Abstract
Oxidative stress (OS) is brought on by heat stress (HS), which weakens antioxidant defense and initiates OS. Since mitochondria are the primary source of reactive oxygen species (ROS), HS-mediated OS may be lessened by targeting mitochondria with particular antioxidants. The purpose of this study was to investigate the effect of oral coenzyme Q10 (CoQ10) supplementation on the reproductive performance of goat bucks under HS conditions. Ten mature bucks were randomly separated into two groups and housed in an environment with a high-temperature humidity index (THI: 88.3 to 94.8; summer season). The first group (n = 5) got the baseline diet while the second group (n = 5) received supplemental oral CoQ10 (3 mg/kg BW; CoQ10 group) daily for six weeks. Testicular blood flow parameters (TBF), testicular volume (TV) and echogenicity (TE), nitric oxide (NO), seminal alanine aminotransferase (ALT) and catalase (CAT) activities, total antioxidant capacity (TAC), malondialdehyde (MDA) content, and semen quality traits were all measured. The examinations started a week before (W-1), on the first supplementation day (W0), and weekly for eight consecutive weeks (W1-W8). There were marked (P < 0.05) increases in TBF (W3-W6) and TV, and a decrease in TE (W3-W5) in the CoQ10 group compared to the CON group. Similarly, testosterone (T) and NO levels (W3-W5) in the CoQ10 group were higher (P < 0.05) than those of the control group. The CoQ10 group demonstrated significant (P < 0.05) increases in seminal CAT (W4-W8) and TAC (W2-W6) activities and decreases in ALT (W4-W7) activity and MDA (W5-W8) concentration as compared to the control group. The CoQ10 group showed improvements (P < 0.05) at W3-W6 for sperm progressive motility, viability, and normal morphology and at W6-W8 for sperm concentration. In conclusion, oral CoQ10 supplementation improved testicular hemodynamics, testosterone production, semen quality, and antioxidant capacity in goat bucks during summer heat stress conditions.
Keywords: Antioxidants, Coenzyme Q10, Goats, Heat stress, Reproductive hormones, Semen, Testicular blood flow
Introduction
Recently, there have been raising concerns regarding the global warming issue, which could affect livestock production and reproduction (Jamal et al. 2021). With the elevation of the planet's temperatures, animals would be exposed to environmental heat (HS) stress (Belhadj Slimen et al. 2016). Several studies have reported adverse effects of HS on male reproductive functions through reduced sperm quality and production, steroid production and libido, and potential fertility (Al-Kanaan et al. 2015). HS has an immediate adverse effect on sperm properties such as morphology, plasma membrane integrity, and fertility (Shahat et al. 2020). In addition, in hot and humid conditions, reproductive hormones such as gonadotropins (FSH and LH) and steroids (testosterone and estrogen) play important roles in controlling testicular processes such as spermatogenesis and sexual behavior. (Gündoğan 2007; Morrell 2020). In addition, testicular blood flow (TBF) decreases during the hot summer months as one of the most important determinants of testicular functions due to the supply of essential nutrients and oxygen (Samir et al. 2018; Hedia et al. 2020).
The goats are considered to be more tolerant to temperature fluctuations than other ruminants such as cows and sheep, via an increase in water conservation, respiratory and cardiac rates, and a decrease in metabolic rate (Lu 1989); however, this claimed adaptation is based mainly on the territory, breed under examination, and individual responses to HS (Lu 1989; Sharma et al. 2013; Salama et al. 2014). Baladi goats are more prone to heat stress than crossbreeds under subtropical conditions (Teama and El-Tarabany 2016; Al‐Dawood 2017; El-Tarabany et al. 2017). Temperature-humidity index (THI) has become the main tool to evaluate the HS status of the examined animal (Habeeb et al. 2018). It has been reported that ruminants reared in THI over 75 were considered under HS conditions (West 2003).
The deteriorating effect of HS on testicular functions is primarily caused by the production of free radicals [reactive oxygen species (ROS) and nitrogen species (RNS)] during mitochondrial oxidative phosphorylation processes and the loss of compensatory antioxidant defenses and subsequent oxidative stress (OS) (El-Tohamy et al. 2012). Hedia et al. (2020) reported a marked decrease in blood and semen antioxidant enzymes [superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase CAT)] and an increase in malondialdehyde (MDA, lipid peroxidation biomarker) accompanied by lower TBF during summer months in rams. Recently, mitochondria-targeted antioxidants such as coenzyme Q10 (CoQ10) have been artificially involved in improving OS-induced cell damage, with better results than cytosolic counterparts (Elokil et al. 2019; Tiwari et al. 2021).
CoQ10 is a fat-soluble, vitamin-like, mitochondrial origin, and bio-energetic molecule, structured with the quinone group and existing ubiquitously in all cell types in both animals and plants; this is why it is called ubiquinone. CoQ10 exists heavily in the mitochondria and is implemented in the electron transport chain for ATP production. Moreover, CoQ10 has strong antioxidant properties that may overcome cellular antioxidants (tocopherol and resveratrol) in its actions against ROS attacks (Tiwari et al. 2021).
CoQ10 was introduced into animal feeds for the treatment of various OS-induced organ dysfunctions, including cattle (Wafa and El-Nagar 2021), rabbits (Elokil et al. 2019), and birds (Sharideh et al. 2020), horses (Nemec Svete et al. 2021), rats (Delkhosh et al. 2021) and humans (Nadjarzadeh et al. 2014). CoQ10 supplementation enhances testicular function and fertility in aged breeding roosters and heat-stressed rabbits by lowering OS, increasing total antioxidant capacity and testosterone levels, and upregulating testicular melatonin receptors (Elokil et al.2019; Sharideh et al.2020).
Evaluation of TBF using colored-Doppler ultrasonography has become a useful, precise, and non-invasive tool for predicting testicular functionality via assessment of the testicular artery’s impedance against blood flow. In an oxidative state, the vascular endothelium becomes more vulnerable to ROS attack especially superoxide anion, which affects nitric oxide (NO, vascular tone regulator) synthesis with a subsequent decrease in testicular blood perfusion. Several studies (Gloria et al. 2020; Samir et al. 2020; Abdelnaby et al. 2021) have reported a strong relation between Doppler indices and testicular functions (steroidogenesis and spermatogenesis).
Based on the strong antioxidant capacity of CoQ10 and the advantages of free diffusion through the mitochondrial membrane and elimination of cradle-generated ROS; we hypothesized that summer oral CoQ10 supplementation could weaken OS induced by heat stress and improve reproductive performance in the bucks. To date, this is the first study to examine the effects of oral CoQ10 supplementation on testicular hemodynamics and semen quality in goat bucks under HS conditions.
Materials and methods
Animals and management
Ten Baladi goat bucks, with average body weight and age (49 ± 2.9 kg; 3.4 ± 0.6 y, respectively), were used in this study. These bucks were thoroughly examined before being used in the experimental procedures to exclude any buck with cardiovascular and andrological problems suck as orchitis, based on general examination of the vital signs and Doppler ultrasound scanning of the reproductive system. They were housed in a paddock throughout the experimental time points, with the availability of a 20 m2 shaded slatted area and exposed to natural daylight, ambient temperature, and relative humidity in the summer season in Egypt. The bucks were offered a balanced diet following the NRC instructions, composed of 400 g/ head/day of ready-to-eat pelleted concentrates (18% crude protein) and 1.25 kg/head/day of green roughage. The feed ingredients based on the manufacturer were yellow corn, wheat bran, cotton cake, sunflower cake, soybean meal, gluten feed of 16% and 22%, molasses, lime, NaCl, mineral salts, vitamins, and anti-mycotoxins; while, the feed chemical compositions were crude protein (18%), fat (2%), ash (9%), fiber (15%), and total digestible nutrients (65%). Fresh water and mineral licks were available for the bucks ad libitum. Periodical vaccination and deworming protocols were implied, following the instructions of the general authority of veterinary services in Egypt.
Heat stress assessment
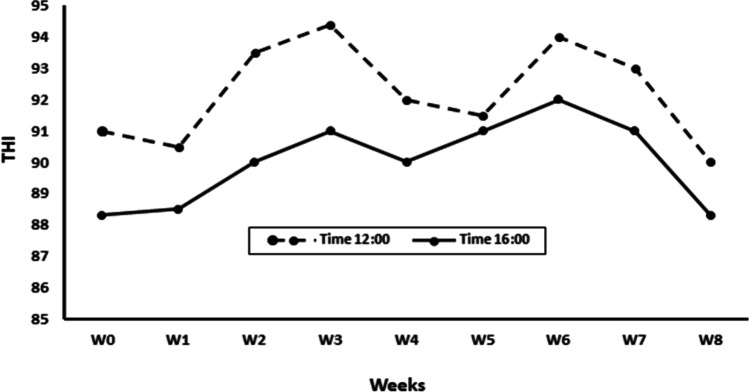
Temperature humidity index (THI) was followed to assess whether the examined bucks were under heat stress conditions or not (Habeeb et al. 2018). THI was calculated based on temperature (T) and relative humidity (RH) records obtained from the Egyptian meteorological authority for the study period (June to August 2021), and territory (Giza city, Giza governorate, Egypt). The obtained T and RH data were for two times/day (12:00 and 16:00) throughout the study timeline (36–40 °C and 60–70%, respectively). The equation proposed according to a previous study (Kendall and Webster 2009) was used to calculate the THI i.e. THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T − 26)]. THI results (Fig. 1) ranged from 88.3 to 94.8 throughout the study. Therefore, the bucks under examination have been considered under severe heat stress conditions (El-Tarabany et al. 2017).
Fig. 1.
Temporal changes of the temperature humidity index (THI: 12:00 and 16:00) throughout the study timeline (W0-W8) in Giza city, Giza governorate, Egypt. The Calculated THI was based on the equation proposed by (Kendall and Webster 2009): THI = (1.8 × T + 32) − [(0.55 − 0.0055 × RH) × (1.8 × T − 26)]
Experimental design
The present work has been carried out at the research farm of the Theriogenology Department, Faculty of Veterinary Medicine, Cairo University. This study was performed in the hottest months in Egypt (June to August). Bucks were divided into two equal groups; the first group (n = 5, Age: 3.6 ± 0.45 y; BW: 50.4 ± 2.2) received the programmed daily diet only (control group, CON), while the other group (n = 5, Age: 3.34 ± 0.35 y; BW: 48.4 ± 1.5) received the programmed daily diet plus oral administration of coenzyme Q10 at a dose of 3 mg/kg BW (CoQ10; Arab company for pharmaceutical and medicinal plants, Heliopolis, Egypt; Coenzyme Q10 Forte 100 mg/soft gelatin capsule) for six consecutive weeks (W1-W6). The CoQ10 dose was designed according to a previous study on goats (El-Ela et al. 2017). The supplemented and control bucks have been examined one week before (W-1) and on the first day of CoQ10 supplementation (W0) and once a week for eight consecutive weeks (W1-W8). The last two examinations (W7-W8) were to evaluate the post-treatment effect after the stoppage of CoQ10 supplementation. During the experimental time points (W-1-W8), the bucks have been examined for testicular hemodynamics [resistive index (RI) and pulsatility index (PI), endpoint of velocity (EDV), peak point of velocity (PSV), and colored area toward the testes/ pixels(CA)], testicular traits [testicular echotexture (TE) and testicular volume (TV)], reproductive hormones (FSH, LH, testosterone (T), estradiol 17β (E2)], nitric oxide (NO) and semen quality parameters [sperm progressive motility (SPM), viability (SV), normal morphology (NS) and sperm cell concentration (SCC)].
Evaluation of testicular blood flow
All the ultrasound scanning procedures throughout the studied time points were carried out by the same qualified operator. Doppler ultrasonography (EXAGO, Echo Control Medical [ECM], France), equipped with a 5–7.5 MHz linear array probe, was used for the assessment of the testicular hemodynamics (TH). Before the ultrasound examination, the animals were controlled without a tranquilizer to eliminate its impact on TH, and the scrotal hair on both sides of the testes and the spermatic cord was clean-shaven. Also, the Doppler sets including gate (0.5 mm), filter (50 Hz), and the angle between the Doppler beam and the longitudinal axis of the supra-testicular (STA) artery (< 60°) were adjusted and kept constant during the study (Samir et al. 2015). Doppler probe was placed vertically on a side of each testis and moved upward for recognition of the vascular network of the spermatic cord. For the STA insight (which appeared convoluted at the proximal pole of the testis) from the venous network, the waveforms (systole and diastole) of the STA clear the issue (Samir et al. 2020). Testicular hemodynamic parameters were estimated including resistive and pulsatile indices (RI and PI), the peak point of velocity (PSV; cm/s), and endpoint of velocity (EDV; cm/s; Fig. 2). The pampiniform vascularization was assessed by measuring the number of colored area/pixels (CA, pixels) either away (blue) or toward (red) the probe, the pre-saved colored Doppler image was operated using Adobe Photoshop cc × 64 software for determining the number of colored areas/pixels (EL-Sherbiny et al. 2022a). The testicular volume was calculated using the following ellipsoid equation = 4/3π abc; where length/2 (a) × width/2(b) × height/2(c) (Love et al. 1991). Echotexture was measured by the Adobe Photoshop CC program as previously described (Brito et al. 2012). Ultrasound measurements were repeated at least three times for results’ validity assurance.
Fig. 2.

Color mode ultrasonograms of the pampiniform plexus showed colored areas. Note: Larger areas were colored blue to represent blood flow toward testes away from the probe, then a pulsed wave Doppler was activated to form the spectral graph. PSV = peak point of velocity, EDV = endpoint of velocity, TAV = time average velocity, RI = resistive index, and PI = pulsatility index
Assaying of circulating hormones and NO levels
Blood samples were collected, just before ultrasound scanning, from the jugular vein into heparinized vacutainer tubes (5 ml). All samples were centrifuged at 3000 rpm for 15 min, and then the harvested plasma was stored (− 20 ℃) until further assessment. Species-specific T, FSH, LH, and E2 were assayed from plasma samples by commercial ELISA (SunLong Biotech Co., LTD CHINA) kits with intra- and inter-assays variation coefficients were ≤ 10 and ≤ 12% for all measured hormones. For NO measuring, 100 µL of serum samples were mixed with equal volumes of Griess reagent [N-(l-naphthyl) ethylenediamine and sulphanilamide] and incubated for 18–20 min at room temperature. The optical density was recorded using a spectrophotometer (543 nm wavelength) and the NO (µM/L) concentration was calculated (Abdelnaby et al. 2021). Hormonal and biochemical analyses were repeated at least two times for valid results assurance.
Semen quality assessment
On the day of ultrasound scanning and blood sampling, semen samples were collected using an artificial vagina (42 °C). The collected samples were transferred immediately to the laboratory and held in a water bath (35 °C) for further evaluation. Each semen sample was divided into two parts, the first portion for sperm microscopical evaluation and the second one for biochemical evaluation of TAC, CAT, ALT, and MDA. To exclude the negative effects of long-time sexual rest, the semen quality data regarding the week before CoQ10 supplementation (W-1) was discarded (Zarazaga et al. 2010).
Microscopical evaluation
Sperm progressive motility (SPM, %), viability (SV, %) and normal morphology (NS, %) were examined subjectively using a heated-stage (38 °C) light microscope (Olympus, Optical co., Ltd., Tokyo, Japan), while, SCC (109 sperm cell/ ml) was recorded using the improved Neubauer hemocytometer (Mahmoud et al. 1997).
For sperm progressive motility, a diluted [sodium citrate dihydrate 2.9% (1:20; v/v)] semen sample was loaded on a pre-warmed glass slide (37 °C) and cover-slipped. The spermatozoa that had forward progressive motility were recorded and expressed in percent. The sperm viability and normal morphology percentages were examined employing the eosin-nigrosin staining (Sprecher and Coe 1996) technique (5 g eosin, 30 g nigrosin, 8.7 g sodium citrate dihydrate dissolved in 300 ml distilled water in a boiling water bath for at least 15 min, and filtered to be homogenous and clear). A diluted (1:20; v/v) semen drop was thoroughly mixed with a pre-warmed (37 °C) stain drop (1:2 v/v) on a pre-warmed glass slide and smeared gently by another slide. The air-dried slide was examined using a light microscope (magnification: 400 ×). For sperm viability assessment (300 sperm were assessed/slide), spermatozoa with defective plasma membranes appeared pink in color; while those with integrated plasma membranes were not stained (colorless). For normal morphology assessment (300 sperm were assessed/slide) using an oil immersion lens (magnification: 1000 ×), spermatozoa that had abnormalities in heads (e.g. detached, pyriform, giant, dwarf, or tapered), midpiece (bowed), cytoplasmic droplet (proximal or distal), and tail (coiled, bent, or double) were considered abnormal. Duplicate smears were followed for all the studied semen parameters.
Seminal plasma oxidative markers (TAC, CAT, and MDA) and ALT activity
For obtaining the seminal plasma (SP), semen samples were centrifuged (4 °C) at 2000 g for 15 min, followed by storage of the harvested SP at -20 °C till later measurement. To exclude the inter-assay variations, all the biochemical evaluations (W0-W8) were performed on the same day after the end of the study. Colormetrically using a spectrophotometer, commercial research kits were used for assaying the levels and activities of TAC (mM/L), CAT (U/L), MDA (mM/mL) (Biodiagnostics, Dokki, Giza, Egypt), and ALT (U/ml; Spectrum-diagnostic, Obour city, Cairo, Egypt) specifically at 510, 520, 534, and 505 nm wavelengths, respectively, following the manufacturer’s instructions (Fathi et al. 2021).
For MDA analysis, the principle of methodology depends on thiobarbituric acid (TBA) reacting with malondialdehyde in an acid medium for 30 min at 95 °C to produce a TBA product (pink product). The absorbance of the product was evaluated at 534 nm. The estimation was done according to the manufacturer’s instructions for the commercial kit following the principle described by Satoh (1978) and Ohkawa et al. (1979).
The estimation of TAC was achieved via the reaction of the sample’s antioxidants with a specified quantity of exogenously supplied hydrogen peroxide (H2O2). The antioxidants in the sample eliminate a certain amount of the supplied H2O2. The residuum H2O2 is estimated colorimetrically by an enzymatic reaction which involves the transformation of 3,5, dichloro –2– hydroxy benzenesulfonate to a colored product that is read against distilled water at 505 nm. TAC was estimated using commercial test kits following the principle described by Koracevic et al. (2001).
Statistical analysis
Firstly, the raw data were checked for normality using the Shapiro–Wilk test. The differences between the right and left testis regarding the Doppler findings were non-significant; therefore, the data of both testes were pooled and the means in each time point were presented. The biological repetition was three times for ultrasound measurements, two times for semen quality, hormonal and biochemical assessments. The differences between control and CoQ10 groups (treatment effect), in terms of testicular hemodynamics values (RI, PI, EV, PV, and CA), circulating hormones (FSH, LH, T, and E2), NO, semen quality traits (SPM, SV, NS, SCC), seminal oxidative biomarkers (TAC, CAT, and MDA), and ALT activity throughout the studied time points (time effect) and combined treatment time effect were analyzed using repeated-measures ANOVA test, followed by Tukey post hoc test. Probability less than 5% was considered a significant result. The values were presented as means ± standard error of the mean (SEM). The Statistical Package for Social Sciences (SPSS®; version 20; Chicago, USA) was used for all statistical analyses.
Results
B- mode ultrasound evaluations
The coenzyme Q10 supplement affected the TV dimensions. Testicular volume (cm3) elevated significantly (P < 0.05) from W 3 (53.24 ± 2.74) till W 5 (50.65 ± 2.55) as shown in (Fig. 3A), while the time and treatment*time interaction had shown a significant (P < 0.01) difference in the TV values, while both TE and pixel heterogeneity (PH) means were affected by the treatment by declination from W 3 (74.148 ± 1.22 for TE, and 18.66 ± 1.01for PH) till W 5 (72.21 ± 0.11 for TE, and 18.33 ± 0.77 for PH), while the time effect did not significantly affect both parameters compared to the CON group (Fig. 3B).
Fig. 3.
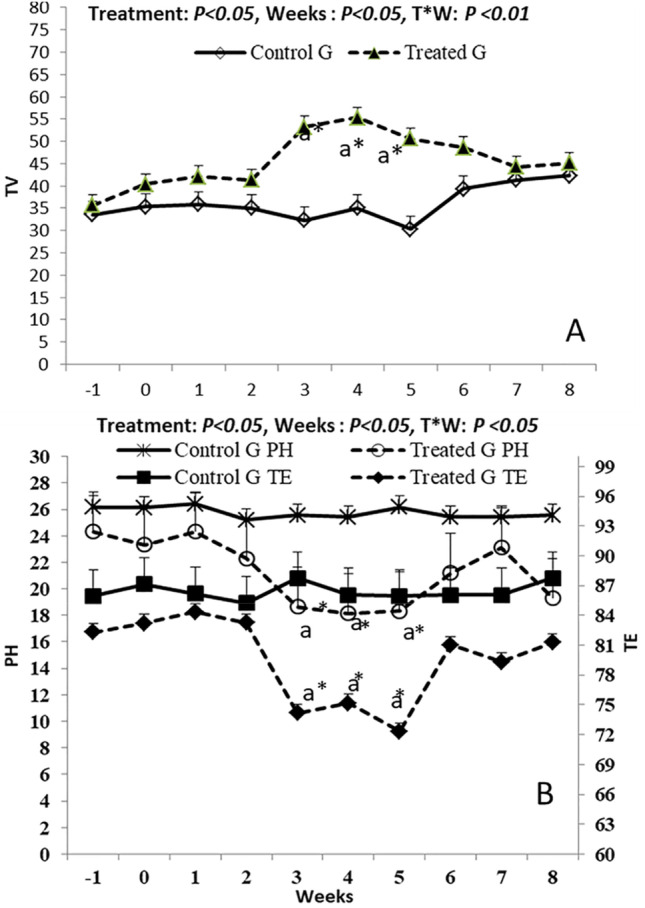
Alterations in testicular volume (cm3; A), and testicular echotexture (B) in form of pixel heterogeneity (PH, SdNPVs) and testicular echogenicity (TE, NPVs). a value is significantly different at P < 0.05 compared with the control and coenzyme Q10 males, while the * value is significantly different at P < 0.05 between the two groups at the same time point
Testicular hemodynamics evaluation
The CoQ10 supplementation affected the main testicular artery vascularity as shown in Table 1. Overall, the treatment effect (CoQ10 vs CON) showed significant (P < 0.01) differences in the values of RI, PI, PSV, and EDV; while the time effect had significant (P < 0.01) differences in RI, PI, PSV, and CA without affecting the EDV. However, the treatment*week interaction had a non-significant effect on all Doppler values. Both Doppler indices (RI and PI) decreased (P < 0.001) from W3 (0.62 ± 0.02 and 0.71 ± 0.02) till W 6 (0.49 ± 0.02 and 0.61 ± 0.02). Concurrently, both PSV and CA markedly increased (P < 0.001) from W3 (19.45 ± 0.05 and 1008.3 ± 6.32) till W6 (20.99 ± 0.07, and 1321.5 ± 6.35), respectively.
Table 1.
Supra testicular artery Doppler parameters including PI, RI, PSV, EDV, and colored areas (CA) away probe in the pampiniform plexus in CoQ10 supplemented (CoQ10; n = 5) versus control (CON; n = 5) group at the studied time points (W0-W8) under heat stress conditions (THI: 88.3–94.4)
| W | PI | RI | PSV cm/s | EDV cm/s | CA away probe toward testes (pixel) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CoQ10 | CON | CoQ10 | CON | CoQ10 | CON | CoQ10 | CON | CoQ10 | CON | |
| W -1 | 0.88 ± 0.01 | 0.92 ± 0.01 | 0.71 ± 0.01 | 0.72 ± 0.01 | 15.58 ± 0.01 | 15.24 ± 0.11 | 4.32 ± 0.04 | 4.33 ± 0.09 | 655.2 ± 22.31 | 593.2 ± 30.69 |
| W0 | 0.91 ± 0.01 | 0.89 ± 0.01 | 0.74 ± 0.02 | 0.72 ± 0.01 | 15.98 ± 0.04 | 15.56 ± 0.05 | 4.51 ± 0.01 | 4.40 ± 0.07 | 645.2 ± 6.32 | 662.6 ± 19.63 |
| W1 | 0.92 ± 0.02 | 0.90 ± 0.01 | 0.73 ± 0.02 | 0.71 ± 0.01 | 15.66 ± 0.04 | 15.56 ± 0.06 | 4.64 ± 0.02 | 4.41 ± 0.07 | 711.3 ± 6.33 | 633.4 ± 34.18 |
| W2 | 0.90 ± 0.01 | 0.89 ± 0.01 | 0.71 ± 0.02 | 0.69 ± 0.01 | 15.64 ± 0.12 | 15.45 ± 0.12 | 4.88 ± 0.04 | 4.78 ± 0.02 | 645.2 ± 3.25 | 692.6 ± 22.83 |
| W3 | 0.71 ± 0.02* | 0.91 ± 0.01 | 0.62 ± 0.02* | 0.72 ± 0.01 | 19.47 ± 0.05* | 15.61 ± 0.05 | 4.45 ± 0.01 | 4.33 ± 0.05 | 1008.3 ± 6.32* | 703.2 ± 23.83 |
| W4 | 0.68 ± 0.02* | 0.87 ± 0.01 | 0.59 ± 0.02* | 0.71 ± 0.01 | 19.58 ± 0.07* | 15.48 ± 0.07 | 4.64 ± 0.03 | 4.40 ± 0.03 | 1142.2 ± 2.36* | 848.4 ± 35.17 |
| W5 | 0.64 ± 0.02* | 0.87 ± 0.01 | 0.54 ± 0.02* | 0.71 ± 0.01 | 20.15 ± 0.05* | 15.48 ± 0.06 | 4.68 ± 0.01 | 4.38 ± 0.05 | 1258.4 ± 5.32* | 855.6 ± 9.37 |
| W6 | 0.61 ± 0.02* | 0.89 ± 0.01 | 0.49 ± 0.02* | 0.72 ± 0.01 | 20.99 ± 0.07* | 15.54 ± 0.07 | 4.64 ± 0.01 | 4.35 ± 0.03 | 1321.5 ± 6.35* | 882.2 ± 21.01 |
| W7 | 0.88 ± 0.01 | 0.87 ± 0.02 | 0.69 ± 0.01 | 0.72 ± 0.01 | 15.21 ± 0.06 | 15.57 ± 0.06 | 4.47 ± 0.02 | 4.40 ± 0.04 | 875.2 ± 15.21 | 886.2 ± 22.43 |
| W8 | 0.91 ± 0.02 | 0.89 ± 0.01 | 0.70 ± 0.01 | 0.71 ± 0.01 | 15.01 ± 0.07 | 15.55 ± 0.07 | 4.66 ± 0.02 | 4.36 ± 0.08 | 888.2 ± 11.32 | 748.2 ± 12.31 |
PI pulsatility index, RI resistive index, PSV peak point of velocity, EDV end point of velocity, W weeks, and CA colored areas. Data presented as mean ± SEM. *Values in each measure are different at least at P < 0.05 between the two groups
Hormonal (T, E2, FSH, and LH), and NO assay
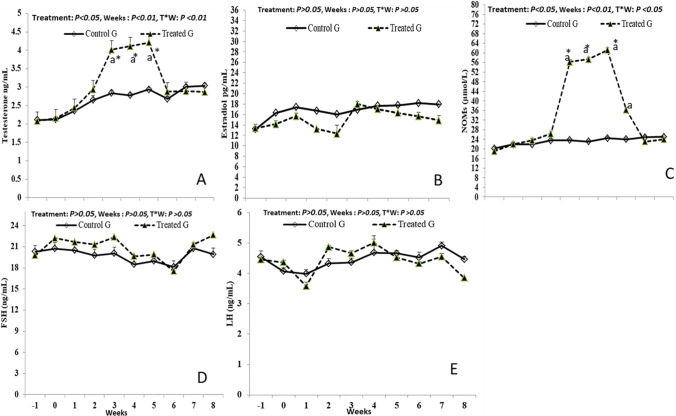
The CoQ10 treatment and time*treatment interaction effects (CoQ10 vs CON) had a non-significant difference in the plasma levels of E2, FSH, and LH (Fig. 4B, D & E). Moreover, The levels of T and NO increased significantly (P < 0.01) in the CoQ10 group compared to the CON group (Fig. 4A & C). The T (ng/mL) and NO (µM/L) values increased significantly with time (P < 0.01) from W3 (4.02 ± 0.14 and 56.25 ± 0.73) till W5 (4.21 ± 0.02 and 61.21 ± 0.08), respectively. Furthermore, the time*treatment interaction had significant differences in T (P < 0.01) and NO (P < 0.05) levels.
Fig. 4.
Alterations in plasma levels of testosterone (T; ng/mL; A), estradiol (E2; pg/mL; B), serum nitric oxide levels (NOMs, µmol/L; C), follicle-stimulating (FSH; ng/mL; D), and luteinizing hormones (LH; ng/mL; E) in male bucks that received coenzyme Q10 compared to control group. Values are presented as means ± SEM. a value is significantly different at P < 0.05 compared with the control and coenzyme Q10 males, while the * value is significantly different at P < 0.05 between the two groups at the same time point
Semen quality parameters
The data showing the effect of supplemental dietary CoQ10 on the semen characteristics of heat-stressed goat bucks (CoQ10 vs CON; W0-W8) is presented in Table 2. The CoQ10 supplementation improved significantly (P < 0.01) the percentages of SPM, SV, and NS starting at W3 till W6. There was a carrying-over effect of treatment on SCC till W8 compared to the CON group.
Table 2.
Semen characteristics (Progressive motility, viability, normal morphology, sperm cell concentration) in CoQ10 supplemented (CoQ10; n = 5) versus control (CON; n = 5) group at the studied time points (W0-W8) under heat stress conditions (THI: 88.3–94.4)
| W | Progressive motility % | Viability % | Normal morphology % | Sperm cell concentration 109/mL | ||||
|---|---|---|---|---|---|---|---|---|
| CoQ10 | CON | CoQ10 | CON | CoQ10 | CON | CoQ10 | CON | |
| W0 | 72.20 ± 1.33 | 70.00 ± 1.84 | 82.40 ± 1.11 | 83.20 ± 1.45 | 83.60 ± 2.66 | 82.60 ± 1.88 | 2.22 ± 0.02 | 2.26 ± 0.55 |
| W1 | 69.00 ± 1.77 | 69.00 ± 1.55 | 76.00 ± 1.44 | 79.80 ± 1.65 | 82.40 ± 0.87 | 82.40 ± 0.88 | 2.45 ± 0.01 | 2.33 ± 0.07 |
| W2 | 73.00 ± 1.65 | 70.00 ± 1.75 | 81.00 ± 0.54 | 79.60 ± 2.33 | 83.00 ± 0.58 | 84.40 ± 0.94 | 2.21 ± 0.01 | 2.24 ± 0.05 |
| W3 | 78.00 ± 1.33* | 73.00 ± 2.04 | 88.60 ± 1.27* | 81.00 ± 1.45 | 89.40 ± 0.84* | 83.60 ± 0.85 | 2.35 ± 0.01 | 2.26 ± 0.07 |
| W4 | 79.00 ± 1.18* | 71.00 ± 1.58 | 90.40 ± 1.22* | 82.60 ± 1.05 | 88.40 ± 0.54* | 83.40 ± 0.88 | 2.29 ± 0.02 | 2.14 ± 0.04 |
| W5 | 80.00 ± 2.14* | 68.00 ± 2.04 | 91.60 ± 1.44* | 81.60 ± 1.97 | 89.60 ± 0.44* | 83.00 ± 0.77 | 2.11 ± 0.01 | 1.97 ± 0.04 |
| W6 | 81.00 ± 1.11* | 72.00 ± 1.52 | 91.60 ± 1.41* | 80.80 ± 1.55 | 91.40 ± 0.54* | 82.60 ± 0.68 | 2.45 ± 0.01* | 1.97 ± 0.05 |
| W7 | 68.20 ± 1.44 | 69.20 ± 1.45 | 81.20 ± 1.21 | 79.80 ± 1.71 | 81.00 ± 0.74 | 83.00 ± 0.55 | 2.52 ± 0.02* | 2.03 ± 0.04 |
| W8 | 73.60 ± 1.01 | 72.40 ± 1.12 | 78.60 ± 1.74 | 80.80 ± 1.57 | 84.40 ± 0.55 | 84.00 ± 0.28 | 2.61 ± 0.01* | 2.15 ± 0.05 |
THI = temperature humidity index, W = weeks. Data obtained as mean ± SEM. *Values in each measure are different at least at P < 0.05 between the two groups. The treatment effect (CoQ10 versus CON) showed a significant difference in sperm progressive motility % (P < 0.001), viability (P < 0.0001), and concentration (P < 0.0001), while the time effect showed a significant (P < 0.01) difference in sperm progressive motility, viability, normal morphology, and concentration
Seminal plasma oxidative markers(MDA, TAC, and CAT) and ALT activity
The seminal plasma levels of MDA and TAC, and activities of CAT and ALT enzymes (CoQ10 vs CON) studied at different time points (W0-W8) under heat stress conditions are presented in Table 3. There were significant (P < 0.01) effects of treatment (CoQ10 vs CON) and time on the values of MDA and TAC, and activities of CAT and ALT. There were marked increases in the levels of TAC (W2-W6) and CAT (W4-W8) activity, and decreases in the values of MDA (W5-W8) and activity of ALT (W4-W7) in the CoQ10 group compared to the CON group.
Table 3.
Seminal plasma levels of malondialdehyde (MDA) and total antioxidant capacity (TAC) and activities of catalase (CAT) and alanine aminotransferase (ALT) enzymes in CoQ10 supplemented (CoQ10; n = 5) versus control (CON; n = 5) group at the studied time points (W0-W8) under heat stress conditions (THI: 88.3–94.4)
| W | MDA (nM/mL) | TAC (mM/L) | CAT (U/L) | ALT (U/mL) | ||||
|---|---|---|---|---|---|---|---|---|
| COQ10 | CON | COQ10 | CON | COQ10 | CON | COQ10 | CON | |
| W0 | 2.76 ± 0.01 | 2.72 ± 0.03 | 0.63 ± 0.01 | 0.60 ± 0.04 | 266.49 ± 24.45 | 267.91 ± 23.72 | 41.13 ± 1.33 | 43.06 ± 0.51 |
| W1 | 2.73 ± 0.05 | 2.78 ± 0.11 | 0.69 ± 0.01 | 0.63 ± 0.05 | 278.74 ± 28.32 | 280.58 ± 24.27 | 45.82 ± 2.41 | 44.34 ± 1.24 |
| W2 | 2.62 ± 0.01 | 2.73 ± 0.01 | 0.84 ± 0.01* | 0.64 ± 0.01 | 247.33 ± 15.97 | 256.01 ± 24.25 | 47.68 ± 2.65 | 43.62 ± 1.54 |
| W3 | 2.69 ± 0.01 | 2.77 ± 0.02 | 1.27 ± 0.01* | 0.64 ± 0.07 | 284.38 ± 26.58 | 296.42 ± 30.74 | 48.74 ± 2.01 | 43.52 ± 2.63 |
| W4 | 2.52 ± 0.02 | 2.71 ± 0.01 | 1.29 ± 0.01* | 0.88 ± 0.07 | 396.87 ± 24.31* | 299.73 ± 46.13 | 36.24 ± 0.97* | 43.36 ± 1.76 |
| W5 | 2.41 ± 0.04* | 2.77 ± 0.03 | 1.33 ± 0.01* | 0.92 ± 0.06 | 446.84 ± 15.88* | 284.40 ± 36.69 | 38.90 ± 1.74* | 47.36 ± 0.84 |
| W6 | 2.11 ± 0.05* | 2.66 ± 0.04 | 1.35 ± 0.02* | 0.96 ± 0.12 | 494.71 ± 24.65* | 309.87 ± 33.29 | 34.48 ± 4.02* | 49.78 ± 1.22 |
| W7 | 2.04 ± 0.04* | 2.68 ± 0.01 | 1.01 ± 0.02 | 0.94 ± 0.08 | 487.27 ± 15.62* | 284.01 ± 33.57 | 34.64 ± 1.22* | 44.16 ± 0.21 |
| W8 | 2.02 ± 0.04* | 2.65 ± 0.06 | 0.79 ± 0.01 | 0.84 ± 0.12 | 529.364 ± 22.31* | 299.29 ± 31.80 | 46.76 ± 2.31 | 43.55 ± 0.22 |
THI temperature humidity index, W weeks. Data obtained as mean ± SEM.*Values in each measure are different at least at P < 0.05 between the two groups. The treatment (COQ10 vs CON) and time effects showed significant (P < 0.01) differences in MDA, TAC, CAT, and ALT
Discussion
Environmental heat stress deteriorates animal productivity and fertility potential (El-Sherbiny et al. 2022b). Since oxidative stress is the main pathway through which HS exerts its destructive impacts on male reproductive functions, numerous studies have reported that dietary supplementation of antioxidants could ameliorate HS-induced male infertility (Elokil et al. 2019; Sharideh et al. 2020; El-Sherbiny et al. 2022c; Fadl et al. 2022c). Mitochondria is the site where free radicals are extensively generated and induce an OS cascade. Therefore, mitochondrial-targeted antioxidants such as CoQ10 could be more beneficial than cytosolic antioxidants.
In the present study, there were marked changes in the STA Doppler indices of the CoQ10 supplemented males compared to the control group. These changes could be interpreted by a decrease in vascular resistance and higher testicular blood perfusion (Gill 1985; Abdelnaby et al. 2022; Hashem et al. 2022). It is well-known that heat-stressed males suffer lower testicular blood flow and functions (Hedia et al. 2020). The hypoxic status of the heat-stressed testicular tissues might be improved by increasing TBF. In the current study, the mechanisms by which CoQ10 nutritional supplements could increase TBF were not specified; however, different perspectives may explain these changes. Firstly, CoQ10 is an indispensable regulator of mitochondrial functions especially the oxidative phosphorylation process and ultimately ATP production (Sandhir et al. 2014). Secondly, the alleviation of heat-stress-induced endothelial dysfunction might be the first explanation for increased TBF in our study and CoQ10 could be accumulated in the vascular endothelium, following an intravenous administration, and induced NO-dependent aortic vasodilatation in rats (Kozaeva et al. 2017). An earlier study also reported that dietary CoQ10 supplements ameliorated diabetes mellitus-induced endothelial dysfunction of the brachial artery in humans (Watts et al. 2002). Improving the endothelial functions in oxidative stress conditions could be explained via its antioxidant capability which indirectly increases NO bioavailability through inactivation of superoxide anion radicals and inhibition of peroxynitrite formation (highly pro-oxidant compound generated via NO and superoxide anion combination). Indeed, CoQ10 administration alleviates the deteriorative impacts of ischemia/reperfusion injury in rats (Arda et al. 2021). The elevated NO levels noted in the present study inferred the protective role of CoQ10 in endothelial functions and synthesis of NO and ultimately higher testicular perfusion.
Electronic measurement of testicular size and its volume has been a reliable tool for male fertility assessment because it provides actual testicular parenchyma morphometric measurements without the need for additional scrotal and epididymal dimensions. In this study, there was a noticeable increase in the testicular volume. This improvement is likely due to the higher blood perfusion that might affect the fluidity of the testicular tissues (EL-Sherbiny et al. 2022d). CoQ10 has a protective effect on the seminiferous tubules' diameter and epithelial height and thickness (Sharideh et al. 2020). A recent study reported the treatment of heat-stressed rats with CoQ10 reversed all the detrimental impacts of heat stress on testicular tissues including irregularity of seminiferous tubules and damaged spermatogonia, spermatocytes, and spermatids as well as decreased testicular weight via its anti-apoptotic and anti-oxidative properties (Delkhosh et al. 2021).
Assessment of echogenicity of the testicular parenchyma provides a realistic information regarding the testicular histomorphology and fluidity (Camela et al. 2019). In this study, there were significant decreases in testicular echotexture (TE) (W3-W5), in the CoQ10 group, followed by gradual increases onward to be non-significant compared to the control group. Interestingly, the echogenic changes of testicular parenchyma were concurrent with the changes noted in the blood flow and cellular part of the testicular tissues. It has been reported that TE is inversely correlated with the blood flow and positively with the cellular matrix of the testes (Giffin et al. 2009). Therefore, higher TBF explains the lower TE values, whereas, the higher sperm cell concentration in the last three weeks of the experiment may interpret the elevated TE values. These outcomes support the concept that TE is affected by testicular dynamics either in blood flow or spermatogenesis and is useful as an indicator in the breeding soundness evaluation of male animals (El-Sherbiny et al. 2022e).
Monitoring the circulating reproductive hormones offers a deep insight into the physiological functions of the testes as well as the pituitary–gonadal axis (Samir et al. 2020; Mandour et al. 2022). In the current study, there were marked increases in the T values of the CoQ10 treated compared to untreated males. This improvement might be linked to CoQ10 properties a bioenergetics compound as it has a pivotal role in the electron transport chain and energy production in mitochondrial membranes of the testicular Leydig cells, which affects its activity and productivity (Delkhosh et al. 2021). Secondly, CoQ10 could protect testosterone-producing cells from oxidative stress via ROS scavenging and membrane lipid peroxidation inhibition (Elokil et al. 2019). Moreover, the CoQ10 can diminish the heat-stress-induced cellular apoptosis which is evident by Bcl2 (anti-apoptotic biomarker) up-regulation and BAX and caspase3 (apoptotic biomarkers) down-regulation (El-Khadragy et al. 2020). Numerous studies support the outcomes of the present study under testicular stress conditions in rats such as lead acetate and lipopolysaccharide toxicity (Song et al. 2017; El-Khadragy et al. 2020), chronic kidney disease (Tsao et al. 2021), and heat stress (Elokil et al. 2019) in rabbits. Unaltered levels of FSH, LH, and E2 during the experimental time points between CoQ10 supplemented males versus control need to be interpreted cautiously taking into consideration its pulsatile secretions. Contrarily, there was evidence that CoQ10 treatment decreased FSH and LH levels concurrent with elevated prolactin levels and these changes were attributed due to the anti-gonadotropic effects of higher prolactin levels (Nadjarzadeh et al. 2014; Alahmar et al. 2021). This discrepancy may be due to the species and conditions variations. Moreover, estradiol 17β is primarily synthesized in the Sertoli cells in the FSH-dependent pathway through testosterone conversion via aromatase activity (Dorrington et al. 1978). The non-significant changes in E2 levels are possibly due to the absence of a direct effect of CoQ10 on E2 synthesis (Sandhir et al. 2014).
Assessment of the seminal plasma oxidative biomarkers provides a clear insight through the sperm membrane integrities (Fadl et al. 2022a). In this study, there were marked increases in seminal plasma CAT activity and TAC, and a decrease in MDA concentration. These results suggest that CoQ10 supplementation improved seminal antioxidant power with subsequent higher membrane integrity evidenced by lower lipid peroxidation profile which is consistent with a previous study (Nadjarzadeh et al. 2014) and a study conducted in human medicine (Alahmar et al. 2021), roosters (Sharideh et al. 2020), and rats (Delkhosh et al. 2021). In addition, our results appear to indicate that CoQ10 rewarded HS-mediated depletion of seminal antioxidant capacity. The assaying of ALT activity considers a useful biomarker for sperm plasma membrane intactness because spermatozoa with damaged membranes release ALT in seminal plasma (Taha et al. 2000; Fadl et al. 2022b). There was a significant decrease in the seminal ALT activity in the present study indicating the higher integrity of the sperm plasma membrane following CoQ10 oral supplementation.
In the present study, there were remarkable improvements in semen quality traits at W3-W6 for sperm progressive motility, membrane integrity, and normal morphology, and at W6-W8 for SCC in the CoQ10 group versus the control group. Since subjective motility evaluation herein might have a wide error range among the examined animals and time points; a further study is recommended using advanced semen quality assessment techniques. Plenty of reports on roosters (Sharideh et al. 2020), rabbits (Elokil et al. 2019), men (Safarinejad 2009; Alahmar et al. 2021), and ducks (Fouda et al. 2021) favor the results of the present study. CoQ10 has a powerful antioxidant capacity and can regenerate endogenous antioxidant systems such as superoxide dismutase enzyme (Navas et al. 2007). Moreover, compared to fertile men, the seminal plasma of infertile men has lower levels of CoQ10 which could be increased via dietary supplementation and improved semen density and quality (Ghanbarzadeh et al. 2014; Nadjarzadeh et al. 2014). It has been reported that heat stress depletes the seminal plasma antioxidant capacity to combat the excess generation of free radicals (Elokil et al. 2019). The elevated TAC and CAT activities and decreased MDA and ALT concentrations noted in the present study inferred the role of CoQ10 in amelioration of heat stress in the perspective of semen quality via enhancement of seminal plasma antioxidant defense systems and lowering sperm membrane lipid peroxidation. Interpretation of the semen quality improvement in the present study, especially before the end of the spermatogenesis (47 days) is complex. However, some explanations may reveal the issue; firstly, daily sperm production in bucks is 20–30 × 106 spermatozoa/g testicular tissue (the highest among domestic species) (Leal et al. 2004; Junior et al. 2011). secondly, average testicular and epididymal (head, body, and tail) sperm reserves were about 44, 8.8, 5.0, and 45.6 × 109 (Daudu 1984). Thirdly, about 15–30% of the expected produced spermatids during the spermatogenesis process are lost especially at the two meiotic stages (Bilaspuri and Guraya 1986; Leal et al. 2004). Therefore, a massive number of spermatozoa already exist in the genital reserve rather than the newly produced ones. Moreover, the sperm maturation process alongside the HS conditions (Shahat et al. 2020) exacerbates the ROS generation which induces more sperm damage during the epididymal journey (Aitken and De Iuliis 2010). A recent study reported that dietary micronutrient administration for 12 days restores sperm OS and enhances semen quality in obese male mice (spermatogenesis ~ 34 days) (McPherson et al. 2019). Based on the above-mentioned evidence, oral coenzyme Q10 supplementation could alleviate the adverse effect of heat stress-mediated oxidative stress and enhance the semen quality (motility, viability, and morphology) in a period less than the whole length of the spermatogenesis process. In the present study, it was noted that the semen quality decreased after the end of CoQ10 supplementation (W7-W8); this decline may be attributed to the HS conditions that affected the sperm quality faster than the cold conditions.
Conclusion
Oral Supplementation of CoQ10 during the summer season ameliorates the adverse effects of heat stress on the testicular hemodynamics and volume, testosterone production and sperm quality, and seminal antioxidant capacity which could improve goat breeding performance. Further studies are needed to investigate the role of CoQ10 supplementation in different male reproductive performance aspects such as sexual activity, more advanced semen quality techniques, and fertility potential.
Authors’ contribution
Hossam R. El-Sherbiny: The notion, experimental design, ultrasound scanning, semen analysis, and manuscript writing; Mohamed Fathi: ultrasound scanning, semen evaluation, manuscript reviewing and editing; Elshymaa A. Abdelnaby: Doppler scanning, hormonal analyses, statistical analysis, reviewing and editing. Noha.Y.Salem, Eman S. Ramadan, and Shimaa G.Yehia performed serum and semen samples’ biochemical and oxidative stress biomarkers analysis, and manuscript writing. All authors approved the final version of the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The data are available by the corresponding author upon a reasonable query.
Declarations
Ethical approval
The experimental procedures and animal handling were approved by the ethical committee for the use and care of animals at Cairo University (vetCU 2305 2022458).
Consent for publication
All authors gave their consent and accredited this study for research publication.
Concent to participate
All authors approved the final version of the manuscript.
Conflict of interest
The authors do have not any conflict of interest to declare.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelnaby E, Yasin N, Abouelela YS, Rashad E, Daghash S, El-Sherbiny H. Ovarian, uterine, and luteal vascular perfusions during follicular and luteal phases in the adult cyclic female rabbits with special orientation to their histological detection of hormone receptor. BMC Vet Res. 2022 doi: 10.1186/s12917-022-03390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelnaby EA, Emam IA, Fadl AM. Assessment of the accuracy of testicular dysfunction detection in male donkey (Equus asinus) with the aid of colour-spectral Doppler in relation to plasma testosterone and serum nitric oxide levels. Reprod Domest Anim. 2021;56:764–774. doi: 10.1111/rda.13916. [DOI] [PubMed] [Google Scholar]
- Aitken R, De Iuliis G. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- Al-Kanaan A, König S, Brügemann K. Effects of heat stress on semen characteristics of Holstein bulls estimated on a continuous phenotypic and genetic scale. Livest Sci. 2015;177:15–24. doi: 10.1016/j.livsci.2015.04.003. [DOI] [Google Scholar]
- Al-Dawood A. Effect of heat stress on adipokines and some blood metabolites in goats from Jordan. Anim Sci J. 2017;88:356–363. doi: 10.1111/asj.12636. [DOI] [PubMed] [Google Scholar]
- Alahmar AT, Sengupta P, Dutta S, Calogero AE. Coenzyme Q10, oxidative stress markers, and sperm DNA damage in men with idiopathic oligoasthenoteratospermia. Clin Exp Reprod Med. 2021;48:150. doi: 10.5653/cerm.2020.04084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arda E, Yuksel I, Akdere H, Akdeniz E, Yalta TD, Aktoz T, Altun GD. Contrary effects of coenzyme Q10 and vitamin E after testicular ischemia/reperfusion in a rat model validated with glucose metabolism imaging. Urol J. 2021;88:56–63. doi: 10.1177/0391560319882232. [DOI] [PubMed] [Google Scholar]
- Belhadj Slimen I, Najar T, Ghram A, Abdrrabba M. Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J Anim Physiol Anim Nutr. 2016;100:401–412. doi: 10.1111/jpn.12379. [DOI] [PubMed] [Google Scholar]
- Bilaspuri G, Guraya S. The seminiferous epithelial cycle and spermatogenesis in rams (ovisaries) Theriogenology. 1986;25:485–505. doi: 10.1016/0093-691x(86)90133-0. [DOI] [PubMed] [Google Scholar]
- Brito L, Barth A, Wilde R, Kastelic J. Testicular ultrasonogram pixel intensity during sexual development and its relationship with semen quality, sperm production, and quantitative testicular histology in beef bulls. Theriogenology. 2012;78:69–76. doi: 10.1016/j.theriogenology.2012.01.022. [DOI] [PubMed] [Google Scholar]
- Camela ES, Nociti RP, Santos VJ, Macente BI, Murawski M, Vicente WR, Bartlewski PM, Oliveira MEF. Changes in testicular size, echotexture, and arterial blood flow associated with the attainment of puberty in Dorper rams raised in a subtropical climate. Reprod Domest Anim. 2019;54:131–137. doi: 10.1111/rda.13213. [DOI] [PubMed] [Google Scholar]
- Daudu CS. Spermatozoa output, testicular sperm reserve and epididymal storage capacity of the Red Sokoto goats indigenous to northern Nigeria. Theriogenology. 1984;21:317–324. doi: 10.1016/0093-691X(84)90417-5. [DOI] [PubMed] [Google Scholar]
- Delkhosh A, Shoorei H, Niazi V, Delashoub M, Gharamaleki MN, Ahani-Nahayati M, Dehaghi YK, Raza S, Taheri MH, Mohaqiq M. Coenzyme Q10 ameliorates inflammation, oxidative stress, and testicular histopathology in rats exposed to heat stress. Hum Exp Toxicol. 2021;40:3–15. doi: 10.1177/0960327120940366. [DOI] [PubMed] [Google Scholar]
- Dorrington J, Fritz I, Armstrong D. Control of testicular estrogen synthesis. Biol Reprod. 1978;18:55–64. doi: 10.1095/biolreprod18.1.55. [DOI] [PubMed] [Google Scholar]
- El-Ela A, Hafez Y, Abdel-Hafez M, El-Ghandour A. Effect of L-carnitine and Co-enzyme Q10 treatments on immune response, productive and reproductive performance of Damascus goats and their offspring. 2-Productive, reproductive performance and some blood metabolites during late pregnancy and lactation periods. Egypt J Sheep Goat Sci. 2017;12:1–22. doi: 10.21608/ejsgs.2017.26311. [DOI] [Google Scholar]
- El-Khadragy M, Al-Megrin WA, AlSadhan NA, Metwally DM, El-Hennamy RE, Salem FEH, Kassab RB, Abdel Moneim AE. Impact of coenzyme Q10 administration on lead acetate-induced testicular damage in rats. Oxid Med Cell Longev. 2020;2020:4981386. doi: 10.1155/2020/4981386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-Sherbiny H, EL-Shahat K, Abo El-Maaty A, Abdelnaby EA. Ovarian and uterine haemodynamics and their relation to steroid hormonal levels in postpartum Egyptian buffaloes. Bulg J Vet Med. 2022;25:262–273. doi: 10.15547/bjvm.2020-0091. [DOI] [Google Scholar]
- El-Sherbiny HR, Samir H, El-Shalofy AS, Abdelnaby EA. Exogenous L-arginine administration improves uterine vascular perfusion, uteroplacental thickness, steroid concentrations, and nitric oxide levels in pregnant buffaloes under subtropical conditions. Reprod Domest Anim. 2022;00:1–10. doi: 10.1111/rda.14225. [DOI] [PubMed] [Google Scholar]
- El-Sherbiny HR, Fathi M, Samir H, Abdelnaby EA. Supplemental dietary curcumin improves testicular hemodynamics, testosterone levels, and semen quality in Baladi bucks in the non-breeding season. Theriogenology. 2022;188:100–107. doi: 10.1016/j.theriogenology.2022.05.020. [DOI] [PubMed] [Google Scholar]
- EL-Sherbiny HR, Shahat A, Hedia M, EL-Shalofy AS. Effect of sexual maturation on testicular morphometry and echotexture and their association with intratesticular blood flow in ossimi rams. Indian J Small Rumin. 2022;28:85–90. doi: 10.5958/0973-9718.2022.00034.4. [DOI] [Google Scholar]
- El-Sherbiny HR, El-Shalofy AS, Samir H. Exogenous L-carnitine administration ameliorates the adverse effects of heat stress on testicular hemodynamics, echotexture, and total antioxidant capacity in Rams. Front Vet Sci. 2022;9:860771. doi: 10.3389/fvets.2022.860771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Tarabany MS, El-Tarabany AA, Atta MA. Physiological and lactation responses of Egyptian dairy Baladi goats to natural thermal stress under subtropical environmental conditions. Int J Biometeorol. 2017;61:61–68. doi: 10.1007/s00484-016-1191-2. [DOI] [PubMed] [Google Scholar]
- El-Tohamy M, Kotp M, El-Nattat W, Mohamed A. Semen characteristics and oxidative/antioxidati in semen and serum of male rabbits supplemented with antioxidants during heat stress. Iran J Appl Anim Sci. 2012;2:175–183. [Google Scholar]
- Elokil AA, Bhuiyan AA, Liu H-Z, Hussein MN, Ahmed HI, Azmal SA, Yang L, Li S. The capability of L-carnitine-mediated antioxidant on cock during aging: evidence for the improved semen quality and enhanced testicular expressions of GnRH1, GnRHR, and melatonin receptors MT 1/2. Poult Sci. 2019;98:4172–4181. doi: 10.3382/ps/pez201. [DOI] [PubMed] [Google Scholar]
- Fadl A, Abdelnaby E, El-seadawy I, Kotp M, El-Maaty AMA, El-Sherbiny H. Eco-friendly synthesized zinc oxide nanoparticles improved frozen-thawed semen quality and antioxidant capacity of Rams. J Adv Vet Res. 2022;12:259–264. [Google Scholar]
- Fadl AM, Abdelnaby EA, El-Sherbiny HR. INRA82 extender enhances semen quality in ram under cooled and cryopreserved stages. Asian Pac J Reprod. 2022;11:100–104. doi: 10.4103/2305-0500.341117. [DOI] [Google Scholar]
- Fadl AM, Abdelnaby EA, El-Sherbiny HR. Supplemental dietary zinc sulfate and folic acid combination improves testicular volume and hemodynamics, testosterone levels and semen quality in rams under heat stress conditions. Reprod Domest Anim. 2022;57:567–576. doi: 10.1111/rda.14096. [DOI] [PubMed] [Google Scholar]
- Fathi M, Salama A, El-Shahat K, EL-Sherbiny HR, Abdelnaby EA. Effect of melatonin supplementation during IVM of dromedary camel oocytes (Camelus dromedarius) on their maturation, fertilization, and developmental rates in vitro. Theriogenology. 2021;172:187–192. doi: 10.1016/j.theriogenology.2021.05.021. [DOI] [PubMed] [Google Scholar]
- Fouda SF, Khattab AA, El Basuini MF, El-Ratel IT. Impacts of different antioxidants sources on semen quality and sperm fertilizing ability of Muscovy ducks under high ambient temperature. J Anim Physiol Anim Nutr. 2021;00:1–12. doi: 10.1111/jpn.13620. [DOI] [PubMed] [Google Scholar]
- Ghanbarzadeh S, Garjani A, Ziaee M, Khorrami A. CoQ10 and L-carnitine attenuate the effect of high LDL and oxidized LDL on spermatogenesis in male rats. Drug Res. 2014;64:510–515. doi: 10.1055/s-0033-1361176. [DOI] [PubMed] [Google Scholar]
- Giffin JL, Franks SE, Rodriguez-Sosa JR, Hahnel A, Bartlewski PM. A study of morphological and haemodynamic determinants of testicular echotexture characteristics in the ram. Exp Biol Med. 2009;234:794–801. doi: 10.3181/0812-RM-364. [DOI] [PubMed] [Google Scholar]
- Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol. 1985;11:625–641. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- Gloria A, Di Francesco L, Marruchella G, Robbe D, Contri A. Pulse-wave Doppler pulsatility and resistive indexes of the testicular artery increase in canine testis with abnormal spermatogenesis. Theriogenology. 2020;158:454–460. doi: 10.1016/j.theriogenology.2020.10.015. [DOI] [PubMed] [Google Scholar]
- Gündoğan M. Seasonal variation in serum testosterone, T3 and andrological parameters of two Turkish sheep breeds. Small Rumin Res. 2007;67:312–316. doi: 10.1016/j.smallrumres.2005.11.005. [DOI] [Google Scholar]
- Habeeb AA, Gad AE, Atta MA. Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int J Biotechnol Recent Adv. 2018;1:35–50. doi: 10.18689/ijbr-1000107. [DOI] [Google Scholar]
- Hashem N, EL-Sherbiny H, Fathi M, Abdelnaby E. Nanodelivery system for ovsynch protocol improves ovarian response, ovarian blood flow Doppler velocities, and hormonal profile of goats. Animals. 2022;12:1442. doi: 10.3390/ani12111442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedia MG, El-Belely MS, Ismail ST, Abo El-Maaty AM. Seasonal variation in testicular blood flow dynamics and their relation to systemic and testicular oxidant/antioxidant biomarkers and androgens in rams. Reprod Domest Anim. 2020;55:861–869. doi: 10.1111/rda.13696. [DOI] [PubMed] [Google Scholar]
- Jamal A, Rashid MA, Malik MI. Effects of sodium bicarbonate and chromium propionate supplementation on growth performance, blood indices of Beetal bucks under heat stress. Trop Anim Health and Prod. 2021;53:1–9. doi: 10.1007/s11250-021-02931-9. [DOI] [PubMed] [Google Scholar]
- Junior AM, Neto AA, Junior AS, Menezes D, Alves F, Sousa A, Carvalho M. Daily sperm production and testicular morphometry in goats according to external scrotal conformation. Anim Reprod Sci. 2011;127:73–77. doi: 10.1016/j.anireprosci.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Kendall P, Webster J. Season and physiological status affects the circadian body temperature rhythm of dairy cows. Livest Sci. 2009;125:155–160. doi: 10.1016/j.livsci.2009.04.004. [DOI] [Google Scholar]
- Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozaeva LP, Gorodetskaya EA, Ruuge EK, Kalenikova EI, Medvedev OS. Beneficial effect of coenzyme Q10 injection on nitric oxide-related dilation of the rat aorta. Eur J Pharmacol. 2017;794:15–19. doi: 10.1016/j.ejphar.2016.11.019. [DOI] [PubMed] [Google Scholar]
- Leal M, Becker-Silva S, Chiarini-Garcia H, Franca L. Sertoli cell efficiency and daily sperm production in goats (Capra hircus) Anim Reprod. 2004;1:122–128. [Google Scholar]
- Love C, Garcia M, Riera F, Kenney R. Evaluation of measures taken by ultrasonography and caliper to estimate testicular volume and predict daily sperm output in the stallion. J Reprod Fertil Supplement. 1991;44:99–105. [PubMed] [Google Scholar]
- Lu C. Effects of heat stress on goat production. Small Rumin Res. 1989;2:151–162. doi: 10.1016/0921-4488(89)90040-0. [DOI] [Google Scholar]
- Mahmoud AM, Depoorter B, Piens N, Comhaire FH. The performance of 10 different methods for the estimation of sperm concentration. Fertil Steril. 1997;68:340–345. doi: 10.1016/S0015-0282(97)81526-9. [DOI] [PubMed] [Google Scholar]
- Mandour AS, Samir H, El-Beltagy MA, Hamabe L, Abdelmageed HA, Watanabe I, Elfadadny A, Shimada K, El-Masry G, Al-Rejaie S. Monthly dynamics of plasma elements, hematology, oxidative stress markers, and hormonal concentrations in growing male Shiba goats (Capra hircus) reared in Tokyo-Japan. Animals. 2022;12:645. doi: 10.3390/ani12050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson NO, Shehadeh H, Fullston T, Zander-Fox DL, Lane M. Dietary micronutrient supplementation for 12 days in obese male mice restores sperm oxidative stress. Nutrients. 2019;11:2196. doi: 10.3390/nu11092196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell JM. Heat stress and bull fertility. Theriogenology. 2020;153:62–67. doi: 10.1016/j.theriogenology.2020.05.014. [DOI] [PubMed] [Google Scholar]
- Nadjarzadeh A, Shidfar F, Amirjannati N, Vafa M, Motevalian S, Gohari M, Nazeri Kakhki S, Akhondi M, Sadeghi M. Effect of Coenzyme Q10 supplementation on antioxidant enzymes activity and oxidative stress of seminal plasma: a double-blind randomised clinical trial. Andrologia. 2014;46:177–183. doi: 10.1111/and.12062. [DOI] [PubMed] [Google Scholar]
- Navas P, Villalba JM, de Cabo R. The importance of plasma membrane coenzyme Q in aging and stress responses. Mitochondrion. 2007;7:34–40. doi: 10.1016/j.mito.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Nemec Svete A, Vovk T, Bohar Topolovec M, Kruljc P. Effects of Vitamin E and Coenzyme Q10 supplementation on oxidative stress parameters in untrained leisure horses subjected to acute moderate exercise. Antioxidants. 2021;10:908. doi: 10.3390/antiox10060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi W, Yagi K. Determination of lipid peroxidation by MDA. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Safarinejad MR. Efficacy of coenzyme Q10 on semen parameters, sperm function and reproductive hormones in infertile men. J Urol. 2009;182:237–248. doi: 10.1016/j.juro.2009.02.121. [DOI] [PubMed] [Google Scholar]
- Salama A, Caja G, Hamzaoui S, Badaoui B, Castro-Costa A, Façanha D, Guilhermino M, Bozzi R. Different levels of response to heat stress in dairy goats. Small Rumin Res. 2014;121:73–79. doi: 10.1016/j.smallrumres.2013.11.021. [DOI] [Google Scholar]
- Samir H, Nyametease P, Elbadawy M, Nagaoka K, Sasaki K, Watanabe G. Administration of melatonin improves testicular blood flow, circulating hormones, and semen quality in Shiba goats. Theriogenology. 2020;146:111–119. doi: 10.1016/j.theriogenology.2020.01.053. [DOI] [PubMed] [Google Scholar]
- Samir H, Nyametease P, Nagaoka K, Watanabe G. Effect of seasonality on testicular blood flow as determined by color Doppler ultrasonography and hormonal profiles in Shiba goats. Anim Reprod Sci. 2018;197:185–192. doi: 10.1016/j.anireprosci.2018.08.027. [DOI] [PubMed] [Google Scholar]
- Samir H, Sasaki K, Ahmed E, Karen A, Nagaoka K, El Sayed M, Taya K, Watanabe G (2015) Effect of a single injection of gonadotropin-releasing hormone (GnRH) and human chorionic gonadotropin (hCG) on testicular blood flow measured by color doppler ultrasonography in male Shiba goats. J Vet Med Sci 14-0633. 10.1292/jvms.14-0633 [DOI] [PMC free article] [PubMed]
- Sandhir R, Sethi N, Aggarwal A, Khera A. Coenzyme Q10 treatment ameliorates cognitive deficits by modulating mitochondrial functions in surgically induced menopause. Neurochem Int. 2014;74:16–23. doi: 10.1016/j.neuint.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Satoh K. Method of lipid peroxidation determination in serum. Clin Chim Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Shahat A, Rizzoto G, Kastelic J. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology. 2020;158:84–96. doi: 10.1016/j.theriogenology.2020.08.034. [DOI] [PubMed] [Google Scholar]
- Sharideh H, Zeinoaldini S, Zhandi M, Zaghari M, Sadeghi M, Akhlaghi A, Peebles ED. Use of supplemental dietary coenzyme Q10 to improve testicular function and fertilization capacity in aged broiler breeder roosters. Theriogenology. 2020;142:355–362. doi: 10.1016/j.theriogenology.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Sharma S, Ramesh K, Hyder I, Uniyal S, Yadav V, Panda R, Maurya V, Singh G, Kumar P, Mitra A. Effect of melatonin administration on thyroid hormones, cortisol and expression profile of heat shock proteins in goats (Capra hircus) exposed to heat stress. Small Rumin Res. 2013;112:216–223. doi: 10.1016/j.smallrumres.2012.12.008. [DOI] [Google Scholar]
- Song M-H, Kim H-N, Lim Y, Jang I-S. Effects of coenzyme Q10 on the antioxidant system in SD rats exposed to lipopolysaccharide-induced toxicity. Lab Anim Res. 2017;33:24–31. doi: 10.5625/lar.2017.33.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprecher D, Coe P. Differences in bull spermiograms using eosin-nigrosin stain, feulgen stain, and phase contrast microscopy methods. Theriogenology. 1996;45:757–764. doi: 10.1016/0093-691X(96)00005-2. [DOI] [PubMed] [Google Scholar]
- Taha T, Abdel-Gawad E, Ayoub M. Monthly variations in some reproductive parameters of Barki and Awassi rams throughout 1 year under subtropical conditions 2. Biochemical and enzymatic properties of seminal plasma. Anim Sci. 2000;71:325–332. doi: 10.1017/S135772980005517X. [DOI] [Google Scholar]
- Teama FEI, El-Tarabany AA. Physiological and biochemical response to Omega-3 plus as a dietary supplement to growing goats under hot summer conditions. Rev Bras de Zootec. 2016;45:174–180. doi: 10.1590/S1806-92902016000400005. [DOI] [Google Scholar]
- Tiwari S, Mohanty T, Bhakat M, Kumar N, Baithalu R, Nath S, Yadav H, Dewry R. Comparative evidence support better antioxidant efficacy of mitochondrial-targeted (Mitoquinone) than cytosolic (Resveratrol) antioxidant in improving in-vitro sperm functions of cryopreserved buffalo (Bubalus bubalis) semen. Cryobiology. 2021;101:125–134. doi: 10.1016/j.cryobiol.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Tsao C-W, Hsu Y-J, Tseng X-T, Chang T-C, Tsao C-H, Liu C-Y. Does Coenzyme Q10 supplementation improve testicular function and spermatogenesis in male mice with chronic kidney disease? Biology. 2021;10:786. doi: 10.3390/biology10080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafa W, El-Nagar H. Reproductive efficiency, milk production, health status, antioxidant capacity, lipid profile, and metabolic hormones of lactating cows treated with coenzyme q10 and l-carnitine. J Anim Health Prod. 2021;9:380–390. doi: 10.17582/journal.jahp/2021/9.4.380.390. [DOI] [Google Scholar]
- Watts G, Playford D, Croft K, Ward N, Mori T, Burke V. Coenzyme Q10 improves endothelial dysfunction of the brachial artery in Type II diabetes mellitus. Diabetologia. 2002;45:420–426. doi: 10.1007/s00125-001-0760-y. [DOI] [PubMed] [Google Scholar]
- West JW. Effects of heat-stress on production in dairy cattle. J Dairy Sci. 2003;86:2131–2144. doi: 10.3168/jds.S0022-0302(03)73803-X. [DOI] [PubMed] [Google Scholar]
- Zarazaga L, Gatica M, Celi I, Guzmán J, Malpaux B. Effect of artificial long days and/or melatonin treatment on the sexual activity of Mediterranean bucks. Small Rumin Res. 2010;93:110–118. doi: 10.1016/j.smallrumres.2010.05.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available by the corresponding author upon a reasonable query.