1,606.63 and 1,415.68 cm −1 indicating interaction of calcium ions and CMC. Additional phosphate groups at 1118.37 cm −1 (5% CAG) and 1,085.43 cm −1 (10% CAG) indicate that the amorphous forms were present in the cement-based CMC/ACP.
Fig. 1.
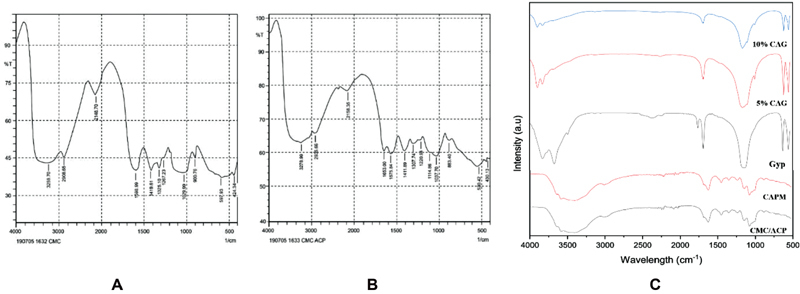
FTIR spectrum of ( A ) CMC, ( B ) CMC/ACP, ( C ) CMC/ACP, CAPM, Gyp, 5% CAG, and 10% CAG comparison.
XRD was used to identify the crystallization phase in the CMC/ACP processed by cryodesiccation and CAPM. The crystallization phase of calcium and dicalcium phosphate dihydrate was evident in CMC/ACP and CAPM. Fig. 2 shows that there is no difference in diffraction spectra among the Gyp, 5% CAG, and 10% CAG groups. The Gyp group showed higher crystal phase of nitratine, gypsum, and bassanite compared with cement-based CMC/ACP (5% CAG and 10% CAG).
Fig. 2.
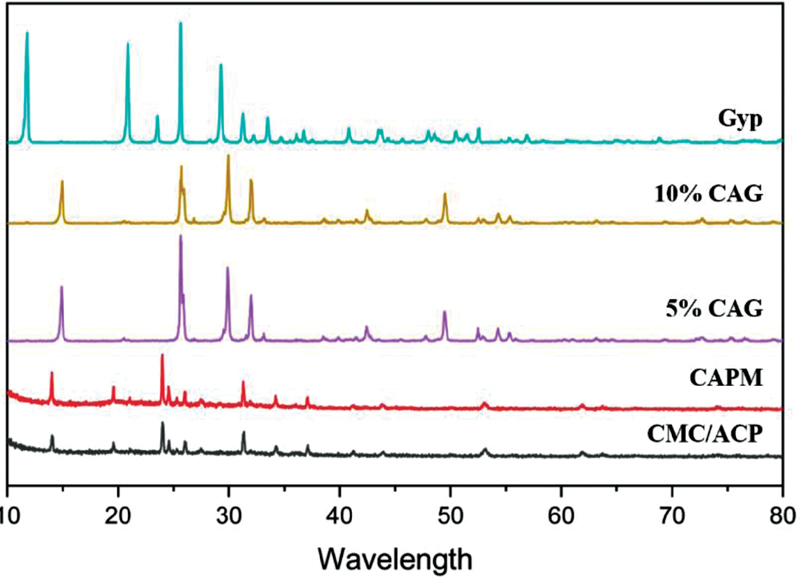
XRD spectrum of CMC/ACP, CAPM, 5% CAG, 10% CAG, and Gyp. The graphic showed no difference in diffraction spectra among the Gyp, 5% CAG, and 10% CAG groups. ACP, amorphous calcium phosphate; CAPM, CMC/ACP post-milled powder; CMC, carboxymethyl chitosan; Gyp, gypsum; XRD, X-ray diffraction.
Morphology
The morphology images of Gyp, 5% CAG, and 10% CAG were shown in Fig. 3a to c . Needle-shaped crystal and intermingled crystalline structures of gypsum were observed in Gyp. The porous structure between crystalline structures was evident in gypsum. The presence of CMC/ACP that filled hollow spaces between the gypsum crystals and the aggregations surrounding the gypsum crystals was evident in the 5% CAG and 10% CAG cement groups. The CMC/ACP showed envelopes and attached to the crystalline structures of the gypsum.
Fig. 3.
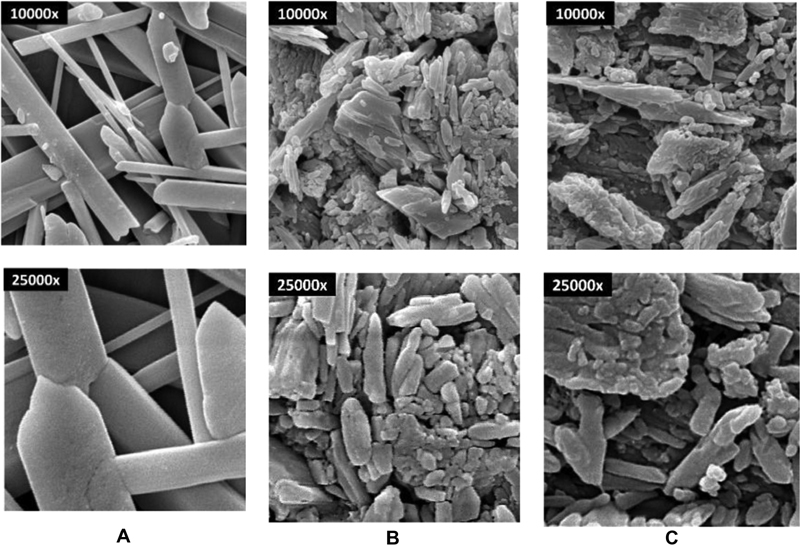
Morphology of ( A ) Gyp, ( B ) 5% CAG, ( C ) 10% CAG at 10.000× and 25.000 × . SEM images revealed that the CMC/ACP cement groups (5% CAG and 10% CAG) showed CMC/ACP cluster filled with hollow spaces between the gypsum crystals and aggregations surrounding the gypsum crystals were evident in the 5% CAG and 10% CAG cement groups. The CMC/ACP showed envelopes and attached to the crystalline structures of the gypsum. ACP, amorphous calcium phosphate; CAPM, CMC/ACP post-milled powder; CMC, carboxymethyl chitosan; Gyp, gypsum; SEM, scanning electron microscopy.
Setting Time
The setting times of the materials are listed in Fig. 4 . The setting times of cement-based CMC/ACP in all groups (5% CAG and 10% CAG) ranged from 389.37 to 424.44 minutes, and the setting times of gypsum (Gyp) ranged from 25.62 to 30.88 minutes. Our findings further showed that the setting time of cement-based CMC/ACP (5% CAG and 10% CAG) in all groups was significantly longer than Gyp ( p < 0.05). This result indicated that setting time was altered in 5% CAG and 10% CAG compared with Gyp.
Fig. 4.
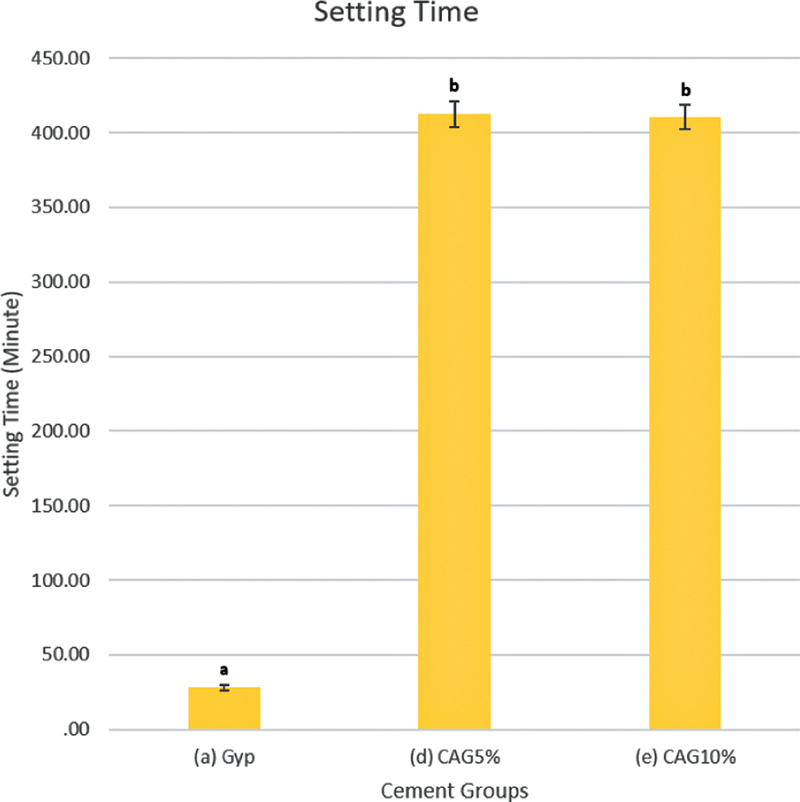
Setting time of Gyp, 5% CAG, and 10% CAG. The error bars represent the standard deviation. Bars with different letters are statistically significant ( n = 6; p < 0.05, Tukey's honestly significant difference). Gyp, gypsum.
Discussion
Chitosan is a natural polymer derived from the exoskeleton of crustaceans and one of the most abundant carbohydrates in nature. 10 18 Chitosan also has desirable characteristics such as biorenewability, biodegradability, biocompatibility, bioadhesivity, antimicrobial activity, nontoxicity, low immunogenicity, low cost, and accessibility. 10 18 The attempt to modify chitosan as a novel material or addition in restoration materials showed potential benefit in restorative dentistry. 19 20 21 22 However, chitosan has several limitations, such as highly ordered crystalline structure arising from solid hydrogen bonds and poor solubility in water. 10 18 23 The limitation of poor solubility of chitosan will limit its effectiveness in various processes, including modification as a cement-based material, but this can be overcome by depolymerization into CMC, which can lead to its preparation as a water-soluble derivatives. 10 18 23 ACP has been shown to be a material that plays a primary role in biomineralization. 24 25 The ACP and its modification could decrease demineralization and provide a potential method for caries management. 5 14 15 24 26 27 28 29 Stabilization of ACP by analogs of noncollagen protein has been studied in bottom-up remineralization strategy. 5 14 24 25 CMC as one of the natural analogs has a proven ability to stabilize ACP and prevent the particles from undergoing crystal transformation. 5 11 14 The attempt to modify the CMC/ACP into a gel has been conducted by Chen et al using lyophilization or a desiccation process in vacuum freeze dryer and has also been confirmed by Annisa et al and Setiati et al. 5 11 13 However, the gel form of CMC/ACP was complex to apply in clinical settings due to lack of handling properties, short duration of use, complex storage, and complicated processing.
Modification of CMC/ACP into a powder form in this study showed that the characteristic of CMC to stabilize ACP was evident in CMC/ACP from the FTIR spectra. The result of Chen et al indicated that different enhanced times of cryodesiccation did not alter this characteristic of CMC/ACP compared with CMC/ACP in gel formed. 5 CMC/ACP was ground into fine particles and the materials were blended using ball milling for 30 minutes. In the pilot study, the ball milling process did not decrease the particle size linearly with increasing times, and the smallest particle was evident in 30 minutes of milling. This result probably indicated that the flexible structure of polymers of CMC enables it to adopt complexation with metal ions, thus allowing the material to withstand the milling process. 10 The interesting finding of this study was a ball milling time of 30 minutes did not alter the functional groups or the mineral phase of CAPM compared with CMC/ACP. The interaction of carboxyl groups, which act as stabilizers of ACP, might be the reason for the stability of CAPM after the ball milling process. 5 10 14 18
Gypsum or calcium sulfate has been used in dentistry because of its biocompatibility and its ability to act as a carrier for other biomaterials. 14 17 The addition of viscous polymer (i.e., carboxymethyl cellulose and hyaluronan) shows better handling characteristics and mechanical properties. 30 The mixture of CMC/ACP as concentrated solution and gypsum on dentin remineralization has been described by Yamin et al and Maharti et al. 14 15 In this study, the porous structure of gypsum was evidently filled with CMC/ACP cluster when the cement was mixed in all groups, and similar phenomenon has been described by Subhi et al using chitosan material. 17 The addition of CAPM did not affect the crystalline phase of calcium sulfate in this research and similar study from Ding showed that chitosan matrix did not affect the crystalline phase of calcium phosphate cement. 31 The evidence of phosphate groups from FTIR spectrum finding in 5% CAG and 10% CAG indicates the presence of amorphous phase is expected to support remineralization process and treatment of decalcification. 5 27 32 33 CMC/ACP cement-based groups (5% CAG and 10% CAG) induce crystallization phase in a form of gypsum, nitratine and bassanite but lacked characteristic diffraction of crystalline hydroxyapatite that may be indicating the ACP phase associated with CMC. 5 Subhi et al stated that chitosan matrix did not affect the crystallization of calcium sulfate. 17 The pattern of CMC/ACP adsorption in 5% CAG and 10% CAG was observed in the SEM images and compared with Gyp ( Fig. 3B, C ). These findings provide concerning evidence from the above statement and by previous research from Low et al. 16 In addition, CMC/ACP cement-based groups (5% CAG and 10% CAG) showed better handling properties when manipulated.
In this study, the setting times of cement-based CMC/ACP groups (5% CAG and 10% CAG) were significantly larger compared with the setting time of Gyp. The results indicate that crystallization was compromised due to Ca 2+ ion stabilization by carboxyl groups from CMC/ACP, resulting in increased setting time. The ratio of powder/water prior to mixing was adapted from Subhi et al. 17 However, compared with the results from the present study in which gypsum hemihydrate is used, the results of Subhi et al are different due to the use of gypsum dihydrate, which is transformed into a hemihydrate phase. The amorphous calcium sulfate phase of gypsum can precipitate from an aqueous solution via hemihydrate. 34 Increased time of setting due to the amorphous phase could be the reason the crystallization of material was compromised. Therefore, the CMC as a polymer can be a strong inhibitor of dihydrate formation, resulting retardation of crystal growth and increase of setting time. 35 The extended time for the material for setting in the clinical scenario might be favorable for dentine remineralization. Kim et al stated that prolonged application time using remineralization agents can promote remineralization of the early carious lesion. 36 The limitation of this study is that this study only assessed the characteristic and setting time of cement-based CMC/ACP (5% CAG and 10% CAG). Future research should include the physical properties, cell viability, and dentin remineralization in the tooth caries model.
Conclusion
The result of our study showed that cement-based CMC/ACP (5% CAG and 10% CAG) demonstrated amorphous characteristics, which can stabilize calcium ions and phosphate group (ACP). In addition, the modification of gypsum using CMC/ACP as cement-based extended the time of setting.
Funding Statement
Funding This study received financial help from Universitas Indonesia through PUTI Grant with contract number BA-080/UN2.RST/PPM.00.03.01/2021.
Footnotes
Conflict of Interest None declared.
References
- 1.Dai L, Liu Y, Salameh Z. Can caries-affected dentin be completely remineralized by guided tissue remineralization? Dent Hypotheses. 2011;2(02):74–82. doi: 10.5436/j.dehy.2010.1.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frencken J E, Peters M C, Manton D J, Leal S C, Gordan V V, Eden E. Minimal intervention dentistry for managing dental caries - a review: report of a FDI task group. Int Dent J. 2012;62(05):223–243. doi: 10.1111/idj.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hargreaves K M, Cohen S, Berman L H. St. Louis, Mo: Mosby Elsevier; 2011. Cohen's pathways of the pulp; pp. 458–460. [Google Scholar]
- 4.Bertassoni L E, Habelitz S, Kinney J H, Marshall S J, Marshall G W., Jr Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43(01):70–77. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Z, Cao S, Wang H. Biomimetic remineralization of demineralized dentine using scaffold of CMC/ACP nanocomplexes in an in vitro tooth model of deep caries . PLoS One. 2015;10(01):e0116553. doi: 10.1371/journal.pone.0116553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varas-díaz N, Rita M, Garbi C. Biomimetic remineralization of dentin. Dent Mater J. 2013;3(01):43–56. [Google Scholar]
- 7.Considerations B.Biomineralization and biomaterial considerations in dentin remineralization Journal of Operative Dentistry & Endodontics 20161(June):7–12. [Google Scholar]
- 8.Onwubu S C, Mdluli P S, Singh S, Bharuth V. Remineralization potential of a modified eggshell-titanium composite-scanning electron microscope study. Eur J Dent. 2019;13(02):187–192. doi: 10.1055/s-0039-1695662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdel-Azem H M, Elezz A FA, Safy R K. Effect of Galla chinensis on remineralization of early dentin lesion. Eur J Dent. 2020;14(04):651–656. doi: 10.1055/s-0040-1716778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourya V K, Inamdar N N, Tiwari A. Carboxymethyl chitosan and its applications. Adv Mater Lett. 2010;1(01):11–33. [Google Scholar]
- 11.Annisa R N, Djauharie N, Suprastiwi E, Avanti N. The effect of carboxymethyl chitosan/amorphous calcium phosphate to guide tissue remineralization of dentin collagen. Int J Appl Pharm. 2019;11(01):181–183. [Google Scholar]
- 12.Xiao Z, Que K, Wang H. Rapid biomimetic remineralization of the demineralized enamel surface using nano-particles of amorphous calcium phosphate guided by chimaeric peptides. Dent Mater. 2017;33(11):1217–1228. doi: 10.1016/j.dental.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Setiati H D, Suprastiwi E, Artiningsih D ANP, Utami L PTB.Concentration dependent effects of carboxymethyl chitosan on dentin remineralization with amorphous calcium phosphate Int J Appl Pharm 202012(Special Issue 2):31–33. [Google Scholar]
- 14.Yamin E, Suprastiwi E, Usman M, Sarmayana S. The effect of gypsum extension on a mixture of carboxymethyl chitosan and amorphous calcium phosphate in dental remineralization. J Stomatol (Brux) 2020;73(02):69–73. [Google Scholar]
- 15.Maharti I D, Suprastiwi E, Setiati H D, Yamin E, Cahyani A N.The effects of mixtures of various concentrations of carboxymethyl chitosan/amorphous calcium phosphate with gypsum on dentin remineralization Int J Appl Pharm 202012(Special Issue 2):13–15. [Google Scholar]
- 16.Low A, Mohd Yusof H, Reza F. Gypsum-based biomaterials: evaluation of physical and mechanical properties, cellular effects and its potential as a pulp liner. Dent Mater J. 2015;34(04):522–528. doi: 10.4012/dmj.2015-029. [DOI] [PubMed] [Google Scholar]
- 17.Subhi H, Reza F, Husein A, Al Shehadat S A, Nurul A A. Gypsum-based material for dental pulp capping: effect of chitosan and BMP-2 on physical, mechanical, and cellular properties. Int J Biomater. 2018;2018:3.804293E6. doi: 10.1155/2018/3804293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shariatinia Z.Carboxymethyl chitosan: properties and biomedical applications Int J Biol Macromol 2018120(Pt B):1406–1419. [DOI] [PubMed] [Google Scholar]
- 19.Perchyonok V T, Zhang S, Grobler S R, Oberholzer T G. Insights into and relative effect of chitosan-H, chitosan-H-propolis, chitosan-H-propolis-nystatin and chitosan-H-nystatin on dentine bond strength. Eur J Dent. 2013;7(04):412–418. doi: 10.4103/1305-7456.120666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senthil Kumar R, Ravikumar N, Kavitha S.Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release Int J Biol Macromol 2017104(Pt B):1860–1865. [DOI] [PubMed] [Google Scholar]
- 21.Husain S, Al-Samadani K H, Najeeb S. Chitosan biomaterials for current and potential dental applications. Materials (Basel) 2017;10(06):1–20. doi: 10.3390/ma10060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim M A, Meera Priyadarshini B, Neo J, Fawzy A S. Characterization of chitosan/TiO 2 nano-powder modified glass-ionomer cement for restorative dental applications . J Esthet Restor Dent. 2017;29(02):146–156. doi: 10.1111/jerd.12282. [DOI] [PubMed] [Google Scholar]
- 23.Liang P, Zhao Y, Shen Q, Wang D, Xu D. The effect of carboxymethyl chitosan on the precipitation of calcium carbonate. J Cryst Growth. 2004;261(04):571–576. [Google Scholar]
- 24.Gu L, Kim Y K, Liu Y. Biomimetic analogs for collagen biomineralization. J Dent Res. 2011;90(01):82–87. doi: 10.1177/0022034510385241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao J, Liu Y, Sun W B, Zhang H. Amorphous calcium phosphate and its application in dentistry. Chem Cent J. 2011;5(01):40. doi: 10.1186/1752-153X-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yassaei S, Aghili H, Shahraki N, Safari I. Efficacy of erbium-doped yttrium aluminum garnet laser with casein phosphopeptide amorphous calcium phosphate with and without fluoride for remineralization of white spot lesions around orthodontic brackets. Eur J Dent. 2018;12(02):210–216. doi: 10.4103/ejd.ejd_265_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Liu Y, Sun W-B, Yang X. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J Dent Sci. 2012;7(04):316–323. [Google Scholar]
- 28.Cantaert B, Beniash E, Meldrum F C. The role of poly(aspartic acid) in the precipitation of calcium phosphate in confinement. J Mater Chem B Mater Biol Med. 2013;1(48):6586. doi: 10.1039/C3TB21296C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uysal T, Amasyali M, Koyuturk A E, Sagdic D. Efficiency of amorphous calcium phosphate-containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Eur J Dent. 2009;3(02):127–134. [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis K N, Thomas M V, Puleo D A. Mechanical and degradation behavior of polymer-calcium sulfate composites. J Mater Sci Mater Med. 2006;17(06):531–537. doi: 10.1007/s10856-006-8936-0. [DOI] [PubMed] [Google Scholar]
- 31.Ding S J. Preparation and properties of chitosan/calcium phosphate composites for bone repair. Dent Mater J. 2006;25(04):706–712. doi: 10.4012/dmj.25.706. [DOI] [PubMed] [Google Scholar]
- 32.Meyer F, Amaechi B T, Fabritius H-O, Enax J. Overview of calcium phosphates used in biomimetic oral care. Open Dent J. 2018;12(01):406–423. doi: 10.2174/1874210601812010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pakravanan K, Rezaee Roknabadi M, Farzanegan F, Hashemzadeh A, Darroudi M. Amorphous calcium phosphate nanoparticles-based mouthwash: preparation, characterization, and anti-bacterial effects. Green Chem Lett Rev. 2019;12(03):278–285. [Google Scholar]
- 34.Wang Y W, Kim Y Y, Christenson H K, Meldrum F C. A new precipitation pathway for calcium sulfate dihydrate (gypsum) via amorphous and hemihydrate intermediates. Chem Commun (Camb) 2012;48(04):504–506. doi: 10.1039/c1cc14210k. [DOI] [PubMed] [Google Scholar]
- 35.Gomes C EM, Sousa A KD, da Silva OliveiraAraujo M E, Ferreira S B, Fontanini P. Mechanical and microstructural properties of redispersible polymer-gypsum composites. Mater Res. 2019;22(03):1–9. doi: 10.1590/1980-5373-MR-2018-0119. [DOI] [Google Scholar]
- 36.Kim H J, Mo S Y, Kim D S. Effect of bioactive glass-containing light-curing varnish on enamel remineralization. Materials (Basel) 2021;14(13):1–13. doi: 10.3390/ma14133745. [DOI] [PMC free article] [PubMed] [Google Scholar]


