Abstract
Emerging evidence suggests that coronavirus disease-2019 (COVID-19) may lead to a wide range of post-acute sequelae outcomes, including new onset of diabetes. The aim of this meta-analysis was to estimate the incidence of newly diagnosed diabetes in survivors of COVID-19. We searched MEDLINE, Scopus, Cochrane Central Register of Controlled Trials and the World Health Organization Global Literature on Coronavirus Disease and clinical trial registries for studies reporting the association of COVID-19 and diabetes. Search dates were December 2019–October 16, 2022. Two investigators independently assessed studies for inclusion. Risk of bias was assessed using the Newcastle–Ottawa Scale. We estimated the effect of COVID-19 on incident diabetes by random-effects meta-analyses using the generic inverse variance method. We identified 8 eligible studies consisting of 4,270,747 COVID-19 patients and 43,203,759 controls. Median age was 43 years (interquartile range, IQR 35–49), and 50% were female. COVID-19 was associated with a 66% higher risk of incident diabetes (risk ratio, 1.66; 95% CI 1.38; 2.00). The risk was not modified by age, sex, or study quality. The median risk of bias assessment was 7. In this systematic review and meta-analysis, COVID-19 was associated with higher risk for developing new onset diabetes among survivors. Active monitoring of glucose dysregulation after recovery from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is warranted.
Subject terms: Medical research, Infectious diseases, Viral infection
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative strain of coronavirus disease 2019 (COVID-19) was first detected in early December 2019 in Wuhan, China. As of October 16, 2022, more than 625 million COVID-19 cases and 6.6 million deaths were reported globally1.
Post-COVID or long COVID-19 conditions are a wide range of new, returning, or ongoing health problems that individuals experience after first being infected with the virus that causes COVID-192. Emerging evidence suggests that COVID-19 may lead to a wide range of post-acute sequelae outcomes, including new onset of diabetes3–8. The exact mechanisms for incident diabetes in survivors of COVID-19 are not well understood, but it is likely that complex interrelated processes are involved, including previous stress hyperglycemia, steroid-induced hyperglycemia, and direct or indirect effects of SARS-CoV-2 on the β-cells of pancreatic islets4,6,7.
A previous study with more than 180,000 veterans found that patients who survived COVID-19 were 40% more likely to develop diabetes than those who were never diagnosed with COVID-199. Moreover, another study found that up to 14% of people hospitalized for COVID-19 were diagnosed with diabetes later10. However, to date, there is no study that has systematically synthesized the available evidence for the association of COVID-19 with new onset diabetes. A previous systematic review and meta-analysis was limited to only a proportion of newly diagnosed diabetes after COVID-19 with no comparison groups10. We aim to fill this critical knowledge gap by conducting a systematic review and meta-analysis to determine the association of COVID-19 with incident diabetes.
Methods
This study is being reported following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 202011. This study was deemed exempt by the Penn State Institutional Review Board.
Data sources and searches
We searched MEDLINE, Scopus, Cochrane Central Register of Controlled Trials and the World Health Organization Global Literature on Coronavirus Disease and clinical trial registries for studies reporting the association of COVID-19 and diabetes without language restriction. Search dates were December 2019–October 16, 2022. The following Medical Subject Headings and keyword search terms were used; [“diabetes” OR type 2 diabetes OR type 1 diabetes OR “type 1 diabetes mellitus” OR “type 2 diabetes mellitus OR “diabetes mellitus”] AND [“SARS-CoV-2” OR “COVID-19” OR “severe acute respiratory syndrome coronavirus-2” OR “coronavirus disease 2019”].
Study selection
Participant (P) Exposure (E) Comparator [C], Outcome (O) Study type (S) [PECOS] criteria was used to select studies12:
Participants Persons of all ages and sex included in studies that investigated incident diabetes in survivors of COVID-19.
Exposure COVID-19.
Comparison Non-COVID-19 group.
Outcome of interest Diabetes.
Study type Observational studies.
Pairs of independent investigators (YZ and DMB) screened the titles and abstracts of all citations and screened the full-text version of eligible studies. Disagreements in the included papers were resolved by discussion and if necessary, a third investigator (PS) was consulted.
Data extraction and quality assessment
Two investigators (YZ and DMB) worked independently to extract study the following date: authors, publication year, country of the study, study design, study-level descriptive statistics (mean (SD)/median (IQR) age in years, proportion (%) female), sample size, number with diabetes, number with COVID-19, outcome assessment, follow-up time, number of controls, risk ratio and 95% confidence interval. Newcastle–Ottawa Scale for observational studies was used to evaluate the risk of bias13. Studies with fewer than 5 stars were considered low quality; 5 to 7 stars, moderate quality; and more than 7 stars, high quality.
Data synthesis and analysis
The primary outcome was incident diabetes in survivors of COVID-19. For studies without measures of associations, a generalized linear mixed model was used to calculate the RR using the number of events and the sample size of each study group14. One study Barret et al. (2022) used two different national databases and reported separate results. Therefore, in this circumstance, we separated the effect estimates from Barret et al. study into two studies as one with IQVIA database and the second one with HealthVerity3. A study by McKeigure and colleagues reported two separate RRs for diabetes associated with COVID-19 at various time points, therefore, a fixed-effects model was utilized to pool the estimate within the study before conducting the random-effect meta-analysis. The pooled RR estimate for diabetes risk from each study was weighted by the inverse of its variance (inter-study plus intra-study variances). Pooled inter-study variance (heterogeneity) was estimated by DerSimonian and Laird (DL) random-effects method15. Heterogeneity between studies was evaluated with the indicator expressed as percent low (25%), moderate (50%), and high (75%)16. Egger’s linear regression and Begg’s rank tests were employed to quantitatively evaluate publication bias17,18 and qualitatively with funnel plots. Statistical significancy was set at p < 0.05. All statistical analyses were performed with R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria) using Meta and Metafor R packages.
Results
Identified studies
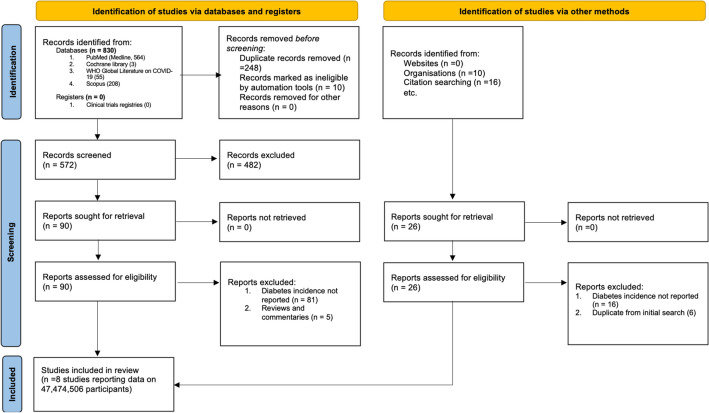
Figure 1 summarizes study selection process. A total of 853 studies were screened. The exclusion process yielded 8 studies3,5,9,19–23 conducted in 3 countries. Barret et al. was reported in this meta-analysis as two independent studies3. The baseline characteristics of the studies included in the systematic review are presented in Table 1. Included studies consisted of patients 47,474,506 participants, with median age of 43 years (IQR 35–49), and 50% were female. The median study quality was 7 (range 5–9).
Figure 1.
PRISMA flow chart of a systematic review of diabetes incidence in survivors of COVID-19. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Meta-analysis characteristics of included cohort studies reporting COVID-19 and risk of diabetes.
| Author (year) | Sample size, N | Female, N (%) | Outcome (diabetes) assessment | Country | Study design | Mean age (y) | Total Case, N | Follow-up periods | Median follow-up time (D) | Reported effect sizes: HR/RR (95% CI) | Covariates in the fully-adjusted model | Quality score | COVID-19 patients | Controls |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rathmann et al. (2022) | 71,730 (35,865 pairs) | 32,732 (45.6%) | ICD-10 codes (E11-E14) | Germany | Retrospective cohort study | 42.6 | 364 | March 2020 to January 2021 | 119 | IRR: 1.28 (1.05, 1.57) | Sex, age, health insurance, index month for Covid-19 and comorbidity (obesity, hypertension, hyperlipidaemia, myocardial infarction, stroke) | 8 | 35,865 | 35,865 |
| Barret et al. (2022) | 485,358 | 243,102 (50.1%) | ICD-10 codes (E08-E13) | US | Retrospective cohort study | 12.3 | 200 | March 2020 to February 2021 | NA | HR: 2.66 (1.98, 3.56) | Matched on age, sex, and month of encounter | 7 | 80,893 | 404,465 |
| Barret et al. (2022) | 878,878 | 440,024 (50.1%) | ICD-10 codes (E08-E13) | US | Retrospective cohort study | 12.7 | 1973 | March 2020 to June 2021 | NA | HR: 1.31 (1.20, 1.44) | Age, sex, and month of encounter | 7 | 439,439 | 439,439 |
| Xie et al. (2022) | 4,299,721 | 485,021 (11.3%) | ICD-10 codes (E08.X to E13.X) or a HbA1c measurement of more than 6·4% (46 mmol/mol) | US | Cohort study | 60.9 | 134,873 | March 2020 to Sept 2021 | 352 | HR: 1.40 (1.36, 1.44) | Age, race, sex, area deprivation index, BMI, smoking status, use of long-term care, number of outpatient and inpatient encounters, and number of HbA1c measurements; comorbidities including cancer, cardiovascular disease, cerebrovascular disease, chronic lung disease, dementia, HIV, hyperlipidaemia, and peripheral artery disease; laboratory test results including estimated glomerular filtration rate (eGFR) and HbA1c; vital signs including systolic and diastolic blood pressure; and medications including the use of steroids | 9 | 181,280 | 4,118,441 |
| Wander et al. (2022) | 2,777,768 | 376,274 (13.5%) |
(1) two or more abnormal laboratory values from plasma or serum (random glucose ≥ 200 mg/dL, fasting glucose ≥ 126 mg/dL, 2-h glucose from an oral glucose tolerance test ≥ 200 mg/dL) or whole blood (A1C ≥ 6.5%); or (2) two outpatient or one inpatient ICD-10 codes of E08–E13; or (3) receipt of an initial and one refill prescription of a glucose-lowering medication |
US | Retrospective cohort study | 59 | 9150 | March 2020 to March 2021 | 120 |
OR for male: 2.56 (2.32, 2.83) OR for female: 1.21 (0.88, 1.68) |
Age, race, ethnicity, BMI, tobacco use, and facility location | 9 | 126,710 | 2,651,058 |
| Daugherty et al. (2021) | 9,247,505 | 4,607,112 (49.8%) | ICD-10 codes | US | Retrospective cohort study | 42.4 | 1886 | January 2020 to October 2020 | 95 | HR: 2.47 (1.14, 5.37) | Propensity score matching with age, sex, socioeconomic status, race, index month, pre-existing comorbidities, total length of stay as an inpatient in the previous year, previous number of visits to a primary care physician, cardiologist, or nephrologist | 5 | 266,586 | 8,980,919 |
| Qeadan et al. (2022) | 27,292,879 | 13,755,616 (54.1%) | ICD-10 codes | US | Retrospective cohort study | 45.4 | 5163 | December 2019 to July 2021 | NA | OR: 1.42 (1.38, 1.46) | Age, gender, race and ethnicity, marital status, and US geographical region | 6 | 2,489,266 | 24,803,613 |
| Kendall et al. (2022) | 571,256 (285,628 matched pairs) | 142,288 (49.8%) | NA | US | Matched Retrospective cohort study | 9.3 | 123 | 2020 to 2021 | NA | HR: 1.83 (1.36, 2.44) | Propensity score matching with age, sex, race, ethnicity, family history of diabetes | 9 | 285,628 | 285,628 |
| McKeigure et al. (2022) | 1,849,411 | 924,706 (50%) | ICD-10 codes (E10–E14) or an outpatient consultation with specialty coded A81 for diabetes | UK | Retrospective cohort study | NA | 1074 | March 2020 to November 2021 | NA |
"RR: 0.86 (0.62, 1.21) for infection > 30 days RR: 2.62 (1.81, 3.78) for infection within 30 days |
Age, sex, and number of vaccine doses at least 14 days before | 7 | 365,080 | 1,484,331 |
Association of COVID-19 and incident diabetes
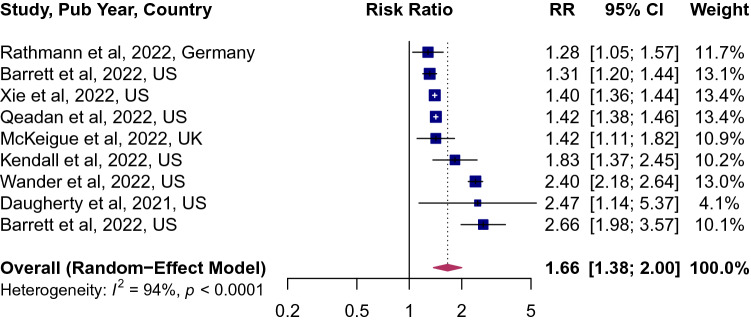
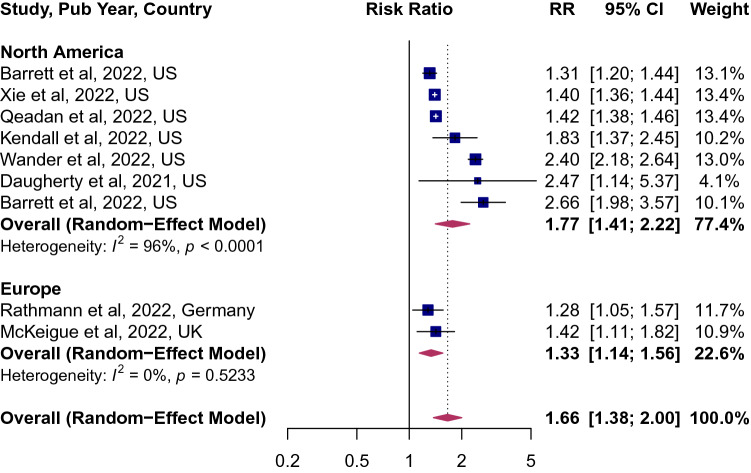
Of the 8 studies that characterized the risk of incident diabetes among survivors of COVID-19, the pooled point estimates was 1.66 (95% CI 1.38; 2.00, Fig. 2), implying a 66% higher risk of diabetes. The between-study variation was high (I2 = 94, p < 0.0001). The risk was not modified by age, sex and study quality (Supplemental Table 1). However, when studies were stratified by geographic region, the risk was higher in studies from the United States 1.77 (95% CI 1.41; 2.22, Fig. 3), compared to those in Europe 1.33 (95% CI 1.14; 1.56).
Figure 2.
Forest plot for the overall pooled estimate for the association of COVID-19 and incident diabetes. Effect size values represent risk ratio and corresponding 95% CI. Blue squares and their corresponding lines are the point estimates of each study and 95% confidence intervals (95% CI). Maroon diamonds represent the pooled estimate (width denotes 95% CI). Heterogeneity (I2 = 94%, p for heterogeneity < 0.0001; 8 studies).
Figure 3.
Forest plot of studies stratified by geographic regions.
Publication bias and study heterogeneity
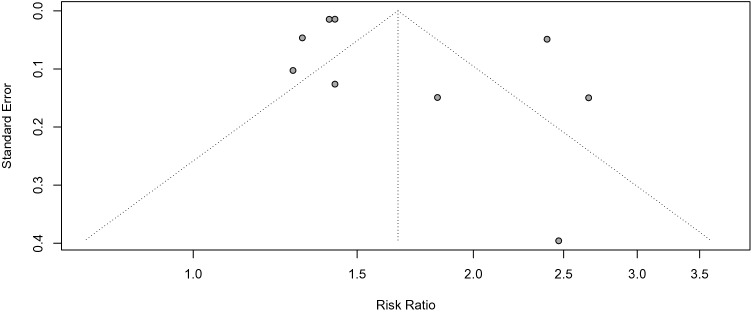
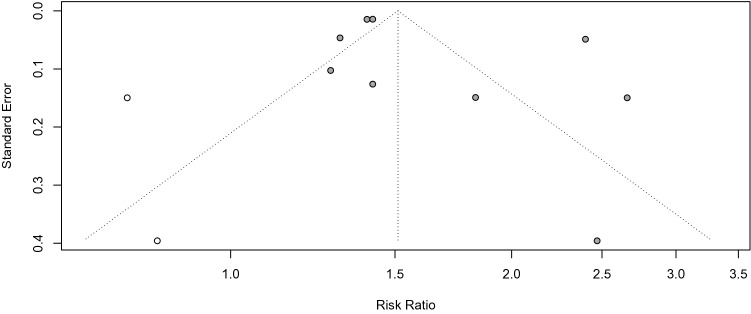
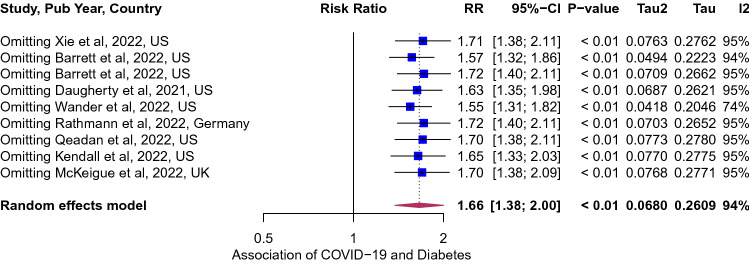
Funnel plot of the included studies (Fig. 4) indicated asymmetry suggesting lack of publication bias. Quantitative analysis of publication bias with Egger’s test (p = 0.053) and Begg's test (p = 0.06) were non-significant. Duval and Tweedie's trim and fill test was conducted to balance the funnel plots and adjust for potential publication bias24. The analysis showed that if publication bias existed, 2 additional studies will be needed to eliminate bias and the overall effect of COVID-19 on incident diabetes changed from 1.66 (95% CI 1.38; 2.00 to 1.51 (1.21; 1.88, Fig. 5). Next, we performed influence sensitivity analyses by excluding and replacing one study at a time from the meta-analysis and calculated the RR for the remaining studies25. No substantial change from any of the pooled RR was observed when other studies were removed in turn, indicating that no individual study had a considerable influence on the pooled estimate. The plots for the analysis estimates are provided in Fig. 6.
Figure 4.
Funnel plots to assess potential for small-study publication bias26. Symmetrical inverted funnel plot suggested absence of publication bias.
Figure 5.
Funnel plots from trim and fill analysis. Duval & Tweedie trim and fill analytical method suggests that the adjusted effect estimates would fall in the range of 1.21 to 1.88, and 2 studies were added24.
Figure 6.
Influence and outlier (leave-one-out meta-analysis) analysis for the association of COVID-19 and incident diabetes27. The results of our outlier and influence analysis show the recalculated pooled point estimate ranged from 1.55 to 1.72 when one study was omitted each time.
Discussion
Principal findings
In this systematic review and meta-analysis of 8 cohort studies including over 47 million participants, COVID-19 was associated with a 66% higher risk of diabetes compared to the controls without COVID-19. The risk was not modified by age, sex, and study quality. The risk of bias assessment was low.
Our findings are consistent with the previous meta-analysis that assessed the proportion of COVID-19 survivors with incident diabetes. A 2021 study by Sathish and colleagues assessed a total of 3711 COVID-19 patients with 492 cases of newly diagnosed diabetes from eight studies10. In the random-effects meta-analysis model, the estimated pooled proportion of incident diabetes was 14.4% (95% CI 5.9–25.8%). They, however, noted a high degree of heterogeneity (I2 98.6%, p < 0.001). The weaknesses of the above study, however, included a lack of a control group and a very small study sample size.
Potential pathophysiological mechanisms of new-onset diabetes among COVID-19 survivors are complex and not fully understood. SARS-CoV-2 binds to angiotensin-converting enzyme 2 and transmembrane serine protease 2 receptors, which are expressed in key metabolic organs and tissues, including pancreatic beta cells, adipose tissue, the small intestine, and the kidneys28–30. Furthermore, it has been demonstrated that SARS-CoV-2 infection attenuates pancreatic insulin levels and secretion and induces β cell apoptosis31,32. Thus, it is plausible that SARS-CoV-2 may cause pleiotropic alterations of glucose metabolism that could lead to incident diabetes or facilitate a rapid transition from the prediabetes state to full-blown diabetes. SARS-CoV-2 is not the only virus associated with diabetes. A significant number of other viruses are associated with type 1 diabetes through molecular mimicry, including Coxsackievirus B, rotavirus, mumps virus, and cytomegalovirus33–35. Furthermore, findings from prospective studies have demonstrated a temporal association between hepatitis C virus and type 2 diabetes36.
Clinical implications of our findings and recommendations
Given the extraordinary number of COVID-19 survivors globally, the modest increase in diabetes risk could correspond to a drastic rise in the number of people diagnosed with the disease worldwide. Therefore, active monitoring of glucose dysregulation after recovery from severe COVID-19 infection is warranted. Additionally, there is a need for studies that determine various social determinants of health associated with new onset diabetes. These factors would be critical to developing effective prevention and management strategies for the disease. Lastly, future research could also focus on employing genomics data to stratify acute COVID-19 patients and predict phenotypes of patients at an increased risk of COVID-19- induced diabetes and uncover novel disease mechanisms.
Limitations
Our study has some limitations worth noting. First, a high degree of heterogeneity was observed, which could have been caused by pooling studies from different sociodemographic populations. Nevertheless, a random effects model was invoked to derive plausible estimates. Second, it is also a possibility that some individuals in the control groups could have had undetected mild or asymptomatic COVID-19 because they had not been tested. Such non-differential misclassification of the exposure may underestimate the strength of the association of COVID-19 with the onset of diabetes. Lastly, due to the limited number of studies included in the present meta-analysis, we did not categorize the risk by the type of diabetes such as type 1 and type 2.
Conclusions
In this systematic review and meta-analysis, COVID-19 was a risk factor for developing new onset diabetes among survivors. Active monitoring of glucose dysregulation after recovery from severe acute respiratory syndrome coronavirus 2 infection is warranted.
Supplementary Information
Author contributions
Designed research (project conception, development of overall research plan, and study oversight): P.S. and D.M.B. Data extraction: Y.Z., P.S., and D.M.B. Analyzed data: P.S. and D.M.B. Performed statistical analysis: P.S. Wrote the first draft of the manuscript: P.S. and D.M.B. Review and editing: P.S., L.W., V.M.C. and D.M.B. All authors have read and approved the final manuscript.
Data availability
All data generated for this study are included in this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24185-7.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet. Infect. Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html. Accessed 25 June 2022.
- 3.Barrett CE, Koyama AK, Alvarez P, et al. Risk for newly diagnosed diabetes >30 days after SARS-CoV-2 infection among persons aged <18 years: United States, March 1, 2020-June 28, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022;71(2):59–65. doi: 10.15585/mmwr.mm7102e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khunti K, Del Prato S, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44(12):2645–2655. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathmann W, Kuss O, Kostev K. Incidence of newly diagnosed diabetes after Covid-19. Diabetologia. 2022;65(6):949–954. doi: 10.1007/s00125-022-05670-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubino F, Amiel SA, Zimmet P, et al. New-onset diabetes in covid-19. N. Engl. J. Med. 2020;383(8):789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh AK, Khunti K. COVID-19 and diabetes. Annu. Rev. Med. 2022;73:129–147. doi: 10.1146/annurev-med-042220-011857. [DOI] [PubMed] [Google Scholar]
- 8.Steenblock C, Schwarz PEH, Ludwig B, et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9(11):786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: A cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–321. doi: 10.1016/S2213-8587(22)00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sathish T, Kapoor N, Cao Y, Tapp RJ, Zimmet P. Proportion of newly diagnosed diabetes in COVID-19 patients: A systematic review and meta-analysis. Diabetes Obes. Metab. 2021;23(3):870–874. doi: 10.1111/dom.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014;14(1):1–10. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Oxford University Press; 2000. [Google Scholar]
- 14.Chang B-H, Hoaglin DC. Meta-analysis of odds ratios: Current good practices. Med. Care. 2017;55(4):328. doi: 10.1097/MLR.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemp. Clin. Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 19.Wander PL, Lowy E, Beste LA, et al. The incidence of diabetes among 2,777,768 veterans with and without recent SARS-CoV-2 infection. Diabetes Care. 2022;45(4):782–788. doi: 10.2337/dc21-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daugherty SE, Guo Y, Heath K, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: Retrospective cohort study. BMJ. 2021;373:n1098. doi: 10.1136/bmj.n1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKeigue PM, McGurnaghan S, Blackbourn L, et al. Relation of incident type 1 diabetes to recent COVID-19 infection: Cohort study using e-health record linkage in Scotland. Diabetes Care. 2022 doi: 10.2337/dc22-0385. [DOI] [PubMed] [Google Scholar]
- 22.Qeadan F, Tingey B, Egbert J, et al. The associations between COVID-19 diagnosis, type 1 diabetes, and the risk of diabetic ketoacidosis: A nationwide cohort from the US using the Cerner Real-World Data. PLoS ONE. 2022;17(4):e0266809. doi: 10.1371/journal.pone.0266809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kendall EK, Olaker VR, Kaelber DC, Xu R, Davis PB. Association of SARS-CoV-2 infection with new-onset type 1 diabetes among pediatric patients from 2020 to 2021. JAMA Netw. Open. 2022;5(9):e2233014–e2233014. doi: 10.1001/jamanetworkopen.2022.33014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: Aa simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 25.Viechtbauer W, Cheung MWL. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods. 2010;1(2):112–125. doi: 10.1002/jrsm.11. [DOI] [PubMed] [Google Scholar]
- 26.Sterne, J. A., Becker, B. J. & Egger, M. The funnel plot. in Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments, 75–98 (2005)
- 27.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: Proposed metrics and empirical evaluation. Int. J. Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C-T, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic & #x3b2; cells and elicits & cell impairment. Cell Metab. 2021;33(8):1565–1576.e1565. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaharuddin SH, Wang V, Santos RS, et al. Deleterious effects of SARS-CoV-2 infection on human pancreatic cells. Front. Cell Infect. Microbiol. 2021;11:678482–678482. doi: 10.3389/fcimb.2021.678482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suresh V, Parida D, Minz AP, Sethi M, Sahoo BS, Senapati S. Tissue distribution of ACE2 protein in syrian golden hamster (Mesocricetus auratus) and its possible implications in SARS-CoV-2 related studies. Front. Pharmacol. 2021;11:330. doi: 10.3389/fphar.2020.579330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Müller JA, Groß R, Conzelmann C, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat. Metab. 2021;3(2):149–165. doi: 10.1038/s42255-021-00347-1. [DOI] [PubMed] [Google Scholar]
- 32.Wu C-T, Lidsky PV, Xiao Y, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565–1576.e1565. doi: 10.1016/j.cmet.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hyöty H, Taylor KW. The role of viruses in human diabetes. Diabetologia. 2002;45(10):1353–1361. doi: 10.1007/s00125-002-0852-3. [DOI] [PubMed] [Google Scholar]
- 34.Pak CY, Eun HM, McArthur RG, Yoon JW. Association of cytomegalovirus infection with autoimmune type 1 diabetes. Lancet. 1988;2(8601):1–4. doi: 10.1016/S0140-6736(88)92941-8. [DOI] [PubMed] [Google Scholar]
- 35.Honeyman MC, Stone NL, Harrison LC. T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: Potential for mimicry with rotavirus and other environmental agents. Mol. Med. 1998;4(4):231–239. doi: 10.1007/BF03401920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang CS, Wang ST, Yao WJ, Chang TT, Chou P. Hepatitis C virus infection and the development of type 2 diabetes in a community-based longitudinal study. Am. J. Epidemiol. 2007;166(2):196–203. doi: 10.1093/aje/kwm061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated for this study are included in this manuscript.