Abstract
Pyroptosis is commonly induced by the gasdermin (GSDM) family and is accompanied by the release of inflammatory cytokines such as IL-1β and IL-18. Recently, increasing evidence suggests that pyroptosis plays a role in respiratory diseases. This review aimed to summarize the roles and mechanisms of pyroptosis in inflammation-related respiratory diseases. There are several pathways involved in pyroptosis, such as the canonical inflammasome-induced pathway, non-canonical inflammasome-induced pathway, caspase-1/3/6/7/GSDMB pathway, caspase-8/GSDMC pathway, caspase-8/GSDMD pathway, and caspase-3/GSEME pathway. Pyroptosis may be involved in asthma, chronic obstructive pulmonary disease (COPD), lung cancer, acute lung injury (ALI), silicosis, pulmonary hypertension (PH), and tuberculosis (TB), in which the NLRP3 inflammasome-induced pathway is mostly highlighted. Pyroptosis contributes to the deterioration of asthma, COPD, ALI, silicosis, and PH. In addition, pyroptosis has dual effects on lung cancer and TB. Additionally, whether pyroptosis participates in cystic fibrosis (CF) and sarcoidosis or not is largely unknown, though the activation of NLRP3 inflammasome is found in CF and sarcoidosis. In conclusion, pyroptosis may play a role in inflammation-related respiratory diseases, providing new therapeutic targets.
Keywords: Pyroptosis, Inflammation, Respiratory diseases, Inflammasomes, Gasdermin, Caspase-1
Introduction
Pyroptosis is a novel programmed cell death (PCD) form induced by the gasdermin (GSDM) family, accompanied by the secretion of inflammatory cytokines such as IL-1β and IL-18 [18, 63]. When activated, pyroptosis results in the elimination of bacterial, fungal, and viral pathogens through the robust inflammatory response which is indispensable for organismal homeostasis [29]. However, pyroptosis may also be detrimental to the body, now that the deterioration of diseases occurs with pyroptosis. It is reported that pyroptosis plays an essential role in several diseases, such as cancers, atherosclerosis, and so on [57, 65, 79]. Respiratory diseases are considered to account for the increased mortality rate [64, 67]. It is well established that respiratory diseases are closely related to inflammation [30, 48]. Increasing evidence indicates that pyroptosis is crucial for inflammatory diseases [65]. Recently, accumulative studies focus on the role of pyroptosis in the inflammatory diseases of the lung [89, 91]. This review aimed to summarize the roles and plausible mechanisms of pyroptosis in inflammatory diseases of the lung.
Molecular mechanism of pyroptosis
As an original PCD form, pyroptosis was discovered after apoptosis and necrosis [79]. In 1992, Zychlinsky et al. first observed this new death form, which was mistakenly depicted as apoptosis in Shigella flexneri-infected macrophages [94]. Later, this new cell death form was termed pyroptosis by Brennan following the observation of Salmonella-mediated death in macrophages [14]. The characteristics of pyroptosis, to a certain extent, are analogous to those of apoptosis, such as nuclear pyknosis and DNA fragmentation. Meanwhile, pyroptosis is also accompanied by some necrosis-like features, including cellular swelling, pore formation, and membrane disruption [57]. A large body of evidence suggests that the GSDM family leads to the formation of membrane channels and then pyroptosis [63]. The GSDM family consists of six members in humans (GSDMA, GSDMB, GSDMC, GSDMD, GSDME which is also called DFNA5, and GSDMF which is also known as DFNB59 or PJVK) [73]. It is reported that GSDMA, GSDMB, GSDMC, GSDMD, and GSDME are closely related to pyroptosis [73]. Most members of the GSDM family share the highly conserved N-terminal domain (NTD) and C-terminal domain (CTD) [93]. The NTD is activated by the dissociation of CTD to form an inner pore on the membrane which interacts with the cytosol and extracellular space directly [38], allowing the leakage of intercellular contents and the inflow of extracellular water. Accordingly, pyroptosis is redefined as the GSDM-mediated PCD [63].
Increasing studies suggest that various signaling pathways participate in pyroptosis [63, 79].
Inflammasome-induced signaling pathway
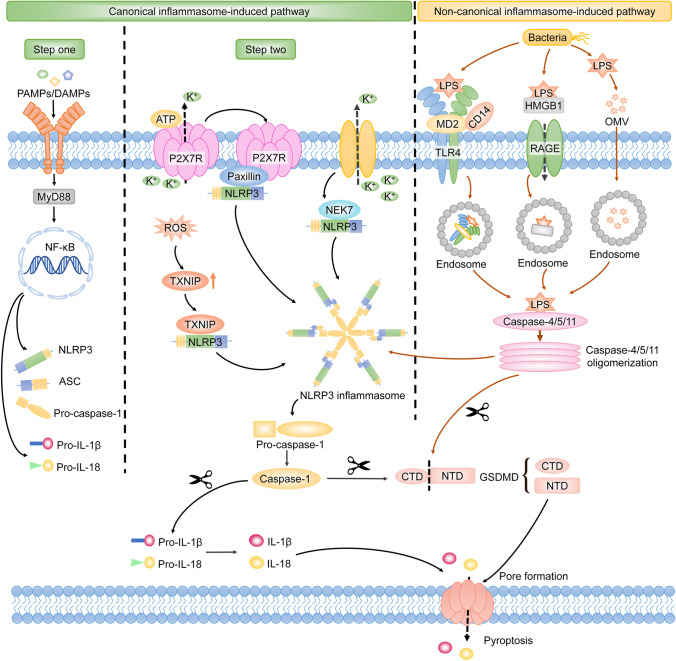
Pyroptosis is originally mediated by the inflammatory caspases, which are activated by the inflammasomes [29]. Inflammasomes are multi-protein complexes that contain certain pattern-recognition receptors (PRRs) [29]. Pyroptosis-related PRRs include membrane-bound Toll-like receptors (TLRs), the nucleotide-binding domain and leucine-rich repeat-containing receptors (NLRs), and the absent in melanoma (AIM)-like receptors (ALRs) [33, 79]. These PRRs sense pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) when host cells suffer from abnormal conditions such as microbial infection, stress, and tissue damage, which lead to the activation of inflammasomes [33]. Subsequently, activated inflammasomes lead to the cleavage of inflammatory caspases, which allows the activation of GSDMD [38]. Consequently, the GSDMD-NTD oligomerizes to form the membrane channel, and then pyroptosis occurs [38]. In light of the different involvement of inflammasomes and distinct kinds of inflammatory caspases, the pyroptotic signaling pathways are divided into canonical and non-canonical inflammasome-induced pathways [43] (Fig. 1).
Fig. 1.
Canonical and non-canonical inflammasome-induced pathways in pyroptosis. The NLRP3 inflammasome is mostly highlighted in the canonical inflammasome-induced pathway. The NLRP3 inflammasome is activated depending on two steps. Step 1 is that the PAMPs or DAMPs stimulate the TLRs/MyD88/NF-κB signaling pathway, leading to the upregulated transcription of NLRP3, ASC, pro-caspase-1, pro-IL-1β, and pro-IL-18. In step 2, the formation of the P2X7R-paxillin-NLRP3 complex activates the NLRP3 inflammasome, which depends on the ATP/P2X7R axis-induced potassium efflux. Simultaneously, NEK7 interacts with NLRP3 in response to the declined intercellular potassium, which contributes to the activation of the NLRP3 inflammasome. In addition, increased TXNIP can also activate the NLRP3 inflammasome by directly binding to NLRP3. The activated NLRP3 inflammasome excites caspase-1. Sequentially, GSDMD is cleaved by caspase-1 and releases GSDMD-NTD to form the membrane pore. Then pyroptosis occurs. Meanwhile, IL-1β and IL-18 are activated by caspase-1 and secreted from the GSDMD-induced pore, which results in the inflammatory cascade downstream. LPS is transferred into the cytosol by the formation of an endosome, which is induced by TLR4/MD2/CD14 complex, HMGB1/RAGE signaling pathway, or OMVs. Intracellular LPS directly activates caspase-4/5/11. Activated caspase-4/5/11 cleaves GSDMD to induce pore formation and pyroptosis. During this procedure, caspase-4/5/11 activates the NLRP3 inflammasome by inducing potassium efflux. Note: MyD88, myeloid differentiation factor 88; NF-κB, nuclear factor kappa-B; ASC, apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain; P2X7R, P2X7 receptor; NEK7, NIMA-related kinase 7; TXNIP, thioredoxin interacting protein; GSDMD, gasdermin D; NTD, N-terminal domain; HMGB1, high mobility group box 1; RAGE, receptor for advanced glycation end-products; OMVs, outer membrane vesicles
Canonical inflammasome-induced pathway
Canonical inflammasomes include AIM2, NLRP1, NLRP3, NLRC4, and pyrin inflammasomes [33, 56]. Canonical inflammasomes play an important role in the canonical inflammasome-induced pathway. They are composed of the sensor acting as PRRs, the effector acting as the pro-caspase-1, and the adaptor acting as the apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain (ASC) which connects the sensor and the effector [56]. The canonical inflammasomes are activated by distinct PAMPs or DAMPs. The AIM2 inflammasome is only activated by cytoplasmic double-stranded DNA (dsDNA) [33]. In addition, NLRP1 and NLRC4 inflammasomes are specially activated by the anthrax lethal toxin and bacterial flagellin, respectively [3, 46]. Meanwhile, the assembly of NRLP3 is triggered by diverse PAMPs, including bacterial pathogens and viral DNA, as well as DAMPs involving ATP, reactive oxygen species (ROS), uric acid crystals, and silica [80]. Currently, more researchers are focusing on the mechanisms of NLRP3 inflammasome in pyroptosis. The NLRP3 inflammasome is activated depending on two steps [80, 84]. Step 1 is that the PAMPs or DAMPs stimulate the TLRs/MyD88/NF-κB signaling pathway, leading to the upregulated transcription of NLRP3, ASC, and pro-caspase-1 [80]. Then, step 2 is the assembly and activation of the NLRP3 inflammasome. In step 2, paxillin recruits NLRP3 to form the P2X7 receptor (P2X7R)-paxillin-NLRP3 complex, which is dependent on the potassium efflux induced by the ATP/P2X7R signaling pathway, leading to the activation of NLRP3 inflammasome [76]. Simultaneously, the NIMA-related kinase 7 (NEK7) facilitates the oligomerization of ASC by interacting with NLRP3 in response to the intercellular decline of potassium concentration [23]. Recently, it has been reported that the thioredoxin-interacting protein (TXNIP) mediated by ROS plays an important role in the activation of the NLRP3 inflammasome. Sufficient ROS may activate endoplasmic reticulum stress (ERS). When ERS is initiated, phosphorylation of protein kinase R-like endoplasmic reticulum kinase (PERK), which is an ER transmembrane sensor, leads to the activation of eukaryotic translation-initiation factor 2α (eIF2α). Activated eIF2α upregulates the expression of CCAAT-enhancer-binding protein homologous protein (CHOP). The activation of the PERK/eIF2α/CHOP signaling pathway results in the upregulation of TXNIP, which activates the NLRP3 inflammasome by directly interacting with NLRP3 [80]. Accordingly, paxillin, NEK7, and TXNIP are important molecules in the activation of the NLRP3 inflammasome. The activated NLRP3 inflammasome excites caspase-1 depending on the ASC speck. Similar to the NLRP3 inflammasome, the AIM2 and pyrin inflammasomes activating caspase-1 are dependent on ASC speck. NLRP1 and NLRC4 inflammasomes activate caspase-1 independent of the ASC speck [3, 46]. Active caspase-1 cleaves GSDMD to promote the formation of the membrane pore [38]. Meanwhile, caspase-1 cleaves pro-IL-1β and pro-IL-18 into mature IL-1β and IL-18 [18]. Subsequently, IL-1β and IL-18 are secreted from the GSDMD-induced pore, resulting in the inflammatory cascade downstream. It is confirmed that IL-1β is involved in various inflammatory diseases via binding to IL-1 receptor and IL-18 participates in the regulation of both T helper cell 1 (Th1)-type and Th2-type inflammatory cytokines [17, 72] (Fig. 1).
Non-canonical inflammasome-induced pathway
Human caspase-4/5 and murine caspase-11 are referred to as non-canonical inflammasomes [28, 43]. It is reported that intracellular lipopolysaccharide (LPS) activating caspase-4/5/11 plays a critical role in the non-canonical inflammasome-induced pathway of pyroptosis [79, 84]. The extracellular LPS is transferred into cells via TLR4/MD2/CD14 receptor-mediated endocytosis [45] or with the help of high mobility group box 1 protein and the receptor for advanced glycation end-products (RAGE) [43, 84], or through outer membrane vesicles (OMVs) [84]. The intracellular LPS will then directly interact with caspase-4/5/11. The activation of caspase-4/5/11 results in the cleavage of GSDMD independent of caspase-1 [28]. Thus, pyroptosis occurs. Different from the canonical inflammasome-induced pathways which is caspase-1-dependent, activated caspase-4/5/11 does not process the precursors of IL-1β and IL-18 but still leads to the secretion of these two cytokines via the GSDMD-NTD channel [28]. Additionally, it is reported that the LPS-activated caspase-11 triggers the activation of the NLRP3 inflammasome by inducing potassium efflux [84]. Accordingly, there may be crosstalk between the canonical and non-canonical inflammasome-induced pathways of pyroptosis (Fig. 1).
Other signaling pathways
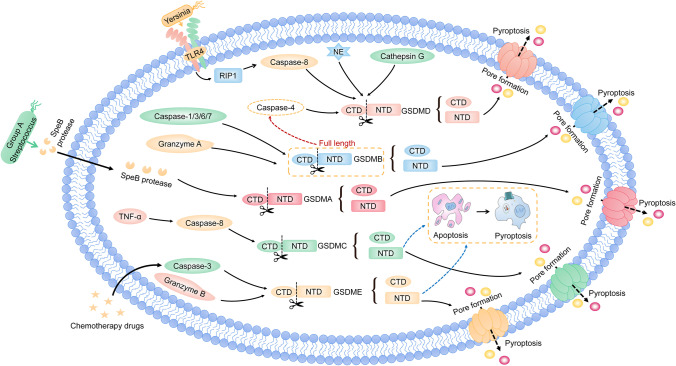
Apart from the inflammasome-induced pathway, there are other signaling pathways in pyroptosis. In the other signaling pathways, GSDM family-induced pyroptosis is independent of the inflammasomes and the activation of the GSDM family plays a critical role in pyroptosis [63]. Currently, the members of the GSDM family, including GSDMA, GSDMB, GSDMC, GSDMD, and GSDME, lead to pyroptosis [93] (Fig. 2).
Fig. 2.
Other signaling pathways in pyroptosis. Most members of the GSDM family play an important role in the pyroptotic signaling pathways. GSDMD can be cleaved by NE, cathepsin G, and caspase-8, which is activated by Yersinia via the TLR4/RIP1 signaling pathway to induce pyroptosis. GSDMB is cleaved by caspase-1/3/6/7 and granzyme A to trigger pyroptosis. Meanwhile, the full length of GSDMB enhances caspase-4 cleaving GSDMD to undergo pyroptosis. The cleavage of GSDMC is executed by caspase-8 to induce pyroptosis. GSDME is cleaved to induce pyroptosis with chemotherapy drugs that initiate caspase-3. Additionally, GSDME can be processed by granzyme B to initiate pyroptosis. Moreover, the caspase-8/GSDMC and caspase-3/GSDME signaling pathways can switch apoptosis to pyroptosis. Note: GSDMD, gasdermin D; NE, neutrophil elastase; RIP1, receptor interacting protein 1; GSDMB, gasdermin B; GSDMA, gasdermin A; GSDMC, gasdermin C; GSDME, gasdermin E
GSDMD is the first member of the GSDM family found to execute pyroptosis [28]. Recently, it is reported that caspase-8, which is activated by Yersinia via TLR4/receptor-interacting protein 1 signaling pathway, exhibits cleaving GSDMD to drive pyroptosis [60]. Furthermore, neutrophil elastase and cathepsin G can also activate GSDMD, which results in pyroptosis [73].
In addition, GSDMA is widely expressed in the upper gastrointestinal tract [93]. Though its domains are similar to GSDMD, the active protease of GSDMA is largely unknown. Recently, it is reported that the GSDMA is specifically cleaved by the SpeB protease of group A Streptococcus to trigger pyroptosis in keratinocytes [16]. However, whether GSDMA can be activated by other proteases remains unclear.
Different from other GSDM family members, both the cleaved and full-length GSDMB can lead to pyroptosis [93]. It is reported that GSDMB is cleaved by the caspase-1/3/6/7 as well as granzyme A to undergo pyroptosis [73, 92]. While the full-length GSDMB enhances caspase-4 cleaving GSDMD, indirectly promoting non-canonical inflammasome-induced pyroptosis [11].
Meanwhile, the cleavage of GSDMC is executed by caspase-8 to induce pyroptosis. In addition, the upregulation of GSDMC mediated by programmed death-ligand 1 (PD-L1) switches apoptosis to pyroptosis in cancer cells [24].
GSDME is highlighted in cancer for its suppressive activity on the tumor. It is suggested that GSDME is cleaved to induce pyroptosis with chemotherapy drugs that initiate caspase-3 [77]. In addition, the apoptosis induced by caspase-3 is turned on to undergo pyroptosis in the GSDME-positive cells. Accordingly, the caspase-3/GSDME signaling pathway balances the switch between apoptosis and pyroptosis [77]. Moreover, GSDME is cleaved by granzyme B to produce GSDME-NTD, and then pyroptosis is triggered by GSDME [73].
Collectively, most GSDMs play an essential role in pyroptosis. However, further research into the specific mechanism and signaling pathways of this pore-forming protein-induced pyroptosis is warranted in the future.
Pyroptosis and inflammation-related respiratory diseases
Pyroptosis and asthma
Asthma is a common chronic respiratory disease that affects more than 300 million individuals worldwide [67]. The main characteristics of asthma include allergic airway inflammation, airway hyperresponsiveness (AHR), and airway remodeling.
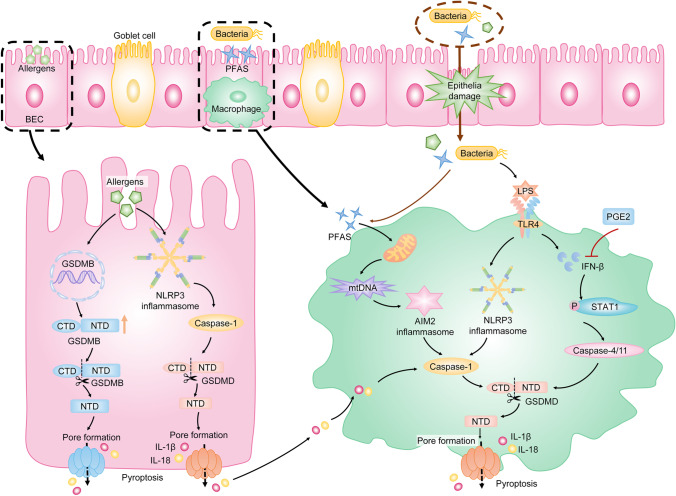
Disruption of bronchial epithelial cell (BEC) barrier is important in the pathogenesis of asthma [70, 91]. Damaged epithelia fail to provide mucociliary clearance and allow various allergens to affect the lung [70]. Meanwhile, loss of epithelial integrity leads to the recruitment of inflammatory cells by releasing cytokines and chemokines [70]. It is reported that exposure to environmental allergens such as Dermatophagoides farina 1 or toluene diisocyanate triggers pyroptosis in BECs by activating the NLRP3/caspase-1/GSDMD signaling pathway and induces airway inflammation [70, 91]. Selective inhibition of NLRP3 inflammasome effectively prevents airway allergic inflammation and AHR [30, 91]. Accordingly, NLRP3/caspase-1/GSDMD-induced pyroptosis of BECs may be involved in the deterioration of asthma. Additionally, GSDMB is an executioner of pyroptosis and is related to the pathogenesis of asthma. The increased level of GSDMB is found in asthmatics [15], and the upregulated expression of GSDMB in BECs increases the susceptibility to asthma [39]. These results suggest that GSDMB-induced pyroptosis may play an important role in asthma.
Macrophages are the main effectors against environmental particles. Inflammation mediated by macrophages via pyroptosis may be an important promoter in the development of asthma [59]. It is reported that perfluoroalkyl substances induce GSDMD-dependent pyroptosis in macrophages by activating the AIM2 inflammasome, which aggravates asthmatic inflammation by enhancing the secretion of IL-4 and IL-1β [75]. Prostaglandin E2 (PGE2) plays a protective role in asthma by inhibiting bronchoconstriction and inflammation [86]. It is reported that PGE2 suppresses pyroptosis in macrophages via inhibiting the IFN-β/signal transducer and activator of transcription 1 (STAT1)/caspase-11/GSDMD signaling pathway, which protects the host from allergic airway inflammation [86]. Furthermore, inhibiting pyroptosis of macrophages reduces airway inflammation and airway remodeling in LPS-induced asthmatic rats via suppressing the NLRP3/caspase-1/GSDMD axis [12].
In summary, these studies directly or indirectly indicate that pyroptosis may play a crucial role in asthma. However, the specific mechanism of pyroptosis in asthma needs to be explored (Fig. 3).
Fig. 3.
Pyroptosis in asthma. Environmental allergens activate the NLRP3/caspase-1/GSDMD signaling pathway to trigger pyroptosis of BECs. Meanwhile, allergens induce the increased transcription of GSDMB, which results in GSDMB-dependent pyroptosis. Pyroptosis of BECs leads to the loss of epithelial integrity and increased secretion of pro-inflammatory cytokines such as IL-1β and IL-18, which promote augmented and persistent pulmonary inflammation downstream. Additionally, macrophages are the main effectors against environmental particles. PFAS triggers AIM2 inflammasome-induced pyroptosis of macrophages by causing mitochondrial damage, which results in the accumulation of mtDNA. Additionally, bacteria induce pyroptosis of macrophages by activating the TLR4/NLRP3/caspase-1/GSDMD pathway. Meanwhile, LPS causes the upregulation of IFN-β, which results in the activation of STAT1. The activation of STAT1 leads to the increased caspase-4/11, which is important in the pyroptotic signaling pathway. PGE2 indirectly inhibits the caspase-4/11-induced pyroptosis in macrophages by suppressing IFN-β to take a protective role in asthma. Note: BECs, bronchial epithelial cells; PFAS, perfluoroalkyl substance; mtDNA, mitochondrial DNA; AIM2, absent in melanoma 2; IFN-β, interferon-beta; STAT1, signal transducer and activator of transcription 1; PGE2, prostaglandin E2
Pyroptosis and chronic obstructive pulmonary disease
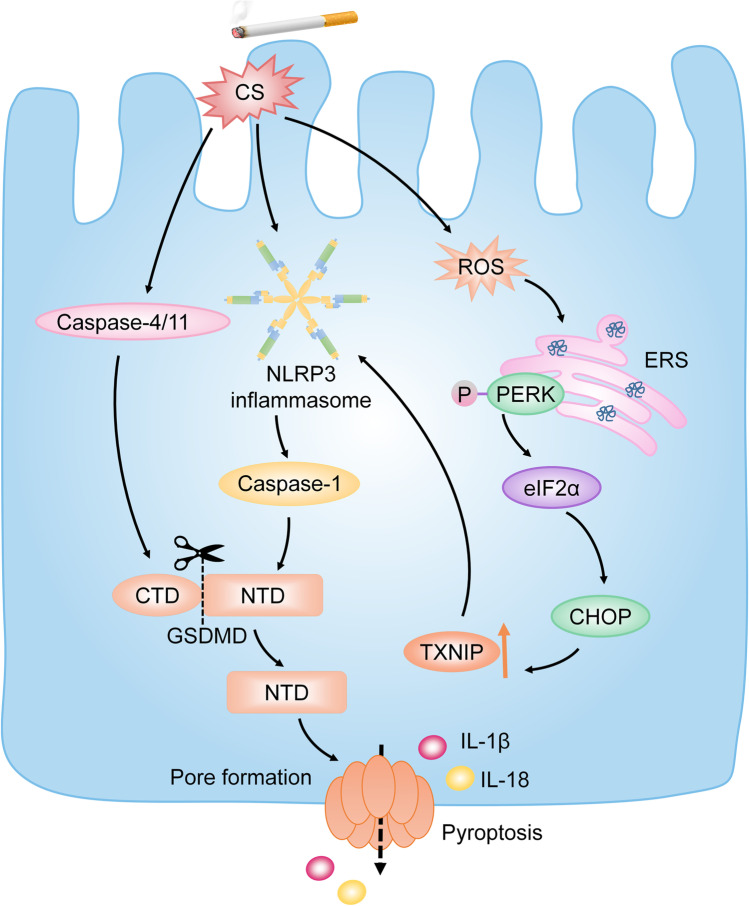
Chronic obstructive pulmonary disease (COPD), regarded as the third most lethal disease in the world, is characterized by consistent airway inflammation and irreversible airflow obstruction [74]. The persistent airway inflammation causes airway remodeling, which is characterized by sloughing of BECs, mucus hypersecretion, and abnormal deposition of collagen [37]. A large body of evidence suggests that cigarette smoke (CS) is the predominant risk factor for COPD [47, 89].
Currently, it is shown that CS extract (CSE) activates the NLRP3/caspase-1/GSDMD signaling pathway and sequentially induces pyroptosis in BECs, which causes the impaired BEC barrier and severe lung injury [74]. Inhibiting the NLRP3 inflammasome results in the downregulation of GSDMD, which causes pyroptosis, as well as the alleviation of pulmonary inflammation [47, 89]. Furthermore, accumulating studies suggest that pyroptosis is activated by CS-induced ERS via ROS and participates in the progression of COPD [41, 89]. CS induces the generation of sufficient ROS to activate ERS and mitochondrial dysregulation [41]. Sequentially, ERS-induced activation of the PERK/eIF2α/CHOP signaling pathway results in increased TXNIP. TXNIP is an important molecule in pyroptosis by activating the NLRP3 inflammasome. It is reported that inhibition of ROS suppresses pyroptosis in CS-treated BEC via inhibiting the ROS/TXNIP/NLRP3/caspase-1 pathway, which leads to the amelioration of COPD both in vitro and vivo [7, 41, 89]. Thus, CS may induce pyroptosis to deteriorate COPD through activating the ROS/TXNIP/NLRP3/caspase-1 signaling pathway.
Additionally, caspase-4, the human analog of murine caspase-11, may be partially responsible for the airway inflammation in COPD [13]. It is reported that caspase-11 knockout in mice treated with CS results in the reduced bronchial deposition of collagen and mucus hypersecretion, which are related to airway remodeling [13]. Therefore, caspase-4/11-induced pyroptosis may participate in CS-induced COPD via the activation of non-canonical inflammasome-induced pathway. However, the specific signaling pathway remains unclear (Fig. 4).
Fig. 4.
Pyroptosis in COPD. CS is the predominant risk factor for COPD. CS triggers pyroptosis of BECs by activating the NLRP3/caspase-1/GSDMD signaling pathway. Meanwhile, CS induces ERS to trigger pyroptosis of BECs via mediating the generation of sufficient ROS. The initiation of CS-induced ERS results in the activation of PERK, which sequentially upregulates TXNIP by activating the PERK/eIF2α/CHOP pathway. Increased TXNIP contributes to the activation of the NLRP3 inflammasome. Consequently, pyroptosis occurs. Additionally, CS induces caspase-4/11-induced pyroptosis in BECs and accelerates airway remodeling. Note: COPD, chronic obstructive pulmonary disease; CS, cigarette smoke; BECs, bronchial epithelial cells; ERS, endoplasmic reticulum stress; PERK, protein kinase R-like endoplasmic reticulum kinase; TXNIP, thioredoxin interacting protein; eIF2α, eukaryotic translation-initiation factor 2α; CHOP, CCAAT-enhancer-binding protein homologous protein
Pyroptosis and lung cancer
Lung cancer is one of the malignant tumors with the highest morbidity and mortality [64]. Long-term exposure to risk factors results in the continuous activation of the inflammatory response, while chronic inflammation plays an essential role in lung cancer. Inflammasomes play a critical role in the host inflammatory response. It is reported that increased NLRP3 inflammasome, AIM2 inflammasome, caspase-1, IL-1β, and IL-18 are found in lung cancer cells [31]. Notably, the expression of NLRP3 inflammasome is significantly upregulated in high-grade lung adenocarcinoma patients who have a higher risk of progression and postoperative recurrence [31]. NLRP3 inflammasome, AIM2 inflammasome, and caspase-1 are important molecules in pyroptosis. Therefore, pyroptosis may play an important role in lung cancer. It is shown the higher expression of GSDMD, which is the classical executioner of pyroptosis, is associated with larger tumor sizes and more advanced tumor-node-metastasis stage in non-small cell lung cancer (NSCLC) patients. Depletion of GSDMD attenuates the proliferation of NSCLC cells and restricts the growth of tumor cells by facilitating caspase-3-induced apoptosis [21]. These studies suggest that pyroptosis may promote tumor growth by providing an inflammatory microenvironment [79].
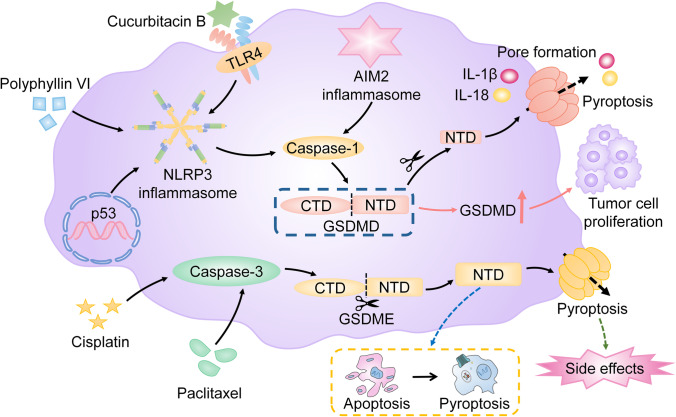
However, pyroptosis may also participate in the elimination of cancer tissues by triggering tumor cell death. Many potent anti-tumor drugs can trigger pyroptosis to inhibit the growth of tumors. It is reported that the NLRP3/caspase-1/GSDMD-dependent pyroptosis is enhanced in NSCLC cells treated with polyphyllin VI, leading to the elimination of tumor cells [68]. Meanwhile, cucurbitacin B induces pyroptosis to inhibit the growth of NSCLC cells by activating the TLR4/NLRP3/caspase-1/GSDMD signaling pathway [85]. Moreover, it is demonstrated that p53, which is a tumor suppressor gene, induces pyroptosis to attenuate the proliferation of NSCLC cells by activating the NLRP3/ASC/caspase-1 signaling pathway [90]. Additionally, GSDME, which is regarded as a potential tumor suppressor, is confirmed to execute pyroptosis after the cleavage of caspase-3. It is shown that the NSCLC patients with lower expression of GSDME in tumor tissues are inclined to have a higher mortality rate after platinum treatment [49]. Further research suggests that the GSDME-induced pyroptosis may enhance the sensitivity of cisplatin to NSCLC cells [49]. Meanwhile, it is reported that the increased level of GSDME activated by caspase-3 with chemotherapeutic drugs such as cisplatin and paclitaxel leads to the switch from apoptosis to pyroptosis in lung cancer cells [49, 87]. Accordingly, GSDME-induced pyroptosis may play an important role in lung cancer. Notably, GSDME expression is higher in normal tissues such as the gastrointestinal tract and kidney than in cancer tissues [77, 93]. It is reported that caspase-3/GSDME-dependent pyroptosis mediated by cisplatin occurs in normal tissue and produces chemotherapy side effects during the cisplatin treatment, while inhibition of the caspase-3/GSDME-mediated pyroptosis enhances the tolerance of normal tissue cells to cisplatin [25]. Therefore, it is suggested that caspase-3/GSDME-mediated pyroptosis may be involved in the cisplatin-based side effects such as digestive tract reactions, intestinal injury, and nephrotoxicity [25], which may provide a novel insight into the prevention of cisplatin-based side effects [25, 87].
Collectively, pyroptosis may have dual effects on lung cancer because it can promote both inflammation and cell death. However, the determinants of pyroptosis to induce inflammation or cell death remain unknown (Fig. 5).
Fig. 5.
Pyroptosis in lung cancer. Increased GSDMD contributes to the proliferation of NSCLC cells. However, many potent anti-tumor drugs, such as polyphyllin VI and cucurbitacin B, induce NLRP3/caspase-1/GSDMD signaling pathway-mediated pyroptosis. Meanwhile, p53 induces pyroptosis to attenuate the proliferation of NSCLC cells by activating the NLRP3/ASC/caspase-1 signaling pathway. Additionally, chemotherapeutic drugs such as cisplatin and paclitaxel trigger caspase-3/GSDME-dependent pyroptosis to eliminate NSCLC cells and lead to the switch from apoptosis to pyroptosis in lung cancer cells. Notably, caspase-3/GSDME-dependent pyroptosis, which is mediated by cisplatin in normal tissues, contributes to the cisplatin-based side effects. Note: GSDMD, gasdermin D; NSCLC, non-small cell lung cancer; ASC, apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain; GSDME, gasdermin E
Pyroptosis and acute lung injury
Acute lung injury (ALI) is a life-threatening hypoxemic respiratory disease. Acute progressive pulmonary inflammation characterized by infiltration of inflammatory cells, increased permeability of the alveolar-capillary barrier, and acute diffuse alveolar edema are commonly found in ALI [10, 36].
Consistent excessive inflammation induced by alveolar macrophages (AMs) is the key to ALI [10]. Increasing studies indicate that pyroptosis of AMs may contribute to the development of ALI by mediating inflammation. It is reported that GSDMD-dependent pyroptosis is triggered by LPS in AMs via activating the TLR4/NLRP3/caspase-1 pathway, which leads to severe lung damage characterized by infiltration of inflammatory cells, pulmonary interstitial edema, hemorrhage, and alveolar wall thickness [27, 36]. Inhibition of NLRP3 inflammasome results in reduced pyroptosis and inflammatory cytokines such as IL-1β and IL-18 in AMs, which is followed by improved lung injury [27, 36, 82]. Additionally, the p38 MAPK signaling pathway, which mediates inflammation, is shown to participate in the pyroptosis of AMs in ALI by mediating the activation of NLRP3 inflammasome. It is reported that a selective inhibitor of the p38 MAPK signaling pathway, SB203580, suppresses the NLRP3/caspase-1-dependent pyroptosis in AMs, which results in the amelioration of lung inflammation [35]. Accordingly, NLRP3/caspase-1/GSDMD-mediated pyroptosis of AMs may participate in the development of ALI. Additionally, pyroptosis mediated by anthrax lethal toxin-activated NLRP1 inflammasome in AMs promotes lung inflammation and results in the exacerbation of ALI [32]. Furthermore, growing evidence suggests that pyroptosis may also trigger the enhanced inflammatory response in ALI by generating pyroptotic bodies (PyrBDs), which are mainly derived from pyroptotic AMs [52]. PyrBDs, which contain substantial DAMPs, trigger vascular interstitial edema and neutrophil recruitment [52].
In addition, loss of pulmonary endothelial integrity, which controls lung vascular permeability, is important in the pathogenesis of ALI [44]. The pulmonary endothelial cells are invariably exposed to LPS or other pathogens circulating in the blood. Therefore, pyroptosis of pulmonary endothelial cells may play an important role in ALI. It is shown that caspase-11/GSDMD-mediated pyroptosis in pulmonary endothelial cells leads to pulmonary edema, while the endothelial-specific deletion of caspase-11 reverses pulmonary inflammation and lung damage [9] (Fig. 6).
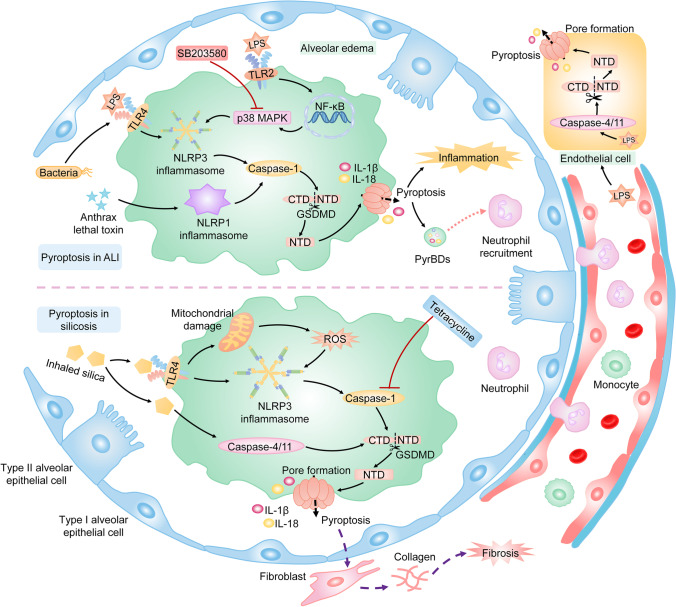
Fig. 6.
Pyroptosis in ALI and silicosis. Exposure to LPS, which is an important component of bacteria, is a common cause of ALI. LPS binds to TLR4 and activates the NLRP3 inflammasome. Meanwhile, LPS activates the p38 MAPK pathway via the TLR2/NF-κB signaling pathway, which results in the activation of the NLRP3 inflammasome. The activated NLRP3 inflammasome excites caspase-1, and active caspase-1 sequentially cleaves GSDMD. Finally, GSDMD-dependent pyroptosis of AMs occurs, which leads to consistent excessive alveolar inflammation and diffuse alveolar edema. Additionally, the lethal toxin-activated NLRP1 inflammasome induces pyroptosis of AMs, contributing to the development of ALI. Furthermore, pyroptosis of AMs aggravates ALI by releasing caspase-1-dependent PyrBDs, which trigger the vascular interstitial edema and neutrophil recruitment. In addition, LPS circulating in the blood triggers pyroptosis of pulmonary endothelial cells via activating the caspase-4/11/GSDMD pathway. Inhaled silica activates the NLRP3 inflammasome, which is dependent or independent of ROS. Activated NLRP3 inflammasome processes caspase-1 which cleaves GSDMD. Additionally, silica activates caspase-4/11, which leads to the release of GSDMD-NTD. Consequently, GSDMD-mediated pyroptosis occurs in AMs and results in chronic inflammation and pulmonary fibrosis. Note: ALI, acute lung injury; LPS, lipopolysaccharide; GSDMD, gasdermin D; AMs, alveolar macrophages; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor kappa-B; PyrBDs, pyroptotic bodies
Pyroptosis and silicosis
Silicosis is considered an occupational lung disease and is characterized by diffuse pulmonary fibrosis and silicon nodules [34]. AMs, which are the first cells that engulf inhaled silica particles, play a considerably essential role in the progression of silicosis [51, 66]. The phagocytosis of silica causes the release of cytokines such as IL-1β and IL-18 from AMs [51]. IL-1β and IL-18 are key molecules in regulating pulmonary inflammation and fibrosis, and the release of these two cytokines is correlated with pyroptosis [2]. Therefore, pyroptosis of AMs may play an important role in silicosis. Recently, it is shown that GSDMD-dependent pyroptosis is triggered in silica-treated AMs by activating the TLR4/NLRP3/caspase-1/GSDMD pathway, which results in aggravated pulmonary inflammation and diffuses silicon nodules [66, 83]. Blocking pyroptosis of macrophages by inhibiting the NLRP3 inflammasome or caspase-1 reduces silica-mediated pulmonary inflammation and fibrosis [50, 83]. Meanwhile, silica induces the generation of ROS, which is important in the activation of the NLRP3/caspase-1 signaling pathway to accelerate pyroptosis in AMs [83]. Additionally, it is shown that caspase-11 is upregulated in mice exposed to silica [66]. Inhibition of caspase-11 reduces GSDMD-induced pyroptosis in silica-treated macrophages [66]. Therefore, it is suggested that caspase-4/5/11/GSDMD-induced pyroptosis is involved in the development of silicosis. However, the specific mechanism is unclear (Fig. 6).
Pyroptosis and pulmonary hypertension
Pulmonary hypertension (PH) is characterized by pulmonary vascular remodeling and increased right ventricular systolic pressure (RVSP) [22, 78, 88].
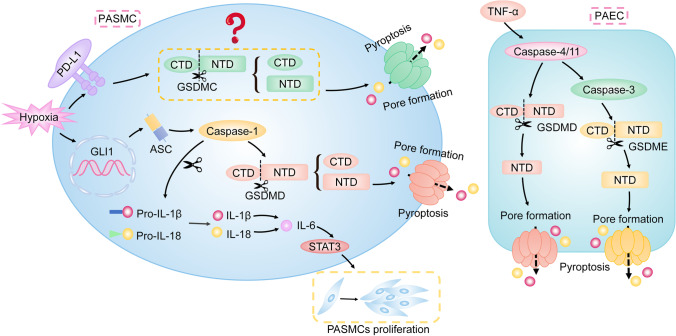
Accumulating evidence suggests that abnormal proliferation of pulmonary artery smooth muscle cells (PASMCs) induced by inflammation results in pulmonary vascular remodeling [22, 71, 88]. Glioma-associated oncogene family zinc finger 1 (GLI1) is a transcription factor that mediates cancer cell proliferation [81]. It is reported that GLI1 induces pyroptosis of PASMCs under hypoxic conditions by upregulating the expression of ASC, which results in vascular remodeling and increased RVSP [22]. Whereas, inhibition of GLI1 reduces pyroptosis of PASMCs and alleviates the progression of hypoxia-induced PH [22]. Additionally, PD-L1 is a transmembrane protein correlated to GSDMC-induced pyroptosis [24]. It is shown that PD-L1 triggers pyroptosis of PASMCs and sequentially contributes to hypoxia-induced PH [88]. However, the specific mechanism of the PD-L1-induced pyroptosis in PASMCs is unclear. Additionally, caspase-1 is an important molecule in the canonical inflammasome-induced pathway of pyroptosis. It is reported that caspase-1 induces the abnormal proliferation of PASMCs in hypoxia by upregulating the IL-1β/IL-18/IL-6/STAT3 pathway, which causes increased pulmonary arterial pressure [71]. Collectively, pyroptosis of PASMCs is highlighted in the progression of hypoxia-induced PH.
In addition, the pulmonary arterial endothelial cells (PAECs) dysfunction caused by enhanced vascular inflammation is an important cause of PH [69]. Wu et al. have elucidated that GSDMD-dependent pyroptosis is triggered by the activation of caspase-4/11 in TNF-α-treated PAECs. Meanwhile, caspase-4 enhances pyroptosis in PAECs by activating the caspase-3/GSDME pathway. Caspase-4/5-mediated pyroptosis in PAECs leads to pulmonary vascular resistance and increased RVSP [78]. While inhibition of caspase-4/11 ameliorates the development of PH and vascular remodeling [78].
Accordingly, pyroptosis is involved in the pathogenesis of PH (Fig. 7).
Fig. 7.
Pyroptosis in PH. The abnormal proliferation of PASMCs leads to pulmonary vascular remodeling. PD-L1 triggers pyroptosis of PASMCs, which contributes to hypoxia-induced PH. Though PD-L1 induces GSDMC-dependent pyroptosis, the specific mechanism of PD-L1-induced pyroptosis in PASMCs is unclear. Additionally, GLI1 induces pyroptosis of PASMCs under hypoxic conditions by increasing the level of ASC, which results in vascular remodeling. Meanwhile, increased IL-1β or IL-18 mediated by caspase-1 upregulates IL-6 to activate STAT3, which contributes to the abnormal proliferation of PASMCs. In addition, TNF-α is a classic pro-PH factor and causes PAECs dysfunction, which is an important cause of PH. It activates the caspase-4/11/GSDMD pathway to induce pyroptosis of PAECs. Meanwhile, caspase-4 also enhances pyroptosis in PAECs by activating the caspase-3/GSDME pathway. Consequently, pyroptosis of PAECs promotes pulmonary vascular resistance. Note: PH, pulmonary hypertension; PASMCs, pulmonary arterial smooth muscle cells; PD-L1, programmed death-ligand 1; GLI1, glioma-associated oncogene homolog 1; ASC, apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain; STAT3, signal transducer and activator of transcription 3; TNF-α, tumor necrosis factor-alpha; PAECs, pulmonary arterial endothelial cells
Pyroptosis and cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive disorder attributed to the mutations of a gene that encodes the cystic fibrosis transmembrane conductance regulator (CFTR) protein [42]. Patients with CF suffer from recurrent and chronic respiratory infections and inflammation, which results in airway obstruction and even respiratory failure [6].
The deficiency of CFTR leads to reduced efflux chloride in epithelial cells, which contributes to bacterial proliferation and excessive inflammation in CF by forming a thick and viscous mucus layer [20]. Recently, it is reported that increased cytosolic chloride caused by dysfunction of CFTR induces the activation of the NLRP3 inflammasome in monocytes by upregulating the expression of P2X7R, which contributes to inflammation in CF. While the CFTR modulators restore the CFTR expression and reduce inflammation in patients with CF by blocking the P2X7R/NLRP3/caspase-1 axis [20]. Additionally, the absence of CFTR leads to the upregulation of epithelial sodium channel (ENaC), which may be partially responsible for the recurrent respiratory infections and chronic inflammation in CF [61]. Recently, it has been reported that the increased activity of ENaCs activates NLRP3/caspase-1 signaling pathway in human BECs with CF-associated mutations by directly increasing potassium efflux, which enhances excessive inflammation in CF [61]. The NLRP3/caspase-1 pathway is important in pyroptosis. Therefore, these results imply a potential role of NLRP3/caspase-1-dependent pyroptosis in CF. However, whether pyroptosis is involved in the pathogenesis of CF is still unknown.
Additionally, Pseudomonas aeruginosa is a common cause of airway inflammation and pulmonary tissue damage in CF. It is shown that P. aeruginosa can activate NLRC4 inflammasome, NLRP3 inflammasome, and caspase-11 [1]. Meanwhile, caspase-11-dependent pyroptosis is triggered in response to P. aeruginosa infection, suggesting that pyroptosis may participate in the inflammatory response against P. aeruginosa in CF patients. However, the specific mechanism is unclear.
Pyroptosis and granulomatous diseases in the respiratory system
Inflammation is the main cause of granulomatous diseases [48]. Accumulating evidence suggests that pyroptosis may play a role in pulmonary granulomatous diseases such as tuberculosis (TB) and sarcoidosis [5, 53].
Tuberculosis
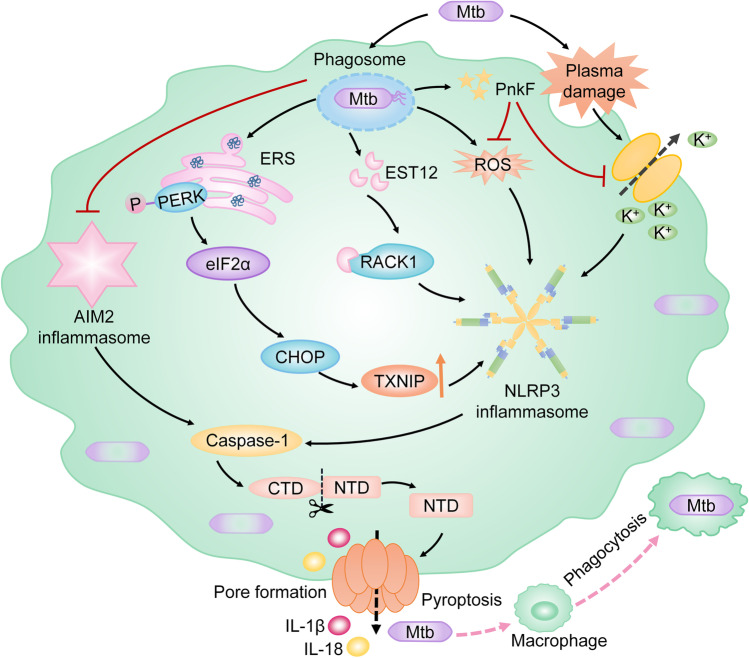
TB is a chronic inflammatory disease characterized by caseous necrotizing granuloma formation [40], which remains the leading cause of death worldwide [53]. The pathogen of TB is Mycobacterium tuberculosis (Mtb), which causes a host inflammatory response via complex mechanisms [54]. Studies show that pyroptosis of macrophages may play an important role in host resistance to Mtb infection by mediating inflammatory cytokines downstream. It is shown that EST12, which is a protein secreted by Mtb, interacts with receptors for activated C kinase 1 and sequentially induces the NLRP3/GSDMD-mediated pyroptosis to enhance mycobacterial clearance. While the EST12-deficient Mtb strain causes decreased pyroptosis and escapes the clearance of host cells in vitro and vivo [53]. Meanwhile, another study demonstrates that Mtb inhibits pyroptosis of macrophages by producing PknF, which is a phosphokinase of Mtb. Pkn inhibits the activation of the NLRP3 inflammasome by suppressing the potassium efflux and generation of ROS. The Pkn-induced inhibition of NLRP3/caspase-1/GSDMD-dependent pyroptosis then allows Mtb to evade the clearance of the host cell [55]. In addition to the NLRP3 inflammasome, the AIM2 inflammasome is highlighted in the host inflammatory response against Mtb. It is reported that Mtb inhibits the activation of AIM2 inflammasome to escape host clearance [62]. Meanwhile, AIM2-deficient mice are more susceptible to Mtb [58]. This research indicates that AIM2 inflammasome may take a protective effect against Mtb by regulating downstream inflammatory cytokines. AIM2 inflammasome is an important molecule in pyroptosis. Nonetheless, whether pyroptosis participates in the AIM2 inflammasome-mediated host inflammatory response against Mtb is unclear.
However, excessive pyroptosis induced by Mtb may lead to augmented destruction of the lung tissue as well as the dissemination of Mtb [4, 19, 40]. It is reported that the Mtb-induced plasma membrane rupture contributes to the NLRP3/caspase-1/GSDMD-dependent pyroptosis by inducing potassium efflux, which leads to bacterial growth and spread [4]. Additionally, a growing body of evidence suggests that the Mtb can trigger pyroptosis to promote the dissemination of bacteria via mediating ERS. Mtb-induced ERS leads to the upregulation of TXNIP by activating the PERK/eIF2α/CHOP signaling pathway [19]. TXNIP plays an important role in the activation of the NLRP3 inflammasome. It has been shown that inhibition of the PERK/eIF2α/TXNIP/NLRP3/caspase-1/GSDMD-mediated pyroptosis in Mtb-infected macrophages alleviates the lung tissue damage and restricts the spread of Mtb [19, 40].
In summary, pyroptosis plays dual roles in TB. On the one hand, it participates in the host defense against Mtb infection. On the other hand, excessive pyroptosis induced by Mtb contributes to lung tissue damage and the spread of bacteria. However, the specific mechanism that determines the role of pyroptosis in TB remains unclear (Fig. 8).
Fig. 8.
Pyroptosis in TB. Pyroptosis can promote the host clearance of Mtb. When Mtb invades the host, it is phagocytosed by macrophages to form a phagosome, which activates inflammatory response and restricts the growth of Mtb. EST12, which is secreted by Mtb, triggers pyroptosis through activating the RACK1/NLRP3/caspase-1/GSDMD signaling pathway, which improves the clearance of intracellular Mtb. Additionally, Mtb produces PnkF to suppress the potassium efflux and generation of ROS. Sequentially, the NLRP3/caspase-1/GSDMD-dependent pyroptosis is inhibited, which leads to the Mtb escaping from host clearance. Furthermore, the phagosome of Mtb inhibits the activation of the AIM2 inflammasome which is an important molecule in the pyroptotic pathway by an unknown mechanism to evade the host elimination. Additionally, Mtb-induced pyroptosis can trigger the dissemination of bacteria. Phagocytosed Mtb causes ERS which leads to the upregulated TXNIP via activating the PERK/eIF2α/CHOP pathway. Increased TXNIP leads to the activation of NLRP3 inflammasome. Meanwhile, Mtb triggers the activation of the NLRP3 inflammasome by inducing plasma damage. The NLRP3/caspase-1/GSDMD-dependent pyroptosis then occurs and results in the growth and spread of Mtb. Note: TB, tuberculosis; Mtb, Mycobacterium tuberculosis; AIM2, absent in melanoma 2; RACK1, receptor for activated C kinase 1; GSDMD, gasdermin D; ERS, endoplasmic reticulum stress; PERK, protein kinase R-like endoplasmic reticulum kinase; TXNIP, thioredoxin interacting protein; eIF2α, eukaryotic translation-initiation factor 2α; CHOP, CCAAT-enhancer-binding protein homologous protein
Sarcoidosis
Sarcoidosis is an inflammatory disease characterized by the formation of non-necrotizing epithelioid granulomas [5]. IL-18 and IL-1β play a potential role in sarcoidosis [26]. The activation of the NLRP3 inflammasome contributes to the maturation and secretion of IL-18 and IL-1β. It is reported that downregulated mRNA levels of miR-223, a direct downregulator of the NLRP3 inflammasome, induce the increased expression of IL-1β in AMs from sarcoidosis patients than in healthy volunteers [26]. Meanwhile, in trehalose 6,6-dimycolate-treated mice, an NLRP3 inflammasome inhibitor reduces IL-1β, which is followed by a reduced number of pulmonary granuloma formation [26]. Additionally, serum amyloid A (SAA), which is highly expressed by macrophages in sarcoidosis granuloma, may play an important role in sarcoidosis via modulating the production of IL-18 and TNF [8]. It is suggested that SAA may activate the NLRP3 inflammasome as a DAMP to participate in the pathogenesis of sarcoidosis [26]. Collectively, the NLRP3 inflammasome may play a critical role in sarcoidosis. However, whether pyroptosis participates in sarcoidosis remains unknown.
Conclusion
Pyroptosis is a novel regulated cell death induced by the GSDM family, which is usually accompanied by the release of active pro-inflammatory cytokines such as IL-1β and IL-18. There are several pathways involved in pyroptosis, such as the canonical inflammasome-induced pathway, non-canonical inflammasome-induced pathway, caspase-1/3/6/7/GSDMB pathway, caspase-8/GSDMC pathway, caspase-8/GSDMD pathway, and caspase-3/GSEME pathway. Although GSDMA is engaged in pyroptosis, its upstream effectors remain unclear.
Pyroptosis may be involved in inflammation-related respiratory diseases such as asthma, COPD, lung cancer, ALI, silicosis, PH, and TB, in which the NLRP3 inflammasome-induced pathway is mostly highlighted. Pyroptosis contributes to the deterioration of asthma, COPD, ALI, silicosis, and PH. In addition, pyroptosis appears to have dual effects in lung cancer and TB, while the molecules that determine the specific roles of pyroptosis in lung cancer and TB remain unclear. Furthermore, how the GSDME, as an executioner of pyroptosis, switches caspase-3-induced apoptosis to pyroptosis is not clear in lung cancer. Additionally, whether pyroptosis participates in CF and sarcoidosis or not is largely unknown, though the activation of NLRP3 inflammasome is found in CF and sarcoidosis. Collectively, the specific mechanisms of pyroptosis in inflammation-related respiratory diseases remain unclear. More studies on pyroptosis in inflammatory respiratory diseases are warranted in the future.
Acknowledgements
The authors would like to acknowledge the assistance provided by Mr. Dong Zhiwu of the Clinical Medicine, Five Class, 2019 Grade, Kunming Medical University, Kunming, China.
Abbreviations
- AHR
Airway hyperresponsiveness
- AIM
Absent in melanoma
- ALI
Acute lung injury
- ALRs
Absent in melanoma-like receptors
- AMs
Alveolar macrophages
- ASC
Apoptosis-associated speck-like protein containing a caspase-activation and recruitment domain
- ATP
Adenosine triphosphate
- BECs
Bronchial epithelial cells
- CF
Cystic fibrosis
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CHOP
CCAAT-enhancer-binding protein homologous protein
- COPD
Chronic obstructive pulmonary disease
- CS
Cigarette smoke
- CSE
Cigarette smoke extract
- CTD
C-terminal domain
- DAMPs
Danger-associated molecular patterns
- dsDNA
Double-stranded DNA
- eIF2α
Eukaryotic translation-initiation factor 2α
- ENaC
Epithelial sodium channel
- ERS
Endoplasmic reticulum stress
- GLI1
Glioma-associated oncogene family zinc finger 1
- GSDM
Gasdermin
- GSDMD
Gasdermin D
- IFN
Interferon
- IL
Interleukin
- LPS
Lipopolysaccharide
- Mtb
Mycobacterium tuberculosis
- NEK7
NIMA-related kinase 7
- NF-κB
Nuclear factor kappa-B
- NLRC
Nucleotide-binding oligomerization domain-like receptor, caspase activation and recruitment domain-containing protein
- NLRP
NOD-like receptor protein
- NLRs
Nucleotide-binding domain and leucine-rich repeat containing receptors
- NSCLC
Non-small cell lung cancer
- NTD
N-terminal domain
- OMVs
Outer membrane vesicles
- PAECs
Pulmonary arterial endothelial cells
- P. aeruginosa
Pseudomonas aeruginosa
- PAMPs
Pathogen-associated molecular patterns
- PASMCs
Pulmonary arterial smooth muscle cells
- PCD
Programmed cell death
- PD-L1
Programmed death-ligand 1
- PERK
Protein kinase RNA-like ER kinase
- PGE2
Prostaglandin E2
- PH
Pulmonary hypertension
- PRRs
Pattern-recognition receptors
- PYD
Pyrin domain
- PyrBDs
Pyroptotic bodies
- RAGE
Receptor for advanced glycation end-products
- ROS
Reactive oxygen species
- RVSP
Right ventricular systolic pressure
- STAT
Signal transducer and activator of transcription
- SAA
Serum amyloid A
- TB
Tuberculosis
- TDI
Toluene diisocyanate
- Th
T helper cell
- TLRs
Toll-like receptors
- TNF-α
Tumor necrosis factor alpha
- TXNIP
Thioredoxin interacting protein
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Yuanyu Feng, Shibo Sun, Min Li, Xiaoting Yangzhong, Xifeng Zhang, Anju Zu, Yunjao Hou, and Lin Li. The first draft of the manuscript was written by Yuanyu Feng, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
This work was supported by the National Natural Science Foundation of China (grant no. 82160007) and the Yunnan Provincial Science and Technology Department (grant no. 2019FE001(-058)).
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Footnotes
Key Points
• Pyroptosis is involved in several inflammation-related respiratory diseases.
• The role of pyroptosis is discrepant in different inflammation-related respiratory diseases.
• The NLRP3 inflammasome-induced signaling pathway is mostly highlighted in pyroptosis.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balakrishnan A, Karki R, Berwin B, Yamamoto M, Kanneganti TD. Guanylate binding proteins facilitate caspase-11-dependent pyroptosis in response to type 3 secretion system-negative Pseudomonas aeruginosa. Cell Death Discov. 2018;4:3. doi: 10.1038/s41420-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes H, Goh NSL, Leong TL, Hoy R. Silica-associated lung disease: an old-world exposure in modern industries. Respirology. 2019;24:1165–1175. doi: 10.1111/resp.13695. [DOI] [PubMed] [Google Scholar]
- 3.Bauer R, Rauch I. The NAIP/NLRC4 inflammasome in infection and pathology. Mol Aspects Med. 2020;76:100863. doi: 10.1016/j.mam.2020.100863. [DOI] [PubMed] [Google Scholar]
- 4.Beckwith KS, Beckwith MS, Ullmann S, Sætra RS, Kim H, Marstad A, et al. Plasma membrane damage causes NLRP3 activation and pyroptosis during Mycobacterium tuberculosis infection. Nat Commun. 2020;11:2270. doi: 10.1038/s41467-020-16143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belperio JA, Shaikh F, Abtin FG, Fishbein MC, Weigt SS, Saggar R, et al. Diagnosis and treatment of pulmonary sarcoidosis: a review. JAMA. 2022;327:856–867. doi: 10.1001/jama.2022.1570. [DOI] [PubMed] [Google Scholar]
- 6.Biswas L, Götz F. Molecular mechanisms of Staphylococcus and Pseudomonas interactions in cystic fibrosis. Front Cell Infect Microbiol. 2021;11:824042. doi: 10.3389/fcimb.2021.824042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Zhou X, Yin Z, Yu X, Yang Q, Guo Q, et al. The anti-inflammatory effect of BML-111 on COPD may be mediated by regulating NLRP3 inflammasome activation and ROS production. Prostaglandins Other Lipid Mediat. 2018;138:23–30. doi: 10.1016/j.prostaglandins.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen ES, Song Z, Willett MH, Heine S, Yung RC, Liu MC, et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med. 2010;181:360–373. doi: 10.1164/rccm.200905-0696OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng KT, Xiong S, Ye Z, Hong Z, Di A, Tsang KM, et al. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. J Clin Invest. 2017;127:4124–4135. doi: 10.1172/jci94495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng P, Li S, Chen H. Macrophages in lung injury, repair, and fibrosis. Cells. 2021;10:426. doi: 10.3390/cells10020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Shi P, Wang Y, Zou D, Wu X, Wang D, et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J Mol Cell Biol. 2019;11:496–508. doi: 10.1093/jmcb/mjy056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, Xiao Z, Jiang Z, Jiang Y, Li W, Wang M. Schisandrin B attenuates airway inflammation and airway remodeling in asthma by inhibiting NLRP3 inflammasome activation and reducing pyroptosis. Inflammation. 2021;44:2217–2231. doi: 10.1007/s10753-021-01494-z. [DOI] [PubMed] [Google Scholar]
- 13.Colarusso C, Terlizzi M, Lamort AS, Cerqua I, Roviezzo F, Stathopoulos G, et al. Caspase-11 and AIM2 inflammasome are involved in smoking-induced COPD and lung adenocarcinoma. Oncotarget. 2021;12:1057–1071. doi: 10.18632/oncotarget.27964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 15.Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc Natl Acad Sci U S A. 2016;113:13132–13137. doi: 10.1073/pnas.1610433113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng W, Bai Y, Deng F, Pan Y, Mei S, Zheng Z, et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature. 2022;602:496–502. doi: 10.1038/s41586-021-04384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans MD, Esnault S, Denlinger LC, Jarjour NN. Sputum cell IL-1 receptor expression level is a marker of airway neutrophilia and airflow obstruction in asthmatic patients. J Allergy Clin Immunol. 2018;142:415–423. doi: 10.1016/j.jaci.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019;26:99–114. doi: 10.1038/s41418-018-0212-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Shen J, Li Y, Liu F, Ning B, Zheng Y, et al. Inhibition of the PERK/TXNIP/NLRP3 axis by baicalin reduces NLRP3 inflammasome-mediated pyroptosis in macrophages infected with Mycobacterium tuberculosis. Mediators Inflamm. 2021;2021:1805147. doi: 10.1155/2021/1805147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabillard-Lefort C, Casey M, Glasgow AMA, Boland F, Kerr O, Marron E, et al. Trikafta rescues CFTR and lowers monocyte P2X7R-induced inflammasome activation in cystic fibrosis. Am J Respir Crit Care Med. 2022;205:783–794. doi: 10.1164/rccm.202106-1426OC. [DOI] [PubMed] [Google Scholar]
- 21.Gao J, Qiu X, Xi G, Liu H, Zhang F, Lv T, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non-small cell lung cancer. Oncol Rep. 2018;40:1971–1984. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Ma C, Zhang L, et al. GLI1-mediated pulmonary artery smooth muscle cell pyroptosis contributes to hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2020;318:L472–l482. doi: 10.1152/ajplung.00405.2019. [DOI] [PubMed] [Google Scholar]
- 23.He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016;530:354–357. doi: 10.1038/nature16959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou J, Zhao R, Xia W, Chang CW, You Y, Hsu JM, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang Z, Zhang Q, Wang Y, Chen R, Wang Y, Huang Z, et al. Inhibition of caspase-3-mediated GSDME-derived pyroptosis aids in noncancerous tissue protection of squamous cell carcinoma patients during cisplatin-based chemotherapy. Am J Cancer Res. 2020;10:4287–4307. [PMC free article] [PubMed] [Google Scholar]
- 26.Huppertz C, Jäger B, Wieczorek G, Engelhard P, Oliver SJ, Bauernfeind FG, et al. The NLRP3 inflammasome pathway is activated in sarcoidosis and involved in granuloma formation. Eur Respir J. 2020;55:1900119. doi: 10.1183/13993003.00119-2019. [DOI] [PubMed] [Google Scholar]
- 27.Jiang R, Xu J, Zhang Y, Zhu X, Liu J, Tan Y. Ligustrazine alleviate acute lung injury through suppressing pyroptosis and apoptosis of alveolar macrophages. Front Pharmacol. 2021;12:680512. doi: 10.3389/fphar.2021.680512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 29.Kesavardhana S, Malireddi RKS, Kanneganti TD. Caspases in cell death, inflammation, and pyroptosis. Annu Rev Immunol. 2020;38:567–595. doi: 10.1146/annurev-immunol-073119-095439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim RY, Pinkerton JW, Essilfie AT, Robertson AAB, Baines KJ, Brown AC, et al. Role for NLRP3 inflammasome-mediated, IL-1β-dependent responses in severe, steroid-resistant asthma. Am J Respir Crit Care Med. 2017;196:283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 31.Kong H, Wang Y, Zeng X, Wang Z, Wang H, Xie W. Differential expression of inflammasomes in lung cancer cell lines and tissues. Tumour Biol. 2015;36:7501–7513. doi: 10.1007/s13277-015-3473-4. [DOI] [PubMed] [Google Scholar]
- 32.Kovarova M, Hesker PR, Jania L, Nguyen M, Snouwaert JN, Xiang Z, et al. NLRP1-dependent pyroptosis leads to acute lung injury and morbidity in mice. J Immunol. 2012;189:2006–2016. doi: 10.4049/jimmunol.1201065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Leung CC, Yu IT, Chen W. Silicosis Lancet. 2012;379:2008–2018. doi: 10.1016/s0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Ren W, Jiang Z, Zhu L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol Med Rep. 2018;18:4399–4409. doi: 10.3892/mmr.2018.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Wang Z, He R, Xiong R, Li G, Zhang L, et al. Buformin alleviates sepsis-induced acute lung injury via inhibiting NLRP3-mediated pyroptosis through an AMPK-dependent pathway. Clin Sci (Lond) 2022;136:273–289. doi: 10.1042/cs20211156. [DOI] [PubMed] [Google Scholar]
- 37.Liu G, Philp AM, Corte T, Travis MA, Schilter H, Hansbro NG, et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther. 2021;225:107839. doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Christenson SA, Modena B, Li H, Busse WW, Castro M, et al. Genetic analyses identify GSDMB associated with asthma severity, exacerbations, and antiviral pathways. J Allergy Clin Immunol. 2021;147:894–909. doi: 10.1016/j.jaci.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Fu Y, Sun J, Shen J, Liu F, Ning B, et al. Tanshinone IIA alleviates NLRP3 inflammasome-mediated pyroptosis in Mycobacterium tuberculosis-(H37Ra-) infected macrophages by inhibiting endoplasmic reticulum stress. J Ethnopharmacol. 2022;282:114595. doi: 10.1016/j.jep.2021.114595. [DOI] [PubMed] [Google Scholar]
- 41.Mahalanobish S, Dutta S, Saha S, Sil PC. Melatonin induced suppression of ER stress and mitochondrial dysfunction inhibited NLRP3 inflammasome activation in COPD mice. Food Chem Toxicol. 2020;144:111588. doi: 10.1016/j.fct.2020.111588. [DOI] [PubMed] [Google Scholar]
- 42.Manti S, Parisi GF, Papale M, Marseglia GL, Licari A, Leonardi S. Type 2 inflammation in cystic fibrosis: new insights. Pediatr Allergy Immunol. 2022;33(Suppl 27):15–17. doi: 10.1111/pai.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Matikainen S, Nyman TA, Cypryk W. Function and regulation of noncanonical caspase-4/5/11 inflammasome. J Immunol. 2020;204:3063–3069. doi: 10.4049/jimmunol.2000373. [DOI] [PubMed] [Google Scholar]
- 44.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122:2731–4270. doi: 10.1172/jci60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazgaeen L, Gurung P. Recent advances in lipopolysaccharide recognition systems. Int J Mol Sci. 2020;21:379. doi: 10.3390/ijms21020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell PS, Sandstrom A, Vance RE. The NLRP1 inflammasome: new mechanistic insights and unresolved mysteries. Curr Opin Immunol. 2019;60:37–45. doi: 10.1016/j.coi.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mo R, Zhang J, Chen Y, Ding Y. Nicotine promotes chronic obstructive pulmonary disease via inducing pyroptosis activation in bronchial epithelial cells. Mol Med Rep. 2022;25:92. doi: 10.3892/mmr.2022.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patterson KC, Queval CJ, Gutierrez MG. Granulomatous inflammation in tuberculosis and sarcoidosis: does the lymphatic system contribute to disease? BioEssays. 2019;41:e1900086. doi: 10.1002/bies.201900086. [DOI] [PubMed] [Google Scholar]
- 49.Peng Z, Wang P, Song W, Yao Q, Li Y, Liu L, et al. GSDME enhances cisplatin sensitivity to regress non-small cell lung carcinoma by mediating pyroptosis to trigger antitumor immunocyte infiltration. Signal Transduct Target Ther. 2020;5:159. doi: 10.1038/s41392-020-00274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peukert K, Steinhagen F, Fox M, Feuerborn C, Schulz S, Seeliger B, et al. Tetracycline ameliorates silica-induced pulmonary inflammation and fibrosis via inhibition of caspase-1. Respir Res. 2022;23:21. doi: 10.1186/s12931-022-01937-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pollard KM. Silica, Silicosis, and Autoimmunity. Front Immunol. 2016;7:97. doi: 10.3389/fimmu.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin X, Zhou Y, Jia C, Chao Z, Qin H, Liang J, et al. Caspase-1-mediated extracellular vesicles derived from pyroptotic alveolar macrophages promote inflammation in acute lung injury. Int J Biol Sci. 2022;18:1521–1538. doi: 10.7150/ijbs.66477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qu Z, Zhou J, Zhou Y, Xie Y, Jiang Y, Wu J, et al. Mycobacterial EST12 activates a RACK1-NLRP3-gasdermin D pyroptosis-IL-1β immune pathway. Sci Adv. 2020;6:eaba4733. doi: 10.1126/sciadv.aba4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rastogi S, Briken V. Interaction of mycobacteria with host cell inflammasomes. Front Immunol. 2022;13:791136. doi: 10.3389/fimmu.2022.791136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rastogi S, Ellinwood S, Augenstreich J, Mayer-Barber KD, Briken V. Mycobacterium tuberculosis inhibits the NLRP3 inflammasome activation via its phosphokinase PknF. PLoS Pathog. 2021;17:e1009712. doi: 10.1371/journal.ppat.1009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rathinam VA, Fitzgerald KA. Inflammasome complexes: emerging mechanisms and effector functions. Cell. 2016;165:792–800. doi: 10.1016/j.cell.2016.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruan J, Wang S, Wang J. Mechanism and regulation of pyroptosis-mediated in cancer cell death. Chem Biol Interact. 2020;323:109052. doi: 10.1016/j.cbi.2020.109052. [DOI] [PubMed] [Google Scholar]
- 58.Saiga H, Kitada S, Shimada Y, Kamiyama N, Okuyama M, Makino M, et al. Critical role of AIM2 in Mycobacterium tuberculosis infection. Int Immunol. 2012;24:637–644. doi: 10.1093/intimm/dxs062. [DOI] [PubMed] [Google Scholar]
- 59.Saradna A, Do DC, Kumar S, Fu QL, Gao P. Macrophage polarization and allergic asthma. Transl Res. 2018;191:1–14. doi: 10.1016/j.trsl.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A. 2018;115:E10888–e10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scambler T, Jarosz-Griffiths HH, Lara-Reyna S, Pathak S, Wong C, Holbrook J, et al. ENaC-mediated sodium influx exacerbates NLRP3-dependent inflammation in cystic fibrosis. Elife. 2019;8:e49248. doi: 10.7554/eLife.49248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shah S, Bohsali A, Ahlbrand SE, Srinivasan L, Rathinam VA, Vogel SN, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-β and AIM2 inflammasome-dependent IL-1β production via its ESX-1 secretion system. J Immunol. 2013;191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 64.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 65.Song D, Li M, Yu X, Wang Y, Fan J, Yang W, et al. The molecular pathways of pyroptosis in atherosclerosis. Front Cell Dev Biol. 2022;10:824165. doi: 10.3389/fcell.2022.824165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song M, Wang J, Sun Y, Pang J, Li X, Liu Y, et al. Inhibition of gasdermin D-dependent pyroptosis attenuates the progression of silica-induced pulmonary inflammation and fibrosis. Acta Pharm Sin B. 2022;12:1213–1224. doi: 10.1016/j.apsb.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stern J, Pier J, Litonjua AA. Asthma epidemiology and risk factors. Semin Immunopathol. 2020;42:5–15. doi: 10.1007/s00281-020-00785-1. [DOI] [PubMed] [Google Scholar]
- 68.Teng JF, Mei QB, Zhou XG, Tang Y, Xiong R, Qiu WQ, et al. Polyphyllin VI induces caspase-1-mediated pyroptosis via the induction of ROS/NF-κB/NLRP3/GSDMD signal axis in non-small cell lung cancer. Cancers (Basel) 2020;12:193. doi: 10.3390/cancers12010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsai YM, Chiang KH, Hung JY, Chang WA, Lin HP, Shieh JM, et al. Der f1 induces pyroptosis in human bronchial epithelia via the NLRP3 inflammasome. Int J Mol Med. 2018;41:757–764. doi: 10.3892/ijmm.2017.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Udjus C, Cero FT, Halvorsen B, Behmen D, Carlson CR, Bendiksen BA, et al. Caspase-1 induces smooth muscle cell growth in hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2019;316:L999–L1012. doi: 10.1152/ajplung.00322.2018. [DOI] [PubMed] [Google Scholar]
- 72.Vecchié A, Bonaventura A, Toldo S, Dagna L, Dinarello CA, Abbate A. IL-18 and infections: is there a role for targeted therapies? J Cell Physiol. 2021;236:1638–1657. doi: 10.1002/jcp.30008. [DOI] [PubMed] [Google Scholar]
- 73.Wang C, Ruan J. Mechanistic insights into gasdermin pore formation and regulation in pyroptosis. J Mol Biol. 2022;434:167297. doi: 10.1016/j.jmb.2021.167297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L, Meng J, Wang C, Wang Y, Yang C, Li Y. Hydrogen sulfide attenuates cigarette smoke-induced pyroptosis through the TLR4/NF-κB signaling pathway. Int J Mol Med. 2022;49:56. doi: 10.3892/ijmm.2022.5112. [DOI] [PubMed] [Google Scholar]
- 75.Wang LQ, Liu T, Yang S, Sun L, Zhao ZY, Li LY, et al. Perfluoroalkyl substance pollutants activate the innate immune system through the AIM2 inflammasome. Nat Commun. 2021;12:2915. doi: 10.1038/s41467-021-23201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang W, Hu D, Feng Y, Wu C, Song Y, Liu W, et al. Paxillin mediates ATP-induced activation of P2X7 receptor and NLRP3 inflammasome. BMC Biol. 2020;18:182. doi: 10.1186/s12915-020-00918-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Y, Gao W, Shi X, Ding J, Liu W, He H, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 78.Wu Y, Pan B, Zhang Z, Li X, Leng Y, Ji Y, et al. Caspase-4/11-mediated pulmonary artery endothelial cell pyroptosis contributes to pulmonary arterial hypertension. Hypertension. 2022;79:536–548. doi: 10.1161/hypertensionaha.121.17868. [DOI] [PubMed] [Google Scholar]
- 79.Xia X, Wang X, Cheng Z, Qin W, Lei L, Jiang J, et al. The role of pyroptosis in cancer: pro-cancer or pro-“host”? Cell Death Dis. 2019;10:650. doi: 10.1038/s41419-019-1883-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang Y, Wang H, Kouadir M, Song H, Shi F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019;10:128. doi: 10.1038/s41419-019-1413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yao Y, Zhou D, Shi D, Zhang H, Zhan S, Shao X, et al. GLI1 overexpression promotes gastric cancer cell proliferation and migration and induces drug resistance by combining with the AKT-mTOR pathway. Biomed Pharmacother. 2019;111:993–1004. doi: 10.1016/j.biopha.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 82.Ying Y, Mao Y, Yao M. NLRP3 inflammasome activation by microRNA-495 promoter methylation may contribute to the progression of acute lung injury. Mol Ther Nucleic Acids. 2019;18:801–814. doi: 10.1016/j.omtn.2019.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin H, Fang L, Wang L, Xia Y, Tian J, Ma L, et al. Acute silica exposure triggers pulmonary inflammation through macrophage pyroptosis: an experimental simulation. Front Immunol. 2022;13:874459. doi: 10.3389/fimmu.2022.874459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yi YS. Functional crosstalk between non-canonical caspase-11 and canonical NLRP3 inflammasomes during infection-mediated inflammation. Immunology. 2020;159:142–155. doi: 10.1111/imm.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan R, Zhao W, Wang QQ, He J, Han S, Gao H, et al. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res. 2021;170:105748. doi: 10.1016/j.phrs.2021.105748. [DOI] [PubMed] [Google Scholar]
- 86.Zasłona Z, Flis E, Wilk MM, Carroll RG, Palsson-McDermott EM, Hughes MM, et al. Caspase-11 promotes allergic airway inflammation. Nat Commun. 2020;11:1055. doi: 10.1038/s41467-020-14945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang CC, Li CG, Wang YF, Xu LH, He XH, Zeng QZ, et al. Chemotherapeutic paclitaxel and cisplatin differentially induce pyroptosis in A549 lung cancer cells via caspase-3/GSDME activation. Apoptosis. 2019;24:312–325. doi: 10.1007/s10495-019-01515-1. [DOI] [PubMed] [Google Scholar]
- 88.Zhang M, Xin W, Yu Y, Yang X, Ma C, Zhang H, et al. Programmed death-ligand 1 triggers PASMCs pyroptosis and pulmonary vascular fibrosis in pulmonary hypertension. J Mol Cell Cardiol. 2020;138:23–33. doi: 10.1016/j.yjmcc.2019.10.008. [DOI] [PubMed] [Google Scholar]
- 89.Zhang MY, Jiang YX, Yang YC, Liu JY, Huo C, Ji XL, et al. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021;269:119090. doi: 10.1016/j.lfs.2021.119090. [DOI] [PubMed] [Google Scholar]
- 90.Zhang T, Li Y, Zhu R, Song P, Wei Y, Liang T, et al. Transcription factor p53 suppresses tumor growth by prompting pyroptosis in non-small-cell lung cancer. Oxid Med Cell Longev. 2019;2019:8746895. doi: 10.1155/2019/8746895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhuang J, Cui H, Zhuang L, Zhai Z, Yang F, Luo G, et al. Bronchial epithelial pyroptosis promotes airway inflammation in a murine model of toluene diisocyanate-induced asthma. Biomed Pharmacother. 2020;125:109925. doi: 10.1016/j.biopha.2020.109925. [DOI] [PubMed] [Google Scholar]
- 92.Zhou Z, He H, Wang K, Shi X, Wang Y, Su Y, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:eaaz7548. doi: 10.1126/science.aaz7548. [DOI] [PubMed] [Google Scholar]
- 93.Zou J, Zheng Y, Huang Y, Tang D, Kang R, Chen R. The versatile gasdermin family: their function and roles in diseases. Front Immunol. 2021;12:751533. doi: 10.3389/fimmu.2021.751533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.