Abstract
Objective
To review cases of acute macular neuroretinopathy (AMN) after COVID-19 vaccination and add a similar case to the literature.
Methods
A thorough PubMed search was conducted, and data from studies describing AMN after COVID-19 vaccination were extracted, tabulated, pooled, and reviewed.
Results
We present a case of AMN in a young woman 5 days after immunization with the BBIBP-CorV (Sinopharm) COVID-19 vaccine. Data from 21 cases were pooled and reviewed. The most frequent vaccines among the cases were recombinant ones (13/21), followed by mRNA-based (6/21) and inactivated vaccines (2/21). Only one patient (5%) was male. Seventeen over twenty-one (81%) were young women, ages 18–33. Most cases (14/21; 67%) reported recent/concurrent use of contraceptive medication. In 90% of cases (19/21), symptoms appeared within 8 days of vaccination. A confined wedge-/oval-shaped lesion morphology was more frequent than a diffuse, semilunar one. Resolution of symptoms took 4 to over 15 weeks.
Conclusion
Attention should be paid to the history of vaccination and contraceptive use in patients with sudden-onset visual symptoms. Optical coherence tomography is integral to the detection of AMN-related abnormalities.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Acute macular neuroretinopathy, Oral contraceptives
Résumé
Objectif
Examiner les cas de neurorétinopathie maculaire aiguë (AMN) après la vaccination contre la COVID-19 et ajouter un cas similaire à la littérature.
Méthodes
Une recherche approfondie sur PubMed a été menée et les données des études décrivant l’AMN après la vaccination contre la COVID-19 ont été extraites, regroupées et examinées.
Résultats
Nous présentons un cas d’AMN chez une jeune femme 5 jours après l’immunisation avec le vaccin BBIBP-CorV (Sinopharm) COVID-19. Les données de 21 cas ont été regroupées et examinées. Les vaccins les plus fréquents parmi les cas étaient les vaccins recombinants (13/21), suivis des vaccins à base d’ARNm (6/21) et des vaccins inactivés (2/21). Un seul patient (5 %) était de sexe masculin. 17/21 (81 %) étaient de jeunes femmes âgées de 18 à 33 ans. La plupart des cas (14/21 ; 67 %) avaient utilisé récemment ou simultanément des médicaments contraceptifs. Dans 90 % des cas (19/21), les symptômes sont apparus dans les 8 jours suivant la vaccination. Une morphologie lésionnelle confinée cunéiforme/ovale était plus fréquente qu’une morphologie semi-lunaire diffuse. La résolution des symptômes peut prendre de 4 à plus de 15 semaines.
Conclusions
Une attention particulière doit être portée aux antécédents de vaccination et d’utilisation de contraceptifs chez les patients présentant des symptômes visuels d’apparition soudaine. La tomographie par cohérence optique fait partie intégrante de la détection des anomalies liées à l’AMN.
Mots clés: COVID-19, SARS-CoV-2, Vaccination, Neurorétinopathie maculaire aiguë, Contraceptifs oraux
Introduction
Mass COVID-19 vaccination programs are still underway to help further immunize at-risk populations and boost the limited/waning protection provided by previous vaccine doses; there are concerns regarding newer SARS-CoV-2 variants of unknown infectivity and virulence [1], [2]. Among WHO-approved vaccines, the BBIBP-CorV COVID-19 vaccine (Sinopharm, China) is the most frequently injected in Iran [3], [4]. It is an inactivated SARS-CoV-2 vaccine and has shown acceptable safety and efficacy [5]. However, some infrequent adverse events after immunization are not detected until vaccines are used widely enough for those events to occur and be documented – Mainly in the form of sporadic reports and observations.
Several cases of ocular adverse events after COVID-19 vaccination have been reported, including acute macular neuroretinopathy (AMN), ophthalmic vein thrombosis, corneal graft rejection, and uveitis [6]. AMN is a rare retinal disease of presumed vascular etiology, typically affecting young females. It presents through acute-onset unilateral/bilateral paracentral scotomata, often preceded by non-specific flu-like symptoms [7]. We reviewed and pooled the data from AMN cases following COVID-19 vaccination and added to the existing literature the case of a young female who developed flu-like symptoms after vaccination and noticed bilateral scotomas shortly afterward.
Methods
PubMed database was searched using a query consisting of the following terms: “acute macular neuroretinopathy”, “AMN”, “COVID-19”, “SARS-CoV-2”, “COVID”, “vaccination”, “vaccine”, and “immunization”. The reference lists of the obtained records were manually searched for additional reports. Articles reporting one/multiple cases of AMN in individuals with recent vaccination against SARS-CoV-2 were included; no inclusion limitation regarding language, publication date, study design/method was set. Extracted data were patients’ demographic information, vaccine type (manufacturer), drug history, background condition, vaccination-to-symptoms time interval, presenting symptoms, findings on imaging studies, and outcome.
Results
Case presentation
An 18-year-old female with a complaint of reading problem was referred for retinal evaluation. Three weeks before our visit, she had taken her first dose of the BBIBP-CorV vaccine (Sinopharm). A day later, she experienced flu-like symptoms – Including headache, fatigue, fever, and chills. Four days later, she noticed some spots of visual defects while reading books. Her medical history was unremarkable. She denied having contracted COVID-19.
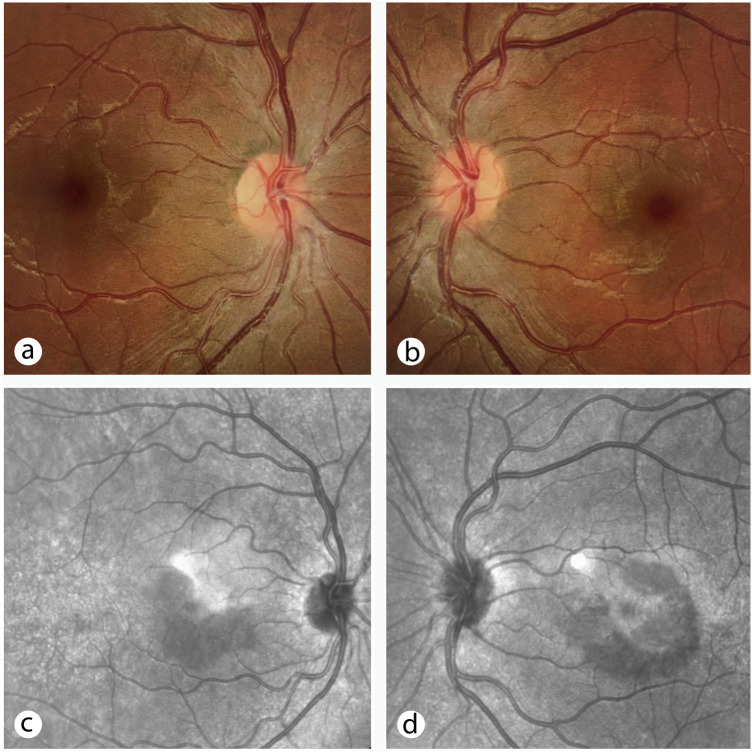
Her clinical examination showed 20/20 visual acuity (OU). Upon slit lamp examination and fundus photography, hypopigmented perifoeval areas (OU) were detected (Fig. 1a and b ). Ishihara test was normal, and no relative afferent pupillary defect was found. No anterior segment abnormality was detected. A Humphrey Visual Field 10-2 indicated paracentral scotomas in both eyes. Infrared reflectance (IR) imaging showed two perifoveal hyporeflective lesions (OD) – One superotemporal (wedge-shaped) and the other inferior and inferotemporal to the fovea (Fig. 1c, Fig. 2a ) – And a diffuse perifoveal semi-circular hyporeflective lesion (OS) extending from the inferonasal axis to the superotemporal axis (Fig. 1d, Fig. 2d). Cross-sectional spectral-domain OCT (SD-OCT) showed OPL thickening, ONL thinning, and disruption of the ellipsoid zone in areas corresponding to the lesions (OU) (Fig. 2b and e). OCT angiography indicated no flow abnormality at superficial/deep retinal vasculature or choriocapillaris. Fluorescein angiography revealed no vascular pathology.
Figure 1.
Baseline color fundus photographs (a and b) showing perifoveal areas of hypopigmentation, corresponding to hyporeflective lesions seen on infrared reflectance images (c and d).
Figure 2.
Cross-sectional optical coherence tomography scans at baseline (b and e; OD and OS), showing areas of outer plexiform layer thickening, outer nuclear layer thinning, and disruption of ellipsoid zone, and upon 2-week follow-up visit (c and f; OD and OS), showing partial improvement of abnormalities (c and f; OD and OS), corresponding to the section shown on en face scans (a and d; OD and OS, respectively).
A diagnosis of AMN was made, and the patient was put on oral prednisolone 25 mg/day for ten days. On the follow-up visit, 14 days later, the visual field test indicated improvement of scotomas in both eyes; notably, the patient stated that her perceived visual disturbance had resolved. SD-OCT scan also showed partial improvement at the pathology sites (Fig. 2c and f).
Search results
In the literature, we found 18 articles that together reported 20 cases of AMN in people with recent COVID-19 vaccination. Patients’ demographic information, vaccine type (manufacturer), drug history, background condition(s), vaccination-to-symptoms interval, presenting symptoms, findings on imaging studies, and outcome were extracted and summarized in Table 1 .
Table 1.
Identified cases of acute macular neuroretinopathy after COVID-19 vaccination in the literature.
| Case No./Author | Age/sex | Vaccine | Drug history/background illness | Interval (days) | Presenting symptoms | Imaging features | Outcome |
|---|---|---|---|---|---|---|---|
| #1 Fekri et al. | 18/F | BBIBP-CorV [Sinopharm] | Unremarkable | 5 | Bilateral paracentral scotomas | IR: wedge-shaped (OD) and diffuse semi-circular (OS) perifoveal lesions | Subjective resolution of visual defects and partial improvement of lesions on OCT and IR images at 2 weeks follow-up |
| OCT: OPL thickening – ONL thinning – EZ disruption | |||||||
| #2 Book et al. [31] | 21/F | AZD1222 [AstraZeneca] | OCP/– | 3 | Bilateral paracentral scotomas | IR: Bilateral confined paracentral lesions | NR |
| OCT: OPL thickening – EZ disruption | |||||||
| #3 Drüke et al. [17] | 23/F | AZD1222 [AstraZeneca] | OCP, steroids/JIA & recurrent iritis | 1 | Bilateral paracentral scotomas | IR: Bilateral confined parafoeveal lesions | Despite initial improvement, scotomas persisted with slow regression of lesion (in 15 weeks) |
| OCT: OPL thickening – ONL thinning – Disruption of EZ, IZ, and ELM | |||||||
| #4 Girbardt et al. [25] | 21/F | AZD1222 [AstraZeneca] | – | 3 | Paracentral scotoma (OS) | IR: Bilateral confined paracentral lesions | NR |
| OCT: subtle outer retinal alterations, more pronounced in the left eye | |||||||
| #5 Mambretti et al. [16] | 22/F | AZD1222 [AstraZeneca] | OCP/– | 2 | Scotoma (OD) | IR: Tear drop-shaped perifoveal lesions | NR |
| OCT: hyperreflectivity of OPL and ONL – EZ disruption | |||||||
| #6 Mambretti et al. [16] | 28/F | AZD1222 [AstraZeneca] | OCP/– | 2 | Paracentral scotoma (OD) | IR: wedge-shaped lesion in papillo-macular bundle | NR |
| OCT: hyperreflectivity of OPL and ONL – EZ attenuation | |||||||
| #7 Pichi et al. [18] | NR | BBIBP-CorV [Sinopharm] | CSCR (OU)–chronic serous PED (OS)–Choroidal thickness | 5 | Acute vision loss (OS) | OCT: hyperreflectivity of OPL, HFL, and ONL – EZ attenuation | BCVA improvement & resolution of OCT findings (at 2 months follow-up) |
| OCTA: Semilunar area of signal absence at DCP | |||||||
| #8/Valenzuela et al. [26] | 20/F | BNT162b2 [Pfizer-BioNTech] | CVR/– | 2 | Photopsia and bilateral scotomas | OCT: parafoveal hyperreflective foci at ONL–EZ granularity | Complete resolution of scotomas (at 1 week follow-up) |
| #9 Bøhler et al. [29] | 27/F | AZD1222 [AstraZeneca] | OCP/– | 2 | Paracentral scotoma (OS) | OCT: Teardrop-shaped macular lesion (en face view)/hyperreflectivity of OPL and ONL – EZ disruption (cross-sectional view) | NR |
| #10 Chen et al. [32] | 21/F | BNT162b2 [Pfizer-BioNTech] | OCP/– | 3 | Paracentral scotoma (OS) | IR: 2 parafoeval oval hyporeflective lesions (OS) | Gradual reduction of scotoma intensity over 10 weeks |
| OCT: focal areas of paracentral OPL and ONL hyperreflectivity – EZ disruption | |||||||
| #11 Diafas et al. [19] | 54/M | BNT162b2 [Pfizer-BioNTech] | NR/T2DM, low myopia | 21 | Photopsia and small scotoma (OS) | OCT: OPL hyperreflective band – EZ disruption | Remained symptomatic at 2 months follow-up |
| #12 Franchi et al. [20] | 19/F | AZD1222 [AstraZeneca] | OCP (DC 2 months before presentation)/asthma | 1 | fortification (OS) | NIR: multiple wedge-shaped hyporeflective lesions (OU) | Persisting visual symptoms and photoreceptor layer derangement, despite resolution of hyperreflective bands, at 8 weeks follow-up |
| OCT: parafoveal OPL & ONL hyperreflective bands – EZ disruption (OU) | |||||||
| #13 Franchi et al. [20] | 31/F | AZD1222 [AstraZeneca] | OCP/– | 2 | fortification and paracentral scotoma (OD) | NIR: small wedge-shaped hyporeflective lesions (OU) | Persisting visual symptoms and photoreceptor layer derangement, despite resolution of hyperreflective bands, at 15 weeks follow-up |
| OCT: OPL & ONL hyperreflective bands – EZ disruption (OU) | |||||||
| #14 Gabrielle et al. [33] | 25/F | AZD1222 [AstraZeneca] | OCP/– | 1 | Bilateral paracentral scotomas | NIR: multiple hyporeflective, wedge-shaped, parafoveal lesions | NR |
| OCT: OPL hyperreflectivity and thickening – ONL thinning – Disruption of EZ | |||||||
| #15 Michel et al. [34] | 21/F | AZD1222 [AstraZeneca] | OCP/– | 2 | 4 central scotomas (OS) | NIR: multiple hyporeflective, oval-shaped lesions around the fovea (OS) | Improvement of symptoms, visual field, and OCT biomarkers at 6 weeks follow-up |
| OCT: foci of OPL and ONL hyperreflectivity – ONL thinning – EZ disruption | |||||||
| #16 Ishibashi et al. [21] | 33/F | BNT162b2 [Pfizer-BioNTech] | NR/HTN, Alport syndrome, ESRD | 8 | Visual field defect (OS) | OCT: hyperreflective foci of OPL and ONL – EZ disruption OCTA: signal absence at DCP in the corresponding area |
NR |
| #17 Patel et al. [35] | 26/F | Ad26.COV2.S [Janssen] | OCP/– | 2 | Bilateral paracentral scotomas | NIR: wedge-shaped parafoveal lesions (OS) | NR |
| OCT: parafoveal hyperreflective bands w/o retinal thickening (OU) | |||||||
| #18 Priluck et al. [22] | 20/F | mRNA-1273 [Moderna] | OCP, fluticasone, loratadine/allergic rhinitis, myopia | 8 | Bilateral central scotomas | NIR: hyporeflective wedge-shaped lesions (OU) | Progressive subjective improvement of scotomas w/o complete resolution |
| OCT: EZ disruption at corresponding lesion locations | |||||||
| #19 Sanjay et al. [23] | 25/F | AZD1222 [AstraZeneca] | -/β thalassemia trait | 3 | Blurry vision and Shadow (OD) – one week later, a new scotoma was noticed | NIR: normal | Resolution of AMN (OD) and development of Similar symptoms and findings (OS) a month later |
| OCT: hyperreflective area at OPL and ONL | |||||||
| #20 Zaheer et al. [36] | 22/F | AZD1222 [AstraZeneca] | NA | < 7 | NA | NA | NA |
| #21 Jalink et al. [24] | 42/F | mRNA-1273 [Moderna] | OCP/- | NAa | Barely noticeable scotoma, temporal to the center (OS) | OCT: subtle changes of the ONL and photoreceptor layers | Scotoma was still present but had diminished in size (at 1-month follow-up) |
AMN: acute macular neuroretinopathy; BCVA: best-corrected visual acuity; CSCR: central serous chorioretinopathy; CVR: contraceptive vaginal ring; DC: discontinued; DCP: deep capillary plexus; ELM: external limiting membrane; ESRD: end-stage renal disease; EZ: ellipsoid zone; F: female; HFL: Henle fiber layer; HTN: hypertension; IR: infrared reflectance; IZ: interdigitation zone; JIA: juvenile idiopathic arthritis; M: male; NA: not available; NIR: near-infrared reflectance; NR: not reported; OCP: oral contraceptive pills; OCT: optical coherence tomography; OCTA: optical coherence tomography angiography; OD: oculus dexter; ONL: outer nuclear layer; OPL: outer plexiform layer; OS: oculus sinister; OU: oculus uterque; PED: pigment epithelial detachment; T2DM: type 2 diabetes mellitus.
The exact time of symptom onset was unclear, but the time from her last vaccine dose to realizing her visual field defect was ∼ 45 days; her scotoma was hard to perceive and would be noticed only when she closed her right eye.
Discussion
AMN etiopathogenesis
The pathophysiology of AMN remains to be elucidated. Environmental triggers most frequently associated with AMN are non-specific flu-like symptoms, oral contraceptive use, epinephrine/ephedrine exposure, trauma, dehydration, and hypovolemia [7]. The retinal layers at which AMN-related structural pathologies are distributed (i.e., OPL, ONL, EZ) implies a vascular compromise and/or ischemic insult occurring at the deep capillary plexus (DCP) to be involved in AMN pathogenesis; each of the triggers above may induce such microvasculopathies in its own way [7].
AMN and COVID-19
Several cases of AMN have been reported during or after the COVID-19 infection course, with or without concurrent paracentral acute middle maculopathy (PAMM) [8], [9], [10]. AMN can also surface as the first manifestation of COVID-19 infection [11]. The increased number of AMN cases identified in some ophthalmic care settings during the pandemic, compared to the pre-COVID era, raised suspicion of a causal link [12]. Viral illnesses have already been associated with AMN [7], and COVID-19 infection can impair the retinal microvasculature through mid- to long-term processes [13], [14], further potentiating speculations on its association with AMN [15].
Pooled data from AMN cases after COVID-19 Vaccination
Rarely has AMN been reported after vaccination against pathogens other than SARS-CoV-2 (e.g., H1N1 and Neisseria meningitides) [16]. Amid the ongoing COVID-19 vaccination programs worldwide, reports of AMN after immunization have recently been described (Table 1).
Vaccine type
From the 21 cases described, 12 (57%) had taken the AZD1222 (AstraZeneca) and 1 (5%) the Ad26.COV2.S recombinant vaccines, 4 (19%) had received the BNT162b2 (Pfizer-BioNTech) and 2 (9%) the mRNA-1273 (Moderna) mRNA-platform vaccines, and 2 (9%; including our patient) had taken the BBIBP-CorV (Sinopharm) inactivated vaccine.
Demographic information and background conditions
Only one patient (5%) was male; 17 (81%) were young females aging between 18–33. Fourteen (67%) had concurrent/recent use of contraceptive medication (oral pills or vaginal rings).
Thirteen (62%) had no remarkable past medical history; among the background conditions reported in cases were juvenile idiopathic arthritis [17], recurrent iritis [17], choroidal thickness (and a history of central serous chorioretinopathy) [18], type 2 diabetes mellitus [19], asthma [20], hypertension and end-stage renal disease [21], allergic rhinitis [22], myopia [19], [22], and β thalassemia trait [23].
Time from vaccination to symptom onset
In most cases (90%; 19/21), the onset of symptoms was less than 8 days apart from the vaccination day, except for a 54-year-old male in whom symptoms manifested 21 days after immunization (case No. 10) [19] and a 42-year-old female whose scotoma was so peripheral that it was barely detectable – She first noticed it on day 45th after vaccination (case No 21) [24].
Imaging findings
The morphology of lesions showed variation among cases; bilateral lesions in our case showed both circumscribed, wedge-shaped, and diffuse, semi-circular patterns. The most frequent lesion morphology was confined wedge-/oval-shaped; a diffuse macular lesion was rarely documented (cases 1 and 7 [18]).
In all cases, SD-OCT was able to detect abnormal structural findings – In some instances, even in the fellow eyes, from which no subjective visual defect was perceived [20], [25]. SD-OCT, in conjunction with near-infrared reflectance (NIR), is suggested as the modality of choice in the detection of AMN-related abnormalities not spotted upon clinical examination and color fundus photography (7).
Outcome
Only twelve reports included follow-up data. Follow-up periods were quite heterogeneous, and so were the outcomes and the time it took for scotomas to resolve. While scotomas became subjectively unnoticeable within 4 weeks in some cases (e.g., cases No. 1 and 8 [26]), some patients were still symptomatic at 15 weeks follow-up (e.g., cases No. 3 [17], 13 [20]) – These were the two ends of the spectrum.
Causality assessment and possible pathomechanisms
The Naranjo probability scale may prove helpful in evaluating adverse drug reactions more objectively [27]. Based on ten criteria, a probability score is assigned (≥ 9, definite; 5–8, probable; 1–4, possible, and ≤ 0, doubtful). Our case was assigned a score of 7, based on the criteria below, and thus, deemed a “probable” vaccine-associated event:
-
•
“Are there previous conclusive reports on this reaction?” – Yes (+ 1);
-
•
“Did the adverse event appear after the suspected drug was administered?” – Yes (+ 2);
-
•
“Did the adverse event improve when the drug was discontinued, or a specific antagonist was administered?” – Yes (+ 1); the patient refrained from the second dose vaccination and has experienced no further retinal ischemic event;
-
•
“Are there alternative causes that could on their own have caused the reaction?” – No (+ 2);
-
•
“Was the adverse event confirmed by any objective evidence?” – Yes (+ 1).
Naranjo scores of all cases reviewed in Table 1 are interpreted as either “possible” or “probable” and cannot exceed 8 because some algorithm criteria are not applicable in this context. For instance, “dose adjustment”, “toxic serum concentrations”, “past exposure to similar drugs”, and “placebo administration” are not relevant concepts in the case of COVID-19 vaccines administered routinely.
However, hormonal contraception bears an established risk of microvascular endothelial dysfunction [28], and a positive history of OCP use, present in 14/21 (67%) of the reviewed cases, may argue against pointing the finger at vaccines with certainty [16]. On the other hand, some explanatory mechanisms are proposed regarding how vaccines may be associated with microvasculopathic events, as discussed below.
Bøhler et al. suspected vaccine-induced thrombotic thrombocytopenia (VITT) in their patient [29]. VITT has recently been described as primarily associated with the AZD1222 adenoviral vector-based vaccine. Like heparin-induced thrombocytopenia, it involves autoantibodies that activate platelets and induce thrombotic events in arterial or venous circulations, ultimately resulting in consumptive coagulopathy [30]. However, the clinical picture of VITT is discordant with AMN; it often occurs around 14 days after inoculation (the time needed for seroconversion) and most often involves simultaneous large vessel thromboses at different sites (e.g., cerebral venous sinus thrombosis, deep vein thrombosis, and pulmonary embolism) [30]. Bøhler et al. found no serological evidence of VITT [29]. Other more plausible mechanisms include induction of an inflammatory milieu by the adenoviral vector, giving rise to arterial endothelial vasomotor dysfunction and/or worsening of an already-thrombophilic status (because of hormonal contraceptive use) [16].
Conclusion
Vaccination remains our most effective means of protection against emerging SARS-CoV-2 variants, and one would expect further doses to be advised in the near future. Thus, the number of reported AMN cases in the temporal proximity of vaccination may continue to grow. Regardless of a causative association, it is necessary to pay attention to COVID-19 vaccination and OCP use history in patients with sudden-onset visual symptoms, such as scotomas. In many instances, clinical examination and fundus color photography may be of lower diagnostic yield than SD-OCT and NIR in diagnosing AMN.
Disclosure of interest
The authors declare that they have no competing interest.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
The authors thank the patient for cooperating and allowing the publication of her case.
References
- 1.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Me. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magen O., Waxman J.G., Makov-Assif M., Vered R., Dicker D., Hernán M.A., et al. Fourth dose of BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azadmanesh K. Iran hopes to defeat COVID with home-grown crop of vaccines [Internet]. 2021 [cited 2022 Jul 17]. Available from: https://www.nature.com/articles/d41586-021-02216-z.
- 4.Hosseinzadeh A., Sahab-Negah S., Nili S., Aliyari R., Goli S., Fereidouni M., et al. COVID-19 cases, hospitalizations and deaths after vaccination: a cohort event monitoring study, Islamic Republic of Iran. Bull World Health Organ. 2022;100:474–483. doi: 10.2471/BLT.22.288073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al Kaabi N., Zhang Y., Xia S., Yang Y., Al Qahtani M.M., Abdulrazzaq N., et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ng X.L., Betzler B.K., Testi I., Ho S.L., Tien M., Ngo W.K., et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216–1224. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhavsar K.V., Lin S., Rahimy E., Joseph A., Freund K.B., Sarraf D., et al. Acute macular neuroretinopathy: a comprehensive review of the literature. Surv Ophthalmol. 2016;61:538–565. doi: 10.1016/j.survophthal.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Virgo J., Mohamed M. Paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye (Lond) 2020;34:2352–2353. doi: 10.1038/s41433-020-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascon P., Briantais A., Bertrand E., Ramtohul P., Comet A., Beylerian M., et al. COVID-19-Associated retinopathy: a case report. Ocul Immunol Inflamm. 2020;28:1293–1297. doi: 10.1080/09273948.2020.1825751. [DOI] [PubMed] [Google Scholar]
- 10.Zamani G., Ataei Azimi S., Aminizadeh A., Shams Abadi E., Kamandi M., Mortazi H., et al. Acute macular neuroretinopathy in a patient with acute myeloid leukemia and deceased by COVID-19: a case report. J Ophthalmic Inflamm Infect. 2021;10:39. doi: 10.1186/s12348-020-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preti R.C., Zacharias L.C., Cunha L.P., Monteiro M.L.R. Acute macular neuroretinopathy as the presenting manifestation of COVID-19 infection. Retin Cases Brief Rep. 2022;16:12–15. doi: 10.1097/ICB.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed W., Suri A., Ahmed A. COVID-19 and acute macular neuroretinopathy – An underlying association? Ann Med Surg. 2022;78:103847. doi: 10.1016/j.amsu.2022.103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S., Wang J., Hu J., Wang N. Retinal microvascular impairment in COVID-19 patients: a meta-analysis. Immun Inflamm Dis. 2022;10:e619. doi: 10.1002/iid3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nourinia R., Ghassempour M., Ahmadieh H., Abtahi S.H. Branch retinal vein occlusion after COVID-19. J Fr Ophtalmol. 2021;44:e441–e443. doi: 10.1016/j.jfo.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capuano V., Forte P., Sacconi R., Miere A., Mehanna C.J., Barone C., et al. Acute macular neuroretinopathy as the first stage of SARS-CoV-2 infection. Eur J Ophthalmol. 2022;112067212210906 doi: 10.1177/11206721221090697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mambretti M., Huemer J., Torregrossa G., Ullrich M., Findl O., Casalino G. Acute Macular Neuroretinopathy following Coronavirus Disease 2019 vaccination. Ocul Immunol Inflamm. 2021;29:730–733. doi: 10.1080/09273948.2021.1946567. [DOI] [PubMed] [Google Scholar]
- 17.Drüke D., Pleyer U., Hoerauf H., Feltgen N., Bemme S. Acute macular neuroretinopathy (AMN) following COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101207. doi: 10.1016/j.ajoc.2021.101207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichi F., Aljneibi S., Neri P., Hay S., Dackiw C., Ghazi N.G. Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. 2021;139:1131–1135. doi: 10.1001/jamaophthalmol.2021.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diafas A., Ghadiri N., Beare N., Madhusudhan S., Pearce I., Tan S.Z. Comment on: paracentral acute middle maculopathy and acute macular neuroretinopathy following SARS-CoV-2 infection. Eye. 2022;36:1507–1509. doi: 10.1038/s41433-021-01709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franchi A., Rauchegger T., Palme C., Frede K., Haas G., Blatsios G., et al. Two cases of acute macular neuroretinopathy associated with the adenovirus-based COVID-19 vaccine Vaxzevria (Astrazeneca) Ocul Immunol Inflamm. 2022;20:1–6. doi: 10.1080/09273948.2022.2027463. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi K., Yatsuka H., Haruta M., Kimoto K., Yoshida S., Kubota T. Branch retinal artery occlusions, paracentral acute middle maculopathy and acute macular neuroretinopathy after COVID-19 vaccinations. OPTH. 2022;16:987–992. doi: 10.2147/OPTH.S357359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Priluck A.Z., Arevalo J.F., Pandit R.R. Ischemic retinal events after COVID-19 vaccination. Am J Ophthalmol Case Rep. 2022;26:101540. doi: 10.1016/j.ajoc.2022.101540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanjay S., Gadde S.G.K., Kumar Yadav N., Kawali A., Gupta A., Shetty R., et al. Bilateral sequential acute macular neuroretinopathy in an Asian Indian female with β Thalassemia trait following (Corona Virus Disease) COVID-19 vaccination and probable recent COVID infection- multimodal imaging study. Ocul Immunol Inflamm. 2022;20:1–6. doi: 10.1080/09273948.2022.2026978. [DOI] [PubMed] [Google Scholar]
- 24.Jalink M.B., Bronkhorst I.H.G. A sudden rise of patients with acute macular neuroretinopathy during the COVID-19 pandemic. Case Rep Ophthalmol. 2022;13:96–103. doi: 10.1159/000522080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girbardt C., Busch C., Al-Sheikh M., Gunzinger J.M., Invernizzi A., Xhepa A., et al. Retinal vascular events after mRNA and adenoviral-vectored COVID-19 vaccines – A case series. Vaccines (Basel) 2021;9:1349. doi: 10.3390/vaccines9111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valenzuela D.A., Groth S., Taubenslag K.J., Gangaputra S. Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. 2021;24:101200. doi: 10.1016/j.ajoc.2021.101200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naranjo C.A., Busto U., Sellers E.M., Sandor P., Ruiz I., Roberts E.A., et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 28.Williams J.S., MacDonald M.J. Influence of hormonal contraceptives on peripheral vascular function and structure in premenopausal females: a review. Am J Physiol Heart Circ Physiol. 2021;320:H77–H89. doi: 10.1152/ajpheart.00614.2020. [DOI] [PubMed] [Google Scholar]
- 29.Bøhler A.D., Strøm M.E., Sandvig K.U., Moe M.C., Jørstad Ø.K. Acute macular neuroretinopathy following COVID-19 vaccination. Eye. 2022;36:644–645. doi: 10.1038/s41433-021-01610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klok F.A., Pai M., Huisman M.V., Makris M. Vaccine-induced immune thrombotic thrombocytopenia. Lancet Haematol. 2022;9:e73–e80. doi: 10.1016/S2352-3026(21)00306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Book B.A.J., Schmidt B., Foerster A.M.H. Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. 2021;139:e212471. doi: 10.1001/jamaophthalmol.2021.2471. [DOI] [PubMed] [Google Scholar]
- 32.Chen S., Hodge C. Comment on: acute macular neuroretinopathy following COVID-19 vaccination. Eye. 2022;36:1513–1514. doi: 10.1038/s41433-021-01781-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrielle P.H., Baudin F., Ben Ghezala I., Meillon C., Bron A.M., Arnould L., et al. Bilateral acute macular neuroretinopathy in a young woman after the first dose of Oxford-AstraZeneca COVID-19 vaccine. Am J Ophthalmol Case Rep. 2022;25:101281. doi: 10.1016/j.ajoc.2022.101281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michel T., Stolowy N., Gascon P., Dupessey F., Comet A., Attia R., et al. Acute macular neuroretinopathy after COVID-19 vaccine. J Fr Ophtalmol. 2022;S0181-5512:00162. doi: 10.1016/j.jfo.2022.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel S.N., Yonekawa Y. Acute macular neuroretinopathy after SARS-COV-2 vaccination. Retin Cases Brief Rep. 2022;16:5–8. doi: 10.1097/ICB.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 36.Zaheer N., Renju M.P., Chavan R. Acute macular neuroretinopathy after COVID-19 vaccination. Retin Cases Brief Rep. 2022;16:9–11. doi: 10.1097/ICB.0000000000001196. [DOI] [PubMed] [Google Scholar]