Abstract
Vasculogenic mimicry (VM) is closely related to cancer progression and metastasis, contributing to poor prognosis in patients with cancer. Resveratrol (RES) is well known to possess anti-cancer activity. This study explored the new role of RES in VM incidence in human prostate cancer (PCa) PC-3 cells. The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, transwell invasion, and three-dimensional culture VM tube formation assays were performed to check the cell viability, invasive ability, and vessel-like networks formation, respectively. VM-related proteins were detected by Western blots. The activity of metalloproteinase-2 (MMP-2) was identified by gelatin zymography. Vascular endothelial cadherin (VE-cadherin) mRNA was assessed by reverse transcriptase-polymerase chain reaction. Nuclear twist expression was observed by immunofluorescence assay. RES reduced serum-induced invasion and VM formation. Serum-induced phosphorylation of erythropoiethin-producing hepatoceullular A2 (EphA2) and the expression of VE-cadherin at the protein and mRNA levels were decreased after RES treatment. RES inhibited serum-induced expression and nuclear localization of twist. Serum-activated AKT signaling pathway, including MMP-2 and laminin subunit 5 gamma-2, was impaired by RES. These results suggested that RES may have an anti-VM effect through suppressing the EphA2/twist-VE-cadherin/AKT signaling cascade in PCa PC-3 cells.
Subject terms: Cancer, Drug discovery
Introduction
Metastasis is the leading cause of death from cancer and occurs through the circulatory system, including the lymphatic system1. The blood vessels around cancer supply nutrients and oxygen that play important roles in cancer growth and metastasis2. To grow beyond 2–3 mm in diameter, new blood vessel formation from pre-existing ones called “angiogenesis” is necessarily required3,4. Since anti-angiogenic drugs target endothelial cells (ECs), it has been proposed that inhibiting angiogenesis can prevent tumor growth and metastasis by destroying blood vessels. However, numerous clinical trials and animal studies have reported that anti-angiogenic therapies have little or no beneficial efficacy, and resistance to these therapies can happen5–8. These results indicated that sufficient blood is supplied to the cancer cells through alternative perfusion pathways, even without ECs.
Vasculogenic mimicry (VM) was first reported in 1999 as a unique process by which highly aggressive and metastatic cancer cells generate de novo matrix-rich vascular-like channels in the absence of ECs.4,9–11. It effectively mimics the normal blood vessels formed by ECs and is considered as a diagnostic indicator of aggressive and metastatic cancers12. In an animal study, anti-angiogenic therapy initially had an inhibitory effect on tumor growth. However, tumor regrowth occurred over a long treatment period. This phenomenon is because tumor cells supplant damaged EC by anti-angiogenic therapy. Moreover, this therapy did not show any effects on tumor growth in VM-competent tumor-bearing mice compared with that in VM-incompetent mice8. According to a meta-analysis, the 5-year overall survival (OS) of patients with VM-positive cancer is lower than that of those with VM-negative13. VM is closely associated with PCa invasion and metastasis. VM formation has a strong relationship with the Gleason score, lymph node metastasis, and distant metastasis in patients with high-risk PCa. Patients with VM-positive PCa showed lower OS and disease-free survival than those with VM-negative PCa.14,15. These reports indicate that the occurrence of VM predicts poor outcomes in patients with cancer. Thus, targeting VM may contribute to overcoming the resistance to anti-angiogenic therapies or may have a synergistic anti-cancer effect by co-administration with anti-angiogenic therapies. Most of all, it would be perfect if drugs had dual effects on targeting VM and angiogenesis.
As naturally occurring compounds, phytochemicals have been studied widely for their beneficial effects, including anti-cancer effects due to their safety. Curcumin, genistein and luteolin have an ability to inhibit VM structure through regulating multiple pathways associated with VM formation16. Epigallocatechin-3-gallate (EGCG) in green tea blocks VM process in PC-3 cells17. Resveratrol (3,5,4′-trihydroxy-trans-stilbene, RES) is one of the most famous phytochemicals found in red wine, grapes, berries, and peanuts, and is a powerful antioxidant that is helpful in various human diseases, such as cardiovascular diseases and cancer18. Although numerous studies have demonstrated that RES has potent anti-cancer properties in various types of cancer18–20, only one study has reported that RES suppresses the formation of melanoma VM by inhibiting vascular endothelial growth factor (VEGF) and, VEGF-receptors 1 and 221. RES suppresses proliferation and migration by inhibiting epithelial-mesenchymal transition mediated by TNF-receptor-associated factor 6 in PCa22. The anti-metastatic effect of RES has been shown by impairing the AKT/microRNA-21 pathway23. Several studies have demonstrated the PCa growth inhibitory effects of RES in in vitro and animal models24. In a recent study, it has been announced that serum promotes VM formation in PC-3 cells25. Therefore, this study examined whether RES plays a decisive role in inhibiting serum-induced VM in human PCa PC-3 cells, focusing on the EphA2/VE-cadherin/AKT pathway.
Methods
Chemicals, antibodies and reagents
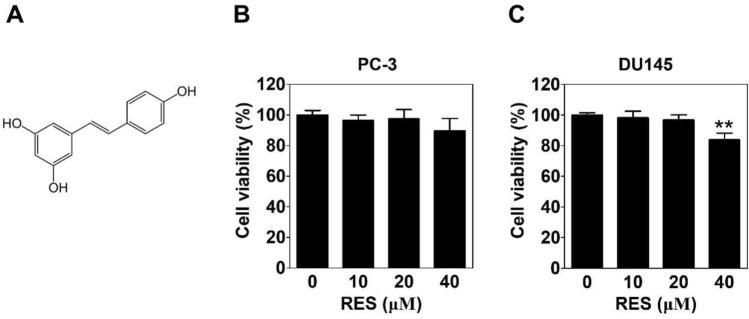
Resveratrol (RES) (Purity: ≥ 99% as determined by HPLC, Fig. 1A), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), primary antibody for β-actin and propidium iodide (PI) solution were obtained from Sigma-Aldrich (St Louis, MO, USA). Antibodies specific for p-EphA2 (6347), EphA2 (6997), p-AKT (4060) and AKT (4691) were purchased from Cell Signaling Technology (Beverly, MA, USA), VE-cadherin (AP2724) was obtained from Abgent (San Diego, CA, USA), m-lgGk BP-FITC (sc-516140) was purchased Santa Cruz Biotechnologies (Danvers, MA, USA), LAMC2 (ab96327), MMP-2 (ab86607) and twist (ab50887) were from Abcam (Cambridge, MA, USA). Zymogram-PAG 10% pre-cast gel and developing buffer were purchased from LABISKOMA (Seoul, Korea). All other chemicals were from Sigma-Aldrich.
Figure 1.
Effect of resveratrol on the cell viability against prostate cancer cells. (A) Chemical structure of resveratrol. PC-3 (B) or DU145 (C) cells were treated with various concentrations of RES for 24 h followed by the MTT assay. Data present as the means ± SD of triplicate determinations.
Cell culture
Human PCa PC-3 and DU145 cells were purchased from Korean Cell Line Bank (KCLB, Seoul) and were cultured in RPMI 1640 (Welgene, Daegu) with 10% fetal bovine serum (FBS, Welgene, Daegu) and 1% antibiotics (Welgene, Daegu) in a humidified incubator at 37 °C containing 5% CO2.
Cell viability assay
Cells (1 × 104) were seeded on a 96-well plate, and treated with RES (10, 20 and 40 μM) for 24 h in a serum-free culture medium. The MTT assay was performed to determine the cytotoxic effect of RES as described previously26–28. Absorbance was measured at 570 nm using a microplate reader (Sunrise RC, TECAN, Mannedorf, Switzerland) and then cell viability was calculated.
Transwell invasion assay
Cell invasion assay was carried out using a transwell25. Costar® Transwell® cell culture inserts (8 μM pore size; Corning Inc., NY) were used after coating with diluted matrigel matrix (1:20 dilution, BD Biosciences, San Jose, CA) to estimate the effects of RES on the invasion of PCa cells. Cells (2 × 105) with RES were seeded on the upper chamber, and the lower chambers were filled with serum for 24 h at 37 °C. Serum-treated cells with or without RES were fixed, stained, and washed, and the cells on the upper chamber were wiped off. The invading cells into the down area were imaged using an inverted light microscope Ts2_PH at 200 × magnification (Nikon, Tokyo, Japan) and counted.
Three-dimensional (3D) culture VM tube formation assay
VM tube formation was assessed as described previously25. A 24-well plate was coated with 100 μl of matrigel at 37 °C for 1 h. Cells (3.2 × 105) were seeded on a matrigel-polymerized plate and treated with serum with or without RES for 16 h at 37 °C. Tubular shapes were imaged using an inverted light microscope Ts2_PH at 40 × magnification and the number of VM structures was counted.
Western blot analysis
Serum-treated cells (3.2 × 105) on a 6-well plate with or without RES for 24 h at 37 °C were lysed. The protein samples (25–30 μg) from cell lysates were separated by (8–12%) SDS-PAGE at 80 V of constant voltage and transferred onto a membrane (Pall Corporation, Port Washington, NY) for 90 min at 330 mA. After incubation in blocking buffer (5% skim milk or bovine serum albumin [BSA]) for 90 min, the membrane was probed with p-EphA2 (1:1000), EphA2 (1:1000) , VE-cadherin (1:1000), twist (1:200), p-AKT (1:1000), AKT (1:3000), MMP-2 (1:1000), LAMC2 (1:500) and β-actin (1:20,000) antibodies overnight at 4 °C followed by specific secondary antibodies for 2 h at room temperature (RT). Each protein bands were detected using an enhanced chemiluminescence reagent (GE Healthcare, Chicago, IL, USA) and quantified using the ImageJ 1.40 g software (National Institute of Health, Bethesda, MD, USA).
Gelatin zymography
The conditioned medium (CM) was collected from serum-treated cells with or without RES for 24 h. Equal amounts of CM were separated on Zymogram-PAG 10% pre-cast gel followed by washing with 2.5% triton X-100. And then, the gel was incubated in a developing buffer for 36 h at 37 °C. After staining and destaining, bands were photographed and quantified using the ImageJ 1.40 g software.
Isolation of RNA and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA extraction was done in serum-treated cells (3.2 × 105) on a 6-well plate with or without RES for 24 h at 37 °C. cDNA synthesis and PCR were then performed. Primers used in this study are listed in Table 1. The PCR products were separated on 2% agarose gel and each PCR product bands were quantified using the ImageJ 1.40 g software.
Table 1.
Primers used in this study.
| mRNA | Primer sequences | Size | Annealing temperature |
|---|---|---|---|
| β-actin |
S: GAGAAGATGACCCAGATCATGT AS : ACTCCATGCCCAGGAAGGAAGG |
463 | 60 |
| VE-cadherin |
S : GCACCAGTTTGGCCAATATA AS : GGGTTTTTGCATAATAAGCAGG |
149 | 60 |
Immunofluorescence assay
Serum-treated cells (1.5 × 105) on an 8-well chamber slide with or without RES for 24 h at 37 °C were fixed with 500 µl of 3.7% formaldehyde and permeabilized with 0.2% Triton-X 100 for 10 min, respectively. The cells were incubated in blocking buffer (5% BSA) for 1 h at RT and probed with twist antibody (1:50) overnight at 4 °C followed by fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100) for 1 h at RT. The slide was mounted in 30% glycerol after counterstaining with PI solution. Images were captured using an ECLIPS Ts2-FL microscope (Nikon, Tokyo, Japan) at 400 × magnification.
Statistical analysis
All results are expressed as the means ± standard deviation (SD) from at least three independent experiments. Student’s t-test was performed using the Sigma plot software (Systat Software Inc., San Jose, CA, USA) to determine statistical significance (p < 0.05).
Results
Effect of resveratrol on the cell viability against prostate cancer cells
To determine the cytotoxicity of RES (Fig. 1A), the MTT assay was performed in RES (10, 20, and 40 μM)-treated PC-3 or DU145 cells. RES treatment at a dose of 40 μM resulted in a slight decrease in the viability of PC-3 cells, which was not statistically significant (Fig. 1B). However, treatment with 40 μM RES significantly decreased in the viability of DU145 cells (Fig. 1C). We conducted subsequent experiments at RES concentrations of 10 and 20 μM.
Resveratrol suppresses serum-induced invasion and VM tube formation in prostate cancer cells
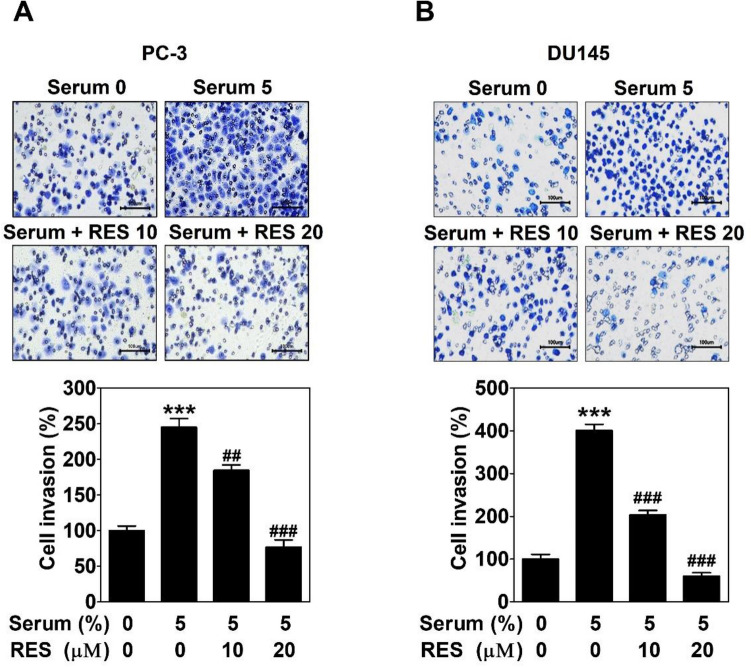
Transwell invasion assay was carried out to investigate whether RES influences the invasive capacity of PC-3 and DU145 cells. Cells were seeded on a matrigel-coated up-chamber and treated with RES. Serum was added to the down-chamber as a chemoattractant. After 24 h, the induction of cell invasion was significantly increased in response to serum exposure. RES inhibited dose-dependently serum-induced cell invasion in both cell lines (Fig. 2).
Figure 2.
Resveratrol suppresses serum-induced invasion in prostate cancer cells. PC-3 (A) or DU145 cells (B) with RES seeded on a matrigel-coated up chamber of transwell and serum was filled in a down chamber. After a 24 h-incubation, the cells were fixed and stained. The images were taken with an inverted microscope at 200 × magnification. Scale bar = 100 μm. The number of invading cells was counted and expressed as a percentage of the untreated control group. Data present as the mean ± SD of three independent experiments by analysis of Student’s t-test. *** p < 0.001 versus untreated control; ##p < 0.01 and ###p < 0.001 versus serum-treated control.
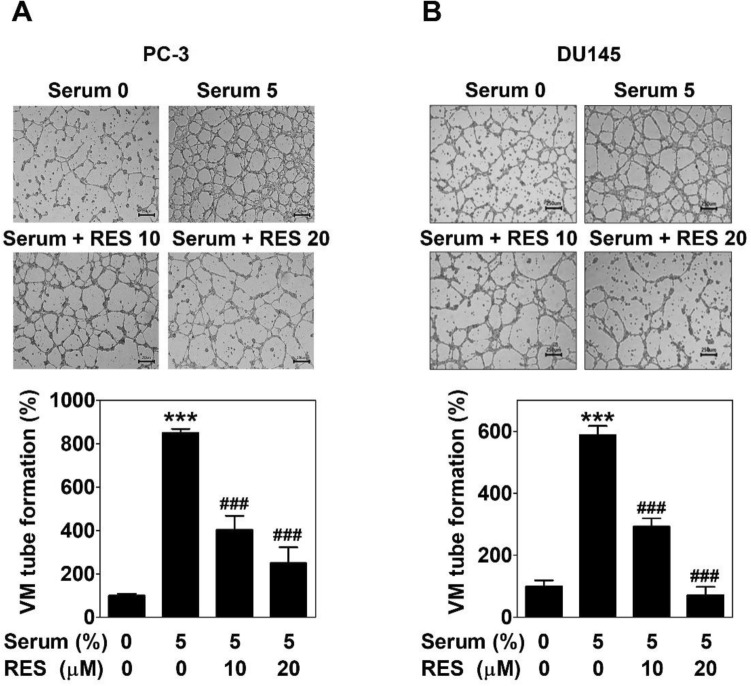
To examine the effect of RES on vessel-like networks formation in PC-3 and DU145 cells, 3D culture VM formation assay was performed on a matrigel-coated plate after treating the cells with serum with or without RES for 16 h. Serum greatly facilitated the formation of perfect tubular shapes. This phenomenon was effectively impeded by RES at 10 and 20 μM in both the cell lines (Fig. 3).
Figure 3.
Resveratrol decreases serum-induced VM tube formation in prostate cancer cells. PC-3 (A) or DU145 cells (B) were seeded on a matrigel-coated a 24-well plate and treated with serum with or without RES. After a 16 h-incubation, the images were taken with an inverted microscope at 40 × magnification. Scale bar = 250 μm. The number of VM tube formation was counted and expressed as a percentage of the untreated control group. Data present as the mean ± SD of three independent experiments by analysis of Student’s t-test. ***p < 0.001 versus untreated control; ###p < 0.001 versus serum-treated control.
Taken together, these results demonstrate that RES shows both anti-invasive and anti-VM activities in PCa cells.
Resveratrol inhibits serum-induced the activation of EphA2 in PC-3 cells
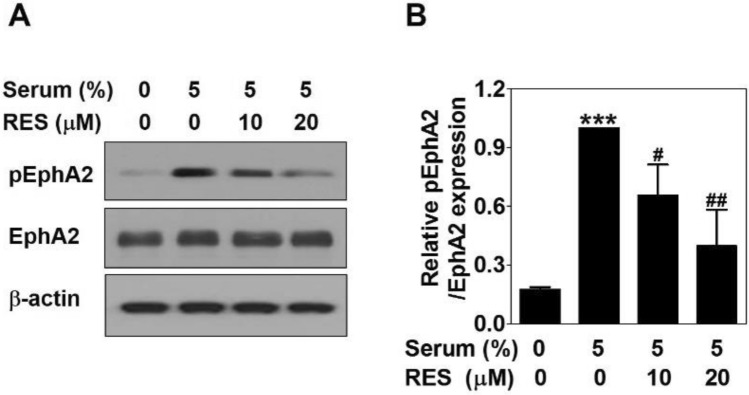
To reveal whether RES affects the activation of erythropoiethin-producing hepatoceullular A2 (EphA2) to suppress serum-induced VM formation, Western blot was conducted in serum-treated PC-3 cells with or without RES for 24 h. The phosphorylation of EphA2 in response to serum was effectively decreased after RES treatment in a dose-dependent manner. However, EphA2 expression levels were not changed by serum or RES (Fig. 4). These results imply that RES causes a marked inhibition of serum-induced activation of EphA2 in PC-3 cells.
Figure 4.
Resveratrol inhibits serum-induced the activation of EphA2 in PC-3 cells. Cells were treated with serum with or without RES for 24 h. (A) The same amount of proteins (25–30 μg) were analyzed by Western blot using the phospho-EphA2 and EphA2 antibodies. As a loading control, β-actin was used. (B) The bands of proteins were quantified. Data present as the means ± SD of three independent experiments by analysis of Student’s t-test. ***p < 0.001 versus untreated control; #p < 0.05 and ##p < 0.01 versus serum-treated control.
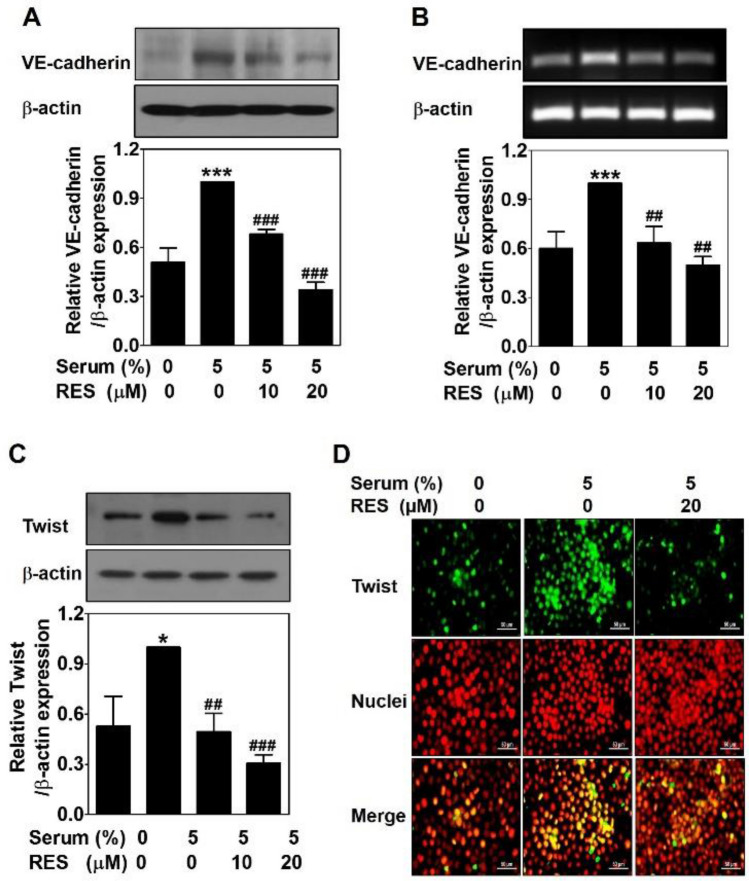
Resveratrol down-regulates serum-induced VE-cadherin expression through decreasing the nuclear twist expression in PC-3 cells
To clarify the inhibitory effects of RES on vascular endothelial cadherin (VE-cadherin) protein and mRNA levels were detected by Western blot and RT-PCR in serum-treated PC-3 cells with or without RES for 24 h, respectively. Serum caused an effective upregulation of VE-cadherin protein level, which was significantly downregulated by RES dose-dependently (Fig. 5A). Consistent with the protein expression levels, RES effectively downregulated VE-cadherin mRNA levels induced by serum (Fig. 5B). These results indicate that RES controls VE-cadherin expression at the transcriptional level.
Figure 5.
Resveratrol down-regulates serum-induced VE-cadherin expression through decreasing the nuclear twist expression in PC-3 cells. Cells were treated with serum with or without RES for 24 h. (A) The same amount of proteins (25–30 μg) were analyzed by Western blot using the VE-cadherin antibody. As a loading control, β-actin was used. (B)) The mRNA levels were analyzed by RT-PCR using the VE-cadherin primer. As a loading control, β-actin primer was used. (C) The same amount of proteins (25–30 μg) were analyzed by Western blot using the twist antibody. β-actin was used as a control. Data present as the means ± SD of three independent experiments by analysis of Student’s t-test. *p < 0.05 and ***p < 0.001 versus untreated control; ##p < 0.01 and ###p < 0.001 versus serum-treated control. (D) The serum-treated cells with or without RES for 24 h were fixed, permeabilized and blocked. After probing with Twist antibody followed by incubation with fluorescein isothiocyanate (FITC)-conjugated secondary antibody, the cells were counterstained with propidium iodide (PI). The images were taken with a fluorescence microscope at 400 × . Scale bar = 50 μm.
To identify the control mechanism of RES in VE-cadherin expression, twist, a transcription factor of VE-cadherin, was detected by Western blot in serum-treated PC-3 cells with or without RES for 24 h. As expected, serum upregulated twist expression levels, which was drastically reduced by RES treatment (Fig. 5C). To confirm this effect, immunofluorescence analysis was conducted under the same conditions. As shown in Fig. 5D, RES reduced serum-increased twist expression observed in the nucleus.
Taken together, these results indicate that RES downregulates serum-induced twist expression in the nucleus, contributing to a decrease in VE-cadherin expression in PC-3 cells.
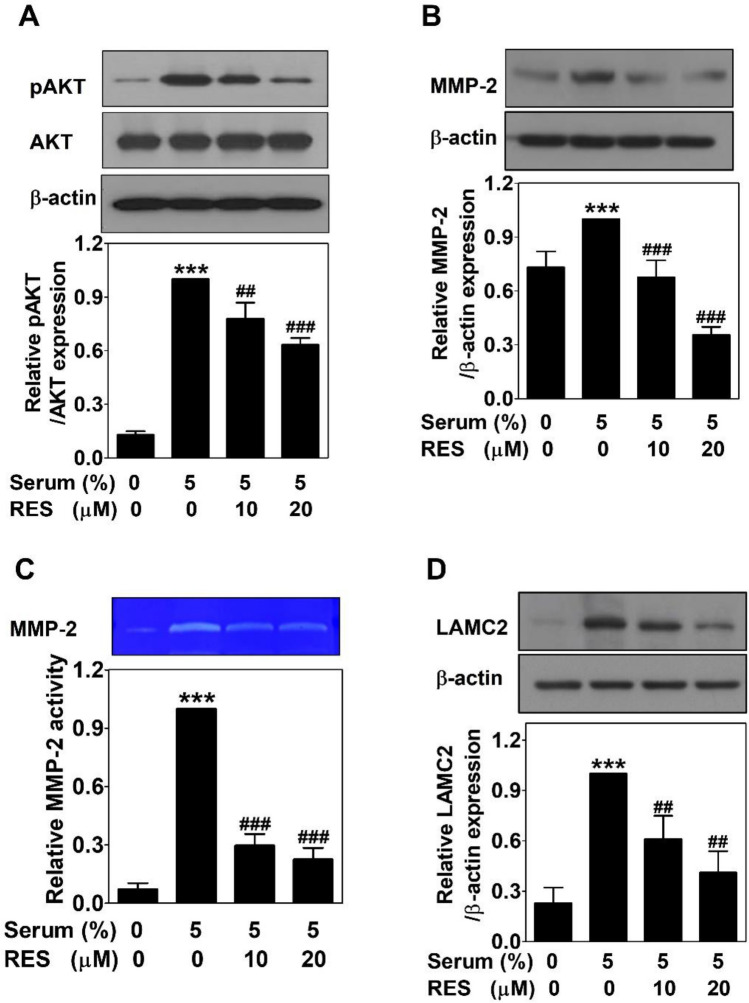
Resveratrol inactivates serum-induced the AKT signaling pathway in PC-3 cells
The AKT pathway was explored by Western blots in serum-treated PC-3 cells with or without RES for 24 h to assess whether this pathway is associated with the anti-VM effect of RES. Serum increased the phosphorylation of AKT but not the expression of AKT. RES treatment blocked dose-dependently the phosphorylation of AKT by serum without affecting AKT expression (Fig. 6A). The expression of matrix metalloproteinase-2 (MMP-2) was upregulated by serum. This effect also inhibited by RES treatment dose-dependently (Fig. 6B). In addition, to assess the activity of MMP-2, gelatin zymography was performed using CM from serum-treated PC-3 cells with or without RES for 24 h. RES effectively impaired the serum-induced activity of MMP-2 (Fig. 6C). Serum-upregulated laminin subunit 5 gamma-2 (LAMC2) was also inhibited by RES treatment dose-dependently (Fig. 6D). These results suggeste that RES suppresses serum-induced VM formation by inactivating the AKT/MMP-2/LAMC2 signaling pathway in PC-3 cells.
Figure 6.
Resveratrol inactivates serum-induced the AKT signaling pathway in PC-3 cells. Cells were treated with serum with or without RES for 24 h. The same amount of proteins (25–30 μg) were analyzed by Western blot using the phospho-AKT, AKT (A), MMP-2 (B) and LAMC2 (D) antibodies. As a loading control, β-actin was used. (C) Gelatin zymography was performed using the CM. Data present as the means ± SD of three independent experiments by analysis of Student’s t-test. ***p < 0.001 versus untreated control; ##p < 0.01 and ###p < 0.001 versus serum-treated control.
Discussion
As a powerful antioxidant, RES has anti-cancer effects by inhibiting angiogenesis and metastasis and inducing apoptosis in various types of cancer cells18–20. However, there is insufficient evidence of a link between RES and blocking of VM formation. In a recent experiment, we demonstrated the following results: (1) serum activates EphA2 and (2) upregulates VE-cadherin expression through increasing nuclear twist expression, (3) which in turn activates the AKT/MMP-2/LAMC2 pathway, (4) contributing to the formation of VM in PCa PC-3 cells25. Based on these findings, we explored whether and how RES affects serum-induced VM in PC-3 cells.
As an alternative perfusion pathway, VM is the formation of matrix-rich blood vessel-like shapes by aggressive and metastatic cancer cells9,10. It can easily be identified in PCa cells, such as PC-3 cells15,29. There is a strong relationship between VM and cancer cell motility30. RES suppresses the motility, such as the migration and invasion of PCa31,32. As expected, RES inhibited the invasion of serum-treated PCa cells (Fig. 2). In addition, RES blocked complete tubular channels induced by serum (Fig. 3). All the effects were observed at non-cytotoxic concentrations (Fig. 1B and 1C). These results show that RES plays a novel role in inhibiting VM formation in PCa.
EphA2 is a tyrosine kinase-containing transmembrane glycoprotein receptor. A high expression of EphA2 increases the invasion of PCa cells33. EphA2 has been considered as a key driver of VM process in various types of cancers, including PCa34. In in vitro and in vivo models, EphA2 contributes to tumor growth and VM formation35. Its expression and phosphorylation levels are closely related to VM formation15,34. Thus, EphA2 is an attractive biomarker for targeting VM. The anti-VM effect of microRNA-141 results from downregulation of EphA2 expression36. In this study, the activation of EphA2 by phosphorylation in serum-treated cells was effectively reduced without affecting EphA2 expression after RES treatment (Fig. 4). This result indicate that RES can block VM by controlling EphA2.
The activity of EphA2 is controlled by VE-cadherin that is a main adhesion receptor exclusively expressed in endothelium10,30,37. It contributes to blood vessel formation and maintains and controls ECs contacts and vascular permeability. However, aberrant overexpression of VE-cadherin has been observed in highly aggressive and metastatic cancer cells38–40. VE-cadherin is one of the first molecules involved in VM signaling pathway. In the absence of VE-cadherin, VM formation does not occur10,40. Genistein41 and ginsenoside Rg342 inhibit VM formation by downregulating VE-cadherin expression. RES inhibited serum-upregulated VE-cadherin at both the protein (Fig. 5A) and mRNA (Fig. 5B) levels, indicating that RES controls VE-cadherin expression at the transcriptional level. Twist is well known as a transcription factor of VE-cadherin43,44. It promotes tumor progression by regulating cancer cell growth, metastasis, differentiation, and angiogenesis45. Previous studies have shown that twist can induce VM formation by regulating VE-cadherin expression levels43,46. The anti-VM effects of EGCG are mediated by inhibiting twist and VE-cadherin expressions17. Polyphyllin I inhibits twist binding to the VE-cadherin promotor, leading to anti-VM activity47. RES suppressed serum-upregulated twist expression in the nucleus (Fig. 5D). Taken together, the VM blocking effect of RES is related to the downregulation of VE-cadherin expression by inhibiting nuclear twist expression.
EphA2 co-localizes with VE-cadherin at sites with cell-to-cell junctions. This interaction results in the activation of the phosphoinositide 3-kinase (PI3K)/AKT pathway that is important in regulating cancer progression, such as survival, proliferation, angiogenesis and metastasis10,30,37,48. The PI3K/AKT pathway also participates in extracellular matrix remodeling and VM process through activating MMP-14 and -2, facilitating the cleavage of LAMC2, thereby promoting VM-related motility of cancer cells10,30,37. EGCG shows anti-VM activities in PC-3 cells through inhibiting the AKT signaling17. Also, phytochemicals, such as curcumin, honokiol, and norcantharidin have anti-VM effects through suppressing the AKT pathway16. In this study, serum-activated AKT levels were significantly reduced by RES treatment (Fig. 6A). Highly invasive and aggressive cancer cells overexpress MMP-14 and -2 and LAMC2, which help to form a vascular structure lined by cancer cells49. The AKT/MMP-2/9 pathway is required for the regulation of VM formation50. Serum upregulated the expression of MMP-2 and LAMC2, which was effectively decreased after treating with RES (Fig. 6B and 6D). In addition, the activity of MMP-2 by serum was effectively impaired by RES (Fig. 6C). Taken together, these results verify that RES effectively suppresses the AKT/MMP-2/LAMC2 cascades, contributing to the anti-VM activity of RES.
Conclusion
This study demonstrates a new role for RES in anti-cancer effects. RES suppressed VM structure formation in PCa PC-3 cells at non-cytotoxic concentrations. This effect is mediated by inactivating EphA2 and reducing twist-mediated VE-cadherin expression, which in turn inactivates the AKT/MMP-2/LAMC2 signaling pathway. This study provides new insights into the functions of RES. However, further work, including in vivo studies, is required.
Abbreviations
- BSA
Bovine serum albumin
- ECs
Endothelial cells
- EGCG
Epigallocatechin-3-gallate
- EphA2
Erythropoiethin-producing hepatoceullular A2
- FITC
Fluorescein isothiocyanate
- LAMC2
Laminin subunit 5 gamma-2
- MMP-2
Matrix metalloproteinase-2
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- OS
Overall survival
- PCa
Prostate cancer
- PI
Propidium iodide
- PI3K
Phosphoinositide 3-kinase
- RES
Resveratrol
- RT
Room temperature
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- VE-cadherin
Vascular endothelial cadherin
- VEGF
Vascular endothelial growth factor
- VM
Vasculogenic mimicry
Author contributions
Conceptualization: E.O.L. Methodology: D.S.H. Software: D.S.H. Validation: D.S.H. Formal analysis: D.S.H. and E.O.L. Investigation: D.S.H. Resources: E.O.L. Data curation: D.S.H., H.J.L. and E.O.L. Writing—original draft preparation: D.S.H. Writing—review and editing: E.O.L. Visualization: D.S.H. Supervision: E.O.L. Project administration: E.O.L. Funding acquisition: E.O.L. The authors read and approved the final manuscript.
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (NRF-2018R1D1A1B07040506) and the Ministry of Science and ICT (NRF-2021R1A2C1005373).
Data availability
The data was available from corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Woodhouse EC, Chuaqui RF, Liotta L. General mechanisms of metastasis. Cancer. 1997;80(8 Suppl):1529–1537. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1529::AID-CNCR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J, Cotran R. Relation of vascular proliferation to tumor growth. Int. Rev. Exp. Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- 4.Folberg R, Hendrix MJ, Maniotis AJ. Vasculogenic mimicry and tumor angiogenesis. Am J Pathol. 2000;156(2):361–381. doi: 10.1016/S0002-9440(10)64739-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vasudev NS, Reynolds AR. Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions. Angiogenesis. 2014;17(3):471–494. doi: 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itatani Y, Kawada K, Yamamoto T, Sakai Y. Resistance to anti-angiogenic therapy in cancer-alterations to anti-VEGF pathway. Int. J. Mol. Sci. 2018;19(4):1232. doi: 10.3390/ijms19041232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis LM, Fidler IJ. Finding the tumor copycat. therapy fails patients don’t. Nat. Med. 2010;16(9):974–975. doi: 10.1038/nm0910-974. [DOI] [PubMed] [Google Scholar]
- 8.Dunleavey JM, Xiao L, Thompson J, Kim MM, Shields JM, Shelton SE, Irvin DM, Brings VE, Ollila DW, Brekken RA, et al. Vascular channels formed by subpopulations of PECAM1+ melanoma cells. Nat. Commun. 2014;5:5200. doi: 10.1038/ncomms6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folberg R, Maniotis AJ. Vasculogenic mimicry. APMIS. 2004;112(7–8):508–525. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. [DOI] [PubMed] [Google Scholar]
- 10.Paulis YW, Soetekouw PM, Verheul HM, Tjan-Heijnen VC, Griffioen AW. Signalling pathways in vasculogenic mimicry. Biochim. Biophys. Acta. 2010;1806(1):18–28. doi: 10.1016/j.bbcan.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Racordon D, Valdivia A, Mingo G, Erices R, Aravena R, Santoro F, Bravo ML, Ramirez C, Gonzalez P, Sandoval A, et al. Structural and functional identification of vasculogenic mimicry in vitro. Sci. Rep. 2017;7(1):6985. doi: 10.1038/s41598-017-07622-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seftor RE, Hess AR, Seftor EA, Kirschmann DA, Hardy KM, Margaryan NV, Hendrix MJ. Tumor cell vasculogenic mimicry: From controversy to therapeutic promise. Am. J. Pathol. 2012;181(4):1115–1125. doi: 10.1016/j.ajpath.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Z, Bao M, Miele L, Sarkar FH, Wang Z, Zhou Q. Tumour vasculogenic mimicry is associated with poor prognosis of human cancer patients: A systemic review and meta-analysis. Eur. J. Cancer. 2013;49(18):3914–3923. doi: 10.1016/j.ejca.2013.07.148. [DOI] [PubMed] [Google Scholar]
- 14.Liu R, Yang K, Meng C, Zhang Z, Xu Y. Vasculogenic mimicry is a marker of poor prognosis in prostate cancer. Cancer Biol. Ther. 2012;13(7):527–533. doi: 10.4161/cbt.19602. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Lin H, Pan J, Mo C, Zhang F, Huang B, Wang Z, Chen X, Zhuang J, Wang D, et al. Vasculogenic mimicry in prostate cancer: The roles of EphA2 and PI3K. J. Cancer. 2016;7(9):1114–1124. doi: 10.7150/jca.14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haiaty S, Rashidi MR, Akbarzadeh M, Maroufi NF, Yousefi B, Nouri M. Targeting vasculogenic mimicry by phytochemicals: A potential opportunity for cancer therapy. IUBMB Life. 2020;72(5):825–841. doi: 10.1002/iub.2233. [DOI] [PubMed] [Google Scholar]
- 17.Yeo C, Han DS, Lee HJ, Lee EO. Epigallocatechin-3-gallate suppresses vasculogenic mimicry through inhibiting the twist/VE-Cadherin/AKT pathway in human prostate cancer PC-3 cells. Int. J. Mol. Sci. 2020;21(2):439. doi: 10.3390/ijms21020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24(5A):2783–2840. [PubMed] [Google Scholar]
- 19.Ye M, Tian H, Lin S, Mo J, Li Z, Chen X, Liu J. Resveratrol inhibits proliferation and promotes apoptosis via the androgen receptor splicing variant 7 and PI3K/AKT signaling pathway in LNCaP prostate cancer cells. Oncol. Lett. 2020;20(5):169. doi: 10.3892/ol.2020.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishayee A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009;2(5):409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 21.Vartanian AA, Burova OS, Stepanova EV, Baryshnikov AY. Lichinitser MR Melanoma vasculogenic mimicry is strongly related to reactive oxygen species level. Melanoma Res. 2007;17(6):370–379. doi: 10.1097/CMR.0b013e3282f1d2ec. [DOI] [PubMed] [Google Scholar]
- 22.Khusbu FY, Zhou X, Roy M, Chen FZ, Cao Q, Chen HC. Resveratrol induces depletion of TRAF6 and suppresses prostate cancer cell proliferation and migration. Int. J. Biochem. Cell Biol. 2020;118:105644. doi: 10.1016/j.biocel.2019.105644. [DOI] [PubMed] [Google Scholar]
- 23.Sheth S, Jajoo S, Kaur T, Mukherjea D, Sheehan K, Rybak LP, Ramkumar V. Resveratrol reduces prostate cancer growth and metastasis by inhibiting the Akt/MicroRNA-21 pathway. PLoS ONE. 2012;7(12):e51655. doi: 10.1371/journal.pone.0051655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chhabra G, Singh CK, Ndiaye MA, Fedorowicz S, Molot A, Ahmad N. Prostate cancer chemoprevention by natural agents: Clinical evidence and potential implications. Cancer Lett. 2018;422:9–18. doi: 10.1016/j.canlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeo C, Lee HJ, Lee E. Serum promotes vasculogenic mimicry through the EphA2/VE-cadherin/AKT pathway in PC-3 human prostate cancer cells. Life Sci. 2019;221:267–273. doi: 10.1016/j.lfs.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Kang CG, Kim SH, Lee EO. Rhapontigenin suppresses cell migration and invasion by inhibiting the PI3K-dependent Rac1 signaling pathway in MDA-MB-231 human breast cancer cells. J. Nat. Prod. 2014;77(5):1135–1139. doi: 10.1021/np401078g. [DOI] [PubMed] [Google Scholar]
- 27.Im E, Yeo C, Lee HJ, Lee EO. Dihydroartemisinin induced caspase-dependent apoptosis through inhibiting the specificity protein 1 pathway in hepatocellular carcinoma SK-Hep-1 cells. Life Sci. 2018;192:286–292. doi: 10.1016/j.lfs.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Kang CG, Lee HJ, Kim SH, Lee EO. Zerumbone suppresses osteopontin-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung cancer A549 cells. J. Nat. Prod. 2016;79(1):156–160. doi: 10.1021/acs.jnatprod.5b00796. [DOI] [PubMed] [Google Scholar]
- 29.Qin L, Ren Y, Chen AM, Guo FJ, Xu F, Gong C, Cheng P, Du Y, Liao H. Peroxisome proliferator-activated receptor gamma ligands inhibit VEGF-mediated vasculogenic mimicry of prostate cancer through the AKT signaling pathway. Mol. Med. Rep. 2014;10(1):276–282. doi: 10.3892/mmr.2014.2198. [DOI] [PubMed] [Google Scholar]
- 30.Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, Yu X, Tian Y. Advanced research on vasculogenic mimicry in cancer. J. Cell Mol. Med. 2015;19(2):315–326. doi: 10.1111/jcmm.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K, Chen Z, Shi J, Feng Y, Yu M, Sun Y, Zhuang Q, Liang B, Luo G, Xu X, et al. Resveratrol inhibits the tumor migration and invasion by upregulating TET1 and reducing TIMP2/3 methylation in prostate carcinoma cells. Prostate. 2020;80(12):977–985. doi: 10.1002/pros.24029. [DOI] [PubMed] [Google Scholar]
- 32.Hsieh TC, Wu JM. Resveratrol suppresses prostate cancer epithelial cell scatter/invasion by targeting inhibition of hepatocyte growth factor (HGF) secretion by prostate stromal cells and upregulation of E-cadherin by prostate cancer epithelial cells. Int. J. Mol. Sci. 2020;21(5):1760. doi: 10.3390/ijms21051760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen P, Huang Y, Zhang B, Wang Q, Bai P. EphA2 enhances the proliferation and invasion ability of LNCaP prostate cancer cells. Oncol. Lett. 2014;8(1):41–46. doi: 10.3892/ol.2014.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitra D, Bhattacharyya S, Alam N, Sen S, Mitra S, Mandal S, Vignesh S, Majumder B, Murmu N. Phosphorylation of EphA2 receptor and vasculogenic mimicry is an indicator of poor prognosis in invasive carcinoma of the breast. Breast Cancer Res. Treat. 2020;179(2):359–370. doi: 10.1007/s10549-019-05482-8. [DOI] [PubMed] [Google Scholar]
- 35.Lu XS, Sun W, Ge CY, Zhang WZ, Fan YZ. Contribution of the PI3K/MMPs/Ln-5gamma2 and EphA2/FAK/Paxillin signaling pathways to tumor growth and vasculogenic mimicry of gallbladder carcinomas. Int. Oncol. 2013;42(6):2103–2115. doi: 10.3892/ijo.2013.1897. [DOI] [PubMed] [Google Scholar]
- 36.Li G, Huang M, Cai Y, Ke Y, Yang Y, Sun X. miR141 inhibits glioma vasculogenic mimicry by controlling EphA2 expression. Mol. Med. Rep. 2018;18(2):1395–1404. doi: 10.3892/mmr.2018.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: Vasculogenic mimicry in tumor cells: Diagnostic and therapeutic implications. Clin. Cancer Res. 2012;18(10):2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: Molecular basis and pathological implications. Dev. Cell. 2009;16(2):209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Vestweber D. VE-cadherin: The major endothelial adhesion molecule controlling cellular junctions and blood vessel formation. Arterioscler. Thromb. Vasc. Biol. 2008;28(2):223–232. doi: 10.1161/ATVBAHA.107.158014. [DOI] [PubMed] [Google Scholar]
- 40.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: Role in vasculogenic mimicry. Proc. Natl. Acad. Sci. U S A. 2001;98(14):8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cong R, Sun Q, Yang L, Gu H, Zeng Y, Wang B. Effect of Genistein on vasculogenic mimicry formation by human uveal melanoma cells. J. Exp. Clin. Cancer Res. 2009;28:124. doi: 10.1186/1756-9966-28-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo JQ, Zheng QH, Chen H, Chen L, Xu JB, Chen MY, Lu D, Wang ZH, Tong HF, Lin S. Ginsenoside Rg3 inhibition of vasculogenic mimicry in pancreatic cancer through downregulation of VEcadherin/EphA2/MMP9/MMP2 expression. Int. J. Oncol. 2014;45(3):1065–1072. doi: 10.3892/ijo.2014.2500. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Zhu DM, Zhou XG, Yin N, Zhang Y, Zhang ZX, Li DC, Zhou J. HIF-2alpha promotes the formation of vasculogenic mimicry in pancreatic cancer by regulating the binding of Twist1 to the VE-cadherin promoter. Oncotarget. 2017;8(29):47801–47815. doi: 10.18632/oncotarget.17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of twist1 in hepatocellular carcinoma: Its role in vasculogenic mimicry. Hepatology. 2010;51(2):545–556. doi: 10.1002/hep.23311. [DOI] [PubMed] [Google Scholar]
- 45.Rahme GJ, Israel MA. Id4 suppresses MMP2-mediated invasion of glioblastoma-derived cells by direct inactivation of Twist1 function. Oncogene. 2015;34(1):53–62. doi: 10.1038/onc.2013.531. [DOI] [PubMed] [Google Scholar]
- 46.Han H, Du L, Cao Z, Zhang B, Zhou Q. Triptonide potently suppresses pancreatic cancer cell-mediated vasculogenic mimicry by inhibiting expression of VE-cadherin and chemokine ligand 2 genes. Eur. J. Pharmacol. 2018;818:593–603. doi: 10.1016/j.ejphar.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Xiao T, Zhong W, Zhao J, Qian B, Liu H, Chen S, Qiao K, Lei Y, Zong S, Wang H, el. Polyphyllin I suppresses the formation of vasculogenic mimicry via Twist1/VE-cadherin pathway. Cell Death Dis. 2018;9(9):906. doi: 10.1038/s41419-018-0902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma. Cancer Res. 2001;61(17):6322–6327. [PubMed] [Google Scholar]
- 50.Liang X, Sun R, Zhao X, Zhang Y, Gu Q, Dong X, Zhang D, Sun J, Sun B. Rictor regulates the vasculogenic mimicry of melanoma via the AKT-MMP-2/9 pathway. J. Cell Mol. Med. 2017;21(12):3579–3591. doi: 10.1111/jcmm.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data was available from corresponding author upon reasonable request.