Abstract
Chronic inflammation is a continuous low-grade activation of the systemic immune response. Whereas downstream inflammatory markers are associated with atrial fibrillation (AF), upstream inflammatory effectors including eicosanoids are less studied. To examine the association between eicosanoids and incident AF. We used a liquid chromatography-mass spectrometry for the non-targeted measurement of 161 eicosanoids and eicosanoid-related metabolites in the Framingham Heart Study. The association of each eicosanoid and incident AF was assessed using Cox proportional hazards models and adjusted for AF risk factors, including age, sex, height, weight, systolic/diastolic blood pressure, current smoking, antihypertensive medication, diabetes, history of myocardial infarction and heart failure. False discovery rate (FDR) was used to adjust for multiple testing. Eicosanoids with FDR < 0.05 were considered significant. In total, 2676 AF-free individuals (mean age 66 ± 9 years, 56% females) were followed for mean 10.8 ± 3.4 years; 351 participants developed incident AF. Six eicosanoids were associated with incident AF after adjusting for multiple testing (FDR < 0.05). A joint score was built from the top eicosanoids weighted by their effect sizes, which was associated with incident AF (HR = 2.72, CI = 1.71–4.31, P = 2.1 × 10–5). In conclusion, six eicosanoids were associated with incident AF after adjusting for clinical risk factors for AF.
Subject terms: Biomarkers, Cardiology, Medical research
Introduction
Increased global life expectancy and longer survival with chronic conditions, including atrial fibrillation (AF), have prompted a keen interest in preventing or postponing age-related common chronic diseases and preserving wellness in the population1–3. Chronic inflammation is a continuous low-grade activation of the systemic immune response. Inflammation is a major feature of biological aging across multiple organ systems, including the cardiovascular system. Also, inflammation has been associated with “accelerated aging” phenotypes and reduced lifespan4,5. The majority of research analyzing inflammation has focused on downstream markers of inflammatory activity such as cytokines (e.g., tumor necrosis factors, interferons) and acute phase reactants (e.g., C-reactive protein, interleukins)6. Chronic elevation of downstream markers is associated with multiple age-related disease states and shorter lifespan7. However, evidence for a clinically important, causal role of these biomarkers is inconsistent8,9. It has been suggested that causative actors in the inflammatory arena are more likely to be upstream effectors10.
Data from small studies suggest that select eicosanoids are related to risk factors such as obesity11, diabetes12, and smoking13,14. Eicosanoids are biologically active lipid mediators derived from 20-carbon polyunsaturated fatty acids (PUFAs)15. Eicosanoids have significant activities in the regulation of normal physiological processes and disease pathogenesis in the human body16,17. Eicosanoids have pleiotropic roles in inflammation and immunity18,19. Many studies have focused on strategies for inhibiting the formation of inflammatory mediators that may contribute to risk of AF20. In contrast, epoxyeicosatrienoic acids act mainly as autocrine and paracrine effectors in the cardiovascular system and kidney, mediating vasorelaxation, anti-inflammatory, and pro-fibrinolytic processes, as well as several cardiovascular protective effects21. Therefore, blocking the process of metabolizing epoxyeicosatrienoic acids into diverse pro-inflammatory compounds (e.g., 1,2-diols, dihydroxyeicosatrienoic acids), leads to increases in the titers of epoxyeicosatrienoic acids, which in turn may contribute to the prevention of AF through attenuation of atrial structure and electrical remodeling22–24.
However, the role of eicosanoids as upstream biomarkers of incident AF is largely unknown. We hypothesized that increased plasma levels of eicosanoids are associated with increased rates of incident AF in the community.
Methods
All data and materials are available at the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center and can be requested at https://biolincc.nhlbi.nih.gov/studies/framcohort/. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Framingham Heart Study protocol was approved by the Boston University Medical Center Institutional Review Board (Approval Number H-32132) and all participants (or proxies) signed informed consent.
Study population
We analyzed data from the Framingham Heart Study (FHS). The FHS Original cohort (n = 5209) was established in 1948 to investigate risk factors for cardiovascular disease25. In the early 1970s, the children of the Original cohort participants and their spouses were enrolled into the Offspring cohort (n = 5124)26. A study sample (n = 2676 participants) was drawn from the FHS Offspring cohort who attended exam eight (2005–2008, total n = 3021 exam attendees) with archived blood specimens available for eicosanoid profiling.
Follow-up and covariate measurement
Participants in the Offspring cohort were routinely evaluated by history and physical examination in the research center by FHS clinicians approximately every 4–8 years. Participants were under surveillance during inter-exam periods for cardiovascular outcomes through review of outside medical records and clinician visits adjudicated by FHS investigators. Incident AF (atrial fibrillation and atrial flutter) was diagnosed if it occurred after the eighth examination by electrocardiogram at a FHS examination or if it was documented in the participants’ outside medical records, interim hospitalizations, and Holter monitor results. Prevalent AF cases, defined as those that were diagnosed with AF at or before their eighth clinical examination, were excluded from the analyses. Participants were followed from the date of their eighth FHS examination until the occurrence of AF, death, loss to follow-up, or December 31, 2018, whichever occurred first.
Age, sex, diabetes, blood pressure, self-reported smoking status, and antihypertensive medication use were recorded during each examination. Hospital records were reviewed to evaluate history of heart failure and myocardial infarction by 2–3 clinicians (the Framingham Endpoint Review Committee) between FHS follow-up exams prior to AF diagnosis. Heart failure was diagnosed based on the simultaneous presence of at least two major criteria or one major and two minor criteria as previously described27. History of myocardial infarction was designated if there were at least two of three findings: (1) symptoms indicative of ischemia; (2) changes in blood biomarkers of myocardial necrosis; (3) serial changes in electrocardiogram. Deaths were documented by death certificates. Additional information was obtained from records supplied by hospital, attending physician, pathologist, medical examiner, or family members.
Measurement of eicosanoids
Details of eicosanoid profiling have been described in detail previously28,29. In brief, eicosanoids and eicosanoid-related metabolites were analyzed using liquid chromatography-mass spectrometry (LC–MS), using a Vanquish UPLC coupled to high resolution, Q Exactive Orbitrap mass spectrometer (Thermo Fisher, Waltham, MA, USA). Metabolites were measured using a Phenomenex Kinetex C18 column, for the measurement of 161 eicosanoids and eicosanoid-related metabolites. Each eicosanoid is represented by MZ/RT, in which MZ is the mass-to-charge ratio (to 5 decimals) and RT is the retention time (to 4 decimals). QC/QA analysis was performed, and spectral data were extracted as previously described. We imputed missing values with the minimum value for each eicosanoid. Metabolites measurements were loge transformed and adjusted for age and sex. Residuals of the regressions were normalized using the median absolute deviation of each eicosanoid, as in a previous study30.
Assay of Eicosanoid Markers of Inflammation
Eicosanoid biomarker assays were performed in the (University of California San Diego, Dr. Jain laboratory) by the use of mass spectrometry (MS) after separation of lipids by gas chromatography (GC) or liquid chromatography (LC). The MS infrastructure used in this study includes an AB SCIEX QTRAP 6500 MS interfaced with a Waters Acquity UPLC system, an AB SCIEX QTRTAP 4000 MS coupled to a Shimadzu HPLC system, and an Agilent 6890 N Gas Chromatograph equipped with an Agilent 5975 Mass Selective Detector. Eicosanoids (> 75 in total) were isolated by solid phase extraction and quantified by reverse phase LC–MS using electrospray ionization (ESI) and multiple reaction monitoring (MRM). The eicosanoid markers included prostaglandins (e.g. PGF1beta, PGF1alpha, PGG2), leukotrienes (e.g. 20-carboxy-LTB4), and clavulones (e.g. clavulone I). A sample of 150 µl of EDTA plasma was required for each participant per exam.
Measurements of inflammation biomarkers
Blood samples were collected after an overnight fast and stored at − 80 °C until assayed. A detailed measurement protocol has been described previously31. CRP measurements were performed in serum using immunoturbidimetry (Roche Diagnostics Latex High Sensitivity Assay), and Interleukin 6 (IL-6) was analyzed using commercially available enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN, USA) as previously described32–34. Both CRP and IL-6 concentrations were measured at the same examination when eicosanoids were profiled. Detailed information regarding biomarker assessments can be found at the FHS website: https://framinghamheartstudy.org/files/2017/08/Inflammatory-Biomarker-Protocol-Offspring-Exam-8-and-Omni-1-Exam-3.pdf.
Statistical analysis
Descriptive statistics were calculated using means and standard deviations for continuous variables, or frequency counts and percentages for dichotomous variables. The association of each eicosanoid with incident AF was assessed using Cox proportional hazards models with robust sandwich estimators (to account for the relatedness of some participants), with follow-up times censored at the last follow-up time or death. Participants who were diagnosed with AF before examination 8 (prevalent AF, n = 210) were excluded from the Cox models. The primary models were adjusted for previously reported AF-related clinical factors35, including age, sex, height, weight, systolic and diastolic blood pressure, current smoking, use of antihypertensive medication, diabetes, history of myocardial infarction, and history of heart failure. The proportional hazard assumption was examined using visual analyses of the curves and Schoenfeld’s test36.
We further categorized eicosanoids into three tertile groups and examined their difference in terms of incident AF risk. In the exploratory models, we additionally adjusted for the concentration of CRP and IL-6 to understand the influence of downstream inflammatory biomarkers on the association between eicosanoids and incident AF.
We adjusted for multiple testing using false discovery rate (FDR)37. Eicosanoids with FDR-adjusted p value < 0.05 were considered significant. Sex- and age-stratified analyses were also performed. We tested for effect modification by sex and age (< 65 vs. ≥ 65 years old) in relation to incident AF risk by including interaction terms in the Cox models. A joint score was also built to represent a weighted combination of top eicosanoids (FDR < 0.05). The score for sample i was defined as , in which n is the number of top eicosanoids associated with incident AF, βj is the estimate of effect size for eicosanoid j, and Vij is the normalized level of eicosanoid j for sample i. Association of the joint score with incident AF was also tested using the Cox proportional hazards model and adjusted for clinical risk factors as the primary models.
In secondary analyses, we also examined the association of each eicosanoid with prevalent AF using generalized estimating equations. We adjusted for the same covariates as those used for the primary models. Eicosanoids with FDR < 0.05 were considered significant. All statistical analyses were performed using R software version 4.0.3 (https://www.r-project.org/).
Results
Study population
We included 2676 individuals without diagnosed AF (mean age 66 ± 9 years, 56% females) in the analysis of the association between eicosanoids and incident AF. Participants were followed for an average of 10.8 ± 3.4 years and a total of 351 participants were diagnosed with new-onset AF during the follow-up period. Baseline characteristics of the study population are presented in Table 1 and the baseline characteristics of subgroups are presented in sTable 1.
Table 1.
Baseline characteristics of study sample.
| Variable | Participants without prevalent AF n = 2676 |
|---|---|
| Age (years) | 66 ± 9 |
| Women | 1488 (55.6%) |
| Height (cm) | 167 ± 10 |
| Weight (kg) | 79 ± 18 |
| Current smoking | 249 (9.3%) |
| Systolic blood pressure (mmHg) | 129 ± 17 |
| Diastolic blood pressure (mmHg) | 74 ± 10 |
| Antihypertensive medication use | 1359 (50.8%) |
| Diabetes mellitus | 342 (12.8%) |
| Prevalent heart failure | 22 (0.8%) |
| Prevalent myocardial infarction | 100 (3.7%) |
Values are represented as n (%) for dichotomous variables or mean ± standard deviation (SD) for continuous variables.
Association of eicosanoids with incident AF
As shown in Table 2, we identified six eicosanoids that were significantly associated with incident AF after adjusting for AF-related clinical risk factors35, including 9-oxoODE, EIC_33, 12(R) HETE, 9-oxoOTrE, 15 oxoEDE, and HETrE [M-H]. The full association results are depicted in sTable 2. For each eicosanoid, we provide the hazard ratio (HR) and 95% confidence interval (CI) per standard deviation of the eicosanoid. Increased concentrations of each of the six top eicosanoids were associated with increased risk of incident AF (HR ranging from 1.16 to 1.22). These associations remained significant after additional adjustment for inflammatory biomarkers CRP and IL-6. We further categorized each eicosanoid into tertiles, and displayed their association with incident AF through Kaplan–Meier plots (sFigs. 1–6).
Table 2.
Eicosanoids associated with incident AF (FDR < 0.05).
| Eicosanoids# | Putative identity | Primary model* | Exploratory model* | ||||
|---|---|---|---|---|---|---|---|
| HR† | 95% CI† | P value† | HR† | 95% CI† | P value† | ||
| 293.21136/5.1237 | 9-oxoODE | 1.22 | 1.10–1.36 | 1.8 × 10–4 | 1.20 | 1.08–1.34 | 8.2 × 10–4 |
| 299.25921/5.5568 | EIC_33 | 1.16 | 1.07–1.27 | 6.9 × 10–4 | 1.25 | 1.04–1.25 | 4.2 × 10–3 |
| 265.17938/3.7720 | 12(R) HETE | 1.19 | 1.07–1.32 | 8.8 × 10–4 | 1.32 | 1.07–1.32 | 1.2 × 10–3 |
| 291.19445/4.3834 | 9-oxoOTrE | 1.19 | 1.07–1.31 | 9.8 × 10–4 | 1.31 | 1.07–1.31 | 1.5 × 10–3 |
| 321.24360/6.1182 | 15 oxoEDE | 1.18 | 1.07–1.29 | 1.0 × 10–3 | 1.28 | 1.05–1.28 | 4.0 × 10–3 |
| 321.24387/5.4933 | HETrE [M-H] | 1.17 | 1.06–1.29 | 1.6 × 10–3 | 1.27 | 1.04–1.27 | 5.8 × 10–3 |
#Each eicosanoid is represented by MZ/RT; MZ is the mass-to-charge ratio (to 5 decimals) and RT is the retention time (to 4 decimals).
*Primary model was adjusted for age, sex, height, weight, systolic and diastolic blood pressure, current smoking, use of antihypertensive medication, diabetes, history of myocardial infarction, and history of heart failure; Exploratory model was additionally adjusted for CRP and IL-6 concentration.
†HR Hazard ratio expressed per standard deviation of log transformed normalized eicosanoid concentration, CI Confidence interval; P value was not adjusted for multiple testing.
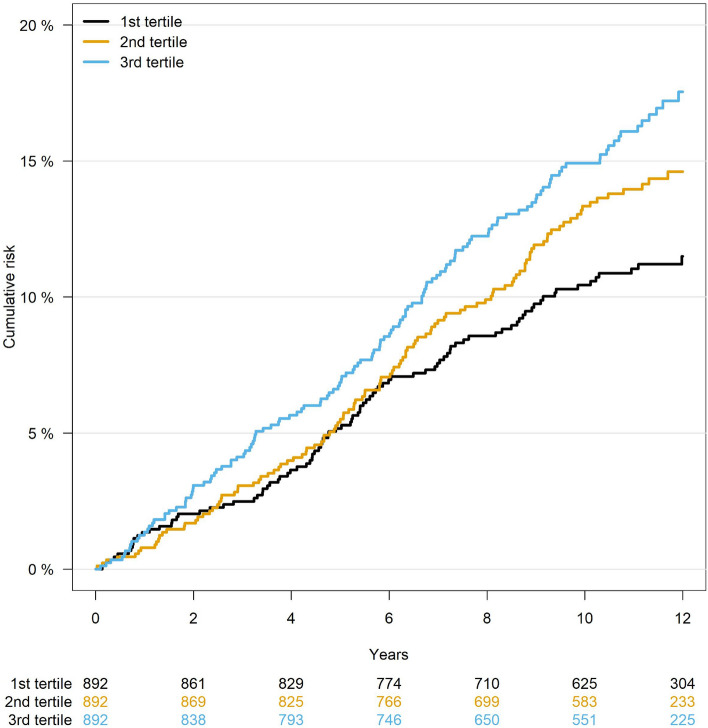
To assess the combined association of multiple eicosanoids with incident AF, we built a joint model that included the six top eicosanoids weighted by the effect size of individual eicosanoids, As displayed in Fig. 1, the score was significantly associated with incident AF after adjusting for known clinical risk factors (HR = 2.72, CI = 1.71–4.31, P = 2.1 × 10–5).
Figure 1.
Cumulative risk curve of AF in tertile groups for the eicosanoid composite score. The score represented a weighted combination of six eicosanoids associated with incident AF. Participants were divided into three tertile groups based on their scores. Lower panel shows the number of participants at risk during the study period.
We also performed sex- and age-stratified analyses. As shown in sTable 3, only one eicosanoid (299.25921/5.5) was nominally significant for women, but all were significant in men. None of eicosanoids showed evidence of significant effect modification by sex. The associations of eicosanoids with incident AF also showed different patterns between older adults (≥ 65 years old) and younger people (< 65 years old) (sTable 4). Two eicosanoids, 293.21136/5.1237 and 321.24387/5.4933, showed relatively lower association in older adults compared to younger adults (P value for interaction = 0.02 and 0.01, respectively).
Finally, in sTable 5 we analyzed the association of each top eicosanoids with CRP and IL-6. We observed modest correlations, ranging from − 0.03 (9-oxoOTrE) to 0.13 (9-oxoODE) for CRP, and from − 0.4 (9-oxoOTrE) to 0.12 (9-oxoODE) for IL-6. Prevalent and incident AF cases tended to have higher levels of CRP and IL-6 compared with referents.
Association of eicosanoids with prevalent AF
We also examined the association of eicosanoids with prevalent AF (n = 210). As depicted in sTable 2, five eicosanoids were significantly associated with prevalent AF after adjusting for AF-related clinical risk factors (FDR < 0.05)35. However, none of these were significantly associated with incident AF after accounting for multiple testing. Among the top six eicosanoids associated with incident AF, 9-oxoODE, EIC_33 and HETrE [M-H] were nominally associated with prevalent AF (P = 0.03, 0.02, and 0.009, respectively).
Discussion
Eicosanoids are biologically active lipid mediators originated mostly from the omega-6 (n-6) PUFA, arachidonic acid, but also from substrates such as the (n−6) PUFA dihomo-γ-linolenic acid, and the omega-3 (n−3) PUFA eicosapentaenoic acid. Arachidonic acid, a key polyunsaturated fatty acid, is a precursor of many pro- and anti-inflammatory signaling molecules, including eicosanoids. Metabolomic profiling of arachidonic acid and its metabolites has improved our understanding of several cardiovascular diseases24. In the current study, we analyzed the association between eicosanoids and incident AF among FHS participants. We identified six eicosanoids—9-oxoODE, 12(R) HETE, 9-oxoOTrE, 15 oxoEDE, and HETrE, as well as an unknown eicosanoid29 (EIC_33)—that were significantly associated with incident AF after adjusting for known AF-related clinical risk factors. Three of these were nominally associated with prevalent AF.
Eicosanoids associated with incident AF
Eicosanoid profiling in human plasma represents an opportunity to identify novel pathophysiologic biomarkers associated with AF initiation and progression. Relatively few investigations have assessed the distribution or abundance of plasma eicosanoids, and even fewer have sought to link this association with incident and prevalent AF.
A previous study described a directed non-targeted mass spectrometry approach for the discovery of eicosanoids and related oxylipins29. Members of the reported eicosanoid and related oxylipin metabolites described in this study were closely associated with markers of systemic inflammation. In our study, using similar approaches, we identified six eicosanoids that were significantly associated with incident AF. Interestingly, one of these eicosanoids (Mass-to-Charge Ratio/Retention Time: 293.21136/5.1237, Accurate Mass: 296.2351) has been identified as an epoxy fatty acid, main class: octadecanoids (LipidMaps ID: LMFA02000038; common name: 12,13 EpOME). For more details on this lipid, please see the link https://pubchem.ncbi.nlm.nih.gov/compound/5356421 and https://www.lipidmaps.org/data/LMSDRecord.php?LMID=LMFA0200003829. The 12,13-epoxyoctadecenoic acid (12,13-EpOME) is produced through the cytochrome P450 dependent metabolism and is known as isoleukotoxin38. The physiologic concentration of EpOME is reported to be dependent on the regulation of biosynthetic pathways and dietary intake of their relevant fatty acid, linoleic acid, which is the most abundantly consumed PUFA in the human diet39. In a study focused on the roles of linoleic acid metabolites in post-ischemic myocardial recovery, investigators showed that 12,13-EpOME and 12,13-DiHOME treated murine hearts exhibited reduced cardiac functional recovery after ischemia40.
Oxo-octadecadienoic acid (9-OxoODE)
In a study on key regulatory processes promoting remarkable longevity in a representative Italian cohort, investigators have suggested that enhanced anti-oxidative response mechanisms might be activated. They observed decreased circulating levels of 9-oxoODE, a marker of lipid oxidative products of linoleic acid41. In contrast, obese individuals with nonalcoholic fatty liver disease have higher concentrations of plasma oxidized linoleic acid metabolites including 9- and 13-oxoODEs42,43. Obesity is a critical risk factor for AF, and a dietary study in obese youth showed that a 12 week treatment with a diet characterized by a low n6:n3 polyunsaturated fatty acids (PUFA) ratio decreased hepatic fat levels, including triglycerides and LDL, with evidence of improved insulin sensitivity. Oxidized linoleic acid metabolites including 9-oxoODE were reduced44. Atrial fatty infiltration contributes to abbreviation of action potential duration and a substrate for AF, and oxidized lipid metabolites likely contribute to this process45.
Hydroxyeicosatetraenoic acids (HETEs)
HETEs are metabolites of arachidonic acid produced in lipoxygenase pathway. Arachidonic acid is involved in several physiological and pathophysiological processes including development of cardiovascular diseases46, hypertension47, cardiac hypertrophy47,48, and inflammatory disease conditions49,50. 12-HETE has been reported to act as a vasoconstrictor and implicated in heart failure by induction of cardiac fibrosis51. Increased levels of the eicosanoid 12-HETE have been observed in the serum of patients with newly-diagnosed Type 1 diabetes52 and heart failure with preserved ejection fraction in patients with type 2 diabetes53. Huang et al.54 observed that the incidence of future acute myocardial infarction was more frequently reported in patients with higher baseline levels of numerous HETEs including the 12-HETE when compared to their counterparts. Finally, there is an evidence of pro-inflammatory property of HETEs55. It has been reported that, the levels of 12-HETE and 5-HETE were significantly increased in individuals with low-grade inflammation and obesity. Importantly, the levels of 12-HETE, 5-HETE, and TNF-α significantly decreased after weight reduction55.
Isolevuglandins in hypertension and AF
In the setting of increased oxidative stress, arachidonic acid and its metabolites are readily oxidized by free radicals, leading to formation of highly reactive electrophiles (isolevuglandins) that can form adducts with proteins with which they interact and modify their function56. These protein adducts are reported to have increased abundance in plasma from patients with atherosclerosis and end-stage renal disease56 as well as hypertension and AF57–59. Quantitation of isolevuglandins requires a different protocol than the approach used to quantify eicosanoids in the current study57. A clinical trial is underway to test the hypothesis that isolevuglandin scavengers can prevent the recurrence of AF following catheter ablation (NCT04433091)60.
Altogether, the results of our study as well as the previous reports may support the notion that specific eicosanoids may serve as biomarkers for prediction of AF incidence. Studies that clarify the role of these lipids in AF may provide novel insights into the aging-related role of these lipids, and guide the selective targeting of patients for whom scavenging these compounds may be helpful.
Clinical implications and future directions
Our study reported the detection of eicosanoids in human plasma represent biomarkers predictive for incident AF. Detected eicosanoids could initially exist in the plasma due to systemic inflammatory response associated with pathologies and risk factors such as cardiovascular diseases, diabetes, aging, etc., that may predispose to incident AF. However, it may also be possible that eicosanoids are generated within the diseased atrial tissues, and therefore, persistent inflammatory mediators may be released and detected in the plasma. Other eicosanoids and metabolites below measurable range remain to be metabolized and therefore need to be localized and measured in the diseased atrial tissues. Therefore, comparative studies of eicosanoid levels in individual-based paired-samples in plasma versus atrial tissues from healthy individuals and AF patients at various stages of disease could provide valuable insights into these metabolites' potential contributions to the initiation and progression of AF.
Finally, in conjunction with the advancement of recent approaches, profiling studies may facilitate integration of clinical information with multiomics data61. Combining these data with genomic62,63, transcriptomic64, proteomic65,66, and metabolomics67 datasets may provide deeper insights into the underlying mechanisms of AF, from initiation to recurrence to progression, and expedite the design of targeted intervention and therapeutic strategies. Future studies may aim to characterize the eicosanoid metabolites and identify their precise involvement in signaling pathways, i.e., their production, degradation, and activities that may lead to pro-, anti-, or resolving-inflammatory processes more deeply. Such efforts are critical in advancing our understanding the role of eicosanoids mediating AF initiation and progression.
Limitations
We acknowledge several epidemiological, clinical, laboratory, and statistical limitations. First, the vast majority of the study participants were of European ancestry and the mean age was 66 years; therefore the generalizability of the results to other races/ethnicities and younger individuals is unknown. Secondly, silent, paroxysmal AF was very likely unrecognized, leading to some misclassification of prevalent and incident AF. Also, we had limited information about AF type (paroxysmal, persistent, longstanding-persistent). In addition, although we describe associations, we cannot determine causal relations or rule out residual confounding. Laboratory limitations include possible measurement errors in biomarker profiles. Statistical limitations include that subgroup analyses (men vs. women and younger (< 65 years) vs. older (≥ 65 years) may be affected by lack of statistical power. Given the short half-life of eicosanoids, evaluation of the abundance of more stable (and pro-inflammatory) metabolites (e.g., 1,2-diols, and dihydroxyeicosatrienoic acids) may be easier to detect than the anti-inflammatory eicosanoids. Finally, replication of our results in other trial cohorts is needed.
Conclusions
In our study, six eicosanoids were significantly associated with incident AF after adjust for multiple testing, of which three were nominally associated with prevalent AF. Eicosanoids are oxidized lipid metabolites that are associated with impaired atrial insulin sensitivity and changes in atrial structure and electrophysiology. Thus, eicosanoid metabolomics profiling may enhance our understanding of the abundance of risk-associated eicosanoid metabolites and facilitate the development of inhibitors of eicosanoid synthesis and activity. A deeper understanding of eicosanoids in relation to AF may have significant clinical implications for AF prevention and therapeutic development. Future studies are needed to replicate and examine the biological bases of our findings.
Supplementary Information
Acknowledgements
We acknowledge the dedication of the FHS study participants without whom this research would not be possible.
Author contributions
J.K.: Conceptualization, Methodology, Investigation, Writing—Original draft preparation, Funding acquisition. M.A.Q.: Methodology, Writing—Original draft preparation, Writing—Reviewing and Editing. M.A.: Methodology, Writing—Reviewing and Editing. D.R.V.W.: Methodology, Writing—Reviewing and Editing. J.D.W.: Writing—Reviewing and Editing. L.T.: Writing—Reviewing and Editing. S.R.P.: Writing—Reviewing and Editing. D.K.: Writing—Reviewing and Editing. M.J.: Methodology, Writing—Reviewing and Editing. E.J.B.: Conceptualization, Methodology, Investigation, Writing—Reviewing and Editing, Funding acquisition, Supervision, Resources. S.C.: Methodology, Investigation, Writing—Reviewing and Editing, Funding acquisition, Resources, Data curation. H.L.: Conceptualization, Methodology, Investigation, Writing—Reviewing and Editing, Supervision, Resources, Data curation.
Funding
The Framingham Heart Study acknowledges the support of contracts NO1-HC-25195, HHSN268201500001I and 75N92019D00031 from the National Heart, Lung and Blood Institute. Dr. Kornej received funding from the Marie Sklodowska-Curie Actions under the European Union’s Horizon 2020 research and innovation programme (Agreement No. 838259). Dr. Qadan is supported by American Heart Association (18SFRN34170442). Dr. Van Wagoner acknowledges the support by NIH R01 HL111314 and American Heart Association (18SFRN34170442). Dr. Trinquart is supported by the American Heart Association (18SFRN34150007). Dr. Preis received funding from NIH grant 5R01HL128914-04. Dr. Ko is supported by American College of Cardiology Foundation/Merck Research Fellowship in Cardiovascular Diseases and Cardiometabolic Disorders. Dr. Jain was supported by NIH 5R01ES027595. Dr. Watrous was supported by NIH 5K01DK116917. Dr. Benjamin received funding from NIH 2R01 HL092577; 2U54HL120163; 1R01AG066010; R01 AG028321; American Heart Association, AHA_18SFRN34110082. Dr. Cheng is supported by NIH R01HL131532; R01HL151828. Dr. Lin acknowledges the support by the European Commission Grant (Agreement No. 847770).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Susan Cheng and Honghuang Lin.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-21786-0.
References
- 1.Kornej J, Börschel C, Benjamin E, Schnabel R. Epidemiology of atrial fibrillation in the 21st century: Novel methods and new insights. Circ. Res. 2020;27:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DD, Newton-Cheh C, Lubitz SA, Magnani JW, Ellinor PT, et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: Triumphs, concerns, and opportunities. N. Engl. J. Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 4.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 5.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zacharia E, Papageorgiou N, Ioannou A, Siasos G, Papaioannou S, Vavuranakis M, Latsios G, Vlachopoulos C, Toutouzas K, Deftereos S, et al. Inflammatory biomarkers in atrial fibrillation. Curr. Med. Chem. 2019;26:837–854. doi: 10.2174/0929867324666170727103357. [DOI] [PubMed] [Google Scholar]
- 7.Fontes JD, Yamamoto JF, Larson MG, Wang N, Dallmeier D, Rienstra M, Schnabel RB, Vasan RS, Keaney JF, Jr, Benjamin EJ. Clinical correlates of change in inflammatory biomarkers: The Framingham Heart Study. Atherosclerosis. 2013;228:217–223. doi: 10.1016/j.atherosclerosis.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, Erdmann J, Braund P, Engert JC, Bennett D, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferreira RC, Freitag DF, Cutler AJ, Howson JM, Rainbow DB, Smyth DJ, Kaptoge S, Clarke P, Boreham C, Coulson RM, et al. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444. doi: 10.1371/journal.pgen.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brunner EJ, Kivimaki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, Miller M, Lowe GD, Rumley A, Casas JP, et al. Inflammation, insulin resistance, and diabetes–Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graziani F, Biasucci LM, Cialdella P, Liuzzo G, Giubilato S, Della Bona R, Pulcinelli FM, Iaconelli A, Mingrone G, Crea F. Thromboxane production in morbidly obese subjects. Am. J. Cardiol. 2011;107:1656–1661. doi: 10.1016/j.amjcard.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 12.Davi G, Ciabattoni G, Consoli A, Mezzetti A, Falco A, Santarone S, Pennese E, Vitacolonna E, Bucciarelli T, Costantini F, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: Effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–229. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 13.Terres W, Becker P, Rosenberg A. Changes in cardiovascular risk profile during the cessation of smoking. Am. J. Med. 1994;97:242–249. doi: 10.1016/0002-9343(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 14.Seet RC, Lee CY, Loke WM, Huang SH, Huang H, Looi WF, Chew ES, Quek AM, Lim EC, Halliwell B. Biomarkers of oxidative damage in cigarette smokers: Which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011;50:1787–1793. doi: 10.1016/j.freeradbiomed.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Calder PC. Eicosanoids. Essays Biochem. 2020;64:423–441. doi: 10.1042/EBC20190083. [DOI] [PubMed] [Google Scholar]
- 16.Sonnweber T, Pizzini A, Nairz M, Weiss G, Tancevski I. Arachidonic acid metabolites in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2018 doi: 10.3390/ijms19113285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br. J. Pharmacol. 2019;176:1038–1050. doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Curr. Top. Med. Chem. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- 19.Samuchiwal SK, Boyce JA. Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology. J. Allergy Clin. Immunol. 2018;141:1182–1190. doi: 10.1016/j.jaci.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Sirish P, Li N, Timofeyev V, Zhang XD, Wang L, Yang J, Lee KS, Bettaieb A, Ma SM, Lee JH, et al. Molecular mechanisms and new treatment paradigm for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016;9:e003721. doi: 10.1161/CIRCEP.115.003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): Metabolism and biochemical function. Prog. Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Liu JY, Timofeyev V, Qiu H, Hwang SH, Tuteja D, Lu L, Yang J, Mochida H, Low R, et al. Beneficial effects of soluble epoxide hydrolase inhibitors in myocardial infarction model: Insight gained using metabolomic approaches. J. Mol. Cell Cardiol. 2009;47:835–845. doi: 10.1016/j.yjmcc.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sirish P, Li N, Timofeyev V, Zhang XD, Wang L, Yang J, Lee KS, Bettaieb A, Ma SM, Lee JH, et al. Molecular mechanisms and new treatment paradigm for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2016;9(9):e003721. doi: 10.1161/CIRCEP.115.003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Liu JY, Qiu H, Harris TR, Sirish P, Hammock BD, Chiamvimonvat N. Use of metabolomic profiling in the study of arachidonic acid metabolism in cardiovascular disease. Congest. Heart Fail. 2011;17:42–46. doi: 10.1111/j.1751-7133.2010.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: The Framingham Study. Am. J. Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am. J. Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 27.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N. Engl. J. Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 28.Lagerborg KA, Watrous JD, Cheng S, Jain M. High-throughput measure of bioactive lipids using non-targeted mass spectrometry. Methods Mol. Biol. 2019;1862:17–35. doi: 10.1007/978-1-4939-8769-6_2. [DOI] [PubMed] [Google Scholar]
- 29.Watrous JD, Niiranen TJ, Lagerborg KA, Henglin M, Xu YJ, Rong J, Sharma S, Vasan RS, Larson MG, Armando A, et al. Directed non-targeted mass spectrometry and chemical networking for discovery of eicosanoids and related oxylipins. Cell Chem. Biol. 2019;26:433–442.e4. doi: 10.1016/j.chembiol.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmu J, Watrous JD, Mercader K, Havulinna AS, Lagerborg KA, Salosensaari A, Inouye M, Larson MG, Rong J, Vasan RS, et al. Eicosanoid inflammatory mediators are robustly associated with blood pressure in the general population. J. Am. Heart Assoc. 2020;9:e017598. doi: 10.1161/JAHA.120.017598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N. Engl. J. Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 32.Schnabel R, Larson MG, Dupuis J, Lunetta KL, Lipinska I, Meigs JB, Yin X, Rong J, Vita JA, Newton-Cheh C, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–1657. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF, Jr, Wang TJ, et al. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arterioscler .Thromb. Vasc. Biol. 2013;33:1728–1733. doi: 10.1161/ATVBAHA.112.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schnabel RB, Lunetta KL, Larson MG, Dupuis J, Lipinska I, Rong J, Chen MH, Zhao Z, Yamamoto JF, Meigs JB, et al. The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ. Cardiovasc. Genet. 2009;2:229–237. doi: 10.1161/CIRCGENETICS.108.804245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, Sinner MF, Sotoodehnia N, Fontes JD, Janssens AC, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: The CHARGE-AF consortium. J. Am. Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodological) 1995;57:289–300. [Google Scholar]
- 38.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: Their roles and interactions with lipid metabolism. Prog. Lipid Res. 2005;44:1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Hildreth K, Kodani SD, Hammock BD, Zhao L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020;86:108484. doi: 10.1016/j.jnutbio.2020.108484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bannehr M, Löhr L, Gelep J, Haverkamp W, Schunck WH, Gollasch M, Wutzler A. Linoleic acid metabolite DiHOME decreases post-ischemic cardiac recovery in murine hearts. Cardiovas. Toxicol. 2019;19:365–371. doi: 10.1007/s12012-019-09508-x. [DOI] [PubMed] [Google Scholar]
- 41.Collino S, Montoliu I, Martin FP, Scherer M, Mari D, Salvioli S, Bucci L, Ostan R, Monti D, Biagi E, et al. Metabolic signatures of extreme longevity in northern Italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS ONE. 2013;8:e56564. doi: 10.1371/journal.pone.0056564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feldstein AE, Lopez R, Tamimi TA, Yerian L, Chung YM, Berk M, Zhang R, McIntyre TM, Hazen SL. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 2010;51:3046–3054. doi: 10.1194/jlr.M007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santoro N, Caprio S, Feldstein AE. Oxidized metabolites of linoleic acid as biomarkers of liver injury in nonalcoholic steatohepatitis. Clin. Lipidol. 2013;8:411–418. doi: 10.2217/clp.13.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Name MA, Savoye M, Chick JM, Galuppo BT, Feldstein AE, Pierpont B, Johnson C, Shabanova V, Ekong U, Valentino PL, et al. A Low ω-6 to ω-3 PUFA ratio (n-6:n-3 PUFA) diet to treat fatty liver disease in obese youth. J. Nutr. 2020;150:2314–2321. doi: 10.1093/jn/nxaa183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suffee N, Baptista E, Piquereau J, Ponnaiah M, Doisne N, Ichou F, Lhomme M, Pichard C, Galand V, Mougenot N, et al. Impacts of a high fat diet on the metabolic profile and the phenotype of atrial myocardium in mice. Cardiovasc. Res. 2021 doi: 10.1093/cvr/cvab367. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins CM, Cedars A, Gross RW. Eicosanoid signalling pathways in the heart. Cardiovasc. Res. 2009;82:240–249. doi: 10.1093/cvr/cvn346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maayah ZH, El-Kadi AO. The role of mid-chain hydroxyeicosatetraenoic acids in the pathogenesis of hypertension and cardiac hypertrophy. Arch. Toxicol. 2016;90:119–136. doi: 10.1007/s00204-015-1620-8. [DOI] [PubMed] [Google Scholar]
- 48.Maayah ZH, El-Kadi AO. 5-, 12- and 15-Hydroxyeicosatetraenoic acids induce cellular hypertrophy in the human ventricular cardiomyocyte, RL-14 cell line, through MAPK- and NF-κB-dependent mechanism. Arch. Toxicol. 2016;90:359–373. doi: 10.1007/s00204-014-1419-z. [DOI] [PubMed] [Google Scholar]
- 49.Issan Y, Hochhauser E, Guo A, Gotlinger KH, Kornowski R, Leshem-Lev D, Lev E, Porat E, Snir E, Thompson CI, et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. 2013;100–101:15–21. doi: 10.1016/j.prostaglandins.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiaro CR, Patel RD, Perdew GH. 12(R)-Hydroxy-5(Z),8(Z),10(E),14(Z)-eicosatetraenoic acid [12(R)-HETE], an arachidonic acid derivative, is an activator of the aryl hydrocarbon receptor. Mol. Pharmacol. 2008;74:1649–1656. doi: 10.1124/mol.108.049379. [DOI] [PubMed] [Google Scholar]
- 51.Kayama Y, Minamino T, Toko H, Sakamoto M, Shimizu I, Takahashi H, Okada S, Tateno K, Moriya J, Yokoyama M, et al. Cardiac 12/15 lipoxygenase-induced inflammation is involved in heart failure. J. Exp. Med. 2009;206:1565–1574. doi: 10.1084/jem.20082596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hennessy E, Rakovac Tisdall A, Murphy N, Carroll A, O'Gorman D, Breen L, Clarke C, Clynes M, Dowling P, Sreenan S. Elevated 12-hydroxyeicosatetraenoic acid (12-HETE) levels in serum of individuals with newly diagnosed Type 1 diabetes. Diabet. Med. 2017;34:292–294. doi: 10.1111/dme.13177. [DOI] [PubMed] [Google Scholar]
- 53.Otto M, Brabenec L, Müller M, Kintrup S, Hellenthal KEM, Holtmeier R, Steinbuch SC, Karsten OS, Pryvalov H, Rossaint J, et al. Development of heart failure with preserved ejection fraction in type 2 diabetic mice is ameliorated by preserving vascular function. Life Sci. 2021;284:119925. doi: 10.1016/j.lfs.2021.119925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang CC, Chang MT, Leu HB, Yin WH, Tseng WK, Wu YW, Lin TH, Yeh HI, Chang KC, Wang JH, et al. Association of arachidonic acid-derived lipid mediators with subsequent onset of acute myocardial infarction in patients with coronary artery disease. Sci. Rep. 2020;10:8105. doi: 10.1038/s41598-020-65014-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Möller K, Ostermann AI, Rund K, Thoms S, Blume C, Stahl F, Hahn A, Schebb NH, Schuchardt JP. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukot. Essent. Fatty Acids. 2016;106:39–49. doi: 10.1016/j.plefa.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 56.Salomon RG, Batyreva E, Kaur K, Sprecher DL, Schreiber MJ, Crabb JW, Penn MS, DiCorletoe AM, Hazen SL, Podrez EA. Isolevuglandin-protein adducts in humans: Products of free radical-induced lipid oxidation through the isoprostane pathway. Biochim. Biophys. Acta. 2000;1485:225–235. doi: 10.1016/s1388-1981(00)00038-x. [DOI] [PubMed] [Google Scholar]
- 57.Yermalitsky VN, Matafonova E, Tallman K, Li Z, Zackert W, Roberts LJ, 2nd, Amarnath V, Davies SS. Simplified LC/MS assay for the measurement of isolevuglandin protein adducts in plasma and tissue samples. Anal. Biochem. 2019;566:89–101. doi: 10.1016/j.ab.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Davies SS, May-Zhang LS, Boutaud O, Amarnath V, Kirabo A, Harrison DG. Isolevuglandins as mediators of disease and the development of dicarbonyl scavengers as pharmaceutical interventions. Pharmacol. Ther. 2020;205:107418. doi: 10.1016/j.pharmthera.2019.107418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benjamin EJ, Al-Khatib SM, Desvigne-Nickens P, Alonso A, Djoussé L, Forman DE, Gillis AM, Hendriks JML, Hills MT, Kirchhof P, et al. Research priorities in the secondary prevention of atrial fibrillation: A national heart, lung, and blood institute virtual workshop report. J. Am. Heart Assoc. 2021;10:e021566. doi: 10.1161/JAHA.121.021566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Neill MJ, Yoneda ZT, Crawford DM, Ye F, Ao M, Pitchford LM, Rathmacher JA, Murray KT, Akers WS, Roden DM, et al. 2-Hydroxybenzylamine (2-HOBA) to prevent early recurrence of atrial fibrillation after catheter ablation: Protocol for a randomized controlled trial including detection of AF using a wearable device. Trials. 2021;22:576. doi: 10.1186/s13063-021-05553-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kornej J, Hanger VA, Trinquart L, Ko D, Preis SR, Benjamin EJ, Lin H. New biomarkers from multiomics approaches—improving risk prediction of atrial fibrillation. Cardiovasc. Res. 2021;117:1632–1644. doi: 10.1093/cvr/cvab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roselli C, Rienstra M, Ellinor PT. Genetics of atrial fibrillation in 2020: GWAS, genome sequencing, polygenic risk, and beyond. Circ. Res. 2020;127:21–33. doi: 10.1161/CIRCRESAHA.120.316575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hsu J, Gore-Panter S, Tchou G, Castel L, Lovano B, Moravec CS, Pettersson GB, Roselli EE, Gillinov AM, McCurry KR, et al. Genetic control of left atrial gene expression yields insights into the genetic susceptibility for atrial fibrillation. Circ. Genom. Precis. Med. 2018;11:e002107. doi: 10.1161/CIRCGEN.118.002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin H, Dolmatova EV, Morley MP, Lunetta KL, McManus DD, Magnani JW, Margulies KB, Hakonarson H, del Monte F, Benjamin EJ, et al. Gene expression and genetic variation in human atria. Heart Rhythm. 2014;11:266–271. doi: 10.1016/j.hrthm.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Souza AI, Camm AJ. Proteomics of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2012;5:1036–1043. doi: 10.1161/CIRCEP.112.973008. [DOI] [PubMed] [Google Scholar]
- 66.Ko D, Benson MD, Ngo D, Yang Q, Larson MG, Wang TJ, Trinquart L, McManus DD, Lubitz SA, Ellinor PT, et al. Proteomics profiling and risk of new-onset atrial fibrillation: Framingham Heart Study. J. Am. Heart Assoc. 2019;8:e010976. doi: 10.1161/JAHA.118.010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ko D, Riles EM, Marcos EG, Magnani JW, Lubitz SA, Lin H, Long MT, Schnabel RB, McManus DD, Ellinor PT, et al. Metabolomic profiling in relation to new-onset atrial fibrillation (from the Framingham Heart Study) Am. J. Cardiol. 2016;118:1493–1496. doi: 10.1016/j.amjcard.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.