Abstract
Plasmodium falciparum malaria is an important cause of morbidity and mortality in children. Factors that determine the development of mild versus severe malaria are not fully understood. Since host-derived nitric oxide (NO) has antiplasmodial properties, we measured NO production and NO synthase (NOS) activity in peripheral blood mononuclear cells (PBMC) from healthy Gabonese children with a history of prior mild malaria (PMM) or prior severe malaria (PSM) caused by P. falciparum. The PMM group had significantly higher levels of NOS activity in freshly isolated PBMC and higher NO production and NOS activity in cultured PBMC. The investigation of NO-modulating cytokines (e.g., interleukin 12, gamma interferon, tumor necrosis factor alpha [TNF-α], and transforming growth factor β1) as an explanation for differing levels of NOS activity revealed that plasma levels of TNF-α were significantly higher in the PSM group. Our results suggest that NOS/ NO and TNF-α are markers for prior disease severity and important determinants of resistance to malaria.
Malaria occurs at a rate of 300 to 500 million infections per year, resulting in 1.5 to 2.7 million deaths, with the highest mortality in children less than 5 years of age (32). In areas where Plasmodium falciparum is hyperendemic, such as Lambaréné, Gabon, adults develop a semi-immune state following frequent episodes of malaria during childhood (31). However, P. falciparum malaria in children (who are largely nonimmune) may lead to severe malaria with hyperparasitemia and clinical complications such as severe anemia, hypoglycemia, and cerebral malaria (33). The molecular determinants that regulate the development of mild versus severe P. falciparum malaria are largely unknown, with the possible exception of the sickle cell trait.
The ability of the human host to produce nitric oxide (NO) may be important for regulating disease severity in malaria. A protective role for NO in malaria is supported by results from in vitro studies and reports on experimental malaria (8, 11, 19, 20, 22, 25). Studies in Gabonese adults and children show that levels of NO metabolites (nitrite plus nitrate [NOx]) in plasma increase with disease severity and are associated with accelerated clinical cure and parasitological clearance (12). Levels of NOx in plasma and urine and expression of inducible nitric oxide synthase type 2 (NOS2) in peripheral blood mononuclear cells (PBMC) are inversely related to disease severity in Tanzanian children infected with P. falciparum (2).
NO production during a malarial infection is likely regulated by a balance of pro- and anti-inflammatory cytokines. While proinflammatory cytokines (e.g., interleukin 12 [IL-12], gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) generally increase NOS2-derived NO synthesis during an inflammatory response, anti-inflammatory cytokines (e.g., transforming growth factor β1 [TGF-β1] and IL-10) decrease NOS2-promoted NO production (21, 27). In addition, there are also reciprocal interactions between cytokines and NO production in which NO can influence cytokine secretion. The development of endotoxin-induced desensitization in murine macrophages is characterized by increased formation of NO that decreases TNF-α production (3). The down-regulatory effect of NO on TNF-α synthesis has also been established in animal models of endotoxemia (4, 9, 26).
To further investigate the role of NO in the pathogenesis of malaria, we measured baseline and cytokine-promoted NO production and NOS enzyme activity in cultured PBMC and NOS activity in freshly isolated (noncultured) PBMC from healthy Gabonese children with a history of either mild or severe P. falciparum malaria. Levels of cytokines that might regulate NO production (i.e., IL-12, IFN-γ, TNF-α, and TGF-β1) in plasma were also analyzed.
Study participants.
All participants (n = 28; age, 2 to 8 years) were healthy and were recruited from an ongoing longitudinal prospective study at the Albert Schweitzer Hospital in Lambaréné, Gabon, an area where P. falciparum malaria is endemic (13). Subjects were divided into two groups: children with prior mild malaria (PMM) (n = 18; nine males and nine females; mean age of 6 years 5 months) and children with prior severe malaria (PSM) (n = 10; six males and four females; mean age of 6 years 4 months). During the malarial episode, the classification of mild and severe malaria was defined according to World Health Organization guidelines with inclusion criteria for severe cases including hyperparasitemia (>250,000 parasites/μl) and/or severe anemia (hemoglobin level, <5.0 g/dl; packed cell volume, <25%) (33). None of the participants had previously suffered from cerebral malaria. After physical examination, a thick blood smear was used to verify that participants were parasite free. Healthy children were selected for the present study because differences detected between the two groups of children during an active malarial infection may reflect differences in the disease state rather than underlying host immunologic factors. Subjects were excluded from the study if they had a thick blood film positive for malaria or if they had experienced malaria or any other severe illnesses within the last 4 months. Children were selected so that the number of previous malarial infections and the time from the last malarial infection were equivalent in the two groups. Informed consent was obtained from the parents of participating children. This study was approved by the ethics committee of the International Foundation of the Albert Schweitzer Hospital and the Duke University Medical Center Investigational Review Board.
NO production and NOS enzyme activity in cultured PBMC.
To determine if NO production differs in children who develop mild versus severe malaria, venous blood (5 ml) was drawn from the two groups of children into EDTA-containing vials, plasma was separated, and PBMC were prepared by using Ficoll-Hypaque as described earlier (30). Freshly isolated mononuclear cells were plated at a density of 1.5 × 106 per well into 48-well tissue culture plates in 0.5 ml of Dulbecco’s modified Eagle medium containing 10% pooled human serum (heat inactivated at 56°C for 30 min). Cells were cultured for 7 days with IFN-α (50 U/ml; Schering-Plough, Kenilworth, N.J.) or a combination of TNF-α (10 ng/ml) and IFN-γ (500 U/ml; R & D Systems, Minneapolis, Minn.) since these cytokines can enhance human monocyte NO production (23, 24, 28). Because NO is rapidly converted to nitrite (NO2−) and nitrate (NO3−), these catabolites (NOx) were measured in culture supernatants to determine NO production levels as described previously (5, 6). In brief, NO3− was converted to NO2− in a reaction mixture containing 1.0 M Tris-HCl, 0.2 mM NADPH (Sigma Chemical Co., St. Louis, Mo.), 5 mM glucose-6-phosphate, 10.0 U of glucose-6-phosphate dehydrogenase per ml, and 1.0 U of nitrate reductase from Aspergillus species (Boehringer, Mannheim, Germany) per ml.
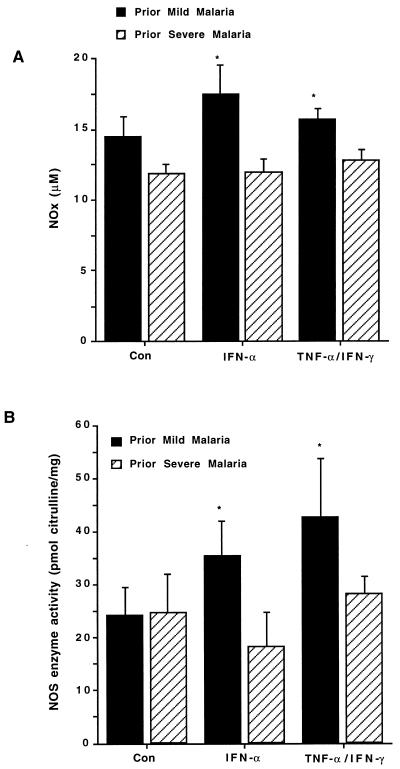
In the absence of stimulation (controls), NOx levels were elevated in the PMM group relative to the PSM group, with the results approaching significance (P = 0.06; Fig. 1A). In addition to NO measurements, NOS enzyme activity was analyzed by measuring the conversion of l-[14C]arginine to l-[14C]citrulline (NEN, Boston, Mass.) in cell extracts prepared by scraping the cells and rapidly freezing the PBMC pellets according to previously described methods (29). Analysis of NOS enzyme activity under control conditions showed that NOS enzyme was equivalent in both groups of children (P = 0.4; Fig. 1B). Stimulation of cultures with IFN-α2b increased NOx and NOS activity levels in the PMM group (P = 0.36 and P = 0.05, respectively; Fig. 1A & B) but not in the PSM group (P = 0.67 and P = 0.56, respectively; Fig. 1A and B). IFN-α-promoted NOx levels and NOS activity were significantly different between the two groups (P = 0.02 and P = 0.04, respectively; Fig. 1A and B). Culturing with TNF-α and IFN-γ did not significantly affect activity in the PMM group (P = 0.36) or the PSM group (P = 0.89) (Fig. 1A). Relative to control conditions, however, culturing cells with TNF-α and IFN-γ significantly increased NOS activity in the PMM group (P = 0.03) but not in the PSM group (P = 0.16) (Fig. 1B). The treatment of PBMC with TNF-α and IFN-γ generated higher levels of NOx (P = 0.04) and NOS activity (P = 0.04) in the PMM group than in the prior PSM group (Fig. 1A and B). Taken together, these results show that cultured PBMC from healthy children with PMM produce higher levels of NO and have greater NOS enzyme activity than those from children with PSM.
FIG. 1.
NOx and NOS enzyme activity in cultured PBMC. PBMC were prepared from healthy children who previously had mild P. falciparum malaria (n = 18) and from healthy children who previously had severe P. falciparum malaria (n = 10). Cultures were incubated for 7 days with medium alone (controls), INF-α2b (50 U/ml), or TNF-α (10 ng/ml) and IFN-γ (500 U/ml). Culture supernatants were removed and assayed in triplicate for NOx (micromolar). PBMC were harvested, lysates were prepared, and NOS enzyme activity (picomoles of citrulline/milligram of protein) was determined in triplicate by measuring the conversion of l-[14C]arginine to l-[14C]citrulline. The graphs show the means + standard errors of the mean (error bars) for each of the two groups. Comparisons between the PMM and PSM groups were made by using the Wilcoxon rank sum and Mann-Whitney U tests (statistical significance [P < 0.05]). ∗, P < 0.05 (for the PMM versus the PSM groups).
NOS enzyme activity in freshly isolated (noncultured) PBMC.
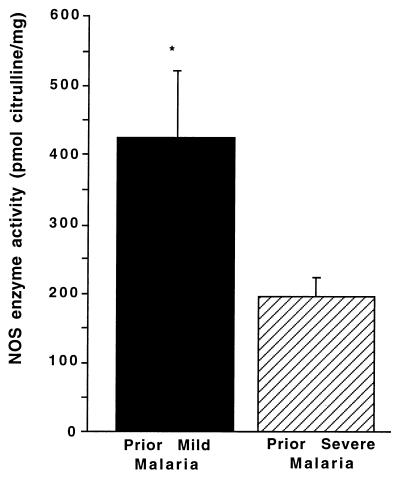
NOS activity was also evaluated in freshly isolated (noncultured) PBMC from the two groups since this activity more closely reflects the in vivo NOS enzyme activity without the influence of in vitro culture. For this analysis, freshly isolated cells were quickly frozen, and NOS activity was determined at a later date by measuring the conversion of l-[14C]arginine to l-[14C]citrulline (29). NOS enzyme activity was significantly higher in freshly isolated PBMC from the PMM group (P = 0.05; Fig. 2). Blood monocytes have a relatively short half-life; thus, elevated levels of NOS activity long after a malarial infection has been resolved suggest that there may be continued immune activation (e.g., cytokines) or genetic factors influencing the NOS activity. Previous studies in Gabonese children with P. falciparum malaria show that a NOS2 promoter polymorphism (G-954C) is associated with decreased severity of disease and increased time to reinfection (14). Although the sample size in the present study limits the power of analysis, the frequency of this NOS2 promoter polymorphism was not significantly different in the two groups (P = 0.76), suggesting that this genetic variable did not account for our results (data not presented).
FIG. 2.
NOS enzyme activity in noncultured PBMC. PBMC were prepared from healthy Gabonese children with PMM (n = 18) or PSM (n = 10). Cell lysates were prepared from noncultured PBMC, and NOS enzyme activity (picomoles of citrulline/milligram of protein) was determined by measuring the conversion of l-[14C]arginine to l-[14C]citrulline. The graph shows the means + standard errors of the mean (error bars) for each of the two groups. Comparisons between the PMM and PSM groups were made by using the Wilcoxon rank sum and Mann-Whitney U tests (statistical significance [P < 0.05]). ∗, P < 0.05 (for the PMM versus the PSM groups).
Levels of potential NOS-regulating cytokines in plasma.
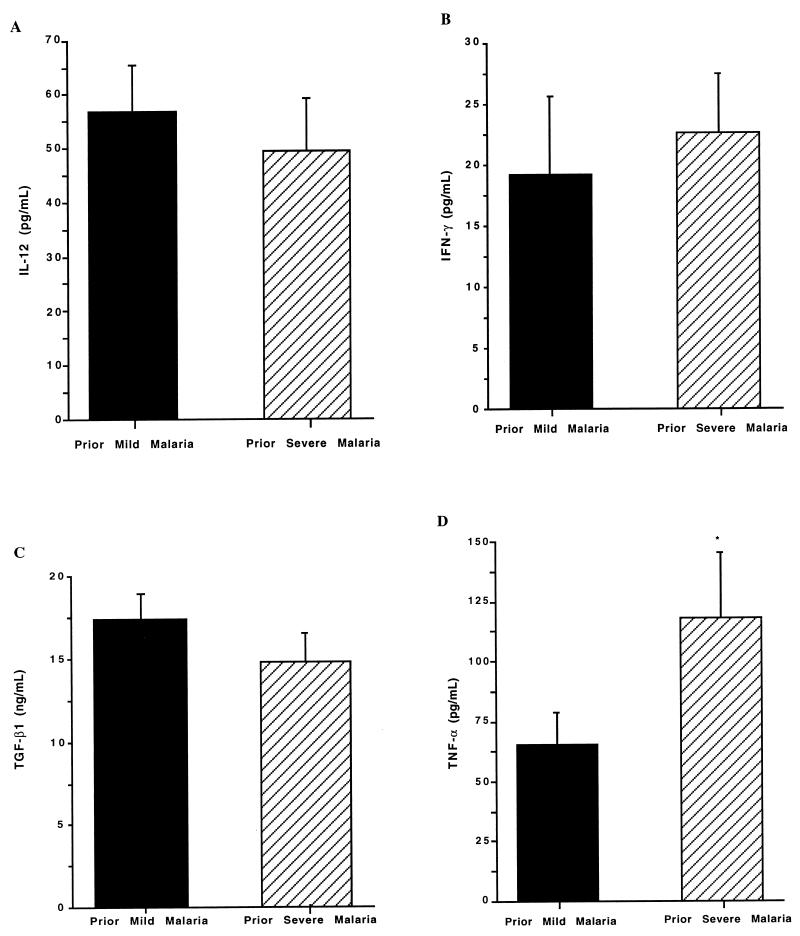
To determine if potential NOS-regulating cytokines could account for the varying degrees of PBMC NOS enzyme activity and NO production in the PMM and PSM groups, levels of selected proinflammatory (IL-12, IFN-γ, and TNF-α) and anti-inflammatory (TGF-β1) cytokines in plasma were determined by the quantitative sandwich enzyme immunoassay technique with commercially available reagents (R & D Systems). For the measurement of IL-12, a monoclonal antibody that recognizes the active heterodimer was used. TGF-β1 was determined in platelet-poor samples that were generated by centrifugation at 10,000 × g for 30 min. In addition, samples for TGF-β1 analysis were activated by incubation with 2.5 N acetic acid–10 N urea for 10 min followed by neutralization with 2.7 N NaOH–1 M HEPES. Levels of IL-12, IFN-γ, and TGF-β1 in plasma were comparable in the two groups (Fig. 3A to C), but TNF-α levels were significantly higher in the PSM group than in the PMM group (P = 0.03; Fig. 3D). The association of elevated concentrations of TNF-α in plasma and severe malaria (especially in cases of fatal disease) is well established (7, 10, 15). Previous reports show that polymorphisms in the promoter region of the TNF-α gene are associated with malarial severity in certain populations (17, 18). There were no major differences in TNF-α production between individual subjects within the groups in the present study, and the frequencies of the TNF-1, -2, -3, -4, and -5 alleles were equivalent in the two groups (P = 0.63; data not presented). However, the small sample size limits the power of analysis. Previous studies show that NO can decrease TNF-α synthesis in vitro and in vivo (4, 9, 26). It is therefore possible that lower PBMC NO production in Gabonese children with PSM contributes to higher levels of TNF-α in plasma. Thus, in addition to a direct antiparasitic effect, NO may exert antidisease effects in malaria by regulating proinflammatory cytokine production (1, 16).
FIG. 3.
Plasma cytokine concentrations. Venous blood was collected into EDTA-containing vials from healthy children with PMM (n = 18) or PSM (n = 10). Plasma was separated, and quantitative sandwich enzyme immunoassays were used to determine concentrations of IL-12 (P = 0.43) (A), IFN-γ (P = 0.23) (B), TGF-β1 (P = 0.6) (C), and TNF-α (P = 0.03) (D) in plasma. The graph shows the means + standard errors of the mean (error bars) for each of the two groups. Comparisons between the PMM and PSM groups were made by using the Wilcoxon rank sum and Mann-Whitney U tests (statistical significance [P < 0.05]). ∗, P < 0.05 (for the PMM versus the PSM groups).
In summary, we show that cultured PBMC from healthy children with PMM have a greater capacity to produce NO and express NOS in vitro than those from children with PSM. Likewise, freshly isolated noncultured PBMC from children with PMM have higher NOS enzyme activity. We postulate that enhanced NO production in children with PMM may allow a more effective reduction in parasitemia and prevent progression to severe disease. The observed differences in NO/NOS and TNF-α may be related to baseline (perhaps unexamined genetic) differences, prior differences in the degree of immune stimulation, or a combination of genetic and environmental factors.
Acknowledgments
We thank the staff members of the Albert Schweitzer Hospital in Lambaréné, Gabon, for their cooperation and technical assistance: Anita van den Biggelaar, Judith Jans, Hanna Knoop, Doris Luckner, Barbara Moritz, Anselme Ndzengue, Marcel Nkeyi, and Milena Sovric. In addition, we thank Nick Anstey, Menzies School for Health Research, Darwin, Australia, for careful review of the manuscript.
REFERENCES
- 1.Anstey N, Granger D, Weinberg J. Nitric oxide in malaria. In: Fang F, editor. Nitric oxide and infection. New York, N.Y: Plenum Press; 1999. pp. 311–341. [Google Scholar]
- 2.Anstey N M, Weinberg J B, Hassanali M, Mwaikambo E D, Manyenga D, Misukonis M A, Arnelle D R, Hollis D, McDonald M I, Granger D L. Nitric oxide in Tanzanian children with malaria. Inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184:557–567. doi: 10.1084/jem.184.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fahmi H, Charon D, Mondange M, Chaby R. Endotoxin-induced desensitization of mouse macrophages is mediated in part by nitric oxide production. Infect Immun. 1995;63:1863–1869. doi: 10.1128/iai.63.5.1863-1869.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florquin S, Amraoui Z, Dubois C, Decuyper J, Goldman M. The protective role of endogenously synthesized nitric oxide in staphylococcal enterotoxin B-induced shock in mice. J Exp Med. 1994;180:1153–1158. doi: 10.1084/jem.180.3.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Granger D L, Hibbs J, Jr, Broadnax L M. Urinary nitrate excretion in relation to murine macrophage activation. Influence of dietary l-arginine and oral NG-monomethyl-l-arginine. J Immunol. 1991;146:1294–1302. [PubMed] [Google Scholar]
- 6.Granger D L, Miller W C, Hibbs J B., Jr . Methods of analyzing nitric oxide production in the immune response. In: Feelisch M, Stamler J S, editors. Methods in nitric oxide research. New York, N.Y: John Wiley & Sons Ltd.; 1996. pp. 603–617. [Google Scholar]
- 7.Grau G E, Taylor T E, Molyneux M E, Wirima J J, Vassalli P, Hommel M, Lambert P H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989;320:1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 8.Gyan B, Troye-Blomberg M, Perlmann P, Bjorkman A. Human monocytes cultured with and without interferon-gamma inhibit Plasmodium falciparum parasite growth in vitro via secretion of reactive nitrogen intermediates. Parasite Immunol. 1994;16:371–375. doi: 10.1111/j.1365-3024.1994.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Iuvone T, Dacquisto F, Carnuccio R, Dirosa M. Nitric oxide inhibits LPS-induced tumor necrosis factor synthesis in vitro and in vivo. Life Sci. 1996;59:PL207–PL211. doi: 10.1016/0024-3205(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 10.Kern P, Hemmer C J, Van Dame J, Gruss H J, Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated malaria. Am J Med. 1989;87:139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- 11.Kremsner P, Neifer S, Chaves M, Rudolph R, Bienzle U. Interferon-γ induced lethality in the late phase of Plasmodium vinckei malaria despite effective parasite clearance by chloroquine. Eur J Immunol. 1992;22:2873–2878. doi: 10.1002/eji.1830221118. [DOI] [PubMed] [Google Scholar]
- 12.Kremsner P G, Winkler S, Wildling E, Prada J, Bienzle U, Graninger W, Nussler A K. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90:44–47. doi: 10.1016/s0035-9203(96)90476-9. [DOI] [PubMed] [Google Scholar]
- 13.Kun J, Schmidt-Ott R, Lehman L, Lell B, Luckner D, Greve B, Matousek P, Kremsner P. Merozoite surface antigen 1 and 2 genotypes and rosetting of Plasmodium falciparum in severe and mild malaria in Lambarene, Gabon. Trans R Soc Trop Med Hyg. 1998;92:110–114. doi: 10.1016/s0035-9203(98)90979-8. [DOI] [PubMed] [Google Scholar]
- 14.Kun J F J, Mordmuller B, Lell B, Lehman L G, Luckner D, Kremsner P G. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet. 1998;351:265–266. doi: 10.1016/S0140-6736(05)78273-8. [DOI] [PubMed] [Google Scholar]
- 15.Kwiatkowski D, Hill A V, Sambou I, Twumasi P, Castracane J, Manogue K R, Cerami A, Brewster D R, Greenwood B M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990;336:1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 16.Kwiatkowski D, Molyneux M, Stephens S, Curtis N, Klein N, Pointaire P, Smit M, Allan R, Brewster D, Grau G, et al. Anti-TNF therapy inhibits fever in cerebral malaria. Q J Med. 1993;86:91–98. [PubMed] [Google Scholar]
- 17.McGuire W, Hill A V, Allsopp C E, Greenwood B M, Kwiatkowski D. Variation in the TNF-alpha promoter region associated with susceptibility to cerebral malaria. Nature. 1994;371:508–510. doi: 10.1038/371508a0. [DOI] [PubMed] [Google Scholar]
- 18.McGuire W, Knight J, Hill A, Allsopp C, Greenwood B, Kwiatkowski D. Severe malarial anemia and cerebral malaria are associated with different tumor necrosis factor promoter alleles. J Infect Dis. 1999;179:287–290. doi: 10.1086/314533. [DOI] [PubMed] [Google Scholar]
- 19.Mellouk S, Hoffman S L, Liu Z-Z, de la Vega P, Billiar T R, Nussler A K. Nitric oxide-mediated antiplasmodial activity in human and murine hepatocytes induced by gamma interferon and the parasite itself: enhancement by exogenous tetrahydrobiopterin. Infect Immun. 1994;62:4043–4046. doi: 10.1128/iai.62.9.4043-4046.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nussler A, Drapier J-C, Renia L, Pied S, Miltgen F, Gentilini M, Mazier D. l-Arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin-6 stimulation. Eur J Immunol. 1991;21:227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- 21.Oswald I P, Wynn T A, Sher A, James S L. NO as an effector molecule of parasite killing: modulation of its synthesis by cytokines. Comp Biochem Physiol Pharmacol Toxicol Endocrinol. 1994;108:11–18. doi: 10.1016/1367-8280(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 22.Rockett K A, Awburn M M, Cowden W B, Clark I A. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect Immun. 1991;59:3280–3283. doi: 10.1128/iai.59.9.3280-3283.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharara A I, Perkins D J, Misukonis M A, Chan S U, Dominitz J A, Weinberg J B. Interferon (IFN)-alpha activation of human blood mononuclear cells in vitro and in vivo for nitric oxide synthase (NOS) type 2 mRNA and protein expression—possible relationship of induced NOS2 to the anti-hepatitis C effects of IFN-alpha in vivo. J Exp Med. 1997;186:1495–1502. doi: 10.1084/jem.186.9.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spitsin S V, Koprowski H, Michaels F H. Characterization and functional analysis of the human inducible nitric oxide synthase gene promoter. Mol Med. 1996;2:226–235. [PMC free article] [PubMed] [Google Scholar]
- 25.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 26.Tiao G, Rafferty J, Ogle C, Fischer J, Hasselgren P. Detrimental effect of nitric oxide synthase inhibition during endotoxemia may be caused by high levels of tumor necrosis factor and interleukin-6. Surgery. 1994;116:332–338. [PubMed] [Google Scholar]
- 27.Vodovotz Y, Bogdan C, Paik J, Xie Q W, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med. 1993;178:605–613. doi: 10.1084/jem.178.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg J B. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol Med. 1998;4:557–591. doi: 10.1007/BF03401758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg J B, Granger D L, Pisetsky D S, Seldin M F, Misukonis M A, Mason S N, Pippen A M, Ruiz P, Wood E R, Gilkeson G S. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weinberg J B, Muscato J J, Niedel J E. Monocyte chemotactic peptide receptor. Functional characteristics and ligand-induced regulation. J Clin Investig. 1981;68:621–630. doi: 10.1172/JCI110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilding E, Winkler S, Kremsner P G, Brandts C, Jenne L, Wernsdorfer W H. Malaria epidemiology in the province of Moyen Ogooue, Gabon. Trop Med Parasitol. 1995;46:77–82. [PubMed] [Google Scholar]
- 32.World Health Organization. WHO fact sheet. Vol. 94. Geneva, Switzerland: World Health Organization; 1995. pp. 1–3. [Google Scholar]
- 33.World Health Organization. Severe and complicated malaria. Trans R Soc Trop Med Hyg. 1990;84(Suppl. 2):1–65. [PubMed] [Google Scholar]