Abstract
Cellular apoptosis is a key pathological mechanism contributing to neuronal death following ischemic stroke. The pro-apoptotic Bcl-2 family protein, Bim, is an important regulator of apoptosis. In this study we investigated the effect of Bim expression on post-stroke functional outcomes, brain injury and inflammatory mechanisms. Wild type (WT) and Bim-deficient mice underwent 1-h middle cerebral artery occlusion (MCAO) followed by 23 h of reperfusion. At 24-h post-stroke, we assessed functional deficit, infarct volume, immune cell death, as well as the number of infiltrating immune cells in the brain and circulating immune cells. Bim deficiency did not affect infarct volume (P > 0.05), but resulted in less motor impairment (~ threefold greater latency to fall in hanging grip strength test, P < 0.05) and a lower median clinical score than WT mice (P < 0.05). Additionally following MCAO, Bim-deficient mice exhibited fewer myeloid cells (particularly neutrophils) in the ischemic brain hemisphere and less apoptosis of CD3+ T cells in the spleen and thymus compared with WT (all P < 0.05). After MCAO, Bim-deficient mice also tended to have more M2-polarised macrophages in the brain than WT mice. In sham-operated mice, we found that Bim deficiency resulted in greater numbers of circulating total CD45+ leukocytes, Ly6Clo+ monocytes and CD3+ T cells, although MCAO did not affect the number of circulating cells at 24 h in either genotype. Our findings suggest that Bim deficiency modulates post-stroke outcomes, including reductions in motor impairment, brain inflammation and systemic post-stroke leukocyte apoptosis. Bim could therefore serve as a potential therapeutic target for stroke.
Keywords: Apoptosis, Stroke, Middle cerebral artery occlusion, Inflammation, Mouse
Introduction
Ischemic stroke is a leading cause of death and disability worldwide. The clot-buster drug, recombinant tissue plasminogen activator, is still the only pharmacological therapy available for ischemic stroke and so a greater understanding of pathophysiological mechanisms in ischemic stroke is vital to identifying potential targets and develop novel therapies (Katan & Luft, 2018). Cell death, be it necrosis or apoptosis, following ischemic stroke is a key pathological mechanism contributing to infarct development. Whilst cells in the ischemic core die within minutes to hours of the event, those in the peri-infarct area may die several hours to days later (Deng et al., 2016; Radak et al., 2017). Although necrosis accounts for a large proportion of early neuronal death post-ischemia, apoptosis is an important cell death mechanism within the peri-infarct area, particularly at later stages (Sairanen et al., 2006). Apoptosis following ischemic stroke can be initiated by several stimuli, including excessive intracellular Ca2+, reactive oxygen species and DNA damage. This occurs mainly through the intrinsic mitochondrial pathway with additional contribution by the extrinsic death receptor-mediated pathway (Broughton et al., 2009). The intrinsic pathway of apoptosis is regulated by the Bcl-2 family proteins, consisting of anti- and pro-apoptotic members, which induce apoptosis when the balance between shifts toward the latter (Doerflinger et al., 2015).
Bcl-2-like protein 11, known as Bim, is a member of the pro-apoptotic BH3-only protein family (O'Connor et al., 1998). It is upregulated in response to a variety of stress stimuli, resulting in the activation of caspases through the intrinsic apoptotic pathway (Lee et al., 2013; Puthalakath et al., 2007). Bim is known to play a role in both physiological processes (e.g. embryonic development, T-cell selection) and disease states (e.g. diabetes mellitus, sepsis) (Doerflinger et al., 2015), including a key regulatory role in β-adrenoreceptor-mediated apoptosis (Lee et al., 2013). Indeed, deletion of Bim leads to an accumulation of lymphocytes due to a reduction in apoptosis (Chougnet et al., 2011). Specifically, Bim-deficient mice have increased numbers of memory T cells post-infection due to less apoptosis once the infection is resolved (Wojciechowski et al., 2006, 2007). Bim-knockout mice have improved outcomes in diseases, such as heart failure and sepsis (Doerflinger et al., 2016; Lee et al., 2013). Furthermore, depletion of CHOP, a transcriptional regulator of Bim, improves outcomes in a rat model of subarachnoid haemorrhage (He et al., 2012). However, the role of Bim in ischemic stroke has not been directly investigated.
It is well established that leukocytes enter the brain from the circulation after stroke. Subpopulations of these cells may exert either pro- or anti-inflammatory actions, resulting in opposing effects on the ongoing brain injury (Zhang et al., 2021). We and others have reported effects of several different leukocyte subtypes (e.g. monocytes, B cells, T cells) in the pathogenesis of ischemic stroke (Benakis et al., 2016; Chu et al., 2015, 2016; Hurn et al., 2007; Yilmaz et al., 2006). Whether Bim regulates the survival of pro- and/or anti-inflammatory leukocytes after stroke is unknown. Therefore, in the present study we have utilised Bim-deficient mice to determine the effect of Bim expression on post-stroke brain and systemic inflammation, brain injury and functional outcome measures.
Methods
Animals
All procedures were approved by the La Trobe University Animal Ethics Committee. Eight- to ten-week-old male C57Bl/6 wild type (WT; n = 38; 29 ± 6 g) and Bim-deficient (Bim−/−; n = 38; 26 ± 5 g) mice were used for experimentation. Bim−/− mice were generated previously (Bouillet et al., 1999). Mice were excluded from the study if they (i) died during the surgical procedure (n = 1), (ii) experienced subarachnoid haemorrhage during intracerebral artery filament insertion (n = 12) or (iii) were euthanized due to not meeting inclusion criteria; i.e. < 65% reduction in regional cerebral blood flow (rCBF) during middle cerebral artery occlusion (MCAO) or < 50% recovery of rCBF within 5 min of reperfusion (n = 2).
Middle Cerebral Artery Occlusion
Ischemia was induced through the occlusion of the right middle cerebral artery (MCA) by an intraluminal filament, as previously described (Kim et al., 2012). Mice were anesthetised by intraperitoneal injection of ketamine–xylazine (100 and 10 mg/kg, respectively) and body temperature was monitored by a rectal thermometer and maintained at 37.5 ± 0.5 °C using a heat lamp. To induce the ischemic event, the right proximal common carotid artery was clamped and a 6–0 nylon monofilament with a silicone-coated tip (Doccol Corporation) was inserted and advanced into the distal internal carotid artery and the Circle of Willis to occlude the origin of the MCA. The resulting occlusion of the MCA was confirmed by transcranial laser Doppler flowmetry (Perimed), where an approximately 80% reduction was observed in the area of the cerebral cortex supplied by the MCA. Once in position, the filament was tied in place and the clamp was removed to allow ischemia to persist for 1 h. The monofilament was then removed to allow for reperfusion for 23 h. Regional CBF (rCBF) levels were monitored for 30-min post-ischemia and reperfusion was confirmed by a return of rCBF to pre-ischemic levels within 5 min. The surgical wound was closed, and the animal was allowed to recover. Mice undergoing sham operation were anesthetised and their right carotid bifurcation exposed and separated from surrounding tissue, without the insertion of the monofilament. Post-surgery, all mice received 1 mL of 0.9% saline subcutaneously. Mice were provided with gel nectar (Able scientific), as well as their usual chow food and water, in individual cages on heat pads until euthanasia by CO2 asphyxiation the following day.
Clinical Neurological Score Assessment
Prior to euthanasia, mice were clinically assessed by an investigator blinded to the experimental conditions and scored using a six-point scoring system. The scoring system was as follows: 0. normal motor function; 1. flexion of torso and contralateral forelimb when mouse is lifted by its tail; 2. circling when mouse is held by its tail on a flat surface; 3. leaning to one side at rest; 4. no spontaneous motor activity and 5. death within the 23-h reperfusion period. Additionally, a hanging grip test was performed to assess forelimb grip strength. Animals were suspended by their forelimbs on a wire at a height of 30 cm for up to 180 s, and the average latency to fall (s) from the wire from three trials interspaced by 5 min was calculated.
Cerebral Infarct Volume
After euthanasia, mice were decapitated, brains were removed and immediately snap-frozen with liquid nitrogen. Thirty-micrometre-thick coronal sections separated by 420 µm were stained with 0.1% thionin to measure the infarct volume. Thionin-stained sections were imaged using TCapture (Version 5.1, Tucsen Photonics). Infarct volume is quantified using ImageJ software (NIH) using the following equation:
where CIV is the corrected infarct volume, RIA is the right hemisphere infarct area, RHA is the right hemisphere area and LHA is the left hemisphere area.
Oedema volume is estimated according to the formula:
where EV is the oedema volume.
Flow Cytometry
Blood was collected by cardiac puncture, after which the mouse was intracardially perfused with PBS. The brain, spleen and thymus were then collected. Isolation of leukocytes from blood was performed using red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 3 mM EDTA). Spleen and thymus cell suspensions were made by mechanical dissociation through a 100 µm cell strainer into FACS buffer (1% bovine serum albumin in PBS), after which spleen cell suspensions were treated with red blood cell lysis buffer. Hemispheres of the brain were separated after removal of the cerebellum and olfactory bulb, which were mechanically dissociated in digestion buffer (125 U/ml collagenase type XI, 60 U/ml hyaluronidase, 450 U/ml collagenase type I-S in Ca2+/Mg2+-containing PBS) and incubated at 37 °C for 45 min in a shaking incubator (550 rpm). The suspension was passed through a 70 µm cell strainer and washed with PBS by centrifugation at 350 RCF and 4 °C for 10 min. After washing, the pellet was resuspended in 3 mL of 30% Percoll (GE Healthcare), beneath which an underlay of 2 mL 70% Percoll was pipetted and the sample was centrifuged at 1400 RCF at room temperature for 20 min with the brake off. The cells at the interphase of the Percoll concentrations were collected and washed in FACS buffer by centrifugation at 350 RCF for 10 min at 4 °C. Cells were counted and 1 × 106 cells was used for subsequent analysis.
Cells are stained with the antibodies listed in Tables 1 and 2. Samples stained with the antibody panel in Table 1 are incubated with their respective combination on ice for 30 min. After their initial stain, all cells were stained with AlexaFluor 680-conjugated Annexin V (made in-house) for a further 1 h on ice. Following the second staining incubation, the cells were washed with FACS buffer by centrifugation at 485 RCF for 5 min at 4 °C. Samples stained with the antibody panel in Table 2 are first incubated with LIVE/DEAD™ Fixable Aqua Dead Cell Stain (Invitrogen) for 15 min at 4 °C. Cells were then washed with FACS buffer by centrifugation at 350 RCF and 4 °C for 5 min. An antibody cocktail containing those directed toward cell surface proteins (see Table 2) was then prepared, with 50 µL incubated on each sample for 25 min at 4 °C. The samples were again washed in FACS buffer by centrifugation. All cells were then fixed and permeabilised with FIX & PERM Cell Fixation & Cell Permeabilization Kit (Invitrogen), incubating them for 20 min at 4 °C. After incubation, the samples were washed with Permeabilization Wash (Invitrogen) diluted in dH2O by centrifugation. For intracellular staining, the FoxP3 antibody was diluted in Permeabilization Wash and applied to each of the samples and incubation at room temperature for 15 min. The samples were washed with Permeabilization Wash by centrifugation and resuspended in 1% formalin in FACS buffer. Cells were analysed using FACS Canto flow cytometer (BD Systems).
Table 1.
Antibodies used for detection of apoptosis of immune cells in the spleen and thymus
| Antigen | Tag | Target cells | Host/isotype | Clone | Supplier |
|---|---|---|---|---|---|
| F4/80 | PE-Cy7 | Macrophages | Rat IgG2a, κ | BM8 | eBioscience |
| CD11b | AF488 | B cells | Rat IgG2b | M1/70 | BD Biosciences |
| Ly6G | PE | Neutrophils | Lewis IgG2a, κ | 1A8 | BD Biosciences |
| CD45R (B220) | V450 | B cells | Rat IgG2a, κ | RA3-6B2 | eBioscience |
| CD3e | FITC | T cells | Armenian Hamster IgG | 145-2C11 | eBioscience |
| CD4 | PE | CD4+ T cells | Rat IgG2b, κ | GK1.5 | eBioscience |
| CD8 | BV510 | CD8+ T cells | Louvain IgG2a, κ | 3–6.7 | BD Biosciences |
Table 2.
Antibodies used for flow cytometry
| Antibody | Tag | Target cells | Host/isotype | Clone | Supplier |
|---|---|---|---|---|---|
| CD45 | A700 | Leukocytes | Rat IgG2b, κ | 30-F11 | BioLegend |
| CD3 | APC | T cells | Armenian Hamster IgG | 145-2C11 | BioLegend |
| CD4 | BV605 | CD4+ T cells | Rat IgG2a, κ | RM4-5 | BioLegend |
| FoxP3 | PE-Cy5.5 | Regulatory T cells | Rat IgG2a, κ | FJK-16s | eBioscience |
| CD11b | BV421 | Myeloid cells | Rat IgG2b, κ | M1/70 | BioLegend |
| CD206 | PE | M2 macrophages | Rat IgG2a, κ | RA-6B2 | BioLegend |
| F4/80- | APC-Cy7 | Microglia/macrophages | Rat IgG2a, κ | BM8 | BioLegend |
| Ly6C | FITC | Monocytes | Rat IgG2c, κ | HK1.4 | BioLegend |
| Ly6G | PE-Cy7 | Neutrophils | Rat IgG2a, κ | 1A8 | BioLegend |
Statistics
Outliers were removed from data using the ROUT method (Q = 1%). Statistical analyses of flow cytometry, spleen weight and thymus weight were performed using two-way ANOVA with Bonferroni multiple comparisons test between the means of relevant groups in R 4.1.0. All other experiments (except for the clinical score) were tested for statistical significance using an unpaired t test, assuming Gaussian distribution and equal variance between populations. Clinical score comparison was analysed using the Mann–Whitney test. Statistical significance was taken at P < 0.05.
Results
Bim-Deficient Mice have Less Severe Functional Outcomes After Stroke
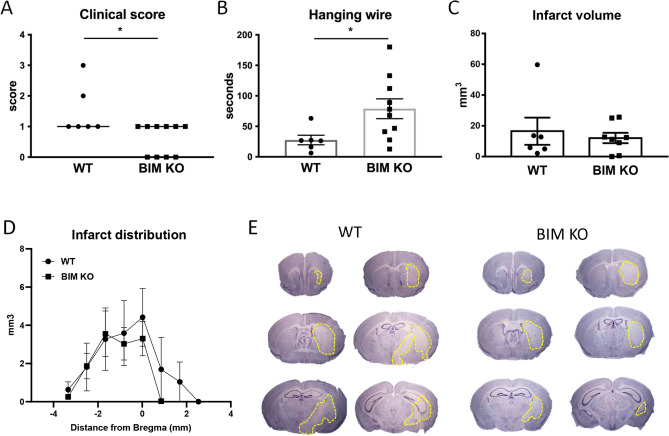
Neurological impairment, as measured by median clinical score, was reduced in Bim−/− compared to WT mice at 24 h after transient MCAO (Fig. 1A; P < 0.05). Furthermore, latency to fall in the hanging grip test was increased by approximately threefold in Bim−/− compared with WT mice (Fig. 1B; P < 0.05). These improvements in functional outcome were evident despite no differences in infarct size or distribution in WT and Bim−/− mice (Fig. 1C–E).
Fig. 1.
Effect of Bim deletion on functional outcomes and infarct volume. A Clinical severity score, B hanging wire latency to fall, C–E infarct volume and distribution in wild type (WT) and Bim-deficient (BIM KO) mice at 24 h after stroke. A Data are presented as median, *P < 0.05 compared with WT, Mann–Whitney test. B, D Data are presented as mean ± SEM, *P < 0.05 compared with WT, unpaired t test, n = 6–10
Bim-Deficient Mice Exhibit Less Apoptosis of Immune Cells In Spleen and Thymus After Stroke
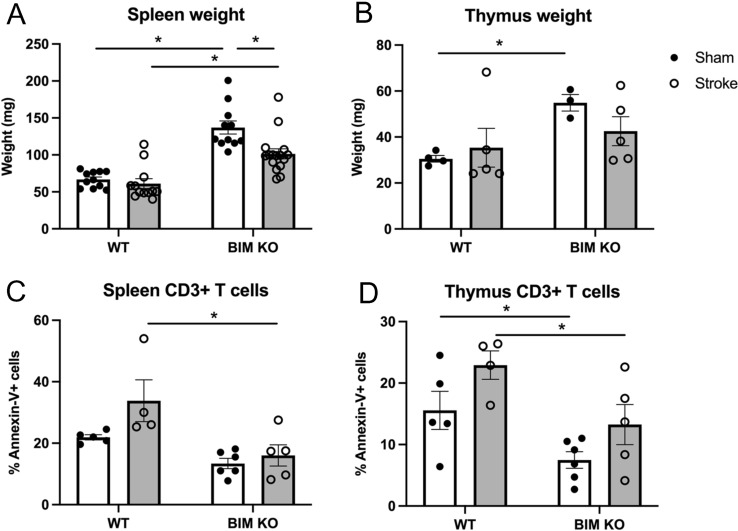
We found that sham-operated Bim−/− mice had larger spleens and thymuses than sham-operated WT mice (Fig. 2A, B), whilst MCAO resulted in a reduced spleen weight in Bim−/− mice only (Fig. 2A; P < 0.05). After stroke, there were fewer Annexin V+ apoptotic CD3+ T cells in spleens and thymuses of Bim−/− mice compared with WT mice (Fig. 2C, D).
Fig. 2.
Effect of Bim deletion on apoptosis in peripheral immune organs. A Spleen and B thymus weight and the number of Annexin V+ CD3+ T cells in the C spleen and D thymus in wild type (WT) or Bim-deficient mice 24 h after sham or stroke surgery. Data are presented as mean ± SEM, *P < 0.05 compared with WT, two-way ANOVA with Bonferroni multiple comparisons test, n = 3–16
Bim-Deficient Mice have Less Infiltration of Immune Cells in the Brain After Stroke
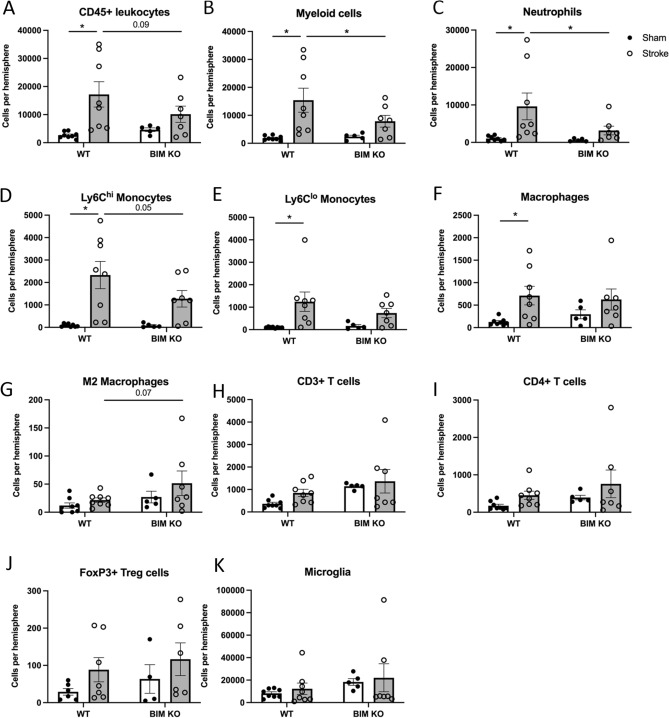
In the brain, Bim deficiency did not affect the total number of leukocytes or any individual leukocyte subset in sham-operated mice (Fig. 3). After stroke, the total number of CD45+ leukocytes was increased by sixfold in the ischemic hemisphere of WT mice, and this increase tended to be smaller in Bim−/− mice (Fig. 3A). Similar profiles, with smaller post-stroke increases in Bim−/− mice, were observed for myeloid cells (CD11b+) and neutrophils (Ly6G+) (Fig. 3B, C). There was also a trend for fewer Ly6Chi monocytes (Fig. 3D) and more M2 (CD206+)-polarised macrophages (Fig. 3G) in the brain of Bim−/− mice than WT mice after stroke, but no strain-dependent differences in number of Ly6Clo monocytes or macrophages (Fig. 3E, F). Neither surgery nor Bim expression had any effect on the number of CD3+ T cells, CD4+ T cells or FoxP3+ T cells (T-regulatory cells) (Fig. 3H–J). The number of microglia (CD45med CD11b+) was similar in all groups (Fig. 3B).
Fig. 3.
Effect of Bim deletion on leukocytes in the brain after stroke. The number of A total leukocytes (CD45+), B myeloid cells (CD11b+), C neutrophils (Ly6G+), D Ly6Chi monocytes, E Ly6Clo monocytes, F macrophages (F4/80+), G M2-polarised macrophages (CD206+), H T cells (CD3+), I CD4+ T cells, J T-regulatory cells (FoxP3+) and K microglia (CD45med CD11b+), C in the ischemic brain hemisphere at 24 h after stroke. Data are presented as mean ± SEM, *P < 0.05 compared with WT, two-way ANOVA with Bonferroni multiple comparisons test, n = 4–8
Bim-Deficient Mice have More Circulating Immune Cells After Stroke
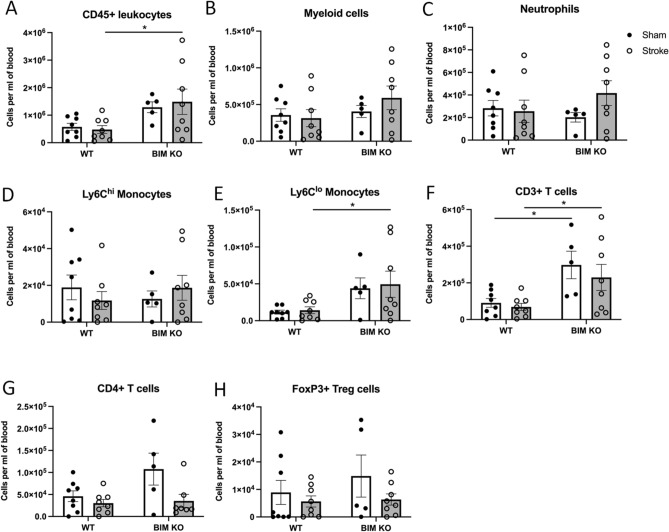
In the blood of sham-operated mice, there was a tendency for more CD45+ leukocytes in Bim−/− than WT mice (Fig. 4A). At 24 h after stroke, there were more circulating CD45+ leukocytes in Bim−/− mice compared with WT mice (Fig. 4A). There were more circulating Ly6Clo monocytes and CD3+ T cells in Bim−/− mice compared with WT mice, with no effect of stroke (Fig. 4E, F). Neither stroke nor deletion of Bim affected the number of circulating myeloid cells, neutrophils, Ly6Chi monocytes, CD4+ T cells, or FoxP3+ T cells (Fig. 4B–D, G–H).
Fig. 4.
Effect of Bim deletion on circulating leukocytes after stroke. The number of A total leukocytes (CD45+), B myeloid cells (CD11b+), C neutrophils (Ly6G+), D Ly6Chi monocytes, E Ly6Clo monocytes, F T cells (CD3+), G CD4+ T cells and H T-regulatory cells (FoxP3+) per mL of blood 24 h after stroke. Data are presented as mean ± SEM, *P < 0.05 compared with WT, two-way ANOVA with Bonferroni multiple comparisons test, n = 5–8
Discussion
Apoptosis is known to be a key pathological mechanism following stroke. As well as directly affecting the brain parenchyma, apoptosis may influence post-stroke outcomes by regulating immune cell survival. As an important regulator of the pro-apoptotic pathway, it is plausible that Bim may influence stroke outcomes. In this study, we utilised mice deficient in Bim to determine the effect of its expression on outcomes after ischemic stroke. In comparison to WT mice, we found that Bim−/− mice had milder functional impairment at 24 h following ischemic stroke despite a similar infarct volume. Bim deficiency also reduced lymphocyte apoptosis in peripheral immune organs and infiltration of select immune cell subtypes into the brain after stroke. Our findings suggest that Bim−/− mice have improved functional outcomes after ischemic stroke compared with WT mice, and this may be attributable to reduced immune cell apoptosis and infiltration into the brain.
The ultimate goal of stroke therapy is to improve functional outcomes. Studies have shown that functional improvements after ischemic stroke are not necessarily simply related to a reduced infarct volume (Kim et al., 2014; Zhang et al., 2018). Thus, whilst neuroprotective agents—for example—may be a focus of research efforts to limit infarct growth, a failure to improve functional outcomes will result in limited or no clinical utility. In the present study, we show that genetic deletion of Bim significantly reduces clinical neurological severity score and improves forelimb grip strength compared with WT mice despite no difference in infarct volume or distribution.
Bim plays a key role in regulating leukocyte apoptosis. Bim deletion has been shown to result in increased numbers of neutrophils and T cells in the spleen and thymus (Bouillet et al., 2002; Chougnet et al., 2011; Herold et al., 2014; Villunger et al., 2003). Consistent with these findings, we found that Bim−/− mice had enlarged spleens, which was associated with reduced Annexin V+ staining of CD3 cells. Bim−/− mice also had reduced apoptosis in the thymus after stroke compared with WT mice. The reduced level of T-cell apoptosis in Bim−/− mice may contribute to the increased number of circulating T cells that we observed in all Bim−/− mice. Therefore, peripheral leukocyte apoptosis may be an important factor in stroke outcomes. Indeed, simvastatin treatment has been shown to reduce post-stroke splenocyte apoptosis and spleen atrophy, which was associated with reduced brain injury and functional impairment (Jin et al., 2013). Thus, preventing splenic atrophy may lead to improved stroke outcomes.
The role of inflammation in the pathogenesis of ischemic stroke is of significant interest. Due to the fact that inflammation occurs over hours to days, and potentially chronically, after the ischemic event, inflammatory mechanisms may represent an opportunity for therapeutic intervention that is much more feasible than the 4.5-h window for clot-buster therapy (Powers et al., 2018). It is important to note that infiltrating immune cells can exert either pro- or anti-inflammatory effects in the brain following the onset of ischemia (Zhang et al., 2021). This complex inflammatory response to ischemic stroke is highlighted by the finding that administration of a broad-specificity chemokine-binding protein is only temporarily effective in delaying inflammation-driven infarct development, indicating that other inflammatory molecules and pathways besides chemokine signalling are involved (Lee et al., 2015). Thus, a clearer understanding of the role of specific immune cell subtypes, chemokines and cytokines is needed to enable the development of immunomodulatory therapies. Consistent with previous studies, MCAO promoted leukocyte infiltration into the brain (Benakis et al., 2016; Chu et al., 2014; Gelderblom et al., 2009; Kim et al., 2014). In transient MCAO, infiltrating immune cells are thought to localise to the infarct core and the peri-infarct region (Beuker et al., 2021). Bim deficiency tended to reduce the overall number of infiltrating cells, and furthermore there were clear changes in the profile of infiltrating cells. In particular, Bim deficiency resulted in fewer infiltrating myeloid cells following stroke, which was primarily due to reduced numbers of neutrophils entering the ischemic hemisphere. Neutrophils are amongst the first cells to enter the brain following an ischemic event and may contribute to worsened outcomes (Chou et al., 2004; Chu et al., 2014; Gelderblom et al., 2009; Huang et al., 2000). Whilst we did not determine whether the infiltrating neutrophils were pro-(N1) or anti-(N2) inflammatory neutrophils, the accompanying reduction in motor deficit would be consistent with fewer infiltrating N1-like neutrophils in Bim−/− mice. In addition, the tendency for an increase in infiltrating M2 macrophages is also consistent with a shift to a more anti-inflammatory profile with Bim deficiency. Different profiles of pro- and anti-inflammatory leukocytes in the post-stroke brain have previously been reported to account for different functional outcomes (Chu et al., 2015; Kim et al., 2014).
Whilst we did not observe an increase in overall T cells, T-helper cell or T-regulatory cell numbers in the ischemic hemisphere of WT mice (Brait et al., 2010; Gelderblom et al., 2009; Kim et al., 2014; Yilmaz et al., 2006), there was a trend for these cells to be increased by 2–3fold after stroke. Like neutrophils and monocytes/macrophages, infiltrating T cells have been shown to be critical mediators of ischemic stroke injury (Hurn et al., 2007; Yilmaz et al., 2006). The reason for these conflicting results is currently unclear.
Whilst the absolute number of T cells infiltrating the brain was similar in WT and Bim−/− mice, it is possible that these cells have a reduced inflammatory capacity. Indeed, Bim deficiency has been reported to impair T-cell activation and cytokine production (Ludwinski et al., 2009). T cells lacking Bim have a reduced production of several pro- (e.g. IL-6, IFN-γ) and anti- (e.g. IL-4, IL-10) inflammatory cytokines, which have been shown to play critical roles in stroke injury (Zhang et al., 2021). Thus, Bim deficiency may reduce post-stroke impairment via shifting infiltrating immune cells to a less inflammatory phenotype and impairing T-cell function. This modulation of the inflammatory response after stroke may be the underlying cause of improved functional outcomes despite no change in infarct volume.
In conclusion, here we have provided direct evidence that Bim expression is an important factor in neurological outcome following ischemic stroke. Bim deficiency resulted in modulation of the post-stroke immune response leading to less motor and neurological deficit despite no change in infarct size. This beneficial effect of Bim deletion for stroke outcome may be at least partly related to a shift to a more anti-inflammatory response to injury in the ischemic brain. We therefore postulate that Bim may be a potential therapeutic target for ischemic stroke.
Acknowledgements
The authors were supported by Grants from the National Health and Medical Research Council of Australia (NHMRC) (Grant Nos. 606488; 1064686; 1085323; 1163282; 2003156).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Jason Glab, Shenpeng Zhang, Michael De Silva and Hyun Ah Kim. The first draft of the manuscript was written by Jason Glab and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
T. Michael De Silva and Hyun Ah Kim—Co-senior authors.
Contributor Information
Jason A. Glab, Email: Jason.glab@monash.edu
Hamsa Puthalakath, Email: H.Puthalakath@latrobe.edu.au.
Shenpeng R. Zhang, Email: S.Zhang@latrobe.edu.au
Antony Vinh, Email: A.Vinh@latrobe.edu.au.
Grant R. Drummond, Email: G.Drummond@latrobe.edu.au
Christopher G. Sobey, Email: C.Sobey@latrobe.edu.au
T. Michael De Silva, Email: T.Desilva@latrobe.edu.au.
Hyun Ah Kim, Email: H.Kim2@latrobe.edu.au.
References
- Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nature Medicine. 2016;22(5):516–523. doi: 10.1038/nm.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuker C, Strecker JK, Rawal R, Schmidt-Pogoda A, Ruck T, Wiendl H, et al. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: A systematic review and meta-analysis. Translational Stroke Research. 2021;12(6):976–990. doi: 10.1007/s12975-021-00887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Köntgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286(5445):1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415(6874):922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- Brait VH, Jackman KA, Walduck AK, Selemidis S, Diep H, Mast AE, et al. Mechanisms contributing to cerebral infarct size after stroke: Gender, reperfusion, T lymphocytes, and Nox2-derived superoxide. Journal of Cerebral Blood Flow and Metabolism. 2010;30(7):1306–1317. doi: 10.1038/jcbfm.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BRS, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Chou WH, Choi DS, Zhang H, Mu D, McMahon T, Kharazia VN, et al. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. The Journal of Clinical Investigation. 2004;114(1):49–56. doi: 10.1172/JCI21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, et al. A major role for Bim in regulatory T cell homeostasis. Journal of Immunology. 2011;186(1):156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HX, Broughton BR, Kim HA, Lee S, Drummond GR, Sobey CG. Evidence that Ly6C(hi) monocytes are protective in acute ischemic stroke by promoting M2 macrophage polarization. Stroke. 2015;46(7):1929–1937. doi: 10.1161/STROKEAHA.115.009426. [DOI] [PubMed] [Google Scholar]
- Chu HX, Kim HA, Lee S, Broughton BRS, Drummond GR, Sobey CG. Evidence of CCR2-independent transmigration of Ly6C(hi) monocytes into the brain after permanent cerebral ischemia in mice. Brain Research. 2016;1637:118–127. doi: 10.1016/j.brainres.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Chu HX, Kim HA, Lee S, Moore JP, Chan CT, Vinh A, et al. Immune cell infiltration in malignant middle cerebral artery infarction: Comparison with transient cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism. 2014;34(3):450–459. doi: 10.1038/jcbfm.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y-H, He H-Y, Yang L-Q, Zhang P-Y. Dynamic changes in neuronal autophagy and apoptosis in the ischemic penumbra following permanent ischemic stroke. Neural Regeneration Research. 2016;11(7):1108–1114. doi: 10.4103/1673-5374.187045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger M, Glab J, Nedeva C, Jose I, Lin A, O'Reilly L, et al. Chemical chaperone TUDCA prevents apoptosis and improves survival during polymicrobial sepsis in mice. Science and Reports. 2016;6:34702–34702. doi: 10.1038/srep34702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger M, Glab JA, Puthalakath H. BH3-only proteins: A 20-year stock-take. FEBS Journal. 2015;282(6):1006–1016. doi: 10.1111/febs.13190. [DOI] [PubMed] [Google Scholar]
- Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43(2):484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold MJ, Stuchbery R, Mérino D, Willson T, Strasser A, Hildeman D, et al. Impact of conditional deletion of the pro-apoptotic BCL-2 family member BIM in mice. Cell Death & Disease. 2014;5(10):e1446–e1446. doi: 10.1038/cddis.2014.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Choudhri TF, Winfree CJ, McTaggart RA, Kiss S, Mocco J, et al. Postischemic cerebrovascular E-selectin expression mediates tissue injury in murine stroke. Stroke. 2000;31(12):3047–3053. doi: 10.1161/01.STR.31.12.3047. [DOI] [PubMed] [Google Scholar]
- Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. Journal of Cerebral Blood Flow and Metabolism. 2007;27(11):1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Zhu X, Liu L, Nanda A, Granger DN, Li G. Simvastatin attenuates stroke-induced splenic atrophy and lung susceptibility to spontaneous bacterial infection in mice. Stroke. 2013;44(4):1135–1143. doi: 10.1161/STROKEAHA.111.000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M, Luft A. Global burden of stroke. Seminars in Neurology. 2018;38(2):208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- Kim HA, Brait VH, Lee S, De Silva TM, Diep H, Eisenhardt A, et al. Brain infarct volume after permanent focal ischemia is not dependent on Nox2 expression. Brain Research. 2012;1483:105–111. doi: 10.1016/j.brainres.2012.09.023. [DOI] [PubMed] [Google Scholar]
- Kim HA, Whittle SC, Lee S, Chu HX, Zhang SR, Wei Z, et al. Brain immune cell composition and functional outcome after cerebral ischemia: Comparison of two mouse strains. Frontiers in Cellular Neuroscience. 2014;8:365. doi: 10.3389/fncel.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Chu HX, Kim HA, Real NC, Sharif S, Fleming SB, et al. Effect of a broad-specificity chemokine-binding protein on brain leukocyte infiltration and infarct development. Stroke. 2015;46(2):537–544. doi: 10.1161/STROKEAHA.114.007298. [DOI] [PubMed] [Google Scholar]
- Lee YY, Moujalled D, Doerflinger M, Gangoda L, Weston R, Rahimi A, et al. CREB-binding protein (CBP) regulates β-adrenoceptor (β-AR)-mediated apoptosis. Cell Death and Differentiation. 2013;20(7):941–952. doi: 10.1038/cdd.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwinski MW, Sun J, Hilliard B, Gong S, Xue F, Carmody RJ, et al. Critical roles of Bim in T cell activation and T cell-mediated autoimmune inflammation in mice. The Journal of Clinical Investigation. 2009;119(6):1706–1713. doi: 10.1172/JCI37619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO Journal. 1998;17(2):384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129(7):1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, et al. Apoptosis and acute brain ischemia in ischemic stroke. Current Vascular Pharmacology. 2017;15(2):115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- Sairanen T, Karjalainen-Lindsberg ML, Paetau A, Ijas P, Lindsberg PJ. Apoptosis dominant in the periinfarct area of human ischaemic stroke–a possible target of antiapoptotic treatments. Brain. 2006;129(Pt 1):189–199. doi: 10.1093/brain/awh645. [DOI] [PubMed] [Google Scholar]
- Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101(6):2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- Wojciechowski S, Jordan MB, Zhu Y, White J, Zajac AJ, Hildeman DA. Bim mediates apoptosis of CD127(lo) effector T cells and limits T cell memory. European Journal of Immunology. 2006;36(7):1694–1706. doi: 10.1002/eji.200635897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, et al. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. Journal of Experimental Medicine. 2007;204(7):1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz G, Arumugam TV, Stokes KY, Granger DN. Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation. 2006;113(17):2105–2112. doi: 10.1161/CIRCULATIONAHA.105.593046. [DOI] [PubMed] [Google Scholar]
- Zhang, S. R., Piepke, M., Chu, H. X., Broughton, B. R., Shim, R., Wong, C. H., Lee, S., Evans, M. A., Vinh, A., Sakkal, S., Arumugam, T. V., Magnus, T., Huber, S., Gelderblom, M., Drummond, G. R., Sobey, C. G., & Kim, H. A. (2018). IL-33 modulates inflammatory brain injury but exacerbates systemic immunosuppression following ischemic stroke. JCI Insight, 3(18), 10.1172/jci.insight.121560. [DOI] [PMC free article] [PubMed]
- Zhang SR, Phan TG, Sobey CG. Targeting the Immune System for Ischemic Stroke. Trends in Pharmacological Sciences. 2021;42(2):96–105. doi: 10.1016/j.tips.2020.11.010. [DOI] [PubMed] [Google Scholar]