Abstract
Background
A comparison between atezolizumab plus bevacizumab (ATEZO/BEVA) and lenvatinib (LEN) for the treatment of hepatocellular carcinoma (HCC) remains unclear.
Objective
This study aimed to compare the therapeutic effects and safety of ATEZO/BEVA and LEN as first-line therapies for HCC.
Patients and Methods
This study was a retrospective analysis of 810 patients with HCC who underwent ATEZO/BEVA (n = 186) or LEN (n = 624) as first-line systemic therapy between March 2018 to March 2022 at 14 facilities. After propensity score matching, 304 patients (ATEZO/BEVA group: n = 152; LEN group: n = 152) were analyzed.
Results
After propensity score matching, although there was no significant difference in objective response rates (ORRs) between the ATEZO/BEVA and LEN groups (ORR 44.8% vs. 46.7%, p = 0.644), the median progression-free survival (PFS) and median overall survival (OS) in the ATEZO/BEVA group were significantly higher than those in the LEN group (median PFS: 8.3 months vs. 6.0 months, p = 0.005; median OS: not reached vs. 20.2 months, p = 0.039). The rates of appetite loss, fatigue, and proteinuria of grade 3 or higher in the ATEZO/BEVA group were lower than those in the LEN group. However, the rate of bleeding of grade 3 or higher in the ATEZO/BEVA group was higher than that in the LEN group. The conversion rate was higher in the ATEZO/BEVA group than that in the LEN group (8.6% vs. 1.9%, p = 0.007).
Conclusions
ATEZO/BEVA showed superiority to LEN in terms of prognosis and conversion rate as first-line therapy. Moreover, ATEZO/BEVA had a lower rate of severe adverse events, except for bleeding, than LEN.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-022-00921-x.
Key Points
| Atezolizumab plus bevacizumab (ATEZO/BEVA) and lenvatinib (LEN) were compared as first-line therapy for hepatocellular carcinoma in this retrospective study. ATEZO/BEVA was superior to LEN in median progression-free survival and median survival time. |
| Although ATEZO/BEVA has relatively lower severe adverse events than LEN, severe bleeding should be carefully monitored in ATEZO/BEVA. |
| ATEZO/BEVA has the potential for a higher conversion rate than LEN. |
Introduction
Systemic treatment of unresectable hepatocellular carcinoma (HCC) has advanced markedly, and patients with unresectable HCC can receive a variety of systemic treatments [1, 2]. After the era of molecular targeted agents (MTAs) that mainly target tumor angiogenesis, atezolizumab plus bevacizumab (ATEZO/BEVA), a combination therapy of immune checkpoint inhibitors and an anti-angiogenic agent, has been approved as a first-line systemic option for unresectable HCC.
ATEZO/BEVA was approved based on the results of the IMbrave 150 phase III clinical trial [3]. ATEZO/BEVA treatment significantly prolonged progression-free survival (PFS) and overall survival (OS) compared with sorafenib (SORA) in patients with unresectable HCC. According to the results of the IMbrave 150 phase III clinical trial, ATEZO/BEVA has been globally approved as first-line systemic therapy for unresectable HCC patients in addition to SORA and lenvatinib (LEN).
LEN has been approved as an MTA for the first-line treatment of patients with unresectable HCC based on the results of the REFLECT trial [4], preceding the approval of Atezo/Beva. LEN targets vascular endothelial growth factor receptors 1–3 and fibroblast growth factor receptors 1–4 [5]. Although LEN showed non-inferiority to SORA for OS, it has the potential for a better response compared with other MTAs [4]. However, IMbrave 150 is a clinical study that compared ATEZO/BEVA and SORA. No clinical trial has directly compared the therapeutic effects and safety between ATEZO/BEVA and LEN. Recently, although some retrospective analyses have compared ATEZO/BEVA and LEN for HCC patients [6, 7], the efficacy and safety of ATEZO/BEVA and LEN remain unclear.
The aim of this study was to compare the therapeutic effects and safety between ATEZO/BEVA and LEN as first-line therapies for patients with unresectable HCC.
Patients and Methods
Study Design
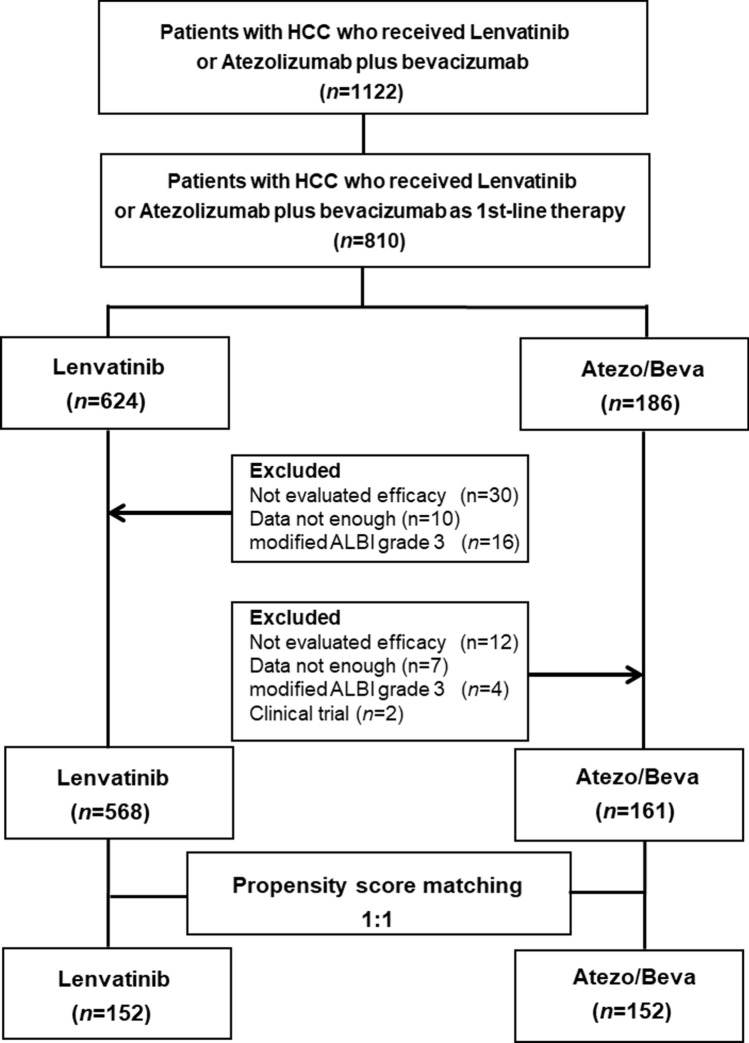
This retrospective study evaluated 810 patients who were treated with ATEZO/BEVA or LEN as first-line therapy between March 2018 and March 2022 across the Kyusyu Liver Cancer Study group, which included 14 institutions. Following the initial evaluation, patients with any of the following exclusion criteria were excluded from the analysis: non-evaluated efficacy (n = 12), insufficient data (n = 7), modified albumin–bilirubin (mALBI) grade 3 (n = 4), or clinical trial (n = 2) in the ATEZO/BEVA group, or non-evaluated efficacy (n = 30), insufficient data (n = 10), or mALBI grade 3 (n = 16) in the LEN group (Fig. 1). The cut-off date for this analysis was September 2022. This study conformed with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kurume University (approval number: 21006). An opt-out approach was used to obtain informed consent from patients, and personal information was protected during data collection.
Fig. 1.
Study design. A total of 810 patients with HCC treated with ATEZO/BEVA or LEN as the first-line systemic treatment were evaluated. In the course of the study, 81 patients were excluded, resulting in 729 patients with HCC being included. Propensity score matching was then performed on the data of the 729 patients with HCC, resulting in 304 patients with HCC being included in the evaluation. HCC hepatocellular carcinoma, ATEZO/BEVA atezolizumab plus bevacizumab, ALBI albumin–bilirubin
Propensity Score Matching (PSM)
Propensity score matching (PSM) overcomes different distributions of covariates among individuals allocated to specific interventions and is generated using potential covariates that could affect group allocation [8]. In this study, the propensity scores of all patients were estimated using a logistic regression model with the following baseline characteristics as covariates: age, sex, etiology of chronic liver disease, body mass index (BMI), ALBI score [9], tumor number, tumor size, α-fetoprotein (AFP) level, and the number of registered patients in each facility. A one-to-one nearest-neighbor matching algorithm with an optimal caliper of 0.2 without replacement was applied to a pair of groups with 152 patients each. Since p-values could be biased by population size, the PSM results were also reported as effect size with |value| < 0.2, |value| < 0.5, |value| < 0.8, and any other value indicated a negligible difference, small difference, moderate difference, and large difference, respectively. The sensitivity and c-statics were 70% and 0.71, respectively (electronic supplementary Fig. 1). Thus, 304 patients (ATEZO/BEVA, n = 152; and LEN, n = 152) were analyzed.
Lenvatinib (LEN) and Atezolizumab Plus Bevacizumab (ATEZO/BEVA) Treatment Protocol and Safety Evaluation
A combination of 1200 mg ATEZO and 15 mg/kg BEVA (Chugai Pharmaceutical Co. Ltd, Tokyo, Japan) was intravenously administered once every 3 weeks, according to the pharmaceutical recommendations. LEN (Eisai Co., Ltd, Tokyo, Japan) was orally administered at a dose of 12 mg/day for patients with a body weight ≥ 60 kg, or 8 mg/day for patients with a body weight < 60 kg. Treatment continued until the development of unacceptable adverse events (AEs) or progressive disease. AEs were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5.0).
Evaluation of Hepatic Functional Reserve and Therapeutic Response
ALBI score and mALBI grade were used to evaluate liver function [10]. In the ATEZO/BEVA group, tumors were assessed by dynamic computed tomography or magnetic resonance imaging every 6 weeks after the start of treatment according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria [11]. In the LEN group, tumors were assessed by dynamic computed tomography or magnetic resonance imaging 4–6 weeks after the start of treatment according to the mRECIST criteria.
Statistical Analysis
All statistical analyses were carried out using statistical analysis software (JMP Pro version 15; SAS Institute Inc., Cary, NC, USA), and all parameters were expressed as median (range) and number. To overcome possible selection bias, we performed one-to-one PSM as previously described [12]. PFS and OS were estimated using the Kaplan–Meier method and analyzed using the log-rank test. Multivariate analyses were conducted using the Cox proportional hazards model to identify risk factors associated with OS. Statistical significance was defined as a two-tailed p value < 0.05.
Results
Patient Characteristics Before PSM
The characteristics of the 729 analyzed patients are summarized in Table 1. The median age was 72 years (range 31–93) and 80.9% (590/729) of patients were males. The median BMI was 23.1 kg/m [2] (range 13.6–38.9). The etiology of HCC was non-viral hepatitis in 44.7% (326/729) of patients. The median ALBI score was −2.44 and ALBI grade 1 was observed in 35.9% (262/729) of patients. The Barcelona Clinic Liver Cancer (BCLC) stages A, B, and C were observed in 4.4%, 47.7%, and 47.9% of patients. Macrovascular invasion and extrahepatic spread were observed in 141 patients (19.3%) and 251 patients (34.4%), respectively. In the entire cohort, 475, 158, and 96 patients were registered with high-, middle-, and low-volume centers (VCs), respectively (Table 1). The ATEZO/BEVA and LEN groups included 161 and 568 patients, respectively. The median age and number of registered patients in each facility were significantly different between the ATEZO/BEVA and LEN groups; however, there were no significant differences in sex, BMI, cause of HCC, ALBI grade, and tumor factors between the two groups. The median observation periods for the ATEZO/BEVA and LEN groups were 12.1 months and 18.0 months, respectively.
Table 1.
Patient characteristics
| Characteristic | All patients | ATEZO/BEVA | LEN | p value |
|---|---|---|---|---|
| N | 729 | 161 | 568 | |
| Age, years | 72 (31–93) | 73 (38–93) | 72 (31–93) | 0.012 |
| Sex (female/male) | 139/590 | 38/123 | 101/467 | 0.103 |
| BMI, kg/m2 | 23.1 (13.6–38.9) | 23.3 (17.0–33.8) | 23.0 (13.6–38.9) | 0.422 |
| Etiology (HBV/HCV/non B, C) | 116/287/326 | 22/63/76 | 94/224250 | 0.617 |
| ALBI score | −2.44 (−3.77 to −1.45) | −2.41 (−3.77 to −1.55) | −2.46 (−3.60 to −1.45) | 0.753 |
| ALBI grade (1/2a/2b) | 262/213/254 | 60/44/57 | 202/169/197 | 0.830 |
| Tumor diameter, mm (<30/≥30) | 355/374 | 72/89 | 283/285 | 0.252 |
| Number of tumors (<5/≥5) | 336/393 | 68/93 | 268/300 | 0.265 |
| BCLC stage (A/B/C) | 32/348/349 | 4/83/74 | 28/265/275 | 0.256 |
| Macrovascular invasion (no/yes) | 588/141 | 126/35 | 462/106 | 0.387 |
| Extrahepatic spread (no/yes) | 478/251 | 114/47 | 364/204 | 0.109 |
| AFP, ng/mL | 32.4 (0.6–907,222) | 28.3 (1.0–907,222) | 32.6 (0.6–252,348) | 0.868 |
| Number of registered patients (high VC/middle VC/low VC) | 475/158/96 | 70/26/65 | 193/178/178 | < 0.001 |
Data are expressed as median (range) or number
ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib, BMI body mass index, HBV hepatitis B virus, HCV hepatitis C virus, ALBI albumin-bilirubin, BCLC Barcelona Clinic Liver Cancer, ALBI albumin-bilirubin score, AFP α-fetoprotein, VC volume center
Comparison of Therapeutic Response and Conversion Rate between the Two Groups before PSM
The therapeutic responses of the two groups are shown in electronic supplementary Table 1. Despite no significant difference in the objective response rate (ORR) between the ATEZO/BEVA and LEN groups (ORR 44.2% vs. 47.5%; p = 0.440), there was a significant difference in the disease control rate (DCR) between the two groups (88.9% vs. 80.7%; p = 0.013). The proportion of patients who received conversion therapy before PSM was significantly higher in the ATEZO/BEVA group compared with the LEN group (8.1% [13/161] vs. 1.6% [9/568]; p = 0.001).
Comparison of Progression-Free Survival (PFS) and Overall Survival (OS) between ATEZO/BEVA and LEN Treatment Before PSM
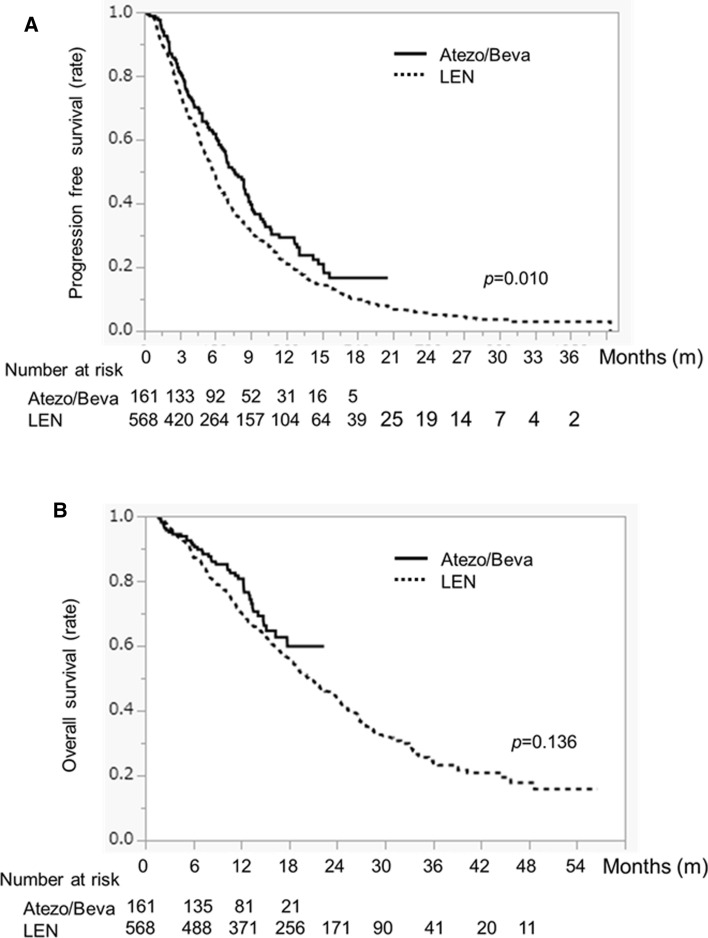
PFS in the ATEZO/BEVA group was significantly higher than in the LEN group (PFS 7.6 months vs. 5.8 months; p = 0.010) (Fig. 2a). In contrast, there was no difference in OS between the ATEZO/BEVA and LEN groups (median survival time (MST) not reached vs. 20.4 months, p = 0.136) (Fig. 2b).
Fig. 2.
a Progression-free survival and b overall survival in HCC patients treated with ATEZO/BEVA or LEN before propensity score matching. The solid black line indicates the ATEZO/BEVA group and the dotted line indicates the LEN group. HCC hepatocellular carcinoma, ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib
Patient Characteristics After PSM
To minimize the effect of confounding factors, we performed PSM using the following factors: age, sex, BMI, etiology, ALBI score, number of tumors, tumor size, macrovascular invasion, extrahepatic spread, AFP level, and number of registered patients in each facility. There were no significant differences between the two groups (Table 2).
Table 2.
Patient characteristics after propensity score matching
| Characteristic | All patients | ATEZO/BEVA | LEN | p value |
|---|---|---|---|---|
| N | 304 | 152 | 152 | |
| Age, years | 74 (31–93) | 73 (51–93) | 75 (31–93) | 0.351 |
| Sex (female/male) | 59/245 | 34/118 | 25/127 | 0.198 |
| BMI, kg/m2 | 23.3 (13.6–38.9) | 23.3 (17.0–33.8) | 23.4 (13.6–38.9) | 0.399 |
| Etiology (HBV/HCV/non B, C) | 47/121/136 | 22/61/69 | 25/60/67 | 0.891 |
| ALBI score | −2.44 (−3.77 to −1.45) | −2.43 (−3.77 to −1.55) | −2.48 (−3.54 to −1.59) | 0.835 |
| ALBI grade (1/2a/2b) | 115/83/107 | 58/41/53 | 57/41/54 | 0.991 |
| Tumor diameter, mm (< 30/≥ 30) | 145/159 | 71/81 | 74/78 | 0.730 |
| Number of tumors (< 5/≥ 5) | 121/183 | 64/88 | 57/95 | 0.412 |
| BCLC stage (A/B/C) | 10/159/135 | 4/81/67 | 6/78/68 | 0.791 |
| Macrovascular invasion (no/yes) | 240/64 | 121/31 | 119/33 | 0.778 |
| Extrahepatic spread (no/yes) | 212/92 | 109/43 | 103/49 | 0.453 |
| AFP, ng/mL | 32.0 (0.9–272,264) | 25.7 (1.0–272,264) | 35.7 (0.9–252,348) | 0.924 |
| Number of registered patients (high VC/middle VC/low VC) | 132/54/60 | 67/25/65 | 65/29/58 | 0.834 |
Data are expressed as median (range), or number
ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib, BMI body mass index, HBV hepatitis B virus, HCV hepatitis C virus, ALBI albumin-bilirubin, BCLC Barcelona Clinic Liver Cancer, AFP α-fetoprotein, VC volume center
Comparison of Therapeutic Response between the Two Groups After PSM
The therapeutic responses of the two groups are shown in electronic supplementary Table 2. Despite no significant difference in ORR between the ATEZO/BEVA and LEN groups (ORR 44.8% vs. 46.7%; p = 0.644), there was a significant difference in DCR between the two groups (90.2% vs. 78.9%; p = 0.006).
Comparison of PFS and OS between ATEZO/BEVA and LEN Treatment After PSM
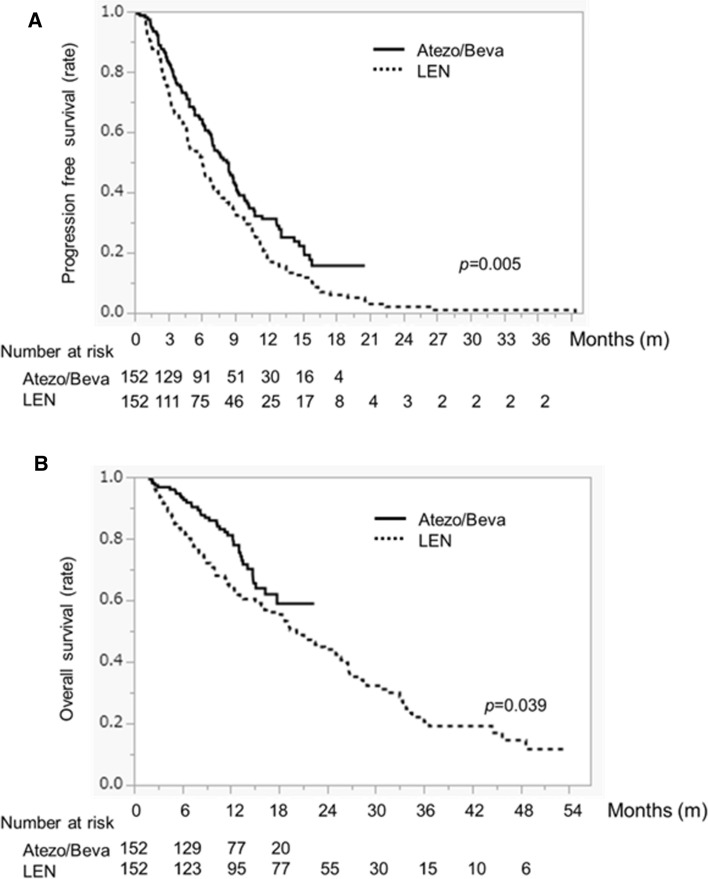
Kaplan–Meier curves for PFS and OS were evaluated after PSM. PFS in the ATEZO/BEVA group was significantly higher than in the LEN group (8.3 months vs. 6.0 months; p = 0.005) (Fig. 3a). In addition, OS in the ATEZO/BEVA group was significantly higher than in the LEN group (MST not reached vs. 20.2 months; p = 0.039) (Fig. 3b). OS rates for the ATEZO/BEVA group were 93.2%, 81.1%, and 58.9% at 0.5, 1, and 1.5 years, respectively, and 82.1%, 64.5%, and 53.9%, respectively, for the LEN group.
Fig. 3.
a Progression-free survival and b overall survival in HCC patients treated with ATEZO/BEVA or LEN after propensity score matching. The solid black line indicates the ATEZO/BEVA group and the dotted line indicates the LEN group. HCC hepatocellular carcinoma, ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib
Univariate and Multivariate Analyses of Factors Associated with OS After PSM
ALBI grade, macrovascular invasion, extrahepatic spread, AFP, and regimen were selected as variables in the univariate analysis. ALBI grade, extrahepatic spread, AFP, and regimen were identified as independent factors for OS in multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analyses of factors for OS after PSM
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| p value | OR | 95% CI | p value | |
| Age (<75 vs. ≥75 years) | 0.479 | |||
| Sex (male vs. female) | 0.079 | |||
| Etiology (HBV vs. HCV vs. non B, C) | 0.176 | |||
| ALBI grade (1 vs. 2) | <0.001 | 0.581 | 0.401–0.842 | <0.001 |
| Maximum tumor diameter, mm (<30/≥30) | 0.056 | |||
| Number of tumors (<5/≥5) | 0.167 | |||
| Macrovascular invasion (yes/no) | 0.046 | 0.715 | 0.488–1.045 | 0.083 |
| Extrahepatic spread (yes/no) | 0.001 | 2.140 | 1.528–3.004 | 0.001 |
| AFP (<200 vs. ≥200 ng/mL) | <0.001 | 0.485 | 0.339–0.694 | <0.001 |
| Regimen (ATEZO/BEVA vs. LEN) | 0.039 | 0.638 | 0.431–0.946 | 0.025 |
OR odds ratio, CI confidence interval, OS overall survival, ALBI, albumin-bilirubin, AFP α-fetoprotein, ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib, PSM propensity score matching
Conversion Rate between the ATEZO/BEVA and LEN Groups
The proportion of patients who received conversion therapy was 8.6% (13/152) and 1.9% (3/152) in the ATEZO/BEVA and LEN groups, respectively. As an additional treatment for conversion therapy, seven, three, one, one, and one patient in the ATEZO/BEVA group underwent surgery, transcatheter arterial chemoembolization (TACE), radiofrequency ablation, radiation, and LEN treatment, respectively. In the LEN group, two patients underwent surgery and one patient underwent TACE (Table 4).
Table 4.
Conversion rate with ATEZO/BEVA and LEN
| Variables | ATEZO/BEVA [n = 152] | LEN [n = 152] | p value |
|---|---|---|---|
| Conversion therapy (yes/no) | 13/142 | 3/149 | 0.007 |
| Conversion rate | 8.6% (13/152) | 1.9% (3/152) | |
| Surgery | 7 | 2 | |
| RFA | 1 | ||
| TACE | 3 | 1 | |
| Radiation | 1 | ||
| LEN | 1 |
ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib, RFA radiofrequency ablation, TACE transarterial chemoembolization
Comparison of Adverse Events between ATEZO/BEVA and LEN Treatment
The prevalence of appetite loss, fatigue, hypothyroidism, and hand-foot syndrome reaction were significantly higher in the LEN group than in the ATEZO/BEVA group (p ≤ 0.001, p = 0.048, p = 0.003, and p ≤ 0.001, respectively). In contrast, the prevalence of bleeding was significantly higher in the ATEZO/BEVA group than in the LEN group (p < 0.001). Regarding grade 3 or higher AEs, the prevalence of hypertension, appetite loss, fatigue, proteinuria, and diarrhea were significantly higher in the LEN group than in the ATEZO/BEVA group (p = 0.004, p = 0.023, p = 0.049, p = 0.046, p = 0.029, respectively); however, the prevalence of bleeding was significantly higher in the ATEZO/BEVA group than in the LEN group (p = 0.031) (Table 5).
Table 5.
Difference in adverse events associated with ATEZO/BEVA and LEN
| Characteristic | ATEZO/BEVA | LEN | p value |
|---|---|---|---|
| N | 152 | 152 | |
| Hypertension | |||
| Any grade | 50 (32.9) | 66 (43.2) | 0.058 |
| Grade 3 or higher | 9 (5.9) | 25 (16.4) | 0.004 |
| Appetite loss | |||
| Any grade | 11 (7.2) | 56 (36.8) | < 0.001 |
| Grade 3 or higher | 1 (0.6) | 7 (4.6) | 0.023 |
| Fatigue | |||
| Any grade | 41 (26.9) | 56 (36.8) | 0.048 |
| Grade 3 or higher | 5 (3.2) | 12 (7.8) | 0.049 |
| Hypothyroidism | |||
| Any grade | 15 (9.9) | 39 (25.6) | 0.003 |
| Grade 3 or higher | 1 (0.6) | 1 (0.6) | NS |
| Proteinuria | |||
| Any grade | 37 (24.3) | 36 (23.6) | 0.893 |
| Grade 3 or higher | 8 (5.2) | 17 (11.8) | 0.046 |
| HFSR | |||
| Any grade | 3 (1.9) | 26 (17.1) | <0.001 |
| Grade 3 or higher | 0 (0.0) | 3 (1.9) | 0.081 |
| Diarrhea | |||
| Any grade | 11 (7.2) | 19 (12.5) | 0.121 |
| Grade 3 or higher | 1 (0.6) | 6 (3.9) | 0.029 |
| Liver disorder | |||
| Any grade | 48 (31.5) | 37 (24.3) | 0.159 |
| Grade 3 or higher | 6 (3.9) | 4 (2.6) | 0.520 |
| Skin disorder | |||
| Any grade | 18 (11.8) | 12 (7.9) | 0.247 |
| Grade 3 or higher | 4 (2.6) | 1 (0.6) | 0.161 |
| Bleeding | |||
| Any grade | 15 (9.9) | 1 (0.6) | < 0.001 |
| Grade 3 or higher | 7 (4.6) | 1 (0.6) | 0.031 |
Data are expressed as n (%)
ATEZO/BEVA atezolizumab plus bevacizumab, LEN lenvatinib, HFSR hand-foot syndrome reaction, NS non-significant
Discussion
We demonstrated that PFS, OS, and conversion rates in the ATEZO/BEVA group were significantly higher than those in the LEN group as first-line systemic therapies following PSM. Moreover, the ATEZO/BEVA regimen was an independent factor associated with OS. However, the rate of bleeding was significantly higher in the ATEZO/BEVA group than in the LEN group.
Although the ORR in the ATEZO/BEVA group was similar to that in the LEN group in this study, the PFS was significantly longer in the ATEZO/BEVA group than in the LEN group. This result suggests that a better therapeutic response was sustained longer with ATEZO/BEVA treatment. It has been reported that the therapeutic response to ATEZO/BEVA treatment is durable [13]. In patients who achieved a better therapeutic response to ATEZO/BEVA, the response remained for at least 6 months in more than half of the responders and for at least 12 months in about one-quarter of responders [13]. In addition to the anti-angiogenic effect of BEVA, activation of tumor immunity by ATEZO/BEVA contributes to the durable activity of ATEZO/BEVA treatment. Moreover, Galle et al. reported that the period until the deterioration of quality of life was significantly longer with ATEZO/BEVA than with SORA. [14] Based on this perspective, the sustained response period and low rate of ATEZO/BEVA AEs might contribute to longer PFS. In contrast, although the therapeutic response of LEN was not inferior to that of ATEZO/BEVA, the discontinuation rate of LEN treatment due to AEs was high. In our previous study, the discontinuation rate of LEN due to AEs was 44% [15]. A high incidence rate of severe AEs can jeopardize the therapeutic response to LEN, resulting in a shortened PFS. Despite these problems in LEN treatment, the ORR rate was 46.7% in the present study, which tended to be slightly better than the ORR rate of 40.6% in the REFLECT trial. We recently reported a useful protocol for LEN involving a 5 days on/2 days off administration schedule (the weekends-off protocol) [16]. The weekends-off protocol for LEN significantly contributed to improvement in the therapeutic response in real-world practice. However, it is unclear why the ORR in the ATEZO/BEVA group was higher than in the IMbrave 150 study; thus, we would like to extend the observation period further to investigate this issue.
Our findings demonstrate that OS was also significantly longer in the ATEZO/BEVA group than in the LEN group. Recently, Hiraoka et al. reported that the prognosis of HCC patients who received ATEZO/BEVA as a first-line treatment is superior to that of HCC patients who received LEN [17]. The REFLECT trial showed that LEN was not inferior to SORA in terms of OS in patients with unresectable HCC, whereas the IMbrave 150 trial showed that ATEZO/BEVA was superior to SORA. Additionally, in this study, conversion rates in the ATEZO/BEVA group were significantly higher than those in the LEN group, especially for curative treatments such as surgical resection and radiofrequency ablation as additional treatments. Considering these points, it is reasonable to conclude that ATEZO/BEVA has a better prognosis than LEN. Moreover, the median observation period of ATEZO/BEVA treatment was longer than in previous studies [6, 7, 17], which we consider a more meaningful result. Additionally, a previous report clarified that the combination of ATEZO/BEVA is superior to LEN based on a matching-adjusted indirect comparison (MAIC) using data on LEN from patients outside of randomized trials, as well as data on the ATEZO/BEVA results derived from the IMbrave150 trial [18]. In the present study, we also found that ATEZO/BEVA is superior to LEN in real-world practice using the group data of two cohorts. However, the median OS in the REFLECT study was 13.6 months, whereas this study improved the median OS in the LEN group to 20.2 months. Currently, sequential therapy with MTAs is considered effective for unresectable HCC [19–21].
Some previous studies have reported that the median OS of patients treated with LEN using sequential therapy was approximately 20 months [7, 21]. Terashima et al. reported that the median post-progression survival was more strongly correlated with OS in advanced HCC patients treated with MTA [22]. In fact, among patients with discontinuation of LEN treatment, 94 patients (63.0%) received subsequent treatment and 59 patients (39.5%) received sequential therapy in that study. Moreover, in the sequential therapy, 27 patients (45.7%) received ATEZO/BEVA. These factors may contribute to the significantly prolonged OS with LEN treatment. In summary, sequential therapy using ATEZO/BEVA as first-line therapy may be expected to further prolong the prognosis of unresectable HCC patients in the future.
In this study, there was a significantly higher conversion rate in the ATEZO/BEVA group than in the LEN group. Kudo reported that when significant tumor reduction occurred, the strategy of ATEZO/BEVA followed by curative conversion (ABC conversion) was possible [23]. In particular, patients with intermediate-stage HCC who are not suitable for TACE may benefit the most [23]. In conversion cases in the ATEZO/BEVA group, nine patients (69.2%) were diagnosed with intermediate-stage HCC and were unsuitable for TACE. Although the observation period of the ATEZO/BEVA group was not sufficient, it is possible that the rate of ABC conversion will increase during the observation period. Thus, the treatment paradigm for HCC was drastically changed by ATEZO/BEVA therapy.
In this study, the incidence rate of the common AEs in the ATEZO/BEVA group was relatively lower than in the LEN group. Among the AEs, the prevalence rates of appetite loss, fatigue, and grade 3 or higher proteinuria in the ATEZO/BEVA group were lower than those in the LEN group. In systemic therapy, appetite loss, fatigue, and proteinuria are common AEs that lead to the discontinuation of treatment [15, 24, 25]. Maesaka et al. reported that the rate of discontinuation due to AEs was lower in the ATEZO/BEVA group than in the LEN group [7]. Regarding safety, ATEZO/BEVA was found to be well tolerated and caused fewer AEs than LEN, which was a particularly interesting aspect of clinical practice. However, an increased risk of severe bleeding has been reported with BEVA treatment [26]. Moreover, Kim et al. reported that it is necessary to have a strong awareness of possible bleeding in the treatment of ATEZO/BEVA [6]. In the present study, the prevalence rate of grade 3 or higher bleeding in the ATEZO/BEVA group was higher than in the LEN group. Therefore, bleeding should be monitored more than usual in patients with HCC undergoing treatment with ATEZO/BEVA.
This study has some limitations. First, it was a retrospective study, and second, the follow-up duration for the ATEZO/BEVA group was not sufficient to assess mortality. Third, the patients introduced selection bias with treatment allocation, and fourth, there is confounding in the OS analysis based on relative dates of enrollment. Lastly, there were differences in the number of registered patients in each facility. To overcome these factors, we used PSM analysis to minimize bias; however, it cannot provide the same level of evidence as randomized controlled trials.
Conclusions
ATEZO/BEVA therapy was superior to LEN treatment as the first-line systemic therapy in terms of PFS and transition conversion rate. Moreover, ATEZO/BEVA therapy had a lower rate of severe AEs, except for bleeding, than LEN in patients with unresectable HCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
This study did not receive any financial support.
Conflicts of interest
Takashi Niizeki, Takayuki Tokunaga, Yuko Takami, Yoshiyuki Wada, Masaru Harada, Michihiko Shibata, Kazuhiko Nakao, Ryu Sasaki, Fumihito Hirai, Satoshi Shakado, Tomoharu Yoshizumi, Shinji Itoh, Hiroshi Yatsuhashi, Shigemune Bekki, Akio Ido, Seiichi Mawatari, Koichi Honda, Rie Sugimoto, Takeshi Senju, Hirokazu Takahashi, Takuya Kuwashiro, Tatsuji Maeshiro, Makoto Nakamuta, Yoshifusa Aratake, Tsutomu Yamashita, Yuichiro Otsuka, Shuichi Matsumoto, Tetsuro Sohda, Shigeo Shimose, Kenta Murotani, and Yasuhito Tanak declare they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical approval and informed consent
This study conformed to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Kurume University (approval number: 21006). An opt-out approach was used to obtain informed consent from patients and personal information was protected during data collection.
Consent to participate
An opt-out approach was used to obtain informed consent from patients.
Consent for publication
Not applicable.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request
Code availability
Not applicable.
Author contributions
TN and SS participated in study conception and design, data acquisition, data interpretation, and manuscript drafting. TT, YT, YW, MH, MS, KN, RS, FH, SS, TY, SI, HY, SB, AI, SM, KH, RS, TS, HT, TK, TM, MN, YA, TY, YO, SM, and TS participated in data acquisition. TN, SS, and KM participated in data analysis, data interpretation, and manuscript drafting. YT participated in the critical revision of the manuscript.
References
- 1.Kudo M. Systemic therapy for hepatocellular carcinoma: latest advances. Cancers (Basel) 2018;10(11):412. doi: 10.3390/cancers10110412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kudo M. Management of hepatocellular carcinoma in Japan: current trends. Liver Cancer. 2020;9(1):1–5. doi: 10.1159/000505370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Matsui J, Matsushima T, Obaishi H, Miyazaki K, Nakamura K, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell. 2014;6:18. doi: 10.1186/2045-824X-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY, et al. Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers (Basel) 2022;14(7):1747. doi: 10.3390/cancers14071747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maesaka K, Sakamori R, Yamada R, Doi A, Tahata Y, Miyazaki M, et al. Comparison of atezolizumab plus bevacizumab and lenvatinib in terms of efficacy and safety as primary systemic chemotherapy for hepatocellular carcinoma. Hepatol Res. 2022;52(7):630–640. doi: 10.1111/hepr.13771. [DOI] [PubMed] [Google Scholar]
- 8.Kitai S, Kudo M, Nishida N, Izumi N, Sakamoto M, Matsuyama Y, et al. Survival benefit of locoregional treatment for hepatocellular carcinoma with advanced liver cirrhosis. Liver Cancer. 2016;5(3):175–189. doi: 10.1159/000367765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiraoka A, Kumada T, Tsuji K, Takaguchi K, Itobayashi E, Kariyama K, et al. Validation of modified ALBI grade for more detailed assessment of hepatic function in hepatocellular carcinoma patients: a multicenter analysis. Liver Cancer. 2019;8(2):121–129. doi: 10.1159/000488778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31(5):1031–1036. doi: 10.1111/jgh.13250. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimose S, Tanaka M, Iwamoto H, Niizeki T, Shirono T, Aino H, et al. Prognostic impact of transcatheter arterial chemoembolization (TACE) combined with radiofrequency ablation in patients with unresectable hepatocellular carcinoma: comparison with TACE alone using decision-tree analysis after propensity score matching. Hepatol Res. 2019;49(8):919–928. doi: 10.1111/hepr.13348. [DOI] [PubMed] [Google Scholar]
- 13.Salem R, Li D, Sommer N, Hernandez S, Verret W, Ding B, et al. Characterization of response to atezolizumab + bevacizumab versus sorafenib for hepatocellular carcinoma: results from the IMbrave150 trial. Cancer Med. 2021;10(16):5437–5447. doi: 10.1002/cam4.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(7):991–1001. doi: 10.1016/S1470-2045(21)00151-0. [DOI] [PubMed] [Google Scholar]
- 15.Shimose S, Iwamoto H, Niizeki T, Shirono T, Noda Y, Kamachi N, et al. Clinical significance of adverse events for patients with unresectable hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Cancers (Basel) 2020;12(7):1867. doi: 10.3390/cancers12071867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwamoto H, Suzuki H, Shimose S, Niizeki T, Nakano M, Shirono T, et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers (Basel) 2020;12(4):1010. doi: 10.3390/cancers12041010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiraoka A, Kumada T, Tada T, Hirooka M, Kariyama K, Tani J, et al. Does first-line treatment have prognostic impact for unresectable HCC?-Atezolizumab plus bevacizumab versus lenvatinib. Cancer Med. 2022 doi: 10.1002/cam4.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casadei-Gardini A, Tada T, Shimose S, Kumada T, Niizeki T, Cascinu S, et al. Is Atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma superior even to lenvatinib? A matching-adjusted indirect comparison. Target Oncol. 2021;16(2):249–254. doi: 10.1007/s11523-021-00803-8. [DOI] [PubMed] [Google Scholar]
- 19.Ogasawara S, Ooka Y, Itokawa N, Inoue M, Okabe S, Seki A, et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Japan. Invest New Drugs. 2020;38(1):172–180. doi: 10.1007/s10637-019-00801-8. [DOI] [PubMed] [Google Scholar]
- 20.Shimose S, Hiraoka A, Nakano M, Iwamoto H, Tanaka M, Tanaka T, et al. First-line sorafenib sequential therapy and liver disease etiology for unresectable hepatocellular carcinoma using inverse probability weighting: a multicenter retrospective study. Cancer Med. 2021;10(23):8530–8541. doi: 10.1002/cam4.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomonari T, Sato Y, Tani J, Hirose A, Ogawa C, Morishita A, et al. Comparison of therapeutic outcomes of sorafenib and lenvatinib as primary treatments for hepatocellular carcinoma with a focus on molecular-targeted agent sequential therapy: a propensity score-matched analysis. Hepatol Res. 2021;51(4):472–481. doi: 10.1111/hepr.13597. [DOI] [PubMed] [Google Scholar]
- 22.Terashima T, Yamashita T, Takata N, Nakagawa H, Toyama T, Arai K, et al. Post-progression survival and progression-free survival in patients with advanced hepatocellular carcinoma treated by sorafenib. Hepatol Res. 2016;46(7):650–656. doi: 10.1111/hepr.12601. [DOI] [PubMed] [Google Scholar]
- 23.Kudo M. Changing the treatment paradigm for hepatocellular carcinoma using atezolizumab plus bevacizumab combination therapy. Cancers (Basel) 2021;13(21):5475. doi: 10.3390/cancers13215475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hatanaka T, Hiraoka A, Tada T, Hirooka M, Kariyama K, Tani J, et al. Association of early bevacizumab interruption with efficacy of atezolizumab plus bevacizumab for advanced hepatocellular carcinoma: a landmark analysis. Hepatol Res. 2022;52(5):462–470. doi: 10.1111/hepr.13748. [DOI] [PubMed] [Google Scholar]
- 25.Rapposelli IG, Tada T, Shimose S, Burgio V, Kumada T, Iwamoto H, et al. Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int. 2021;41(12):2997–3008. doi: 10.1111/liv.15014. [DOI] [PubMed] [Google Scholar]
- 26.Leighl NB, Bennouna J, Yi J, Moore N, Hambleton J, Hurwitz H. Bleeding events in bevacizumab-treated cancer patients who received full-dose anticoagulation and remained on study. Br J Cancer. 2011;104(3):413–418. doi: 10.1038/sj.bjc.6606074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.