Abstract
Evidence suggests that adults with a history of childhood maltreatment, the experience of emotional or physical neglect and/or abuse within the family during childhood, have blunted reward and stress processing, and higher risk of depression. The mu opioid receptor rich nucleus accumbens and amygdala are critical to reward and stress processing respectively. We hypothesized that nucleus accumbens and amygdala mu opioid receptor densities and activity (change in receptor binding due to endogenous opioid release or receptor conformation change) were negatively associated with childhood maltreatment in healthy young adults. Maltreatment was assessed with the Childhood Trauma Questionnaire (CTQ). Healthy participants, n = 75 (52% female) completed [11C]carfentanil positron emission tomography imaging labeling mu opioid receptors. The relationship between CTQ score and binding potential (BPND, proportional to density of unoccupied receptors) was evaluated with a linear mixed effects model. No significant relationship was found between CTQ score and BPND (f = 3.28; df = 1, 73; p = 0.074) or change in BPND (activity) (t = 1.48; df = 198.3; p = 0.14). This is the first investigation of mu opioid receptors in those with childhood maltreatment. We did not identify a significant relationship between mu opioid receptor dynamics and severity of maltreatment in those without psychopathology. Because this cohort has a low CTQ score average, this may indicate that those with low severity of maltreatment may not have associated changes in mu opioid receptor dynamics. Future directions include evaluating a cohort with increased severity of childhood maltreatment.
Keywords: Childhood maltreatment, Neuroimaging, Mu opioid receptors, Positron emission tomography
Introduction
Over 60% of adults in the United States have at least one past adverse childhood experience which may include abuse or neglect (Merrick et al., 2018). Adverse early life experiences are critical determinants of health: as demonstrated by the seminal adverse childhood experience (ACE) study, increased exposure to abuse, neglect or family dysfunction is related to negative health outcomes including cancer, heart disease, and chronic lung disease (Felitti et al., 1998). Childhood maltreatment (CM) is defined here as the experience of emotional or physical neglect and/or abuse within the family (MacDonald et al., 2016). CM negatively impacts mental health; those who have past CM and develop psychiatric disorders have earlier onset, increased symptom severity and increased risk of comorbidity (McCrory et al., 2017). Roughly 30% of all individuals with CM develop major depressive disorder by their late 20’s (Dannlowski et al., 2012). Studying the mu opioid receptor (MOR) system in those with CM may shed light on the biological underpinnings of depression following CM as lower MOR receptor density has been related to depression symptom severity (Nummenmaa et al., 2020). Further, individuals with CM have an increased likelihood of developing opioid use disorder (Merrick et al., 2020; Santo et al., 2021). Because the average age of onset of opioid use is 25.6 years (Naji et al., 2017), evaluating individuals at age 25 and younger is imperative to understand neurobiological effects of CM in healthy young adults before potential onset of psychiatric disorders, including opioid use disorder, to develop appropriate interventions and therapies.
The MOR system plays a key role in perception of reward, stress, and emotional response to rejection (Hsu et al., 2013, 2015). Specifically, increased MOR activity has been observed following reward stimuli and pain stimuli such as social acceptance and rejection respectively (Hsu et al., 2015). However, this response may be altered in those with CM. CM is associated with altered perception and reaction to social feedback, specifically social acceptance, and rejection (Oswald et al., 2021; Puetz et al., 2016; Teicher et al., 2016; van den Berg et al., 2018). Individuals with CM have blunted stress and reward processing, which may be related to altered amygdala and nucleus accumbens (NAc) functioning respectively (Goff et al., 2013; O'Connor et al., 2018; Puetz et al., 2016; Teicher et al., 2016), which supports evidence from preclinical models that have demonstrated MOR alterations after early life stress, specifically in the amygdala and NAc (Chang et al., 2019; Oswald et al., 2021). Using functional MRI (fMRI), human studies have shown CM is consistently associated with decreased signal within the NAc in response to reward anticipation and receipt (Goff et al., 2013; Teicher et al., 2016). Variation in structure and function of the NAc (DeRosse et al., 2020) including reduced size, alterations in developmental trajectory, and blood flow have been reported in adults with a history of CM (Teicher et al., 2016). Additionally, abnormalities in stress processing, specifically decreased amygdala function have been demonstrated in those with CM (O'Connor et al., 2018; Puetz et al., 2016). In CM, social rejection, which induces social stress (Slavich et al., 2010), is associated with decreased activity in the amygdala compared to those without CM as shown through fMRI (Puetz et al., 2016).
In this investigation, an emotional response was elicited with social rejection stimuli, and a reward response with social acceptance stimuli in a simulated dating task shown to induce MOR activity in the amygdala and NAc (Hsu et al., 2013, 2015). MOR dynamics were assessed through Positron Emission Tomography (PET) using the tracer [11C]carfentanil, which reversibly labels MOR in the brain, allowing displacement by endogenous opioid release (Hsu et al., 2013, 2015). Activity is defined here as a change in MOR binding by endogenous opioid release, change in receptor conformational state, or receptor internalization (Hsu et al., 2015). Because endogenous opioid release dampens stress response (Barfield et al., 2010) and modulates reward response (Roth-Deri et al., 2008), and both processes are disrupted in CM (Goff et al., 2013; O'Connor et al., 2018; Puetz et al., 2016; Teicher et al., 2016), it was hypothesized that in healthy individuals, NAc and amygdala MOR density and activity during social feedback is negatively associated with severity of CM.
Additionally, MOR activity in the amygdala downregulates the glucocorticoid cortisol, produced by the hormonal stress response system, the hypothalamic–pituitary– adrenal (HPA) axis (Jaferi & Pickel, 2009), most likely through inhibiting production of corticotropin-releasing factor in the amygdala and hypothalamus (Hsu et al., 2015; Jaferi & Pickel, 2009). When examined in healthy individuals, amygdala MOR activity was negatively related to cortisol release during a similar social rejection task (Hsu et al., 2015). However, this relationship may be disturbed in CM. Those with CM have lower resting cortisol and blunted cortisol release in response to stressful stimuli (Carpenter et al., 2007; O'Connor et al., 2018). One possible mechanism could be that early prolonged periods of stress induces high cortisol release, which later increases sensitivity to negative feedback or glucocorticoids (Fries et al., 2005). Therefore, we hypothesized that in those with lower exposure to CM, MOR amygdala activity has a negative relationship to resting cortisol and cortisol release during rejection, and this association is attenuated in those with more severe exposure to CM.
The present investigation is the first neuroimaging study to employ PET to examine opioid neurotransmission in psychiatrically-healthy individuals with CM who have not yet developed psychopathology (Oswald et al., 2021). Studying this cohort allows for disentanglement of the effects of CM from psychopathology, which frequently co-occurs (Dannlowski et al., 2012). Understanding the neurobiological effects of CM and its relationship with hormonal dysregulation may lead to targeted interventions and improved support for those who experienced abuse and neglect during childhood.
Methods
Participants
Participants were recruited from community advertisements. All participants were never mentally ill based on a structured clinical interview (SCID-IV non-patient version) (First, 2002). Other exclusion criteria included: chronic pain, left-handedness, taking psychotropic medication, hormone medication including contraception, and present smoking. Inclusion criteria included being age 18—25 and being romantically single because previous studies (Hsu et al., 2013, 2015) indicate that being in a romantic relationship impacts the effect of the social feedback task. After receiving a complete description of the investigation, all participants provided written informed consent and the Institutional Review Board of University of Michigan Medical School approved this study. This is the second investigation of MOR binding and activity in this cohort. The first investigation regards the psychological trait rejection sensitivity in relation to MOR binding and activity. This first investigation does not examine childhood maltreatment.
Questionnaires and Stimuli
All participants were administered the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., 1994; Bernstein & Fink, 1998) to assess CM. The CTQ has 25 questions each ranging from possible scores of one—five (excluding the three minimization questions (MacDonald et al., 2016) assessing positive response bias). There are five categories addressed that each have five questions: emotional abuse, physical abuse, sexual abuse, emotional neglect, and physical neglect. Each subscale score ranges from five—25 and the total possible score range is from 25—125.
Social Feedback Task
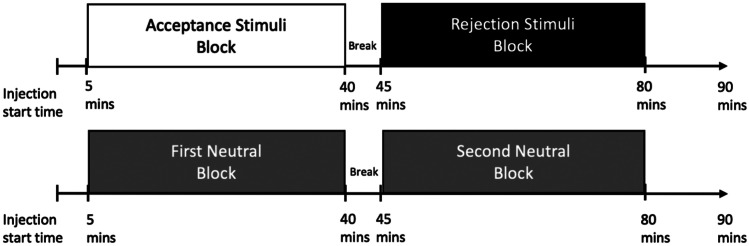
All participants completed the social feedback task during PET scanning. This task was adapted from Hsu et al. (2013). In short, participants chose preferred profiles, from a simulated dating website before the scan day. The simulated website included pictures of individuals, along with description of the interests of each fictional, potential ‘date.’ During the scan day, each participant underwent two PET scans, each with two stimuli blocks. One scan had two feedback blocks (rejection and acceptance) and the other scan had two neutral blocks (Fig. 1). During the rejection feedback block, the participant was presented with six responses from their chosen profiles, indicating that the person they had chosen did not choose the participant. During the acceptance block, the participant was presented with six responses that a simulated individual did like the participant. During the neutral stimuli scan, participants had two blocks where they viewed six response cards that indicated the profiles were “not completed”. The order of each block, and the order of either feedback type (rejection or acceptance) was counterbalanced across subjects with the Latin Squares design (Hsu et al., 2013).
Fig. 1.
Diagram for scan sequence depicting the two scans (neutral and stimuli) each with two blocks along with associated time points in minutes (mins). The two blocks in the stimuli scan are “acceptance” and “rejection” and the two blocks in the neutral scan are first and second neutral
PET and MRI
Techniques for PET and MRI imaging and processing have been reported previously (Hsu et al., 2013, 2015). In short, 10—17 mCi of [11C]carfentanil (maximum mass of 0.05 μg/kg and specific activity > 3000 Ci/mmol) was injected intravenously with a bolus plus infusion technique over the course of each 90-min scan with a Siemens/CTI HR+ scanner at the University of Michigan. Images were reconstructed with an iterative ordered subsets expectation maximization (OSEM) algorithm into a 128 × 128 pixel matrix and a field of view that was 28.8 cm diameters. Both scans occurred on the same day in all but one participant, who completed the second scan on a separate day. Nondisplaceable binding potential (BPND) was calculated with Logan graphical analysis, with the occipital lobe as the reference region (Logan et al., 1996). Voxel MOR binding maps were co-registered to MR images, collected in separate imaging sessions. Coregistered images were normalized to Montreal Neurological Institute (MNI) standard space. Images were smoothed with a three-dimensional Gaussian filter (full width at half maximum (FWHM) 6 mm). The regions of interest (ROIs) were created in a previous study (Hsu et al., 2013) with MarsBaR region of interest toolbox (v. 0.38) for Statistical Parametric Mapping (SPM) v.8 (Wellcome Institute of Cognitive Neurology, London, UK). From the MNI standardized space, ROI masks were applied to PET data after normalization. Regional BPND in bilateral ROI’s were averaged. Baseline BPND indicates the first occurring neutral block in the neutral scan. This is depicted as “First Neutral Block” in Fig. 1.
MOR activity was calculated as the difference in BPND between either acceptance or rejection blocks and its time matched neutral. For example, if the acceptance block occurred first in the feedback scan, it was compared to the first neutral block in the neutral scan (Fig. 1).
Plasma Cortisol Collection and Analysis
For cortisol collection, venous blood samples were collected throughout the PET scan at 0, 20, 30, 40, 60, 70, 80 min post injection and were stored at -80 °C and then analyzed for cortisol using the IMMULITE 1000 system (Siemens Medical Solutions Diagnostic Division, Malvern, PA). Cortisol at baseline and “cortisol release”, which we define here as the difference in cortisol area under the curve, was assessed between rejection and neutral blocks. Additional details may be found in (Hsu et al., 2015). Baseline cortisol was measured in 74 out of 75 participants. Cortisol release was measured in 66 out of 75 participants throughout the PET scan blocks.
Statistics
The relationship between CTQ score and baseline BPND in the amygdala and NAc was evaluated with a linear mixed effects model with log transformed baseline BPND as outcome. Fixed effects included region and log of CTQ score, and the only random effect was participant.
A linear mixed effects model was used to model the relationship between CTQ score and MOR activity in the NAc and amygdala, with log BPND as outcome. Fixed effects included interval (first or second block), region, condition (neutral or rejection/acceptance), log of CTQ score, and the interaction between condition and log of CTQ score. The random effects were participant and scan session that was nested in participant. Within this model we tested whether the difference between neutral and rejection (similarly, the difference between neutral and acceptance) was different across CTQ values. We accomplished this by testing for equality of the slopes.
The relationship between CTQ score, MOR activity, and cortisol measures (baseline cortisol and cortisol release) was assessed using a linear mixed effects model with log of BPND in the amygdala as outcome. The fixed effects were interval, condition (rejection or neutral), log of CTQ score, cortisol measure, and the interaction between log of CTQ score and cortisol measure, and the random effect was subject.
Results
Demographics
All participants (N = 75 (52% female), age range 18–25 years, mean ± standard deviation = 21.1 ± 2.2) had completed at least 12 years of education. The range of CTQ scores were 25–73 with a mean and standard deviation of 32.6 ± 8.2.
Baseline Binding
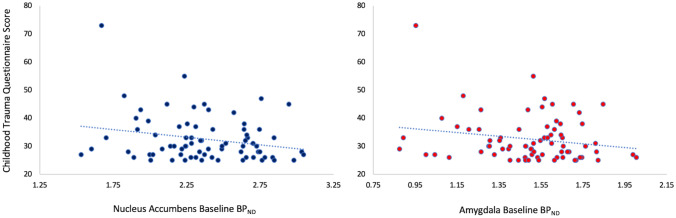
We did not find a significant association between exposure to maltreatment during childhood (CM as measured by the CTQ) and MOR binding in the NAc and amygdala (f = 3.28; df = 1, 73; p = 0.074) as shown in Fig. 2.
Fig. 2.
Association between MOR baseline binding (BPND) in the nucleus accumbens (left panel) and amygdala (right panel) and Childhood Trauma Questionnaire (CTQ) score, which assesses Childhood Maltreatment
Activity With Stimuli
There was no significant relationship identified between severity of exposure to maltreatment (CM as measured by CTQ total score) and MOR activity in the NAc or amygdala during acceptance (t = 1.04; df = 198.3; p = 0.30) or during rejection (t = 1.48; df = 198.3; p = 0.14).
Cortisol Analysis
We did not identify a relationship between MOR activity and the interaction between CTQ score and baseline cortisol (f = 0.23; df = 1, 69; p = 0.63) or MOR activity and the interaction between log of CTQ score and cortisol release (f = 2.73; df = 1, 84.9; p = 0.10).
Discussion
In the present investigation with 75 young adult participants completing two PET scans, MOR binding was examined during neutral stimuli in the NAc and amygdala in relation to severity of childhood maltreatment as measured by the CTQ (Figs. 2 and 3). No significant association between MOR binding and CTQ score was detected (f = 3.28; df = 1, 73; p = 0.074). Because the CTQ scores in the present investigation are low (average ± standard deviation: 32.6 ± 8.2) compared to the total possible score range of 25–125, this may indicate that those with low severity of maltreatment may not have associated changes in MOR density. However, given that the test statistics are not far from significance, evaluating a cohort with a higher average CTQ score in a future investigation may reveal a significant negative association between childhood maltreatment and NAc and amygdala MOR binding.
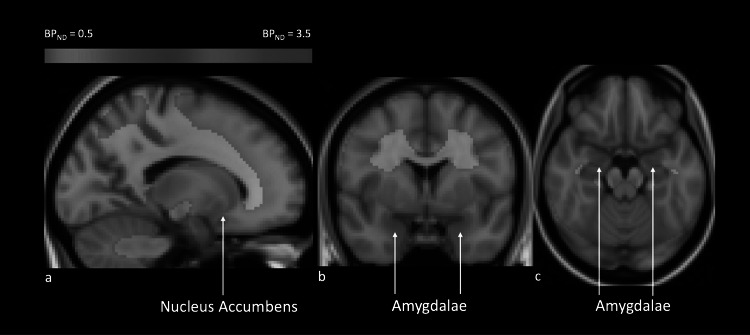
Fig. 3.
Map of MOR baseline binding (BPND). Each voxel represents the average of MOR binding (BPND) across all participants in the regions of interest, nucleus accumbens (NAc) and amygdalae, which are indicated with arrows. a. Sagittal view. b. Coronal view. c. Axial view
A significant linear relationship between CM and MOR binding may support the model that the effect of CM is not all-or-none, rather early life trauma exerts pathological effects in a dose-dependent manner (Oswald et al., 2021). Because binding potential is proportional to density of unoccupied receptors (Innis et al., 2007), a relationship between CTQ score and MOR binding would reflect decreased density of MOR receptors in the amygdala and NAc in individuals with increased CTQ scores. Lower MOR density decreases transduction of signal from endogenous opioid release (Polakiewicz et al., 1998). Because NAc opioidergic neurotransmission plays a role in reward processing (Lutz & Kieffer, 2013), decreased ability to transduce signal from endogenous opioid binding may constitute a mechanism by which CM relates to dampened reward processing. Additionally, since amygdala opioidergic neurotransmission contributes to stress and threat processing (Meier et al., 2021), a negative relationship between amygdala MOR density and CTQ score may be central to altered stress and threat processing in CM (Herzog & Schmahl, 2018; O'Connor et al., 2018; Puetz et al., 2016). Given that development of major depressive disorder is common in individuals with CM (Dannlowski et al., 2012), there is a possibility that decreased MOR binding in amygdala and NAc may precede depression onset since decreased binding in these regions have been shown to be associated with increased symptoms of depression in a cohort with subclinical depression and anxiety (Nummenmaa et al., 2020). Additionally, CM is associated with increased risk for developing opioid use disorder (Merrick et al., 2020; Santo et al., 2021), which is also associated with alterations in MOR density (Greenwald et al., 2003; Zubieta et al., 2000). Therefore, assessing MOR density in a cohort with larger range of CM may be impactful for assessing potential risk for development of psychopathology.
No significant relationship between MOR activity in the NAc or amygdala during acceptance or rejection respectively, was identified. The lack of a significant association may indicate that CM may not affect endogenous opioid release, receptor internalization or alterations in receptor conformational state during social stimuli.
Additionally, we did not observe a significant relationship between MOR activity and the interaction between CM and cortisol measures (baseline cortisol or cortisol release during rejection). Because MOR activity response to rejection varies even in healthy individuals (Hsu et al., 2013), and during rejection, lower MOR activity is related to higher cortisol release (Hsu et al., 2015), we hypothesized that those with lower severity of CM would have an association with cortisol release. Since cortisol release is attenuated in response to stressful stimuli in CM compared to controls (Carpenter et al., 2007; O'Connor et al., 2018), we hypothesized that those with increased severity of CM would have an attenuated relationship between cortisol release and MOR activity. However, there was no association found between cortisol measures, MOR activity and CTQ scores. One possible explanation could be that although the average CTQ score is low compared to the possible score range, this low CM may have an impactful enough exposure of childhood adversity to disrupt the functional relationship between cortisol and MOR activity. To examine this, a post-hoc analysis was performed, and individuals without CM (n = 8) had an association between amygdala MOR activity and cortisol release (f = 7.26; df = 1, 9.91; p = 0.023), which replicated the results of Hsu et al. (2015). Individuals with CM did not exhibit this association (n = 58, f = 0.21; df = 1, 72.3; p = 0.65).
This post-hoc analysis is exploratory and is in need of replication. However, the lack of a significant association between MOR activity and cortisol release in the group with CM indicates that the association between amygdala MOR activity and the HPA axis may be disrupted in those with childhood maltreatment. Because activity at MORs downregulate the HPA axis activity (Jaferi & Pickel, 2009; Jaschke et al., 2021), a disrupted relationship between cortisol release and MOR activity may reflect a failure of amygdala MOR activity to modulate cortisol release. Perhaps because cortisol release is already attenuated in CM, cortisol release is not responsive to inhibitory activity of MORs. However, the ability to modulate cortisol is important. There is evidence to suggest hypocortisolemia increases the likelihood of obesity and metabolic syndrome (Maripuu et al., 2016). As such, the disruption of cortisol modulation may provide a mechanism by which those with adverse childhood experiences have higher prevalence of heart disease (Felitti et al., 1998). Additionally, in individuals with past adverse childhood experiences, lower cortisol levels have been shown to mediate development of depressive symptoms (Iob et al., 2021). Future investigations assessing the impacts of hypocortisolemia may lead to targeted endocrine treatment for those with CM.
Limitations
The present investigation is limited by the low average and small range of CTQ scores. Because there is a high association between CM and psychopathology (Dannlowski et al., 2012; Felitti et al., 1998), recruiting individuals with no psychopathology, but a moderate or severe CM is challenging. The participant with the highest CTQ score is influential to the summary statistics. However, there is no reason to exclude this participant. Because this participant is influential, there is evidence to suggest including individuals with higher CTQ scores may strengthen the relationship between CTQ score and MOR density.
Conclusions
In the present investigation, no significant association between severity of childhood maltreatment and MOR density or activity in the NAc and amygdala was identified. However, since this cohort had a low average severity of maltreatment, this may indicate that those without moderate or severe childhood maltreatment may not have associated changes in MOR density and activity. Future directions include evaluating MOR dynamics in a cohort with increased severity of childhood maltreatment.
Declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. After receiving a complete description of the investigation, all participants provided written informed consent and the Institutional Review Board of University of Michigan Medical School approved this study.
Conflict of Interest Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Barfield ET, Barry SM, Hodgin HB, Thompson BM, Allen SS, Grisel JE. Beta-endorphin mediates behavioral despair and the effect of ethanol on the tail suspension test in mice. Alcoholism, Clinical and Experimental Research. 2010;34(6):1066–1072. doi: 10.1111/j.1530-0277.2010.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bernstein, D.P., Fink, L. (1998). Childhood Trauma Questionnaire: A Retrospective Self Report. San Antonio, Tx.: The Psychological Corporation: Harcourt Brace and Company.
- Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, Price LH. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biological Psychiatry. 2007;62(10):1080–1087. doi: 10.1016/j.biopsych.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Kigar SL, Ho JH, Cuarenta A, Gunderson HC, Baldo BA, Auger AP. Early life stress alters opioid receptor mRNA levels within the nucleus accumbens in a sex-dependent manner. Brain Research. 2019;1710:102–108. doi: 10.1016/j.brainres.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Kugel H. Limbic scars: Long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- DeRosse P, Ikuta T, Karlsgodt KH, Szeszko PR, Malhotra AK. History of childhood maltreatment is associated with reduced fractional anisotropy of the accumbofrontal 'reward' tract in healthy adults. Brain Imaging and Behavior. 2020;14(2):353–361. doi: 10.1007/s11682-020-00265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti, V. J., Anda, R. F., Nordenberg, D., Williamson, D. F., Spitz, A. M., Edwards, V., Marks, J. S. (1998). Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. American Journal Preventive Medicine, 14(4), 245–258. 10.1016/s0749-3797(98)00017-8 [DOI] [PubMed]
- First, M. B., Spitzer, Robert L, Gibbon Miriam, and Williams, Janet B.W. (2002). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York State Psychiatric Institute: New York: Biometrics Research.
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J, Tottenham N. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–138. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald MK, Johanson C-E, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, Zubieta J-K. Effects of Buprenorphine Maintenance Dose on μ-Opioid Receptor Availability, Plasma Concentrations, and Antagonist Blockade in Heroin-Dependent Volunteers. Neuropsychopharmacology. 2003;28(11):2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- Herzog JI, Schmahl C. Adverse Childhood Experiences and the Consequences on Neurobiological, Psychosocial, and Somatic Conditions Across the Lifespan. Front Psychiatry. 2018;9:420. doi: 10.3389/fpsyt.2018.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Walker SJ, Zubieta JK. It still hurts: Altered endogenous opioid activity in the brain during social rejection and acceptance in major depressive disorder. Molecular Psychiatry. 2015;20(2):193–200. doi: 10.1038/mp.2014.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DT, Sanford BJ, Meyers KK, Love TM, Hazlett KE, Wang H, Zubieta JK. Response of the μ-opioid system to social rejection and acceptance. Molecular Psychiatry. 2013;18(11):1211–1217. doi: 10.1038/mp.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow and Metabolism. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Iob E, Baldwin JR, Plomin R, Steptoe A. Adverse childhood experiences, daytime salivary cortisol, and depressive symptoms in early adulthood: A longitudinal genetically informed twin study. Translational Psychiatry. 2021;11(1):420. doi: 10.1038/s41398-021-01538-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaferi A, Pickel VM. Mu-opioid and corticotropin-releasing-factor receptors show largely postsynaptic co-expression, and separate presynaptic distributions, in the mouse central amygdala and bed nucleus of the stria terminalis. Neuroscience. 2009;159(2):526–539. doi: 10.1016/j.neuroscience.2008.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaschke N, Pählig S, Pan YX, Hofbauer LC, Göbel A, Rachner TD. From Pharmacology to Physiology: Endocrine Functions of μ-Opioid Receptor Networks. Trends in Endocrinology and Metabolism . 2021;32(5):306–319. doi: 10.1016/j.tem.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of Cerebral Blood Flow and Metabolism. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Kieffer BL. The multiple facets of opioid receptor function: Implications for addiction. Current Opinion in Neurobiology. 2013;23(4):473–479. doi: 10.1016/j.conb.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald K, Thomas ML, Sciolla AF, Schneider B, Pappas K, Bleijenberg G, Wingenfeld K. Minimization of Childhood Maltreatment Is Common and Consequential: Results from a Large, Multinational Sample Using the Childhood Trauma Questionnaire. PLoS ONE. 2016;11(1):e0146058. doi: 10.1371/journal.pone.0146058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative hypocortisolism is associated with obesity and the metabolic syndrome in recurrent affective disorders. Journal of Affective Disorders. 2016;204:187–196. doi: 10.1016/j.jad.2016.06.024. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, Gerin MI, Viding E. Annual Research Review: Childhood maltreatment, latent vulnerability and the shift to preventative psychiatry - the contribution of functional brain imaging. Journal of Child Psychology and Psychiatry. 2017;58(4):338–357. doi: 10.1111/jcpp.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier IM, van Honk J, Bos PA, Terburg D. A mu-opioid feedback model of human social behavior. Neuroscience and Biobehavioral Reviews. 2021;121:250–258. doi: 10.1016/j.neubiorev.2020.12.013. [DOI] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Haegerich TM, Simon T. Adverse Childhood Experiences Increase Risk for Prescription Opioid Misuse. The Journal of Primary Prevention. 2020;41(2):139–152. doi: 10.1007/s10935-020-00578-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of Adverse Childhood Experiences From the 2011–2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatrics. 2018;172(11):1038–1044. doi: 10.1001/jamapediatrics.2018.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naji L, Dennis BB, Bawor M, Varenbut M, Daiter J, Plater C, Samaan Z. The association between age of onset of opioid use and comorbidity among opioid dependent patients receiving methadone maintenance therapy. Addiction Science & Clinical Practice. 2017;12(1):9. doi: 10.1186/s13722-017-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummenmaa L, Karjalainen T, Isojärvi J, Kantonen T, Tuisku J, Kaasinen V, Rinne J. Lowered endogenous mu-opioid receptor availability in subclinical depression and anxiety. Neuropsychopharmacology. 2020;45(11):1953–1959. doi: 10.1038/s41386-020-0725-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor DB, Green JA, Ferguson E, O'Carroll RE, O'Connor RC. Effects of childhood trauma on cortisol levels in suicide attempters and ideators. Psychoneuroendocrinology. 2018;88:9–16. doi: 10.1016/j.psyneuen.2017.11.00. [DOI] [PubMed] [Google Scholar]
- Oswald, L. M., Dunn, K. E., Seminowicz, D. A., & Storr, C. L. (2021). Early Life Stress and Risks for Opioid Misuse: Review of Data Supporting Neurobiological Underpinnings. Journal Personalized Medicine, 11(4). 10.3390/jpm11040315 [DOI] [PMC free article] [PubMed]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. Journal of Biological Chemistry. 1998;273(36):23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Puetz VB, Viding E, Palmer A, Kelly PA, Lickley R, Koutoufa I, McCrory EJ. Altered neural response to rejection-related words in children exposed to maltreatment. Journal of Child Psychology and Psychiatry. 2016;57(10):1165–1173. doi: 10.1111/jcpp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth-Deri I, Green-Sadan T, Yadid G. Beta-endorphin and drug-induced reward and reinforcement. Progress in Neurobiology. 2008;86(1):1–21. doi: 10.1016/j.pneurobio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Santo T, Jr, Campbell G, Gisev N, Tran LT, Colledge S, Di Tanna GL, Degenhardt L. Prevalence of childhood maltreatment among people with opioid use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence. 2021;219:108459. doi: 10.1016/j.drugalcdep.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Natl Acad Sci U S A. 2010;107(33):14817–14822. doi: 10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nature Reviews Neuroscience. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- van den Berg, L. J. M., Tollenaar, M. S., Pittner, K., Compier-de Block, L., Buisman, R. S. M., van, I. M. H., & Elzinga, B. M. (2018). Pass it on? The neural responses to rejection in the context of a family study on maltreatment. Social Cognitive and Affective Neuroscience, 13(6), 616-627. 10.1093/scan/nsy035 [DOI] [PMC free article] [PubMed]
- Zubieta J, Greenwald MK, Lombardi U, Woods JH, Kilbourn MR, Jewett DM, Johanson CE. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: A preliminary study. Neuropsychopharmacology. 2000;23(3):326–334. doi: 10.1016/s0893-133x(00)00110-x. [DOI] [PubMed] [Google Scholar]