Abstract
Background
The risk of barotrauma associated with different types of ventilatory support is unclear in COVID-19 patients. The primary aim of this study was to evaluate the effect of the different respiratory support strategies on barotrauma occurrence; we also sought to determine the frequency of barotrauma and the clinical characteristics of the patients who experienced this complication.
Methods
This multicentre retrospective case-control study from 1 March 2020 to 28 February 2021 included COVID-19 patients who experienced barotrauma during hospital stay. They were matched with controls in a 1:1 ratio for the same admission period in the same ward of treatment. Univariable and multivariable logistic regression (OR) were performed to explore which factors were associated with barotrauma and in-hospital death.
Results
We included 200 cases and 200 controls. Invasive mechanical ventilation was used in 39.3% of patients in the barotrauma group, and in 20.1% of controls (p<0.001). Receiving non-invasive ventilation (C-PAP/PSV) instead of conventional oxygen therapy (COT) increased the risk of barotrauma (OR 5.04, 95% CI 2.30 - 11.08, p<0.001), similarly for invasive mechanical ventilation (OR 6.24, 95% CI 2.86-13.60, p<0.001). High Flow Nasal Oxygen (HFNO), compared with COT, did not significantly increase the risk of barotrauma. Barotrauma frequency occurred in 1.00% [95% CI 0.88-1.16] of patients; these were older (p=0.022) and more frequently immunosuppressed (p=0.013). Barotrauma was shown to be an independent risk for death (OR 5.32, 95% CI 2.82-10.03, p<0.001).
Conclusions
C-PAP/PSV compared with COT or HFNO increased the risk of barotrauma; otherwise HFNO did not. Barotrauma was recorded in 1.00% of patients, affecting mainly patients with more severe COVID-19 disease. Barotrauma was independently associated with mortality.
Trial registration
this case-control study was prospectively registered in clinicaltrial.gov as NCT04897152 (on 21 May 2021).
Keywords: COVID-19, Acute respiratory failure, Barotrauma, Pneumothorax, High flow nasal cannula, Invasive mechanical ventilation
Abbreviations: C-PAP, continuous positive airway pressure; PSV, pressure support ventilation; COT, conventional oxygen therapy; HFNO, high flow nasal oxygen; C-ARDS, coronavirus acute respiratory distress syndrome; ICU, intensive care unit; NIV, non-invasive ventilation; IMV, invasive mechanical ventilation; NIRS, non invasive respiratory support; P-SILI, patients self-inflicted lung injury; VILI, ventilator induced lung injury; PNX, pneumothorax; PMD, pneumomediastinum; qCSI, Quick COVID-19 Severity Index; ECMO, Extra-Corporeal Membrane Oxygenation; HRCT, high-resolution computed tomography
Introduction
One established complication of mechanical ventilation in critically ill patients is barotrauma.1
The recent pandemic of SARS-CoV2 has drawn attention to the fact that acute respiratory syndrome in COVID-19 patients (C-ARDS) has been disproportionally associated with this complication compared to traditional ARDS during the first wave.2 , 3
A wide range in frequency of barotrauma has been reported in the literature in C-ARDS patients worldwide depending on the hospital setting. In a multicenter study involving up to 72,000 patients in the emergency department in Spain, the frequency reported was 0.56%,4 while in China, the frequency was double and around 1%.5
Conversely, in the United States in intensive care units (ICU), barotrauma frequency reached 15% in mechanically ventilated COVID-19 patients.3
Considering that C-ARDS patients who underwent invasive mechanical ventilation (IMV) have been treated with a protective ventilation strategy as is customarily used in patients with traditional ARDS,6 , 7 the higher percentage of barotrauma in COVID-19 patients could lie outside the ventilatory strategies.
In fact, pneumothorax, pneumomediastinum and subcutaneous emphysema, otherwise known as barotrauma, have also been described in C-ARDS spontaneous breathing patients or those under non-invasive respiratory support (NIRS), which represented the majority of hospitalized patients, and not only during IMV.8 , 9
It is thought that vigorous breathing with uncontrolled effort can increase transpulmonary pressure gradient across lung regions, and global and regional strain, inducing the phenomenon known as patient self-inflicted lung injury (P-SILI).10 At this stage, adequate ventilatory support becomes fundamental to avoiding lung damage, reducing in the meantime ventilator induced lung injury (VILI).
Therefore, in this multicentre study, we aimed to investigate, as a primary aim, the effect of the different respiratory support strategies on barotrauma occurrence and, as secondary aims, the frequency of barotrauma and the clinical characteristics of the patients who experienced this complication.
Materials and methods
Study design
This retrospective case-control observational study was conducted in 9 intensive care units (ICUs) and 15 medical wards in Italy from 1 March 2020 to 28 February 2021. After approval by the Ethics Committee for the coordinating centre (approval number CEUR-2021-3659, Ethics Committee of Friuli-Venezia-Giulia Region, Italy), all local investigators were responsible for obtaining the required permissions in their centres according to the national regulation. The study was prospectively registered on clinicaltrial.gov (NCT04897152).
The study was conducted according to Good Clinical Practice guidelines and the Declaration of Helsinki. Data were anonymously collected using a unique alphanumeric code for each participant. The Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed.11 All data were anonymously collected on the electronic data manager Castor (EDC, 2019, Amsterdam, The Netherlands) in compliance with the European General Data Protection Regulation (GDPR) 2016/679.12
Population
We considered eligible all adult (i.e., ≥18 years/old) patients admitted to hospital for SARS-CoV-2 pneumonia. SARS-CoV-2 infection was ascertained through polymerases chain reaction nasal swab. We included in the study all patients who developed barotrauma during hospital stay; barotrauma was defined as the occurrence of pneumothorax (PNX) and/or pneumomediastinum (PMD), irrespective of the presence or absence of subcutaneous emphysema.
Patients were excluded if one of the following criteria was present: 1) iatrogenic cause of barotrauma (i.e., pneumothorax from central vein catheter insertion or pleural effusion drainage); 2) absence of radiological imaging; or 3) do-not-intubate or do-not-resuscitate order (for palliative care).
Controls were selected among COVID-19 patients without barotrauma and matched 1:1 with cases per period and unit of admission. In other words, controls were included considering patients without barotrauma that were admitted in the same week and in the same treatment unit as the ones experiencing barotrauma, respecting all inclusion and exclusion criteria.
All patients received standard care, according to current clinical practice guidelines and evidence-based recommendations/indications at the time of enrolment.
First and additional aims
The first study aim is to describe the effect of the different respiratory support strategies on barotrauma occurrence.
Additional aims describe the frequency of barotrauma and eventual required treatments.
Finally, the characteristics of respiratory failure, blood tests, infections, hospital length of stay and mortality of patients experiencing barotrauma are compared with those of a matched control group to find possible similarities or important clinical differences.
Data collection
For all patients, we recorded (1) demographic and anthropometric data; (2) comorbidities; (3) severity of COVID-19, stratified as asymptomatic infection, mild, moderate, severe and critical illness, through the World Health Organization (WHO) case definition,13; (4) the Quick COVID-19 Severity Index (qCSI)14; and (5) 4C mortality score.15 We also recorded the arterial partial pressure to inspired oxygen fraction ratio (PaO2/FIO2), the alveolar-to-arterial difference of oxygen (A-a DO2) and the respiratory rate. In addition, whenever available, the following blood tests were recorded: white blood cell (WBC) and lymphocytes counts, C-reactive protein, procalcitonin (PCT), pro-adrenomedullin, interleukin 6 (IL-6), lactate dehydrogenase (LDH) and D-dimer.
We collected the need, the type and the time spent under conventional oxygen therapy (COT), high-flow nasal oxygen (HFNO), continuous positive airway pressure (C-PAP), non-invasive ventilation (NIV), invasive mechanical ventilation (IMV) or Extra-Corporeal Membrane Oxygenation (ECMO), the consecutive modalities together with the need for sedatives, neuromuscular blocking agents and prone position. Notably, COT, HFNO, C-PAP and NIV were defined as non-invasive respiratory support (NIRS).
In every centre, a radiologist reviewed high-resolution computed tomography (HRCT) to compute a severity score, as reported by Salaffi et al.16 In particular, the radiologist evaluated both lungs on three levels and for every single area assigned a score from 0 to 4 according to the nature of abnormalities and another 4-point score according to the percentage of lung area involvement. The scores, according to the abnormalities and their extent, were then multiplied by each other and added to the scores of all 6 levels (3 levels on each side). The final severity score ranged from 0 to 96: the higher the score the more severe was the disease.
The occurrence of co-infections with the need for antibiotic therapy was also recorded.17
Finally, we computed the rate of patients requiring re-intubation after first extubation attempt or tracheostomy, the days from hospital to ICU admission, the hospital length of stay and in-hospital mortality.
Sample size estimation
The primary aim of the study is the effect of the different respiratory support strategies, in particular IMV, on barotrauma. Assuming a proportion of 25% of patients receiving IMV in the control group and of 38% of patients in the case group,18 we calculated a sample size of 392 patients to detect an odds ratio (OR) of 1.890 for barotrauma with an 80% of power and alpha error of 5% with a two-sided Fisher's Exact test.
Statistical analyses
Categorical variables were presented as absolute values (percentages), and continuous variables were described as either mean and standard deviation or median and ranges, according to variable distribution. Normality was assessed using the Shapiro-Wilk Test. Categorical variables were compared using the chi-squared test or Fisher's exact test, while continuous variables were compared using a student t-test or Mann-Whitney U test, according to the distribution of the data. Univariable and multivariable conditional logistic regressions were performed to explore which factors were associated with barotrauma and in-hospital death, stratifying by referral centres. A multiple imputation approach was used to account for missing data, replacing missing values with 50 sets of simulated values and adjusting the obtained parameter estimates for missing-data uncertainty. All clinically relevant variables or those that were significant at p<0.05 in univariable analysis were included in the multivariable analysis, taking into account potential collinearities. Overall survival was described according to the Kaplan-Meier approach. Comparisons among survival distributions were performed using the log-rank test. Two-sided p values of less than 0.05 were determined to be statistically significant. Statistical analyses were performed using Stata/IC 17.0 (StataCorp LP, College Station, USA).
Results
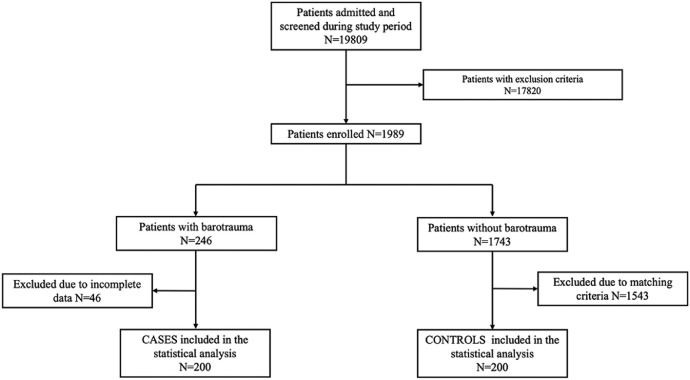
Among 19,809 patients with C-ARDS admitted in the study period, the frequency of barotrauma was 1.00% [95% CI 0.88-1.16]. Forty-six patients were excluded due to exclusion criteria. Finally, 200 cases and 200 matched controls were included for the final statistical analysis (Fig. 1 ).
Fig. 1.
Study flow chart.
Baseline patients’ characteristics were balanced between the two groups, except for age, immunosuppressive therapies and chronic liver disease (Table 1 ). In particular, patients who experienced barotrauma were older (p=0.022), more frequently on domiciliary immunosuppressive therapy (p=0.013) and with less deranged liver function tests (p=0.015).
Table 1.
Baseline patients' characteristics.
| Overall (n=400) | Barotrauma group (n=200) | Controls (n=200) | p-value | |
|---|---|---|---|---|
| Males, n (%) | 295 (73.8) | 155 (77.5) | 140 (70) | 0.088 |
| Age, years, median (IQR) | 68 (59-75) | 69.5 (62-76) | 66.5 (57-75) | 0.022 |
| BMI, median (IQR) | 26.7 (24.5-30.4) | 26.3 (24.5-30.1) | 27.1 (24.4-30.7) | 0.466 |
| Cardiovascular disease, n/N (%) | 196/399 (49.1) | 103 (51.5) | 93/199 (46.7) | 0.341 |
| COPD, n/N (%) | 39/399 (9.8) | 15 (7.5) | 24/199 (12.1) | 0.125 |
| Solid cancer, n(%) | 31/399 (7.8) | 14 (7) | 17/199 (8.5) | 0.565 |
| Haematologic disease, n(%) | 29 (7.3) | 16 (8) | 13 (6.5) | 0.563 |
| Diabetes, n/N (%) | 84 (21) | 42 (21) | 42 (21) | 1.000 |
| CKD, n/N (%) | 37/399 (9.3) | 21 (10.5) | 16/199 (8.0) | 0.397 |
| Iatrogenic immunosuppression, n/N (%) | 29/399 (7.3) | 21 (10.5) | 8/199 (4.0) | 0.013 |
| Liver disease, n(%) | 7 (1.8) | 0 (0) | 7 (3.5) | 0.015 |
Legend. IQR: interquartile range; BMI: Body Mass Index; COPD: Chronic obstructive pulmonary disease: CKD: Chronic kidney disease.
High flow nasal oxygen strategy was more represented in the group with barotrauma than without (42.4% vs. 32.1% respectively, p=0.035). Similarly, C-PAP/PSV was reported in 86% of patients in the barotrauma group and in 50.3% of patients in the group without barotrauma (p<0.001). Invasive mechanical ventilation was used in 39.3% of patients in barotrauma group, a higher frequency compared to the non-barotrauma group, in which only 20.1% of patients received it (p<0.001), as reported in Table 2 .
Table 2.
Ventilation strategies applied during hospital stay.
| Overall (n=400) | Barotrauma (n=200) | No barotrauma (n=200) | p-value | |
|---|---|---|---|---|
| HFNO, n/N (%) | 147/394 (37.3) | 84/198 (42.4) | 63/196 (32.1) | 0.035 |
| CPAP/PSV °, n/N (%) | 257/379 (67.8) | 160/186 (86.0) | 97/193 (50.3) | <0.001 |
| IMV°, n/N (%) | 116/390 (29.7) | 77/196 (39.3) | 39/194 (20.1) | <0.001 |
| Sedation, n/N (%) | 186/300 (62) | 142/196 (72.5) | 44/104 (42.3) | <0.001 |
| Pronation, n/N (%) | 156/298 (52.4) | 127/193 (65.8) | 29/105 (27.6) | 0.001 |
| Curarization, n/N (%) | 187/295 (63.4) | 119/191 (62.3) | 68/104 (65.4) | 0.600 |
| Necessity of re-OTI, n/N (%) | 20/287 (7.0) | 18/189 (9.5) | 2/98 (2.0) | 0.018 |
| Tracheostomy, n/N (%) | 56/290 (19.3) | 53/191 (27.8) | 3/99 (3.0) | <0.001 |
| ECMO, n/N (%) | 10/292 (3.4) | 10/192 (5.2) | 0/100 (0) | 0.017 |
°as intended before barotrauma.
Legend. CPAP/PSV: Continuous positive airway pressure ventilation/Pressure support ventilation, ECMO: extracorporeal membrane oxygenation, HFNO: high flow nasal oxygen; IMV: invasive mechanical ventilation; OTI: orotracheal intubation.
However, considering mean days of ventilation in the barotrauma versus no barotrauma group, adjusted for the type and duration of ventilation, we found a protective effect of CPAP/PSV (5.3±4.6 vs 9.2±7.6 p <0.001), while IMV showed a direct effect (10±10.5 vs 5.3±6.0 p 0.017) and no effect from HFNO (8.1±9.2 vs 7.8±7.3 p 0.955). PEEP level was available in 89 control vs 144 cases, and their value in barotrauma vs no barotrauma was similar (9.2±2.0 vs 9.08±2.2, p=0.621). In addition, patients with barotrauma more frequently received sedatives (p< 0.001) and prone positioning (p< 0.001) and had higher re-intubation (p<0.018) and tracheotomy (p<0.001) rates. ECMO was instituted in 10 (5.2%) patients with barotrauma, whereas none required it in controls (p<0.017).
Additional aims
The frequency of barotrauma was 1.00% [95% CI 0.88-1.16]. Isolated PMD occurred in 90 cases (0.45 [95% CI 0.37-0.56]), whereas isolated PNX occurred in 61 patients (0.31 [95% CI 0.24-0.40]) and combined PMD-PNX occurred in 49 patients (0.25%) – [95% CI 0.18%-0.33%]. PNX was more frequent on the right side (55 of 108 cases; 50.9%), followed by on the left-side (29/108; 26.9%) and bilateral in 24 cases (22.2%).
Hemodynamic instability was reported in 55 patients (27.6%). PNX required draining with a chest tube in 73 cases (66.3%), whereas six patients (5.5%) required surgery and one (0.9%) talc pleurodesis. Detailed data are reported in Table 3 .
Table 3.
Characteristics of barotrauma and barotrauma management.
| Barotrauma findings (n=200) | |
| Pneumomediastinum, n (%) | 90 (45) |
| Pneumothorax, n (%) | 61 (30.5) |
| Both, n (%) | 49 (24.5) |
| Days from hospital admission and barotrauma diagnosis (median, IQR) | 10 (6-18) |
| Pneumothorax side (n=108) | |
| Right sided, n (%) | 55/108 (50.9) |
| Left sided, n (%) | 29/108 (26.9) |
| Bilateral, n (%) | 24/108 (22.2) |
| Barotrauma findings, size (n=122) | |
| Large (≥ 2 cm), n (%) | 83 (68.0) |
| Small (<2 cm), n (%) | 39 (32.0) |
| Haemodinamics (n=199) | |
| Stable, n (%) | 144 (72.4) |
| Unstable, n (%) | 55 (27.6) |
| Pneumothorax management (n=110) | |
| Conservative management, n (%) | 120 (60) |
| Chest tube, n (%) | 73 (36.5) |
| Surgical treatment, n (%) | 6 (3) |
| Pleural talcage, n (%) | 1 (0.5) |
| Days of chest tube maintenance, median (IQR) | 9 (4-19) |
| Barotrauma evolution (n=199) | |
| Spontaneous reabsorption, n (%) | 66 (33.2) |
| Resolution after chest tube/surgery, n (%) | 34 (17.1) |
| Unresolved, n (%) | 82 (41.2) |
| Recurrency, n (%) | 11 (5.5) |
| Unknown, n (%) | 6 (3.0) |
Legend. IQR: interquartile range.
As shown in Supplementary Table 1 (in the ESM), patients with barotrauma were characterized by a more prominent hypoxemia (p=0.004), higher respiratory rate (p=0.001), and higher qCSI and 4C scores at the admission (p<0.001 for both scores) compared to controls.
Patients who experienced barotrauma also showed a higher inflammation profile of serum procalcitonin, pro-adrenomedullin, interleukin-6, LDH and D-dimer than the control group (see Supplementary Table 2 in the ESM).
Table 4 shows univariate and multivariate analyses of risk factors for barotrauma.
Table 4.
Factor associated with barotrauma (univariable and multivariable analysis).
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male sex | 1.51 | 0.95,2.40 | 0.080 | |||
| Age | 1.03 | 1.01,1.04 | 0.005 | 2.00 | 1.13,3.52 | 0.017 |
| BMI | 0.99 | 0.95,1.04 | 0.698 | 1.02 | 1.00,1.04 | 0.029 |
| Cardiovascular disease | 1.21 | 0.81,1.81 | 0.352 | |||
| COPD | 0.55 | 0.27,1.13 | 0.105 | |||
| Solid cancer | 0.81 | 0.39,1.69 | 0.577 | |||
| Haematologic disease | 1.25 | 0.58,2.69 | 0.563 | |||
| Diabetes | 1 | 0.62,1.62 | 1.000 | |||
| CKD | 1.35 | 0.68,2.71 | 0.391 | |||
| Immunosoppression | 2.77 | 1.19,6.41 | 0.018 | |||
| qCSI | 1.13 | 1.07,1.18 | <0.001 | |||
| PaO2/FiO2 ratio ad admission | 0.99 | 0.99,0.99 | 0.004 | |||
| Respiratory rate ad admission | 1.05 | 1.01,1.10 | 0.014 | 1.00 | 0.834 | |
| (A-a)DO2 | 1.00 | 0.99,1.01 | 0.667 | |||
| Ventilation strategies | ||||||
| HFNO vs. COT | 0.55 | -0.44,1.54 | 0.275 | 1.40 | 0.49,4.01 | 0.534 |
| CPAP/PSV vs. COT | 2.06 | 1.35,2.76 | <0.001 | 5.04 | 2.30,11.08 | <0.001 |
| CPAP/PSV vs. HFNO | 1.51 | 0.56,2.46 | 0.002 | 3.00 | 1.09,8.27 | 0.033 |
| IMV vs. COT | 2.33 | 1.63,3.04 | <0.001 | 6.24 | 2.86,13.60 | <0.001 |
| IMV vs. HFNO | 1.78 | 0.84,2.73 | <0.001 | 4.12 | 1.51,11.27 | 0.006 |
| IMV vs. CPAP/PSV | 0.27 | -0.34,0.89 | 0.382 | 1.37 | 0.70,2.70 | 0.359 |
| IMV/CPAP/PSV vs COT/HNFO | 7.81 | 4.44,13.74 | <0.001 | |||
| IMV vs NIRS | 3.52 | 2.08,5.98 | <0.001 | |||
| Extent (%) of lung involvement | ||||||
| 25-49% vs. 0-24% | 1.35 | 0.55,3.32 | 0.514 | 0.93 | 0.33,2.63 | 0.895 |
| 50-74% vs. 0-24% | 3.91 | 1.61,9.52 | 0.003 | 2.07 | 0.75,5.76 | 0.161 |
| 50-74% vs. 25-49% | 2.90 | 1.68,5.02 | <0.001 | 2.31 | 1.22,4.39 | 0.010 |
| ≥75% vs. 0-24% | 4.33 | 1.63,11.52 | 0.003 | 1.80 | 0.57,5.72 | 0.318 |
| ≥75% vs. 25-49% | 3.21 | 1.65,6.23 | 0.001 | 2.01 | 0.92,4.41 | 0.081 |
| ≥75% vs. 50-74% | 1.11 | 0.60,2.04 | 0.743 | 0.87 | 0.43,1.77 | 0.699 |
| White blood cells (n/µL) | 1.00 | 1.00,1.00 | 0.836 | |||
| Lymphocytes count(n/µL) | 1.00 | 1.00,1.00 | 0.472 | |||
| CRP (mg/L) | 1.01 | 1.01,1.01 | 0.014 | 1.00 | 0.99,1.01 | 0.348 |
| Procalcitonin (ng/mL) | 1.12 | 0.96,1.31 | 0.156 | |||
| Proadrenomedullin (mmol/L) | 1.00 | 0.98,1.01 | 0.682 | |||
| Interleukin 6 (pg/mL) | 1.00 | 1.00,1.00 | 0.105 | |||
| LDH (IU/L) | 1.00 | 1.00,1.00 | 0.421 | |||
| D-dimer test (FeU/mL) | 1 | 1.00,1.00 | 0.938 | |||
| Bacterial co-infections | 2.56 | 1.48,4.43 | 0.001 | 1.79 | 0.93,3.46 | 0.081 |
| Fungal co-infections | 5.65 | 2.73,11.66 | <0.001 | 5.27 | 2.10,13.24 | <0.001 |
Legend. BMI: Body Mass Index; COPD: Chronic obstructive pulmonary disease: CKD: Chronic kidney disease, (A-a)DO2: alveolar-arterial gradient; GGO: ground glass; paO2/FiO2 ratio: ratio of arterial oxygen partial pressure (paO2 in mmHg) to the fraction of inspired oxygen (FiO2), CPAP/PSV: Continuous positive airway pressure ventilation/pressure support ventilation, HFNO: high flow nasal oxygen; IMV: invasive mechanical ventilation; NIRS: noninvasive respiratory support; CRP: C reactive protein; PCT: procalcitonin; LDH: lactate dehydrogenase.
In more detail, both invasive and non-invasive ventilation were significantly related to the risk of barotrauma compared to conventional oxygen therapy. In particular, the multivariate analysis showed that receiving C-PAP/PSV versus COT has an OR 5.04 (95% CI 2.30-11.08, p<0.001) for barotrauma, while receiving IMV versus COT has an OR of 6.24 (95% CI 2.86-13.60, p<0.001).
Compared with HFNO, patients with C-PAP/PSV have a 3-times higher risk of barotrauma (OR 3.00, 95% CI 1.09-8.27, p=0.033), while patients receiving IMV had an OR 4.12 for barotrauma (95% CI 1.51-11.27, p=0.006). Patients that underwent only COT, only HFNO, only CPAP/PSV, only IMV versus COT/HFNO, COT/HFNO/CPAP/PSV, COT/HFNO/PSV/IMV intended as an escalation support presented less barotrauma events (p<0.001) as shown in Supplementary Table 3. However, HFNO, compared with COT, did not significantly increase the risk of barotrauma at the uni- and multivariate analysis (OR 1.40, 95 CI 0.49-4.01, p=0.534).
Barotrauma appeared significantly related to the extension of lung involvement (OR 2.31, 95% CI 1.22-4.39, p=0.010) and with thromboembolism at presentation (OR 5.46, IC95% 2.83,10.55, p<0.001).
Fungal infections were significantly associated with barotrauma (OR 5.27, 95% CI 2.10-13.24, p <0.001) as shown in Supplementary Table 4.
Barotrauma was found to be an independent risk factor for death (OR 5.32, 95% CI 2.82-10.03, p<0.001) as shown in Table 5 . Patients receiving C-PAP/PSV versus HFNO have an OR 3.06 (95% CI 0.82-11.44, p=0.097) for in-hospital death. On the other hand, patients under CPAP/PSV versus COT have an OR of 22.22 for in hospital death (95% CI 5.42-91.20, p<0.001).
Table 5.
Indipendent risk factors for in-hospital death.
| Univariable analysis |
Multivariable analysis |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Male sex | 0.65 | 0.39,1.10 | 0.106 | |||
| Age | 1.08 | 1.05,1.10 | <0.001 | 1.09 | 1.06,1.13 | <0.001 |
| PaO2/FIO2 ratio at admission | 1.00 | 0.99,1.00 | 0.400 | |||
| Respiratory rate at admission | 1.01 | 0.98,1.05 | 0.455 | |||
| Barotrauma | 8.21 | 4.77,14.14 | <0.001 | 5.32 | 2.82,10.03 | <0.001 |
| Ventilation strategies | ||||||
| HFNO vs. COT | 3.15 | 0.68,14.61 | 0.143 | 7.27 | 1.25,42.29 | 0.027 |
| CPAP/PSV vs. COT | 24.71 | 7.80,78.30 | <0.001 | 22.22 | 5.42,91.20 | <0.001 |
| CPAP/PSV vs. HFNO | 7.85 | 2.24,27.51 | 0.001 | 3.06 | 0.82,11.44 | 0.097 |
| IMV vs. COT | 29.05 | 9.28,90.95 | <0.001 | 30.70 | 7.49,125.78 | <0.001 |
| IMV vs. HFNO | 9.23 | 2.66,32.04 | <0.001 | 4.22 | 1.17,15.27 | 0.028 |
| IMV vs. CPAP/PSV | 1.18 | 0.63,2.20 | 0.613 | 1.38 | 0.68,2.82 | 0.374 |
| IMV/CPAP/PSV vs HFNO/COT | 18.00 | 7.69,42.16 | <0.001 | |||
| IMV vs NIRS | 3.63 | 2.09,6.31 | <0.001 | |||
| Bacterial co-infections | 1.66 | 0.93,2.94 | 0.085 | |||
| Fungal co- infections | 3.32 | 1.90,5.81 | <0.001 | 1.64 | 0.86,3.13 | 0.132 |
Legend. PaO2/FIO2 ratio: ratio of arterial oxygen partial pressure (paO2 in mmHg) to the fraction of inspired oxygen (FiO2), CPAP/PSV: Continuous positive airway pressure ventilation/pressure support ventilation, HFNO: high flow nasal oxygen; IMV: invasive mechanical ventilation; NIRS: non-invasive respiratory support.
As expected, the barotrauma group showed a longer hospital stay, 27 days versus 12 days, p <0.001. Overall mortality in the barotrauma group compared with patients without was 54.8% versus 17.2%, respectively (p<0.001) (see supplementary Table 4). Of these patients, 64 over 113 (56.6%) received IMV and 77 over 274 who received no NIRS (28.1%) died, p < 0.001.
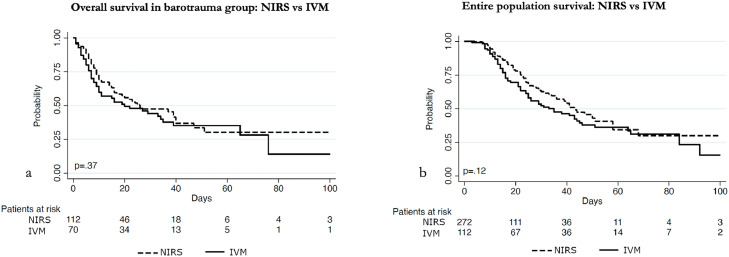
The log-rank test showed no significant difference in survival of patients in the barotrauma group receiving NIRS versus IMV (p=0.37). When analysing the whole population, the log-rank test showed no significant difference in overall survival as well (p=0.12), as shown in Fig. 2 .
Fig. 2.
Survival Kaplan Meier curves.
In Fig. 2 (a) Kaplan-Meier survival curves show overall survival of patients with COVID-19 infection who developed barotrauma when on IMV and on NIRS. Patients in IMV and NIRS were represented by continuous and dotted curves respectively, with no difference in overall survival (p=0.37).
In Fig. 2 (b) Kaplan-Meier survival curves describe overall survival of patients with COVID-19 infection when on IMV, and when in NIRS. Patients in IMV and NIRS were represented by continuous and dotted curves respectively, with no difference in overall survival (p=0.12).
Legend. IMV: invasive mechanical ventilation; NIRS: non-invasive respiratory support
Discussion
The main findings of the investigation are, first, that HFNO compared to COT did not increase the risk of barotrauma. Second, C-PAP/PSV or IMV increased the risk of barotrauma compared with HFNO. Third, barotrauma frequency in this study was lower than in previous reports in COVID-19 cohorts, and we confirm that the right-side was the most affected. Fourth, hemodynamic instability in our study was more frequent than in previous findings, and many patients required chest tube drainage. Fifth, patients with barotrauma exhibited lower PaO2/FIO2 ratio, highest BMI and extended lung disease involvement. Sixth, elevated plasma cytokine concentration was associated with barotrauma. Furthermore, C-PAP/PSV and IMV increased the risk of death compared with COT.
Mirò et al. and Ayazi et al. found that those patients who experienced barotrauma were more tachypnoeic.4 , 19
It could be assumed that high respiratory rates at admission might mirror the increased respiratory effort with the greater risk of developing self-induced positive end-expiratory pressure (auto-PEEP), contributing to barotrauma.20 , 21
HFNO seems to reduce the work of breathing and to improve respiratory mechanics in COVID-19 patients; it provides a homogeneous distribution of tidal volume without any inspiratory assistance; and it promotes alveolar recruitment in not-dependent regions preventing alveolar overdistension.22, 23, 24
Therefore, HFNO might theoretically mitigate the risk of P-SILI during spontaneous breathing and limit the rate of non-invasive treatment failure.
Although both C-PAP and HFNO have been recommended for mild to moderate acute hypoxemic respiratory failure treatment in patients with COVID-19, the RECOVERY-RS trial revealed that only an initial strategy of C-PAP reduced the risk of tracheal intubation or mortality compared with COT and caution is needed because failed NIV carries increased mortality. Yet, there was no significant difference between an initial strategy of HFNO compared with COT.25
In patients with COVID-19 and moderate hypoxia, Crimi et al. showed that HFNO does not significantly reduce the escalation of respiratory support.26 However, this field is still matter of debate, and we can only speculate about the possible role of HFNO in avoiding barotrauma. In our study, in fact, we found that, compared with COT, HFNO did not increase the risk of barotrauma after uni- and multivariate analysis. Moreover, only a few case reports have been published about pneumomediastinum and pneumothorax in spontaneously breathing patients under HFNO.27 , 28 It is therefore difficult to define any pathophysiological correlation.
Concerning C-PAP/PSV and IMV, as expected, both were significantly related to the risk of barotrauma compared to COT.
Comparing our data with the literature, we showed that the frequency of barotrauma is slightly lower than previously reported when considering the entire in-hospital spectrum of COVID-19 disease, as shown in earlier studies from China.5
A recent large multicentre study involving 71,904 COVID-19 patients carried out across 61 emergency departments (ED) in Spain reported an overall pneumothorax incidence at presentation of 0.56%.4 In contrast, McGuiness et al. in 89 mechanically ventilated patients with COVID-19 infection reported an incidence of barotrauma of about 15% in mechanically ventilated COVID-19 patients.3
Wang et al. reported a frequency of pneumothorax of 10% in a monocentric cohort study conducted in ICU.29 A recent meta-analysis of 1,814 invasively ventilated COVID-19 patients found that in one out of six patients (14.7%), there was a barotrauma, and it was associated with increased mortality.30 Probably the 10% and 15% frequencies of barotrauma reported in these ICU studies were related to the denominator (the number of beds available in ICU), and we suppose that many of these patients were admitted due to barotrauma that had developed before ICU admission, leading to overestimation of its frequency in this setting.
In the modern era, all patients admitted to ICU requiring invasive mechanical ventilation should undergo protective ventilation strategy to reduce ventilator-induced lung injury (VILI).31, 32, 33 The current ventilatory approach, which became universally accepted after the ARDS Network trial, is based on reducing the tidal volume to about 6 mL kg−1 of ideal body weight (IBW) while maintaining the airway plateau pressure below 30 cmH2O.34 Following these guidelines, barotrauma has become very rare in the last two decades, and IMV did not worsen the risk of barotrauma in our study. In contrast, an uncontrolled application of NIV could be much more dangerous if not used appropriately in terms of time, volume and pressure, and possible be implicated in Patient-Self-Induced-Lung-Injury (P-SILI) despite the beneficial effects of C-PAP on recruiting collapsed lung's zone.35 , 36
The vigorous breathing on spontaneous ventilation, also due to altered respiratory drive after viral central nervous system involvement, increases transpulmonary pressure, global and regional strain (barotrauma), self-inflicted lung injury (P-SILI), and can be responsible for the higher inflammation profile.37 , 38
To this end, it is rational to think that protective ventilation was present during IMV and absent during spontaneous breathing and NIV use outside the ICU area, especially in the first phase of the pandemic when ICU beds were fewer than required. Nevertheless, barotrauma events in COVID-19 patients are not restricted to patients receiving positive-pressure ventilation.
SARS-COV-2 is known to strongly activate the host's immune system, triggering a deadly cytokine storm, which contributes to alveolar damage in CARDS. In this setting, patients with an active cytokine storm seem naturally more susceptible to stress-induced lung injury. To date, only a single center retrospective analysis on ICU patients found that fungal infections were more frequent in patients experiencing barotrauma (68.4% vs. 18.9%).39 However, there is still lack of evidence to support the hypothesis of a correlation between barotrauma and fungal infections in units other than ICU.
Barotrauma is a potentially life-threatening complication especially in patients on mechanical ventilation and need to be recognized early in relation to the potential impending hemodynamic instability. Patients developing a tension pneumothorax, or a rarer tension pneumomediastinum with hemodynamic instability, require urgent decompression. In the literature, among those patients who developed PM, 46% had simultaneous or new bilateral pneumothorax and most required bilateral chest tube insertion, 38% progressed to intubation and 31% died.40 , 41
Beitler et al. in non-COVID-19 patients with barotrauma reported that these patients required a high vasopressor support to begin prone positioning.42 We found that barotrauma implies a severe grade of acute lung injury to HRCT scan. Another report in a small cohort highlighted the use of ECMO as respiratory and hemodynamic support.43
We found that pro-adrenomedullin and interleukin-6 levels were associated with barotrauma at the univariate analysis. Although the role of P-SILI is not completely clear, the severe and prolonged inflammation caused by the increase in transpulmonary pressure can lead to extensive endothelial disruption, pulmonary vasoconstriction, oedema, atelectasis and alveolar injury, as reflected by increased biomarkers such as those previously mentioned.44, 45, 46
In relation to length of stay, we found an association between patients with barotrauma and longer hospital stay in agreement with previous studies.
Fungal but not bacterial infection was significantly associated with barotrauma, being probably the clinical expression of a more severe disease with a dysregulated host immunity.
Martinelli et al. failed to demonstrate that pneumothorax would be an independent marker of poor prognosis in COVID-19.47 Mirò et al. also failed to attribute the in-hospital mortality increase in patients with barotrauma.4
In contrast, Kangas-Dick et al.48 and Gazivoda et al.49 found that barotrauma was associated with patients’ deaths, and our data support these findings.
To the best of our knowledge, COVI-MIX is the largest multicentre case-control study that investigates patients with COVID-19 and barotrauma throughout the entire clinical spectrum of the disease, including patients from ED to ICU.
However, some limitations of our study should be highlighted: first, the retrospective design; second, the fact that missing data regarding ventilation parameters were not available for all our analysis; and third, we argue that during the 2020 and 2021 peaks of the pandemic surge, COT, HFNO, and IMV were used as escalation therapies. However, at that moment, the hospitals, particularly the Italian ones, used every imaginable type of ventilation, transforming the wards into real war trenches, and we cannot exclude that approaches other than escalation were used.
Conclusions
COVID-19 patients experienced barotrauma, especially during C-PAP/PSV and IMV. HFNO in this study was not demonstrated to increase the risk of pneumothorax or pneumomediastinum. The frequency of barotrauma in our cohort of patients was lower than in other studies in the literature. Patients with barotrauma were sicker, and more had important systemic inflammatory responses.
Declarations
The Italian COVI-MIX Study Group is represented by Prof. Luigi Vetrugno. 1 , 2
COVI-MIX members:
Edoardo De Robertisi,l, Chiara Aldieriee, Lorenzo Ballff,gg, Elisa Baratellaw, Michele Bartolettihh, Annalisa Boscolop, Barbara Burgazziii, Vito Catalanottihh, Paola Confalonieriw, Silvia Corcionell, Francesco Giuseppe De Rosall,mm, Alessandro De Simoniii, Valerio Del Bononn, Roberta Di Triay, Sara Forlanioo, Daniele Roberto Giacobbet,u, Bianca Granozzihh, Laura Labatet, Sara Lococogg, Tommaso Lupiamm, Carola Matellond, Sara Mehrabiqq, Sabrina Morosirr, Silvia Mongodiss, Maddalena Muratt, Stefano Navauu,vv, Riccardo Polaa, Tommaso Pettenuzzop, Nguyen Hoang Quyenqq, Carolina Rescignoww, Elda Righiqq, Barbara Ruarow, Francesco Saltonw, Silvia Scabinill, Angelo Scardaxx, Marcella Sibaniqq, Evelina Tacconelliqq, Gennaro Tartaglionexx, Beatrice Tazzahh, Eleonora Vaniao, Pierluigi Vialehh, Andrea Vianellopp, Alessandro Visentinqq, Umberto Zucconxx, Francesco Meroio, Danilo Buonsensoyy.
eeDivision of Infectious Diseases, Department of Medicine, Hospital Santa Croce e Carle, Cuneo, Italy.
ffAnesthesia and Intensive Care, Ospedale Policlinico San Martino-IRCCS, Genoa, Italy. ggDepartment of Surgical Sciences and Integrated Diagnostics (DISC), University of Genoa, Genoa, Italy.
hhInfectious Diseases Unit, Department of Medical and Surgical Sciences, Policlinico Sant'Orsola, Bologna, Italy.
iiRespiratory Disease and Lung Function Unit, Department of Medicine and Surgery, University of Parma, Parma, Italy.
llDepartment of Medical Sciences, University of Turin, Infectious Diseases, City of Health and Sciences, Turin, Italy.
mmInfectious Diseases Unit, Cardinal Massaia Hospital, Asti, Italy.
nnDivision of Infectious Diseases, Department of Medicine, Hospital Santa Croce e Carle, Cuneo, Italy.
ooPulmonary Medicine Unit, Lodi General Hospital, Lodi.
ppDepartment of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padova, Padova, Italy.
qqInfectious Diseases Division, Diagnostics and Public Health Department, University of Verona, Verona, Italy.
rrDepartment of Infectious Diseases "Santa Maria della Misericordia" Hospital, University of Perugia, Perugia Italy.
ssAnaesthesia and Intensive Care, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
ttAzienda USL Toscana Nord Ovest, U.O. Medicina Interna, Felice Lotti Hospital, Pontedera, Pisa, Toscana, Italy.
uuDepartment of Clinical, Integrated and Experimental Medicine (DIMES), University of Bologna, Bologna, Italy.
vvRespiratory and Critical Care Unit, Sant Orsola University Hospital, Bologna, Italy.
wwUOC Malattie Infettive ad Indirizzo Neurologico, AORN Ospedali dei Colli, P.O. "D. Cotugno", Naples, Italy.
xxRespiratory Disease Unit, "Santa Maria degli Angeli" Hospital, Pordenone, Italy.
yyDepartment of Woman and Child Health and Public Health, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy.
Ethics approval and consent to participate
Ethics Committee of Friuli-Venezia-Giulia Region, Italy, approved the study with approval number CEUR-2021-3659. Consent to participate was waived.
Consent for publication
Not applicable.
Availability of data and materials
The dataset used and analysed during the current study is available from the corresponding author on reasonable request.
Funding
This research was partially funded by PRIN 2017 n.20178S4EK9 “Innovative statistical methods in biomedical research on biomarkers: from their identification to their use in clinical practice”. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Authors’ contributions
LV, NC, AF contributed to conceptualization, data collection, formal analysis and writing the manuscript. CD contributed to conceptualization, formal analysis and writing the manuscript. AC, FL, FF, GC, DLG, PN, SMM, MB, AC, MC, GF, DF, RL, SM, MS, ES, CT, FB and VP contributed to formal analysis and writing the manuscript.
MI, MDM contributed to conceptualization, formal analysis and writing the manuscript. MI, MDM are responsible for statistical analysis. All authors read and approved the final manuscript.
Declaration of Competing Interest
None.
Acknowledgments
Not applicable.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.pulmoe.2022.11.002.
Appendix. Supplementary materials
References
- 1.Anzueto A., Frutos-Vivar F., Esteban A., Alia I., Brochard L., Stewart T., et al. Incidence, risk factors and outcome of barotrauma in mechanically ventilated patients. Intensive Care Med. 2004;30(4):612–619. doi: 10.1007/s00134-004-2187-7. [DOI] [PubMed] [Google Scholar]
- 2.Udi J., Lang C.N., Zotzmann V., Krueger K., Fluegler A., Bamberg F., et al. Incidence of barotrauma in patients with COVID-19 pneumonia during prolonged invasive mechanical ventilation - a case-control study. J Intensive Care Med. 2021;36(4):477–483. doi: 10.1177/0885066620954364. [DOI] [PubMed] [Google Scholar]
- 3.McGuinness G., Zhan C., Rosenberg N., Azour L., Wickstrom M., Mason D.M., et al. Increased incidence of barotrauma in patients with COVID-19 on invasive mechanical ventilation. Radiology. 2020;297(2):E252–EE62. doi: 10.1148/radiol.2020202352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miro O., Llorens P., Jimenez S., Pinera P., Burillo-Putze G., Martin A., et al. Frequency, risk factors, clinical characteristics, and outcomes of spontaneous pneumothorax in patients with coronavirus disease 2019: a case-control, emergency medicine-based multicenter study. Chest. 2021;159(3):1241–1255. doi: 10.1016/j.chest.2020.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020;323(22):2329–2330. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 7.Grasselli G., Cattaneo E., Florio G., Ippolito M., Zanella A., Cortegiani A., et al. Mechanical ventilation parameters in critically ill COVID-19 patients: a scoping review. Crit Care. 2021;25(1):115. doi: 10.1186/s13054-021-03536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun R., Liu H., Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. 2020;21(5):541–544. doi: 10.3348/kjr.2020.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez Vega J.M., Parra Gordo M.L., Diez Tascon A., Ossaba Velez S. Pneumomediastinum and spontaneous pneumothorax as an extrapulmonary complication of COVID-19 disease. Emerg Radiol. 2020;27(6):727–730. doi: 10.1007/s10140-020-01806-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco D.L., Menga L.S., Eleuteri D., Antonelli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85(9):1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.EDC C. Castor Electronic Data Capture. Available at: https://castoredc.com2019.
- 13.WHO. Living guidance for clinical management of COVID-19. 2021: Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-clinical-21-2.
- 14.Haimovich A.D., Ravindra N.G., Stoytchev S., Young H.P., Wilson F.P., van Dijk D., et al. Development and validation of the quick COVID-19 severity index: a prognostic tool for early clinical decompensation. Ann Emerg Med. 2020;76(4):442–453. doi: 10.1016/j.annemergmed.2020.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., et al. Risk stratification of patients admitted to hospital with COVID-19 using the ISARIC WHO clinical characterisation protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salaffi F., Carotti M., Tardella M., Borgheresi A., Agostini A., Minorati D., et al. The role of a chest computed tomography severity score in coronavirus disease 2019 pneumonia. Medicine. 2020;99(42):e22433. doi: 10.1097/MD.0000000000022433. (Baltimore) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garner J.S., Jarvis W.R., Emori T.G., Horan T.C., Hughes J.M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 18.Kahn M.R., Watson R.L., Thetford J.T., Wong J.I., Kamangar N. High incidence of barotrauma in patients with severe coronavirus disease 2019. J Intensive Care Med. 2021;36(6):646–654. doi: 10.1177/0885066621989959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayazi S., Zebarjadi J., Grubic A.D., Tahmasbi H., Ayazi K., Jobe BA. Pneumothorax as the presenting manifestation of COVID-19. J Thorac Dis. 2020;12(12):7488–7493. doi: 10.21037/jtd-20-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corradi F., Isirdi A., Malacarne P., Santori G., Barbieri G., Romei C., et al. Low diaphragm muscle mass predicts adverse outcome in patients hospitalized for COVID-19 pneumonia: an exploratory pilot study. Minerva Anestesiol. 2021;87(4):432–438. doi: 10.23736/S0375-9393.21.15129-6. [DOI] [PubMed] [Google Scholar]
- 21.Corradi F., Vetrugno L., Orso D., Bove T., Schreiber A., Boero E., et al. Diaphragmatic thickening fraction as a potential predictor of response to continuous positive airway pressure ventilation in Covid-19 pneumonia: a single-center pilot study. Respir Physiol Neurobiol. 2021;284 doi: 10.1016/j.resp.2020.103585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieco D.L., Maggiore S.M., Roca O., Spinelli E., Patel B.K., Thille A.W., et al. Non-invasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med. 2021;47(8):851–866. doi: 10.1007/s00134-021-06459-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauri T., Turrini C., Eronia N., Grasselli G., Volta C.A., Bellani G., et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195(9):1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 24.Crimi C., Pierucci P., Renda T., Pisani L., Carlucci A. High-flow nasal cannula and COVID-19: a clinical review. Respir Care. 2022;67(2):227–240. doi: 10.4187/respcare.09056. [DOI] [PubMed] [Google Scholar]
- 25.Perkins G.D., Ji C., Connolly B.A., Couper K., Lall R., Baillie J.K., et al. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. 2022;327(6):546–558. doi: 10.1001/jama.2022.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crimi C., Noto A., Madotto F., Ippolito M., Nolasco S., Campisi R., et al. High Flow nasal oxygen versus conventional oxygen therapy in patients with COVID-19 pneumonia and mild hypoxemia: a randomised controlled trial. Thorax. 2022 doi: 10.1136/thoraxjnl-2022-218806. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Ito T., Suzuki T., Maeda M., Iwamoto S., Hirayama M., Yamada Y., et al. Pulmonary barotrauma following nasal high-flow therapy in a patient with bronchiolitis obliterans syndrome. Am J Case Rep. 2019;20:1619–1622. doi: 10.12659/AJCR.918580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nalewajska M., Feret W., Wojczynski L., Witkiewicz W., Wisniewska M., Kotfis K. Spontaneous pneumothorax in COVID-19 patients treated with high-flow nasal cannula outside the ICU: a case series. Int J Environ Res Public Health. 2021;18(4) doi: 10.3390/ijerph18042191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X.H., Duan J., Han X., Liu X., Zhou J., Wang X., et al. High incidence and mortality of pneumothorax in critically Ill patients with COVID-19. Heart Lung. 2021;50(1):37–43. doi: 10.1016/j.hrtlng.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belletti A., Todaro G., Valsecchi G., Losiggio R., Palumbo D., Landoni G., et al. Barotrauma in coronavirus disease 2019 patients undergoing invasive mechanical ventilation: a systematic literature review. Crit Care Med. 2022;50(3):491–500. doi: 10.1097/CCM.0000000000005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 32.Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., et al. An official american thoracic society/european society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 33.Chiumello D., Brochard L., Marini J.J., Slutsky A.S., Mancebo J., Ranieri V.M., et al. Respiratory support in patients with acute respiratory distress syndrome: an expert opinion. Crit Care. 2017;21(1):240. doi: 10.1186/s13054-017-1820-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acute Respiratory Distress Syndrome N. Brower R.G., Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 35.Cruces P., Retamal J., Hurtado D.E., Erranz B., Iturrieta P., Gonzalez C., et al. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24(1):494. doi: 10.1186/s13054-020-03197-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cammarota G., Vaschetto R., Turucz E., Dellapiazza F., Colombo D., Blando C., et al. Influence of lung collapse distribution on the physiologic response to recruitment maneuvers during noninvasive continuous positive airway pressure. Intensive Care Med. 2011;37(7):1095–1102. doi: 10.1007/s00134-011-2239-8. [DOI] [PubMed] [Google Scholar]
- 37.Deana C., Verriello L., Pauletto G., Corradi F., Forfori F., Cammarota G., et al. Insights into neurological dysfunction of critically ill COVID-19 patients. Trends Anaesth Crit Care. 2021;36:30–38. doi: 10.1016/j.tacc.2020.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rehmani R., Salazar J., Sharma S., et al. COVID-19 associated spontaneous barotrauma: a literature review. F1000Research. 2021;10:412. doi: 10.12688/f1000research.52381.1. [DOI] [Google Scholar]
- 39.Koukaki E., Rovina N., Tzannis K., Sotiropoulou Z., Loverdos K., Koutsoukou A., et al. Fungal infections in the ICU during the COVID-19 Era: descriptive and comparative analysis of 178 patients. J Fungi. 2022;8(8):881. doi: 10.3390/jof8080881. (Basel) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chu C.M., Leung Y.Y., Hui J.Y., Hung I.F., Chan V.L., Leung W.S., et al. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23(6):802–804. doi: 10.1183/09031936.04.00096404. [DOI] [PubMed] [Google Scholar]
- 41.Clancy D.J., Lane A.S., Flynn P.W., Seppelt I.M. Tension pneumomediastinum: a literal form of chest tightness. J Intensive Care Soc. 2017;18(1):52–56. doi: 10.1177/1751143716662665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beitler J.R., Malhotra A., Thompson B.T. Ventilator-induced lung injury. Clin Chest Med. 2016;37(4):633–646. doi: 10.1016/j.ccm.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paternoster G., Bertini P., Belletti A., Landoni G., Gallotta S., Palumbo D., et al. Venovenous extracorporeal membrane oxygenation in awake non-intubated patients with COVID-19 ARDS at high risk for barotrauma. J Cardiothorac Vasc Anesth. 2022;36(8 Pt B):2975–2982. doi: 10.1053/j.jvca.2022.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zantah M., Dominguez Castillo E., Townsend R., Dikengil F., Criner G.J. Pneumothorax in COVID-19 disease- incidence and clinical characteristics. Respir Res. 2020;21(1):236. doi: 10.1186/s12931-020-01504-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinelli A.W., Ingle T., Newman J., Nadeem I., Jackson K., Lane N.D., et al. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J. 2020;56(5):002697.. doi: 10.1183/13993003.02697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kangas-Dick A., Gazivoda V., Ibrahim M., Sun A., Shaw J.P., Brichkov I., et al. Clinical characteristics and outcome of pneumomediastinum in patients with COVID-19 pneumonia. J Laparoendosc Adv Surg Tech A. 2021;31(3):273–278. doi: 10.1089/lap.2020.0692. [DOI] [PubMed] [Google Scholar]
- 49.Gazivoda V.P., Ibrahim M., Kangas-Dick A., Sun A., Silver M., Wiesel O. Outcomes of barotrauma in critically Ill COVID-19 patients with severe pneumonia. J Intensive Care Med. 2021;36(10):1176–1183. doi: 10.1177/08850666211023360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used and analysed during the current study is available from the corresponding author on reasonable request.