Abstract
Canola is one of the important oil crops and is considered the most promising oil source and adapts to reclaimed soil conditions. The current study aimed to evaluate the influence of yeast extract (YE) integrated with nitrogen (N) rates and treatments were arranged as follows: Control (without F0), 95 kg N ha−1 (F1), 120 kg N ha−1 (F2), 142 kg N ha−1 (F3), 95 kg N ha−1 + YE (F4), 120 kg N ha−1 + YE (F5) and 142 kg N ha−1 + YE (F6) on physico-chemical properties, yield and its components for three Canola genotypes i.e. AD201 (G1), Topaz and SemuDNK 234/84 under the sandy soil. In this work, Results reveal that increasing rates of Nitrogen fertilization from 95 kg N ha−1 to 142 kg N ha−1 have a great effect on physicochemical properties yield and its components. The result proved that 142 kg N ha−1 with yeast treatment was the best treatment for three Canola genotypes. Also, the result showed that seed yield was positively correlated with Chl. a/b ratio, plant height, number of branches/plant, number of pods/plant, and number of seeds/pod, and a strong negative correlation was detected between seed oil percentage when the amount of nitrogen fertilization applied without or with yeast extract is increased.
Subject terms: Ecology, Plant sciences
Introduction
Egypt is suffering from a great shortage of edible oils, wherein the gap between the total local production and imported oil is about 92%1. In Egypt, the cultivated area of oil crops is relatively little due to the excessive competition between them and other strategic winter season crops on the limited arable land in the Nile valley and Delta. Cultivation of oil crops such as Canola (Brassica napus L.) may supply a chance to overcome of deficiency of edible oil production in Egypt, because it can cultivate in various regions in comparison with the other oil crops, due to its ability to tolerate abiotic environmental stress such as salinity, drought, etc2. Canola is considered as a promising crop for crude oil production in many countries (14.7% of total edible oil in the world) because it contains high percentage and the good quality oil, wherein oil has a high content of omega 3, vitamin E, lowest saturated fatty acids, erucic acid, and glucosinolates3. Thus, it is recognized as efficient food by medicine4. Furthermore, in industry, oil is used to produce detergents, varnish, cleaning products, leather, rubber component, and biodiesel (rape methyl ester)5, the residual mass after oil extraction is rich in proteins and can be used for animal feeding6.
Chemical fertilizers are the main source of nitrogen (N) input in crop production systems. N plays a critical role in agriculture by increasing crop yield, and it is considered an essential element and occupies a noticeable place in the plant metabolism system; all vital processes in the plant are correlated with protein in which N is a fundamental constituent7. N enhances photosynthetic processes, leaf area production, leaf area duration, and net assimilation rate; consequently, increasing the production of the yield8. Fertilization management practices are one of the mainly important agro-management factors that affect the yield and its components of crops, in particular those grown in the newly reclaimed desert soils9. The optimizing application of nitrogen fertilization rates leads to improve characteristics of the Canola crop, wherein there is positively correlated with soil N level and Canola traits i.e. plant height, number of branches/plant, number of pods/plant, seed yield and oil yield10, yield traits are affected directly by N as a result of increased stem length, a higher number of flowering branches, total plant weight, seeds per pod, number and weight of pods and seeds/pod11. The chemical nitrogen fertilizers, on another hand, have numerous disadvantages such as insufficient supply or adulteration or unavailability of fertilizer at the time of applied. However, excessive N fertilizer use that goes beyond what crops actually need has had unfavorable effects on the quality of the soil, water, and air. Among these include soil acidification, nitrogen leaking into groundwater, and nitrous oxide (N2O) emissions, a strong greenhouse gas that accelerates global warming12. Furthermore, continuous application of chemical fertilizers creates soil contamination effect on the environment and it consumes great energy and cost during the chemical production process13. So, integration between chemical and bio-fertilization is a successful key for these problems. The combination between bio-fertilization and chemical sources of nitrogen provides crops with nitrogen requirements and decreased the pH, which led to enhance the availability of trace elements that improve plant growth14, bio-fertilizers to be a safe alternative to chemical fertilizers to minimize the ecological disturbance, capital and energy of chemical industry process13.
Dry bread yeast (Saccharomyces cerevisiae) is a kind of bio-fertilizers applied to soil or foliar spray for fertilizing the crops15. Dry bread yeast plays a valuable role in vegetative and reproductive growth stages, wherein it contains many nutrients capable to produce growth regulator hormones such as auxins and gibberellins, and it improves the simulative growth compounds that act to enhance the process of photosynthesis16, cell division and growth of the plant17, improve flower formation, enhance nucleic acid protein and accumulation of carbohydrates18. Spraying potato plants in five concentrations of dry bread yeast caused a significant increase in plant height, a number of branches, shoot dry matter, the number of tubers/plant, the rate of tuber weight, and plant yield19. Used dry bread yeast has improved growth and productivity in some vegetable crops19. Yeast has an opportunity to generate a band of enzymes that transform sugars into alcohol and CO2, which is utilized by plants in the photosynthetic process and leads to many plant hormones such as cytokinins, gibberellins, and auxins additionally, vitamins like B1, B2, B6 and B12 similarly, dry yeast possess a stimulatory influence on cell division and expansion, protein, nucleic acid synthesis, and chlorophyll formation. Since yeast is a natural source of cytokinins and protein, the function of yeast extract in enhancing cell division and enlargement of the cell18 so the yeast extract may be responsible for the increase in canola growth and productivity. Therefore, the present study was planned to improve and maximize the productivity of Canola under sandy soil conditions (Nobaria, Behaira Governorate, Egypt) by studying the effect of integration between different rates of nitrogen fertilization and dry bread yeast extract (Saccharomyces cerevisiae) on Canola. Photosynthetic pigments, growth, yield traits, and physico-chemical properties of the oil.
Results and discussion
Photosynthetic pigments
Based on the analysis of variance, data of Photosynthetic pigments as presented in Table 1 indicate that photosynthetic pigments as chlorophyll a (Chl. a) had non-significant for three Canola genotypes AD201 (G1), Topaz (G2) and SemuDNK 234/84 (G3), but chlorophyll b (Chl. b) and chlorophyll a/b ratio (Chl. a/b) had significant difference for three genotypes. Chl. a, Chl. b and Chl. a/b were positively responded to different N application i.e. without nitrogen fertilization (control F0), 95 kg N ha−1 (F1), 120 kg N ha−1 (F2) and 142 kg N ha−1 (F3) (without yeast); and integrated between nitrogen fertilization and yeast extract (YE) treatments as follows: 95 kg N ha−1 + YE (F4), 120 kg N ha−1 + YE (F5) and 142 kg N ha−1 (F6) (with yeast), data indicated that F5 and F6 gave the highest values of Chl. a and Chl. a/b ratio and lowest values of Chl. b Table 1. Interaction data showed that three Canola genotypes that were fertilized with N without yeast or with yeast had a slight difference with statistically significant in chl. a. The highest values of Chl. an obtained by G2 under F5 treatment followed by G1 under F6 treatments. In respect to Chl. a/b ratio, statistical analysis showed that Interaction between Canola genotypes treated with N applications without or with yeast had a significant difference whereas the highest values were recorded when Canola genotypes G3 and G2 fertilized with F6 and F5 with slight differences. While the interaction was significant between N treatments and Canola genotypes for Chl. b. and Canola genotype (G1) gave the highest value when treated with F1. Generally, F6 and F5 improve the contents of chl. a and chl. a/b ratio for three Canola genotypes Table 1. Chl. contents were increased in plants grown under middle and high N conditions as compared with plants grown under low N conditions, which significantly affected photochemical processes20. N is a fundamental element for leaf plants, insufficient N supply lead to decreased photosynthetic rate in plants21, this occurs to many factors such as a decrease in pigment degradation22, reduction in stomatal conductance23 and a decline in the light and dark reaction of photosynthesis. Canola is a nitrophilous plant, wherein a high concentration of NO3 in the culture media results in higher Chl. contents in the plant leave compared with controls20. The Chl. a/b ratio can be a valuable indicator of N element within a leaf because this ratio must be positively related to the ratio of PSII cores to light-harvesting chlorophyll-protein complex (LHCII)24. LHCII contains the majority of Chl. b, consequently it has a lower Chl a/b ratio than other Chl. binding proteins associated with PSII25. Thus, Chl. a/b ratios should increase with decreasing N availability, especially under high light conditions26, the Chl. a/b ratio and the ratio of PSII to Chl. are independent of N availability for spinach27, and lower Chl. a/b ratios were noticed when plants were subjected to low N28, while Kitajima and Hogan29 revealed that the Chl. a/b ratio increased when Chl. content decreased in response to N restriction in photosynthetic cotyledons in leaves of seedlings of four tropical woody species in the Bignoniaceae, and Bungard et al.30 demonstrated that there is a tiny response in Chl. a/b ratios to light or N. The yeast includes bio-regulators i.e. plant growth regulators and endogenous plant hormones, which enhance photosynthesis, also it produces 5-Aminolevulinic acid which is vital to tetrapyrrole biosynthesis and biochemical processes in plants, including heme and Chl. biosynthesis25.
Table 1.
Photosynthetic pigments for the three Canola genotypes under different N applications without and with yeast extract.
| Studied factor | Chlorophyll a | Chlorophyll b | Chl. a/b ratio | |
|---|---|---|---|---|
| Genotypes (G) | ||||
| G1 (AD201) | 4.06 ± 0.132a | 1.56 ± 0.046a | 2.68 ± 0.157b | |
| G2 (Topaz) | 4.11 ± 0.117a | 1.47 ± 0.048b | 2.87 ± 0.167ab | |
| G3 (SemuDNK 234/84) | 4.16 ± 0.092a | 1.44 ± 0.058c | 3.00 ± 0.177a | |
| Fertilizer (F) | ||||
| F0 (control) | 2.96 ± 1.657e | 1.56 ± 0.026d | 1.91 ± 0.087d | |
| F1 (95 kg N ha−1 without yeast) | 3.48 ± 0.123d | 1.73 ± 0.021a | 2.02 ± 0.084d | |
| F2 (120 kg N ha−1 without yeast) | 4.35 ± 0.046bc | 1.66 ± 0.015b | 2.63 ± 0.049c | |
| F3 (142 kg N ha−1 without yeast) | 4.25 ± 0.068c | 1.58 ± 0.020c | 2.70 ± 0.062c | |
| F4 (95 kg N ha−1 with yeast) | 4.42 ± 0.054bc | 1.45 ± 0.060e | 3.07 ± 0.115b | |
| F5 (120 kg N ha−1 with yeast) | 4.70 ± 0.035a | 1.23 ± 0.021f | 3.84 ± 0.066a | |
| F6 (142 kg N ha−1 with yeast) | 4.62 ± 0.150ab | 1.22 ± 0.025f | 3.80 ± 0.135a | |
| Interaction | ||||
| G1 | F0 | 2.68 ± 0.009h | 1.63 ± 0.005de | 1.64 ± 0.027h |
| F1 | 3.16 ± 0.009g | 1.81 ± 0.003a | 1.74 ± 0.007gh | |
| F2 | 4.20 ± 0.012de | 1.71 ± 0.012b | 2.45 ± 0.023de | |
| F3 | 4.34 ± 0.010bcde | 1.51 ± 0.009h | 2.88 ± 0.011c | |
| F4 | 4.59 ± 0.009abcd | 1.61 ± 0.012ef | 2.85 ± 0.017c | |
| F5 | 4.69 ± 0.035abc | 1.31 ± 0.009i | 3.59 ± 0.009b | |
| F6 | 4.76 ± 0.018 ab | 1.32 ± 0.008i | 3.61 ± 0.025b | |
| G2 | F0 | 3.04 ± 0.025gh | 1.51 ± 0.010h | 2.02 ± 0.009fg |
| F1 | 3.58 ± 0.021f | 1.67 ± 0.009c | 2.14 ± 0.006f | |
| F2 | 4.52 ± 0.003abcde | 1.62 ± 0.006de | 2.79 ± 0.009c | |
| F3 | 4.12 ± 0.195e | 1.59 ± 0.009f | 2.59 ± 0.13cd | |
| F4 | 4.35 ± 0.006bcde | 1.52 ± 0.003gh | 2.86 ± 0.009c | |
| F5 | 4.81 ± 0.007a | 1.21 ± 0.003j | 3.97 ± 0.015a | |
| F6 | 4.37 ± 0.472bcde | 1.17 ± 0.003k | 3.75 ± 0.410ab | |
| G3 | F0 | 3.15 ± 0.312g | 1.54 ± 0.009g | 2.05 ± 0.184fg |
| F1 | 3.71 ± 0.307f | 1.71 ± 0.009b | 2.17 ± 0.170ef | |
| F2 | 4.33 ± 0.027bcde | 1.64 ± 0.007d | 2.64 ± 0.026cd | |
| F3 | 4.30 ± 0.062cde | 1.64 ± 0.006d | 2.62 ± 0.045cd | |
| F4 | 4.31 ± 0.110cde | 1.22 ± 0.006j | 3.53 ± 0.076b | |
| F5 | 4.62 ± 0.061abcd | 1.16 ± 0.014k | 3.97 ± 0.061a | |
| F6 | 4.73 ± 0.006abc | 1.17 ± 0.006k | 4.04 ± 0.023a | |
| ANOVA | df | |||
| Genotypes (G) | 2 | < 0.001 | < 0.001 | < 0.001 |
| Fertilizer (F) level | 6 | < 0.001 | < 0.001 | < 0.001 |
| G × F | 12 | < 0.001 | < 0.001 | < 0.001 |
Yield and its attributes
Comparing of mean data through the Duncan Multiple Range Test in the probability level of 5%, data showed significant differences among the Canola genotypes for the highest plant (cm), branches number/plant, and pods number/plant. On contrary, there wasn’t a significant difference for seed number/pods, seed yield (t ha−1), biological yield (t ha−1), and harvest index, wherein G2 gave the highest value for the highest plant (cm). In the same trend, G2 gave the highest values of branches No./plant and pods No./plant followed by G3 for the previous two treats Table 2. All examined N without or with yeast caused a significant difference in yield and its attributes, wherein F6 positively affected on abovementioned traits and gave the highest values on the highest plant (cm), branches No./plant, pods No./plant, seed No./pods, seed yield (t ha−1), and harvest index. While the highest values of biological yield (t ha−1) were obtained with F3, F6, and F5, respectively Table 2.
Table 2.
Growth, yield and its attributes for the three Canola genotypes under different N applications without and with yeast extract.
| Studied factor | Plant height (cm) | Number of branches/plant | Pods number/plant | Number of seed/pods | Seed yield (t ha−1) | Biological yield (t ha−1) | Harvest index | |
|---|---|---|---|---|---|---|---|---|
| Genotypes (G) | ||||||||
| G1 (AD201) | 155.74 ± 3.394b | 4.86 ± 0.389b | 148.68 ± 6.362b | 21.34 ± 1.094a | 2.25 ± 0.138a | 7.71 ± 0.323a | 0.28 ± 0.010a | |
| G2 (Topaz) | 161.33 ± 3.886a | 7.48 ± 0.510a | 158.89 ± 6.870a | 22.40 ± 1.15a | 2.38 ± 0.151a | 7.77 ± 0.289a | 0.30 ± 0.011a | |
| G3 (SemuDNK 234/84) | 151.69 ± 2.442b | 7.05 ± 0.616a | 155.29 ± 6.761a | 21.90 ± 1.308a | 2.35 ± 0.174a | 7.60 ± 0.400a | 0.30 ± 0.014a | |
| Fertilizer (F) | ||||||||
| F0 (control) | 75.88 ± 2.263 g | 2.78 ± 0.57e | 76.87 ± 1.56f. | 9.78 ± 0.670f. | 0.73 ± 0.138e | 3.18 ± 0.359c | 0.23 ± 0.009c | |
| F1 (95 kg N ha−1 without yeast) | 151.78 ± 1.077f. | 4.44 ± 0.338d | 128.11 ± 1.327e | 17.78 ± 0.521e | 1.84 ± 0.126d | 7.28 ± 0.394b | 0.25 ± 0.009c | |
| F2 (120 kg N ha−1 without yeast) | 164.33 ± 2.261 d | 6.22 ± 0.465c | 153.44 ± 1.834c | 20.89 ± 0.309 d | 2.26 ± 0.084c | 7.80 ± 0.284b | 0.29 ± 0.006b | |
| F3 (142 kg N ha−1 without yeast) | 182.89 ± 2.988 b | 7.56 ± 0.294b | 184.78 ± 1.544 b | 27.56 ± 0.648b | 2.99 ± 0.076 b | 9.61 ± 0.180 a | 0.31 ± 0.009b | |
| F4 (95 kg N ha−1 with yeast) | 159.00 ± 1.080 e | 5.44 ± 0.377c | 145.67 ± 1.624d | 20.00 ± 0.500d | 2.11 ± 0.077c | 7.36 ± 0.322b | 0.29 ± 0.007b | |
| F5 (120 kg N ha−1 with yeast) | 170.89 ± 2.282 c | 8.33 ± 0.553b | 182.56 ± 3.520b | 25.89 ± 0.512c | 2.76 ± 0.071b | 9.03 ± 0.493 a | 0.31 ± 0.015b | |
| F6 (142 kg N ha−1 with yeast) | 189.00 ± 2.449a | 10.44 ± 0.766a | 208.56 ± 2.410a | 31.22 ± 0.494a | 3.59 ± 0.090a | 9.60 ± 0.351a | 0.38 ± 0.016a | |
| Interaction | ||||||||
| G1 | F0 | 74.17 ± 2.568i | 1.33 ± 0.425j | 74.40 ± 3.482n | 9.35 ± 0.627i | 0.66 ± 0.147i | 3.04 ± 0.714g | 0.22 ± 0.008i |
| F1 | 148.33 ± 1.453k | 3.33 ± 0.333i | 124.00 ± 2.082l | 17.00 ± 0.577h | 1.66 ± 0.110h | 6.94 ± 0.615ef | 0.24 ± 0.006ghi | |
| F2 | 168.00 ± 2.082f | 4.67 ± 0.333gh | 147.67 ± 1.764i | 21.33 ± 0.333f | 2.52 ± 0.060ef | 8.54 ± 0.511bcd | 0.30 ± 0.012cdefg | |
| F3 | 181.67 ± 1.333 d | 6.67 ± 0.333de | 180.33 ± 2.848e | 26.67 ± 0.333cd | 2.84 ± 0.070de | 9.94 ± 0.340a | 0.29 ± 0.014cdef | |
| F4 | 156.33 ± 2.333 hi | 4.00 ± 0.123hi | 141.00 ± 3.464j | 20.00 ± 0.577fg | 2.11 ± 0.130g | 7.68 ± 0.581def | 0.28 ± 0.009defgh | |
| F5 | 174.00 ± 3.512 e | 6.33 ± 0.333ef | 172.33 ± 2.404f | 24.67 ± 0.333e | 2.52 ± 0.073ef | 8.15 ± 0.743 cde | 0.31 ± 0.020 cdef | |
| F6 | 187.67 ± 0.882c | 7.67 ± 0.333cd | 201.00 ± 2.517c | 30.33 ± 1.202 b | 3.40 ± 0.196b | 9.69 ± 0.577 ab | 0.35 ± 0.030abc | |
| G2 | F0 | 77.00 ± 0.789i | 3.67 ± 0.457hi | 79.20 ± 0.759m | 10.08 ± 0.472i | 0.76 ± 0.092i | 3.33 ± 0.625g | 0.23 ± 0.016hi |
| F1 | 154.00 ± 1.527ij | 5.33 ± 0.333fg | 132.00 ± 0.577k | 18.33 ± 0.333h | 1.90 ± 0.080gh | 7.61 ± 0.543def | 0.25 ± 0.027ghi | |
| F2 | 169.33 ± 0.333f | 7.67 ± 0.333cd | 159.00 ± 2.082g | 21.00 ± 0.5772f | 2.14 ± 0.092g | 7.38 ± 0.315 def | 0.29 ± 0.002 defgh | |
| F3 | 193.67 ± 0.882b | 8.33 ± 0.333bc | 188.6 ± 1.453 d | 29.67 ± 0.882b | 3.25 ± 0.097bc | 9.65 ± 0.273ab | 0.34 ± 0.009bcd | |
| F4 | 162.00 ± 0.577g | 6.33 ± 0.333ef | 149.33 ± 0.882 i | 21.33 ± 0.882f. | 2.25 ± 0.175fg | 7.60 ± 0.684 def | 0.30 ± 0.003cdefg | |
| F5 | 175.33 ± 2.603e | 9.33 ± 0.333b | 188.67 ± 5.897d | 25.33 ± 0.333de | 2.80 ± 0.077de | 9.45 ± 0.107ab | 0.30 ± 0.009 cdefg | |
| F6 | 198.00 ± 0.577a | 11.67 ± 0.333a | 215.33 ± 2.603a | 31.00 ± 0.577ab | 3.57 ± 0.098ab | 9.37 ± 0.636ab | 0.38 ± 0.018ab | |
| G3 | F0 | 76.50 ± 1.333i | 3.33 ± 0.627i | 77.00 ± 2.378mn | 9.90 ± 1.025i | 0.78 ± 0.056i | 3.18 ± 0.256g | 0.24 ± 0.028ghi |
| F1 | 153.0 ± 0.577j | 4.67 ± 0.333gh | 128.33 ± 0.667k | 18.00 ± 1.527h | 1.95 ± 0.387gh | 7.28 ± 1.037ef | 0.26 ± 0.014fghi | |
| F2 | 155.67 ± 0.333hij | 6.33 ± 0.333ef | 153.67 ± 0.882h | 20.33 ± 0.667f | 2.11 ± 0.143g | 7.48 ± 0.443def | 0.28 ± 0.018defgh | |
| F3 | 173.33 ± 0.333e | 7.67 ± 0.333cd | 185.33 ± 0.882d | 26.33 ± 0.882cd | 2.89 ± 0.078d | 9.23 ± 0.265 abc | 0.31 ± 0.012cdef | |
| F4 | 158.67 ± 0.333h | 6.00 ± 0.233ef | 146.67 ± 0.882 i | 18.67 ± 0.333gh | 1.96 ± 0.044gh | 6.81 ± 0.455f | 0.29 ± 0.021defgh | |
| F5 | 163.33 ± 0.333g | 9.33 ± 0.667b | 186.6 ± 5.364d | 27.67 ± 0.667c | 2.95 ± 0.042cd | 9.51 ± 1.328ab | 0.32 ± 0.047cde | |
| F6 | 181.33 ± 0.333d | 12.00 ± 0.567a | 209.33 ± 2.156b | 32.33 ± 0.333a | 3.80 ± 0.097a | 9.73 ± 0.839ab | 0.40 ± 0.039a | |
| ANOVA | df | |||||||
| Genotypes (G) | 2 | < 0.001 | < 0.001 | < 0.001 | ||||
| Fertilizer (F) level | 6 | < 0.001 | < 0.001 | < 0.001 | ||||
| G × F | 12 | < 0.001 | < 0.001 | < 0.001 | ||||
The interaction between the Canola genotype and different N rates without or with yeast extract as shown in Table 2, demonstrated a significant difference. Data showed that the highest values of plant height and pods No./plant were recorded by G2 under F6 and the highest values of branches No./plant, seed No./pods, and seed yield (t ha−1) got by G3 and G2 under F6. There was a slight difference with statistically significant biological yield (t ha−1) and highest values established by G1 under F3 and F6; and G2 and G3 under F3, F5, and F6 respectively; and the highest values of harvest index recorded by G1, G2 and G3. under F6. Generally, data proved that 142 kg N/ h−1 + YE (F6) was enhanced the yield and its components of three Canola genotypes i.e. AD201 (G1), Topaz (G2), and SemuDNK 234/84 (G3). Many researchers reported that there are significant differences among Canola varieties and growth and yield traits are significantly increased by increasing N rates11. Increasing N fertilizer rates significantly increased most of the yield and its components31, N enhances metabolites synthesized by the plant which leads to more transformation of photosynthesis to reproductive parts, and induces different physiological mechanisms to access the nutrient32. Yeast extract as bio-fertilizer had a significant and positive effect on plant height and yield traits of Canola. The role of bread yeast in increasing the growth and yield traits; may be due to the content of yeast to many important nutrients elements i.e. N, Mg, Ca, Zn, Cu, and Fe, and the production of some growth regulators such as Auxin and Gibberellin and cytokinin which is necessary for plant biological processers especially photosynthesis and cell division and elongation33. Also, Yeast extract had stimulatory effects on cell division and enlargement, protein and nucleic acid synthesis, and chlorophyll formation34, in addition to its content of cryoprotective agent, i.e. sugars, protein, amino acids, and also several vitamins35. Consequently, it improves growth, flowering, and fruit set and formation and increases yield34.
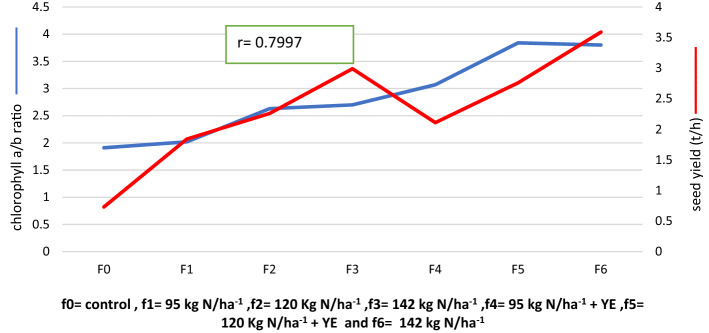
Correlation of Canola seed yield and chlorophyll a/b ratio
Partial correlation coefficients of Canola seed yield and Chl. a/b ratio is given in Fig. 1. This result showed that seed yield was positively correlated with Chl. a/b ratio when the amount of N applied without or with yeast extract is increased. Chl. a/b ratio can be an important indicator of N within a leaf, this ratio must be positively related to photosynthesis and biological processers which reflect on seed yield.
Figure 1.
Correlation of Canola seed yield (t/h) and chlorophyll a/b ratio as affected by different nitrogen rates without and with yeast extract.
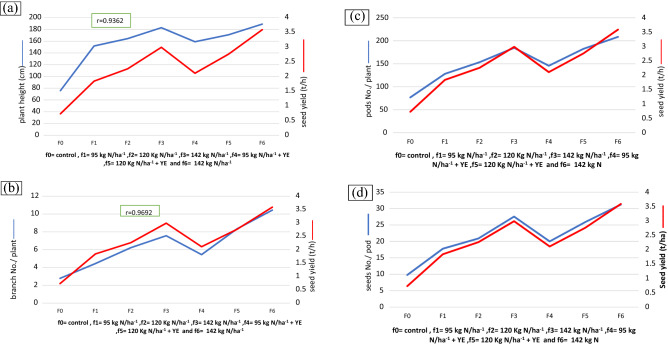
Correlation of Canola seed yield and its attributes
Correlations of seed yield and yield components of Canola are a function of the plant height, number of branches/plant, number of pods/plant, and number of seeds/pod as shown in Fig. 2a–d. These results proved that grain yield was strongly positively correlated with some of the abovementioned traits when N fertilization increased without or with yeast extract. Sufficient N contributes to enhance physiological processes, improves growth, flowering, seed formation, and the seed yield finally.
Figure 2.
(a) Correlation of Canola seed yield (t/h) and plant height (cm) as affected by different nitrogen rates without and with yeast extract, (b) Correlation of Canola seed yield (t/h) and branch No/plant as affected by different nitrogen rates without and with yeast extract, (c) Correlation of Canola seed yield (t/h) and pods No/ plant as affected by different nitrogen rates without and with yeast extract, and (d) Correlation of Canola seed yield (t/h) and seeds No/ pod as affected by different nitrogen rates without and with yeast extract.
Chemical properties
Regarding results of the oil yield (t ha−1), seed oil %, protein %, N % in seed, and N% in straw as presented in Table 3, data showed significant differences among three Canola genotypes; AD201 (G1), Topaz (G2) and SemuDNK 234/84 (G3), excepted oil yield had non-significant difference. G1 was surpassed in oil %; G2, G3 surpassed in protein % and N % in seed, and G3 surpassed in N% in straw. Different N fertilization applies without or with yeast extract had a significant effect on the abovementioned traits, wherein F6 treatment gave the highest oil yield, protein %, N % in seed, and N% in straw, while seed oil % significantly increased with F1 and F4 treatments. There was significant interaction concerning with abovementioned traits, Table 3, as well as the highest values of seed oil yield (t ha−1), protein % in seeds, and nitrogen % in seeds were obtained with G1, G2, and G3 when treated with F6. Wherein the highest values of oil % were obtained by G1 under F1 and F4 treatments. Concerning N% in straw was increased by increasing the rate of N fertilizer application and the highest value was recorded by adding F6 to G336. Seed oil percentage was decreased by increasing nitrogen rates; the effect of interaction between Canola cultivars and nitrogen fertilization treatments was significant on seed oil. % High rates of N led to decreases in seed oil % and increase in protein concentrations in Canola seed37, the increase in seed protein % because N is an integral part of protein and the protein of Canola.
Table 3.
Effect of different N applications without and with yeast extract on oil yield, oil %, protein %, N % in seed and N% in straw for the three Canola genotypes.
| Studied factor | Oil yield (t/ha) | Seed oil % | Protein % | N % in seed | N % in straw | ||
|---|---|---|---|---|---|---|---|
| Genotypes (G) | |||||||
| G1 (AD201) | 938.99 ± 73.7a | 41.97 ± 0.408a | 18.98 ± 0.811b | 3.04 ± 0.130b | 0.57 ± 0.025c | ||
| G2 (Topaz) | 982.88 ± 78.7a | 41.28 ± 0.335b | 19.70 ± 0.797a | 3.15 ± 0.127a | 0.61 ± 0.023b | ||
| G3 (SemuDNK 234/84) | 959.14 ± 78.9a | 41.15 ± 0.488b | 20.00 ± 0.750a | 3.20 ± 0.120a | 0.67 ± 0.024a | ||
| Fertilizer (F) | |||||||
| F0 (control) | 289.80 ± 19.3e | 39.52 ± 0.233e | 12.62 ± 0.159g | 2.02 ± 0.025g | 0.42 ± 0.014g | ||
| F1 (95 kg N ha−1 without yeast) | 805.00 ± 53.6d | 43.91 ± 0.259a | 18.02 ± 0.227f | 2.88 ± 0.036f | 0.55 ± 0.019f | ||
| F2 (120 kg N ha−1 without yeast) | 956.00 ± 36.3c | 42.33 ± 0.248 b | 18.64 ± 0.308e | 2.98 ± 0.049e | 0.59 ± 0.014e | ||
| F3 (142 kg N ha−1 without yeast) | 1210.00 ± 32.8b | 40.46 ± 0.232 d | 22.81 ± 0.195b | 3.65 ± 0.031b | 0.63 ± 0.017c | ||
| F4 (95 kg N ha−1 with yeast) | 918.00 ± 31.8c | 43.59 ± 0.157 a | 19.49 ± 0.229d | 3.12 ± 0.036d | 0.61 ± 0.017d | ||
| F5 (120 kg N ha−1 with yeast) | 1136.00 ± 23.2b | 41.22 ± 0.327 c | 21.28 ± 0.195c | 3.40 ± 0.031c | 0.72 ± 0.020b | ||
| F6 (142 kg N ha−1 with yeast) | 1407.00 ± 35.8a | 39.22 ± 0.346 e | 24.06 ± 0.194a | 3.85 ± 0.031a | 0.76 ± 0.017a | ||
| Interaction | |||||||
| G1 | F0 | 267 ± 18.6g | 40.26 ± 0.159fg | 12.13 ± 0.207i | 1.94 ± 0.0.033i | 0.37 ± 0.011n | |
| F1 | 743 ± 51.6f | 44.73 ± 0.176a | 17.33 ± 0.296g | 2.77 ± 0.047g | 0.49 ± 0.015l | ||
| F2 | 1068 ± 21.6cd | 42.40 ± 0.173cd | 17.67 ± 0.233g | 2.83 ± 0.037g | 0.56 ± 0.027jk | ||
| F3 | 1161 ± 29.5c | 40.87 ± 0.088ef | 22.47 ± 0.549b | 3.60 ± 0.088b | 0.59 ± 0.017i | ||
| F4 | 929 ± 58.2de | 43.87 ± 0.088ab | 18.67 ± 0.145f | 2.99 ± 0.023f | 0.57 ± 0.024jk | ||
| F5 | 1067 ± 39.7cd | 42.33 ± 0.388cd | 20.70 ± 0.306d | 3.31 ± 0.049d | 0.68 ± 0.035ef | ||
| F6 | 1336 ± 78.2ab | 39.30 ± 0.208hi | 23.87 ± 0.521a | 3.82 ± 0.083a | 0.72 ± 0.020d | ||
| G2 | F0 | 297 ± 12.6g | 39.09 ± 0.234hi | 12.62 ± 0.130hi | 2.02 ± 0.021hi | 0.42 ± 0.009m | |
| F1 | 825 ± 35.4ef | 43.43 ± 0.260b | 18.03 ± 0.186fg | 2.89 ± 0.030fg | 0.55 ± 0.012k | ||
| F2 | 889 ± 41.6ef | 41.53 ± 0.273de | 18.77 ± 0.240f | 3.00 ± 0.038f | 0.58 ± 0.015ij | ||
| F3 | 1327 ± 34.9ab | 40.87 ± 0.291ef | 23.07 ± 0.219b | 3.69 ± 0.035b | 0.62 ± 0.012gh | ||
| F4 | 969 ± 69.4de | 43.10 ± 0.289bc | 19.87 ± 0.318e | 3.18 ± 0.051e | 0.61 ± 0.020h | ||
| F5 | 1147 ± 22.4c | 40.93 ± 0.333ef | 21.37 ± 0.260cd | 3.42 ± 0.042cd | 0.71 ± 0.023d | ||
| F6 | 1426 ± 36.1a | 40.00 ± 0.153fgh | 24.20 ± 0.173a | 3.87 ± 0.028a | 0.74 ± 0.026c | ||
| G3 | F0 | 305 ± 59.8g | 39.21 ± 0.397hi | 13.09 ± 0.121h | 2.09 ± 0.019h | 0.47 ± 0.004l | |
| F1 | 847 ± 66.1ef | 43.57 ± 0.441b | 18.70 ± 0.173f | 2.99 ± 0.028f | 0.61 ± 0.005h | ||
| F2 | 910 ± 63.4e | 43.07 ± 0.203bc | 19.50 ± 0.416e | 3.12 ± 0.067e | 0.64 ± 0.003g | ||
| F3 | 1144 ± 25.0c | 39.63 ± 0.219gh | 22.90 ± 0.115b | 3.66 ± 0.018b | 0.69 ± 0.003e | ||
| F4 | 857 ± 18.8ef | 43.80 ± 0.153b | 19.93 ± 0.033e | 3.19 ± 0.005e | 0.66 ± 0.009f | ||
| F5 | 1193 ± 20.0bc | 40.40 ± 0.252fg | 21.77 ± 0.067c | 3.48 ± 0.011c | 0.78 ± 0.007b | ||
| F6 | 1458 ± 64.2a | 38.37 ± 0.833i | 24.10 ± 0.351a | 3.86 ± 0.056a | 0.82 ± 0.007a | ||
| ANOVA | df | ||||||
| Genotypes (G) | 2 | < 0.001 | |||||
| Fertilizer (F) level | 6 | < 0.001 | |||||
| G × F | 12 | < 0.001 | |||||
Correlation of Canola seed yield and seed oil percentage
A strong negative correlation was detected between seed oil percentage as shown in Fig. 3. The result indicates that seed oil percentage decreases with increasing in different N fertilization rates without or with yeast extract. That’s a negative correlation between seed yield and seed oil %; it might be due to N application which results in delaying maturity leading to poor seed filling and a greater proportion of green seed38.
Figure 3.
Correlation of Canola seed yield (t h−1) and oil % as affected by different nitrogen rates without and with yeast extract.
Physico-chemical properties of Canola oil
The effects of different N application rates without or with yeast extract on Canola genotypes on physico-chemical properties i.e. Acid value (mg g−1), saponification number (mg g−1) and peroxide value (mg kg−1) were shown in Table 4. Data of chemical properties of Canola oil showed significant differences among Canola genotypes, the highest acid value and peroxide value were obtained from G2 followed by G1 and G3, respectively, while the highest saponification number was obtained by G3 followed by G1 and G2, respectively.
Table 4.
Oil properties for three Canola genotypes under different N applications without and with yeast extract.
| Studied factor | Acid value (mg g−1) | Saponification number (mg g−1) | Peroxide value (mg kg−1) | ||
|---|---|---|---|---|---|
| Genotypes (G) | |||||
| G1 (AD201) | 1.92 ± 0.111ab | 142.76 ± 4.03b | 7.90 ± 0.187b | ||
| G2 (Topaz) | 2.47 ± 0.163a | 138.94 ± 4.08c | 9.01 ± 0.199a | ||
| G3 (SemuDNK 234/84) | 1.69 ± 0.104b | 144.66 ± 3.75 a | 7.83 ± 0.179b | ||
| Fertilizer (F) | |||||
| F0 (control) | 2.74 ± 0.237a | 177.74 ± .859a | 9.65 ± 0.242a | ||
| F1 (95 kg N ha−1 without yeast) | 2.49 ± 0.215b | 154.56 ± 0.747b | 8.94 ± 0.224b | ||
| F2 (120 kg N ha−1 without yeast) | 2.02 ± 0.171c | 142.44 ± 0.765c | 8.61 ± 0.197c | ||
| F3 (142 kg N ha−1 without yeast) | 1.68 ± 0.143d | 134.00 ± 1.900e | 7.97 ± 0.142d | ||
| F4 (95 kg N ha−1 with yeast) | 2.14 ± 0.211c | 137.44 ± 1.454d | 7.85 ± 0.229d | ||
| F5 (120 kg N ha−1 with yeast) | 1.71 ± 0.116d | 126.89 ± 1.135e | 7.66 ± 0.167e | ||
| F6 (142 kg N ha−1 with yeast) | 1.40 ± 0.112e | 121.78 ± 0.846g | 7.03 ± 0.216f | ||
| Interaction | |||||
| G1 | F0 | 2.53 ± 0.33c | 179.02 ± 1.533a | 9,25 ± 0.259c | |
| F1 | 2.30 ± 0.30cde | 155.67 ± 1.333c | 8.57 ± 0.240de | ||
| F2 | 2.00 ± 0.21defg | 141.33 ± 0.666f | 8.23 ± 0.033e | ||
| F3 | 1.63 ± 0.15ghijkl | 135.67 ± 1.333h | 7.67 ± 0.088fgh | ||
| F4 | 1.97 ± 0.18defgh | 139.33 ± 0.667g | 7.63 ± 0.218fgh | ||
| F5 | 1.67 ± 0.15ghijkl | 127.33 ± 1.667k | 7.37 ± 0.120ghi | ||
| F6 | 1.33 ± 0.19l | 121.00 ± 1.533m | 6.60 ± 0.115j | ||
| G2 | F0 | 3.52 ± 0.06a | 175.57 ± 1.333b | 10.67 ± 0.047a | |
| F1 | 3.20 ± 0.06ab | 152.67 ± 1.667d | 9.78 ± 0.044b | ||
| F2 | 2.33 ± 0.23cd | 141.33 ± 1.000f | 9.35 ± 0.180c | ||
| F3 | 1.93 ± 0.22efghi | 127.00 ± 1.001k | 8.52 ± 0.072de | ||
| F4 | 2.87 ± 0.12b | 132.00 ± 1.333i | 8.68 ± 0.109d | ||
| F5 | 1.90 ± 0.23fghij | 123.67 ± 1.667l | 8.28 ± 0.148e | ||
| F6 | 1.53 ± 0.19jkl | 120.33 ± 0.767m | 7.85 ± 0.132f | ||
| G3 | F0 | 2.16 ± 0.26def | 178.63 ± 0.667a | 9.14 ± 0.095c | |
| F1 | 1.97 ± 0.23defgh | 155.33 ± 0.333c | 8.47 ± 0.088de | ||
| F2 | 1.73 ± 0.29ghijk | 144.67 ± 0.666e | 8.23 ± 0.133e | ||
| F3 | 1.47 ± 0.35kl | 139.33 ± 1.000g | 7.73 ± 0.088fg | ||
| F4 | 1.60 ± 0.25hijkl | 141.00 ± 1.333f | 7.23 ± 0.088i | ||
| F5 | 1.57 ± 0.24ijkl | 129.67 ± 1.333j | 7.33 ± 0.089hi | ||
| F6 | 1.33 ± 0.26l | 124.00 ± 1.000l | 6.63 ± 0.145j | ||
| ANOVA | df | ||||
| Genotypes (G) | 2 | < 0.001 | |||
| Fertilizer (F) level | 6 | < 0.001 | |||
| G × F | 12 | < 0.001 | |||
Where; G1 is AD201, G2 is Topaz, G3 is SemuDNK 234/84, F1 is 95 kg N ha−1, F2 is 120 kg N ha−1, F3 is 142 kg N ha−1 (without bio-fertilizer); F4 is 95 kg N h−1 + yeast extract (YE), F5 is 120 kg N ha−1 + YE and F6 is 142 kg N ha−1 + YE (with bio-fertilizer), G × F is interaction Means in each column with at least one similar letters are not significantly different at the 5% probability leGel using Duncan's new multiple range test.
Data had significant differences among different N application rates without or with yeast extract, by increasing the N rated from F0 to F6 caused decreases in Acid value, Saponification number, and peroxide value. Also, data showed a significant interaction between Canola genotypes and different N application rates without or with yeast extract for all abovementioned traits, wherein the highest values of saponification number were obtained by G1 and G3 under F0 treatment. In addition, the highest values of peroxide value and the acid value were obtained by G2 with F0. The acid value is a physicochemical indicator38, wherein oils which have higher acid value posse poor quality39, on another hand, Low acid value of Canola genotype shows their higher oil quality. The peroxide value varied between 7.1 and 9.06 meq. O2/kg indicates that the tested vegetable oils are fresh, and the lowest initial peroxide value is suitable for consumption40. High saponification value indicated that Canola oil possesses normal triglycerides and may be useful in the production of liquid soap and shampoo41. Saponification number was significantly different among genotypes and a higher nitrogen rate resulted in an increase in the unsaponifiable matter and led to a decrease in oil acid value and saponification value42.
Fatty acids composition percentages in Canola oil
The main values of fatty acids composition percentages in Canola oil were determined and calculated in the second season Table 5. Gas–liquid chromatographic analysis showed that, saturated fatty acids (Palmitic, 16:0, Stearic, 18:0, Arachidic, 20:0, and Behenic, 22:0) represent about 9.1 of the total fatty acids. Palmitic was the dominant acid among the saturated ones. In respect of unsaturated fatty acids i.e., Oleic acid (18:1), Linoleic (18:2), Linolenic (18:3), and Erucic (22:1), they all represent about 90.9% of total fatty acids. Therefore, Oleic acid (18:1) was the major fatty acid in Canola oil (59.43%) followed by Linoleic (20.80%) and Linolenic (9.02%). Erucic acid was less than 2%.
Table 5.
Saturated and unsaturated fatty acids (%) in seeds of the three Canola genotypes and different N applications without and with yeast extract.
| Treatments | Fatty acids (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Saturated | Unsaturated | ||||||||
| Palmitic (16:0) | Stearic (18:0) | Arachidic (20:0) | Behenic (22:0) | Oleic (18:1) | Linoleic (18:2) | Linolenic (18:3) | Erucic (22:1) | ||
| Garieties | AD 201 | 4.79 | 1.54 | 2.06 | 1.15 | 58.36 | 21.33 | 9.16 | 1.61 |
| Topaz | 4.52 | 1.22 | 2.16 | 1.17 | 59.57 | 20.35 | 8.28 | 1.77 | |
| Semu DNK | 4.01 | 1.40 | 2.07 | 1.20 | 60.36 | 20.61 | 8.91 | 1.45 | |
| Fertilizer | 0 k,N h−1 | 3.03 | 2.87 | 4.05 | 2.31 | 57.96 | 18.03 | 7.58 | 2.32 |
| 95 k,N h−1 | 3.59 | 2.00 | 3.18 | 1.51 | 59.68 | 19.92 | 8.26 | 1.87 | |
| 120 k,N h−1 | 4.03 | 1.33 | 2.08 | 1.19 | 60.23 | 20.71 | 8.94 | 1.51 | |
| 142 k,N h−1 | 4.38 | 0.86 | 0.96 | 0.90 | 61.18 | 21.22 | 9.52 | 0.99 | |
| 95 k,N h−1 + yeast | 4.11 | 2.04 | 3.24 | 1.53 | 58.49 | 20.07 | 8.40 | 2.14 | |
| 120 k,N h−1 + yeast | 4.46 | 1.39 | 2.13 | 1.14 | 59.36 | 20.87 | 8.98 | 1.66 | |
| 142 k,N h−1 + yeast | 4.73 | 0.88 | 0.91 | 0.85 | 60.44 | 21.47 | 9.69 | 1.03 | |
Significant values are given in bold and italics.
Data in Table 5, showed slight differences in saturated fatty acids between Canola varieties. AD201(G1) variety contained more amount of Palmitic (4.78%) and Stearic (1.52%) acids followed by Topaz (G2) for Palmitic and SemuDNK 234/84 (V3) for Stearic. However, Behenic acid (1.20%) was higher in G3 than G2 (1.17%), while G2 was the highest in Arachidic acid than G3 variety. These results are in line with those obtained by El Habbasha et al.43. They reported that AD 201, Silvo, and Topas (G2) were different in their oil contents of saturated and unsaturated fatty acids. Canola varieties were also slightly differed in their content of the unsaturated fatty acids Table 5, G3 variety contained more amounts of Oleic (60.36%) acid followed by the G2 variety. G1 recorded the lowest amount of Oleic acid (58.36%) in comparison with the other two varieties. On the other hand, G1 showed a high increment in Linoleic and Linolenic acids followed by G3 for Linoleic and Linolenic acids. The second oil quality breeding objective is to reduce the percentage of Linolenic acid from the percent 8–10% to less than 3% while maintaining or increasing the level of Linoleic acid44. Lower Linolenic acid is desired to improve the storage characteristics of the oil, while higher Linolenic acid content may be nutritionally desirable. Similar observations were reported by Ref.45. Topaz variety recorded the highest value for Erucic acid (1.77%) followed by AD201 variety, whereas Semu DNK gave the lowest value (1.45%). The increase in Erucic acid content in the Topaz variety may be due to the decrease in Oleic acid content46. Stated that the concentrations of Oleic and Erucic acids were negatively correlated and a high Oleic acid concentration (> 50%) was always associated with a low Erucic acid concentration (< 4%).
All saturated fatty acids were slightly affected by N fertilizer rate Table 5. Palmitic acid showed an increase (4.73%) with 142 kg N ha−1 (F6) with yeast followed by 120 kg N ha−1 (F5) with yeast (4.46%), while Stearic, Arachidic, and Behenic acids contents were gradually decreased by increasing N levels up to 142 kg N ha−1 (F3) without or with yeast. The highest value of these acids was recorded with control (F0). In contrast, increasing the N rate up to high-level F3 or F6 increased the Oleic, Linoleic, and Linolenic acids and decreased the percent of Erucic acid in comparison with the other treatments Table 5. The unsaturated fatty acids recorded a slight increment as Canola plants fertilized with N except, Linolenic acid which gave the highest value (9.69%) under F6 Table 5. Oleic acid increased as the N rate increased up to F3 or F6, (61.18%), while Erucic acids recorded a higher increment with F0 followed by F4. These results are similar to those obtained by El kholy et al. and El-Beltagi et al.42,47 The fatty acid composition of Canola oil is mainly under genetic control but can be modified to some extent by N nutrition. Thus, it can be concluded from these observations that the N affected not only the quantity but also the quality of oil, and to obtain higher oil content in seeds and a better fatty acid profile in the oil of Canola varieties, N fertilizer showed to be applied in balanced doses45.
Nitrogen efficiency indexes
Nitrogen uptake can be assessed by nitrogen efficiency indexes such as nitrogen use efficiency (NUE), nitrogen remobilization efficiency (NRE), nitrogen harvest index (NHI), and nitrogen physiological efficiency (NPE) as presented in Table 6. Nitrogen efficiency indexes differed among Canola genotypes, NUE, NHI and NPE differed among G1, G2, and G3 with non-significant, and NRE was higher in G3 and G2 with non-significant. Previous data showed significant differences among different N application without and with yeast extract, F6 showed the highest values of NUE, NRE, and NHI, while F0 showed the highest values of NPE. There was a significant interaction between Canola genotypes and different N application without and with yeast extract. Nitrogen efficiency indexes are important tools to assess the nutritional status of plants; they can be used to evaluate genotypes that make the best use of applied nutrients. It is also possible to improve management techniques to enhance plant production48. Based on Nitrogen efficiency indexes results, the application of nitrogenous nutrients can be improved and, resulting in prevent the overuse of fertilizers, decreasing production costs and environmental damages49,50.
Table 6.
Nitrogen efficiency indexes for three Canola genotypes under different N applications without and with yeast extract.
| Studied factor | NUE | NRE | NHI | NPE | |
|---|---|---|---|---|---|
| Genotypes (G) | |||||
| G1 (AD201) | 21.01 ± 0.592a | 98.51 ± 4.132b | 65.00 ± 2.249a | 22.87 ± 0.812a | |
| G2 (Topaz) | 22.20 ± 0.684a | 106.75 ± 4.469a | 65.76 ± 2.457a | 22.15 ± 0.717a | |
| G3 (SemuDNK 234/84) | 21.79 ± 0.957a | 108.49 ± 5.257a | 64.30 ± 2.375 a | 21.32 ± 0.690a | |
| Fertilizer (F) | |||||
| F0 (control) | – | – | 41.74 ± 1.089e | 29.19 ± 0.683a | |
| F1 (95 kg N ha−1 without yeast) | 19.32 ± 1.332c | 87.15 ± 5.630d | 63.85 ± 1.085d | 22.18 ± 0.479bc | |
| F2 (120 kg N ha−1 without yeast) | 18.97 ± 0.704c | 83.90 ± 2.279d | 67.24 ± 0.759c | 22.60 ± 0.512b | |
| F3 (142 kg N ha−1 without yeast) | 21.06 ± 0.537bc | 106.37 ± 1.880bc | 72.21 ± 0.945b | 19.80 ± 0.313d | |
| F4 (95 kg N ha−1 with yeast) | 22.19 ± 0.810b | 103.23 ± 4.497c | 67.17 ± 0.912c | 21.57 ± 0.383c | |
| F5 (120 kg N ha−1 with yeast) | 23.19 ± 0.597ab | 117.44 ± 5.255b | 67.72 ± 1.533c | 19.92 ± 0.550d | |
| F6 (142 kg N ha−1 with yeast) | 25.27 ± 0.633a | 129.41 ± 3.330a | 75.22 ± 1.229a | 19.54 ± 0.247d | |
| Interaction | |||||
| G1 | F0 | – | – | 42.11 ± 0.848g | 30.53 ± 0.580a |
| F1 | 17.47 ± 1.159f | 75.51 ± 5.857j | 64.27 ± 0.805ef | 23.19 ± 0.421cd | |
| F2 | 21.18 ± 0.506bcdef | 88.30 ± 3.399ghij | 67.88 ± 1.650cdef | 24.01 ± 0.367c | |
| F3 | 20.00 ± 0.494def | 101.33 ± 2.121defghi | 70.92 ± 1.300abcd | 19.77 ± 0.846fg | |
| F4 | 22.28 ± 1.367abcde | 99.96 ± 8.079efghi | 66.81 ± 1.772def | 22.37 ± 0.538cde | |
| F5 | 21.21 ± 0.616bcdef | 102.45 ± 7.315defghi | 68.93 ± 2.589bcdef | 20.84 ± 1.084defg | |
| F6 | 23.94 ± 1.380abcd | 123.54 ± 7.691abcd | 74.07 ± 2.063abc | 19.40 ± 0.523g | |
| G2 | F0 | – | – | 41.66 ± 1.175g | 29.05 ± 1.535ab |
| F1 | 19.20 ± 0.842def | 90.67 ± 0.938fghij | 63.69 ± 1.110f. | 22.07 ± 1.027cdef | |
| F2 | 17.98 ± 0.770ef | 79.43 ± 4.062ij | 68.02 ± 0.269cdef | 22.66 ± 0.379cde | |
| F3 | 22.86 ± 0.683abcd | 112.29 ± 1.790bcdef | 75.10 ± 0.780ab | 20.35 ± 0.291efg | |
| F4 | 23.68 ± 1.844abcd | 110.21 ± 1.446bcdefg | 68.60 ± 0.926cdef | 21.60 ± 0.556cdefg | |
| F5 | 23.56 ± 0.646abcd | 120.45 ± 4.005abcde | 66.90 ± 0.708def | 19.57 ± 0.332fg | |
| F6 | 25.12 ± 0.691ab | 127.42 ± 4.467abc | 76.37 ± 1.102a | 19.73 ± 0.227fg | |
| G3 | F0 | – | – | 41.44 ± 0.838g | 27.98 ± 1.125b |
| F1 | 20.49 ± 2.077cdef | 95.27 ± 5.434fghij | 63.60 ± 1.727f | 21.27 ± 0.774defg | |
| F2 | 17.76 ± 1.202ef | 83.98 ± 3.824hij | 65.83 ± 1.619def | 21.13 ± 0.895defg | |
| F3 | 20.32 ± 0.552cdef | 105.47 ± 2.016cdefgh | 70.61 ± 1.378abcde | 19.27 ± 0.285g | |
| F4 | 20.60 ± 0.460bcdef | 99.53 ± 3.142efghi | 66.10 ± 2.166def | 20.73 ± 0.701defg | |
| F5 | 24.82 ± 0.367abc | 129.44 ± 8.538 ab | 67.33 ± 2.376def | 19.34 ± 1.303g | |
| F6 | 26.74 ± 0.683a | 137.27 ± 1.967a | 75.22 ± 2.441ab | 19.49 ± 0.619g | |
| ANOVA | df | ||||
| Genotypes (G) | 2 | < 0.001 | |||
| Fertilizer (F) level | 6 | < 0.001 | |||
| G × F | 12 | < 0.001 | |||
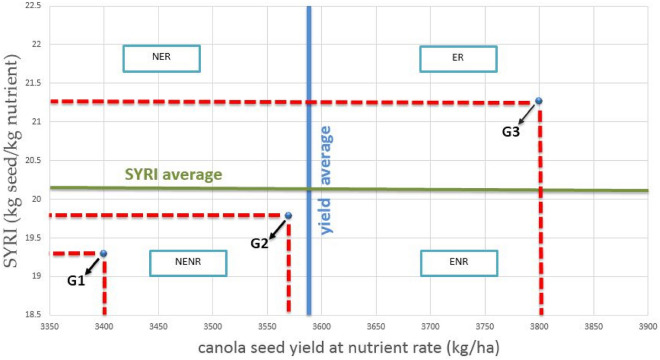
Seed yield response index (SYRI) of Canola
Based on 0 kg N h−1 (control), as a low nutrient rate, and 142 kg N h−1 + yeast extract, as a high nutrient rate of nitrogen, SYRI of Canola was computed. SYRI pointed out the efficient genotype for producing higher seed yield at the low nutrient rate and their response to increasing nutrient fertilizer rates. In this connection, Fig. 4 illustrated that the average Canola seed yield at a low nutrient rate was 3590 kg h−1 as well as the mean SYRI value for 142 kg N h−1 + yeast extract was 20.12 kg seeds kg nutrient h−1. Accordingly, SemuDMK genotype was belonging to efficient and responsive (ER), being exceeded the averages of seed yield at the low nutrient rate and SYRI, while AD 201 and Topaz were neither efficient nonresponsive (NENR) since the seed yield at the low nutrient rate and SYRI were lower than the averages.
Figure 4.
Seed yield response index (SYRI) of the tested Canola genotypes fertilized by nitrogen at a rate of 95 kg N ha−1, 120 kg N ha−1, 142 kg N ha−1, 95 kg N ha−1, + yeast extract, 120 kg N ha−1, + yeast extract, 142 kg N ha−1, + yeast extract. ER efficient and responsive, NENR neither efficient nor responsive.
Conclusion
Productivity and total seed N accumulation differed under different N and yeast extract management practices and canola cultivars. Results revealed that increasing rates of Nitrogen fertilization from 95 to 142 kg N ha−1 have a great effect on physico-chemical properties yield and its components. The result proved that 142 kg N ha−1 with yeast treatment was the best treatment for three canola genotypes. Regarding seed yield response index (SYRI) of canola, data cleared that SemuDMK genotype was belonging to efficient and responsive (ER), being exceeded the averages of seed yield at the low nutrient rate and SYRI, while AD 201 and Topaz were neither efficient nor nonresponsive (NENR) since the seed yield at the low nutrient rate and SYRI were lower than the averages.
Materials and methods
Plant material
The experimental research on plants, including the collection of plant material, complied with the relevant institutional, national, and international guidelines and legislation. Three Canola genotypes AD201 (G1), Topaz (G2), and SemuDNK 234/84 (G3) were used in this experiment, the first and third genotypes are Germany and the second is French. Canola genotypes seeds were secured from the Agricultural Research center (ARC), Ministry of Agriculture, Giza. Egypt.
Experimental site
Two field experiments were carried out at the Experimental Station of the National Research Center, Nobaryia, Behaira Governorate, Egypt, during the winter seasons 2019–2020 and 2020–2021. Mechanical and chemical analysis of the soil experimental site is presented in Table 7 according to Chapman et al.51.
Table 7.
Mechanical and chemical analysis of the experimental site soil.
| Sand | Silt 20-0µ% | Clay < 2µ% | Soil texture | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Course 2000-200µ% | Fine 200-20µ % | 12.66 | 4.18 | Sandy | |||||||
| 47.64 | 36.59 | ||||||||||
| pH 1:2.5 |
EC dSm−1 |
CaCO3 | OM% | Soluble Cations meq/l | Soluble anions meq/l | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg+ | Ca++ | CO3– | HCO3− | Cl− | SO4– | ||||
| 7.50 | 0.13 | 5.3 | 0.06 | 0.59 | 0.14 | 0.95 | 1.0 | 0.0 | 1.27 | 0.46 | 0.87 |
| Available nutrients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Macro element (ppm) | Micro element (ppm) | ||||||||||
| N−3 | P−3 | K+ | Zn+2 | Fe+2 | Mn+2 | Cu+2 | |||||
| 51 | 12.2 | 74 | 0.13 | 1.3 | 0.28 | 0.00 | |||||
Experimental treatments
Treatments were conducted as follows: without nitrogen fertilization (control F0), 95 kg N ha−1 (F1), 120 kg N ha−1 (F2) and 142 kg N ha−1 (F3) (without yeast); and integrated between nitrogen fertilization and yeast extract (YE) treatments as follows: 95 kg N ha−1 + YE (F4), 120 kg N ha−1 + YE (F5) and 142 kg N ha−1 + YE (F6) (with yeast). Nitrogen Fertilization treatments were applied as ammonium nitrate (33% N); it was added in equal twelve portions; the first dose was applied at sowing preparation, and the second was after 15 days from sowing, and the other portions were added weekly with irrigation water requirements. Yeast extract was added as (60 L ha−1) throughout the irrigation system in the same order as nitrogen fertilization. Plants received the recommended doses of Phosphorus and potassium fertilizers before sowing at the rate of (475) and (120) kg ha−1 of calcium supper phosphate (15.5% P2O5) and potassium sulphate (48% K2O), respectively.
Experimental design
The trial design was carried out as a strip plot design with three replications; Fertilizer treatments occupied the vertical main plots and the cultivars were distributed in horizontal ones. The experimental unit area was 16.8 m consisting of 6 ridges 4 m in length and 0.70 m in width and planted one ridge side at 15 cm apart and one plant per hill. The drip irrigation system was installed; the drip lateral had emitters spaced 20 cm apart with a nominal discharge of 4 L h−1.
Yeast extract preparation
Yeast (Saccharomyces cerevisiae) extract was prepared by using a technique that allowed yeast cells (pure active dry yeast 100 g L−1) to be grown and multiplied efficiently during conducive aerobic and nutritional conditions that allowed to produce beneficial bio-constituent i.e. carbohydrates, sugars, proteins, amino acids, fatty acids, hormones, etc52. The chemical analysis of yeast extract was analyzed by Ref.35 as presented in Table 8.
Table 8.
Chemical analysis of yeast extract.
| Amino acid | Vitamins and carbohydrates | ||
|---|---|---|---|
| (mg 100–1 g dry weight) | |||
| Arginine | 1.99 | Vitamin B1 | 2.23 |
| Histidine | 2.63 | Vitamin B2 | 1.33 |
| Isoleucine | 2.31 | Vitamin B6 | 1.25 |
| Leucine | 3.09 | Vitamin B12 | 0.15 |
| Lysine | 2.95 | Thimain | 2.71 |
| Methionine | 0.72 | Riboflavin | 4.96 |
| Phenyl alanine | 2.01 | Insitol | 0.26 |
| Threonine | 2.09 | Biotin | 0.09 |
| Tryptophan | 0.45 | Nicotinic acid | 39.88 |
| Valine | 2.19 | Panthothenic acid | 19.56 |
| Glutamic acid | 2.00 | P amino benzoic acid | 9.23 |
| Serine | 1.59 | Folic acid | 4.36 |
| Aspartic acid | 1.33 | Pyridoxine | 2.90 |
| Cysteine | 0.23 | Total carbohydrates | 23.2 |
| Proline | 1.53 | ||
| Tyrosine | 1.49 | Glucose | 13.33 |
Sampling and collecting data
Samples of guarded Canola plants were randomized and collected in both seasons; the first sample was taken 90 days after sowing, wherein ten plants were collected from each treatment to determine Chlorophyll a and b calorimetrically in fresh leaves (mg g−1 FW) according to the methods described by Ref.53 then calculated Chlorophyll a/b ratio; the second sample ten plants were taken at the harvesting time (180 days after sowing) to measured plant heights (cm), number of branches, pods number/plant and number of seed/pods; All plants (140 plants) in each plot (16.8 m) were harvested to determine the seed yield (kg ha−1), and biological yield (kg ha−1) and Harvest index.
Chemical composition of Canola genotypes
The chemical composition of samples was determined i.e. oil yield, seed oil %, protein %, N % in seed, and N % in straw. Oil % of seed was determined by solvent extraction method according to Dolatabadian et al.54. Protein content in seeds was calculated by multiplying N content by 6.25. Determination of Physico-chemical properties i.e. Acid value (mg g−1), Saponification number (mg g−1) and peroxide value (mg kg−1) were determined as the method described by Dolatabadian et al.54.
Crude oil of seeds (2nd season only) was used as authentic material for identification of the following fatty acids according to Stahl et al.55 The amount of each fatty acid in the oil under investigation was determined according to Nelson et al.56. Note: The statistical analysis does not do on some parameters such as fatty acids composition.
Nitrogen efficiency indexes
Nitrogen efficiency indexes were calculated as follows: Nitrogen use efficiency, NUE = seed yield kg h−1/applied nitrogen kg h−1; Nitrogen remobilization efficiency (NRE) = total N uptake kg h−1 × 100/N applied kg h−1; Nitrogen harvest index (NHI) total N in seeds kg h−1 × 100/total N uptake kg h−1, and nitrogen physiological efficiency (NPE, seed yield (kg h−1)/total N uptake kg h−1) were calculated according to Timsina et al.57.
Seed yield response index
Seed yield response index (SYRI) was calculated for each genotype using formula of Fageria and Barbosa Filho58 as follow: SYRI (kg seeds kg nutrient − 1) = (SY at high nutrient rate − SY at low nutrient rate)/(high nutrient rate − low nutrient rate). Where SY: seed yield kg h−1, Low nutrient rate = 95 kg N h−1, High nutrient rate = 142 kg N h−1 + yeast extract.
According to the SYRI value, genotypes could be classified into four groups: (i) efficient and responsive (ER) that produce high seed yield at low as well as high rates of nutrient fertilizer; (ii) efficient and not responsive (ENR) that produce high seed yield at low nutrient rate with lower response to increase nutrient fertilizer than ER; (iii) not efficient but responsive (NER) that has low seed yield with response to increase nutrient fertilizer; and (iv) neither efficient nor responsive (NENR) that has low seed yield with low response to increase nutrient fertilizer.
Statistical analysis
The obtained data were exposed to the proper statistical analysis according to Snedecor et al.59. Using Costat computer program V 6.303 (2004). Duncan Multiple Range Test60 in the probability level of 5% level of significance was used to differentiate between means, and Correlation coefficient according to Afiah et al.61. Data of both growing seasons were subjected to a homogeneity variance test for running the combined analysis of the data.
Author contributions
All authors contributed to the design of experiments, explanation of data, and writing of the manuscript except E.S.. E.S.contributed to the data analysis, and the revision of the manuscript. M.E. and A.M.S. were involved in all stages, including the conception of the idea, designing experiments, conducting measurements, and explanation of data.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All the data of the current work are included in the submitted article. For further data, please contact the corresponding author (M.E.) via mohamedebaid979@gmail.com.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohamed Ebaid, Email: mohamedebaid979@gmail.com.
Ahmed M. Saad, Email: ahmed.saad@fagr.bu.edu.eg
References
- 1.US Department of Agriculture, A. R. S. USDA national nutrient database for standard reference, release 28. Nutrient data laboratory home page (2011).
- 2.Ghallab K, Sharaan A. Selection in canola (Brassica napus L.) germplasm under conditions of newly reclaimed land. II. Salt tolerant selections. Egypt. J. Plant Breed. 2002;6:15–30. [Google Scholar]
- 3.Yasari, E., Azadgoleh, A. E., Pirdashti, H. & Mozafari, S. Azotobacter and Azospirillum inoculants as biofertilizers in canola (Brassica napus L.) cultivation. Asian J. Plant Sci. (2008).
- 4.Brown, J., Davis, J., Lauver, M. & Wysocki, D. USCA Canola Growers’ Manual. Oregon. P71 (2008).
- 5.Kamel, S., Mahfouz, H., Blal, H., Said, M. & Mahmoud, M. Effects of salicylic acid elicitor and potassium fertilizer as foliar spray on canola production in the reclaimed land in Ismailia Governorate, Egypt. (2016).
- 6.Din J, Khan S, Ali I, Gurmani A. Physiological and agronomic response of canola varieties to drought stress. J. Anim. Plant Sci. 2011;21:78–82. [Google Scholar]
- 7.Massignam A, Chapman S, Hammer G, Fukai S. Physiological determinants of maize and sunflower grain yield as affected by nitrogen supply. Field Crop Res. 2009;113:256–267. doi: 10.1016/j.fcr.2009.06.001. [DOI] [Google Scholar]
- 8.Sultana SR, Ahmad A, Wajid A, Akhtar J. Estimating growth and yield related traits of wheat genotypes under variable nitrogen application in semi-arid conditions. Pak. J. Life Soc. Sci. 2013;11:118–125. [Google Scholar]
- 9.Patel J, Shelke V. Effect of farmyard manure, phosphorus and sulphur on growth, yield and quality of Indian mustard (Brassica juncea) Indian J. Agron. 1998;43:713–717. [Google Scholar]
- 10.Ahmadi M, Bahrani M. Yield and yield components of rapeseed as influenced by water stress at different growth stages and nitrogen levels. Am. Eurasian J. Agric. Environ. Sci. 2009;5:755–761. [Google Scholar]
- 11.Al-Solaimani SG, Alghabari F, Ihsan MZ. Effect of different rates of nitrogen fertilizer on growth, seed yield, yield components and quality of canola (Brassica napus L.) under arid environment of Saudi Arabia. Int. J. Agron. Agric. Res. 2015;6:268–274. [Google Scholar]
- 12.Sainju, U. M., Ghimire, R. & Pradhan, G. P. Nitrogen fertilization I: Impact on crop, soil, and environment. Nitrogen Fixat. (2019).
- 13.Hafeez K, Zhang Y, Malak N. Core competence for sustainable competitive advantage: A structured methodology for identifying core competence. IEEE Trans. Eng. Manag. 2002;49:28–35. doi: 10.1109/17.985745. [DOI] [Google Scholar]
- 14.Salantur A, Ozturk A, Akten S, Sahin F, Donmez F. Effect of inoculation with non-indigenous and indigenous rhizobacteria of Erzurum (Turkey) origin on growth and yield of spring barley. Plant Soil. 2005;275:147–156. doi: 10.1007/s11104-005-8094-z. [DOI] [Google Scholar]
- 15.El-Ghamriny, E., Arisha, H. & Nour, K. Studies on tomato flowering, fruit set, yield and quality in summer season 1-spraying with Thiamine, ascorbic acid and yeast. Zagazig J. Agric. Res. Egypt. (1999).
- 16.Wanas A. Resonance of faba bean (Vicia faba L.) plants to seed soaking application with natural yeast and carrot extracts. Ann. Agric. Sci. Moshtohor. 2002;40:259–278. [Google Scholar]
- 17.Glick BR. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 18.Barnett, J. A., Payne, R. W. & Yarrow, D. Yeasts: characteristics and identification. (1990).
- 19.Sarhan TZ, Mohammed GH, Teli JA. Effect of bio and organic fertilizers on growth, yield and fruit quality of summer squash. Sarhad J. Agric. 2011;27:377–383. [Google Scholar]
- 20.Feng X, An Y, Gao J, Wang L. Photosynthetic responses of canola to exogenous application or endogenous overproduction of 5-aminolevulinic acid (ALA) under various nitrogen levels. Plants. 2020;9:1419. doi: 10.3390/plants9111419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, et al. Low-nitrogen tolerance comprehensive evaluation and physiological response to nitrogen stress in broomcorn millet (Panicum miliaceum L.) seedling. Plant Physiol. Biochem. 2020;151:233–242. doi: 10.1016/j.plaphy.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 22.Vouillot MO, Huet P, Boissard P. Early detection of N deficiency in a wheat crop using physiological and radiometric methods. Agronomie. 1998;18:117–130. doi: 10.1051/agro:19980202. [DOI] [Google Scholar]
- 23.Cruz J, Mosquim P, Pelacani C, Araújo W, DaMatta F. Photosynthesis impairment in cassava leaves in response to nitrogen deficiency. Plant Soil. 2003;257:417–423. doi: 10.1023/A:1027353305250. [DOI] [Google Scholar]
- 24.Terashima I, Hikosaka K. Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ. 1995;18:1111–1128. doi: 10.1111/j.1365-3040.1995.tb00623.x. [DOI] [Google Scholar]
- 25.Evans JR. Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol. 1999;143:93–104. doi: 10.1046/j.1469-8137.1999.00440.x. [DOI] [Google Scholar]
- 26.Hikosaka K, Terashima I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995;18:605–618. doi: 10.1111/j.1365-3040.1995.tb00562.x. [DOI] [Google Scholar]
- 27.Terashima I, Evans JR. Effects of light and nitrogen nutrition on the organization of the photosynthetic apparatus in spinach. Plant Cell Physiol. 1988;29:143–155. doi: 10.1111/j.1365-3040.2005.01453.x. [DOI] [PubMed] [Google Scholar]
- 28.Thompson W, Huang L, Kriedemann P. Photosynthetic response to light and nutrients in sun-tolerant and shade-tolerant rainforest trees. II. Leaf gas exchange and component processes of photosynthesis. Funct. Plant Biol. 1992;19:19–42. doi: 10.1071/PP9920019. [DOI] [Google Scholar]
- 29.Kitajima K, Hogan KP. Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light. Plant Cell Environ. 2003;26:857–865. doi: 10.1046/j.1365-3040.2003.01017.x. [DOI] [PubMed] [Google Scholar]
- 30.Bungard R, Scholes J, Press M. The influence of nitrogen on rain forest dipterocarp seedlings exposed to a large increase in irradiance. Plant Cell Environ. 2000;23:1183–1194. doi: 10.1046/j.1365-3040.2000.00642.x. [DOI] [Google Scholar]
- 31.Abd El-Motaleb H, Gomaa A. Yield response of two canola varieties to nitrogen and biofertilizers under sandy soil conditions. Agric. Res. J. Suez Canal Univ. 2004;4:1–8. [Google Scholar]
- 32.Takahashi CA, Mercier H. Nitrogen metabolism in leaves of a tank epiphytic bromeliad: characterization of a spatial and functional division. J. Plant Physiol. 2011;168:1208–1216. doi: 10.1016/j.jplph.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 33.Tawfiq AA. Estimation levels of Indol acetic acid (IAA) and Gibberellic acid (GA3) from dry bakery yeast Saccharomyces cereviciae. J. Biotechnol. Res. Center. 2010;4:94–100. doi: 10.24126/jobrc.2010.4.2.133. [DOI] [Google Scholar]
- 34.Wanas A. trials for improving growth and productivity of tomato (Lycopersicon esculentum, Mill.) plants grown in winter season. J. Plant Prod. 2007;32:991–1009. [Google Scholar]
- 35.Mahmoued, T. Botanical studies on the growth and germination of mahnolia (Magnolia grandiflora L.) plants. M. Sci, Thesis. Fac. of Agric. Moshtohor, Zagazig University (2001).
- 36.Hocking P, Stapper M. Effects of sowing time and nitrogen fertiliser on canola and wheat, and nitrogen fertiliser on Indian mustard. I. Dry matter production, grain yield, and yield components. Aust. J. Agric. Res. 2001;52:623–634. doi: 10.1071/AR00113. [DOI] [Google Scholar]
- 37.Ahmad G, Jan A, Arif M, Jan M, Khattak R. Influence of nitrogen and sulfur fertilization on quality of canola (Brassica napus L.) under rainfed conditions. J. Zhejiang Univ. Sci. B. 2007;8:731–737. doi: 10.1631/jzus.2007.B0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma D, Pathak D, Atwal A, Sangha M. Genetic variation for some chemical and biochemical characteristics in cotton seed oil. J. Cotton Res. Dev. 2009;23:1–7. [Google Scholar]
- 39.Cornelius J. Some technical aspects influencing the quality of palm kernels. J. Sci. Food Agric. 1966;17:57–61. doi: 10.1002/jsfa.2740170201. [DOI] [Google Scholar]
- 40.Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006;5:169–186. doi: 10.1111/j.1541-4337.2006.00009.x. [DOI] [Google Scholar]
- 41.Gunstone, F. D. Rapeseed and Canola Oil: Production, Processing, Properties and Uses (CRC Press, 2004).
- 42.El Kholy, M., El-Zeky, M., Saleh, S. Z. & Metwaly, S. In Proc. Proceedings of the 12th International Rapeseed Congress (2007). 26–30.
- 43.El Habbasha, E. S. F. & El Salam, M. A. Response of two canola varieties (Brassica napus L.) to nitrogen fertilizer levels and zinc foliar application. (2009).
- 44.Mekki, B. In Proceedings 11th International Rapeseed Congress, Copenhagen. 915–917.
- 45.Mekki B. Yield and quality traits of some canola varieties grown in newly reclaimed sandy soils in Egypt. World Appl. Sci. J. 2013;25:258–263. [Google Scholar]
- 46.Rahman M. Fatty acid composition of resynthesized Brassica napus and trigenomic Brassica void of genes for erucic acid in their A genomes. Plant Breed. 2002;121:357–359. doi: 10.1046/j.1439-0523.2002.00711.x. [DOI] [Google Scholar]
- 47.El-Beltagi HE-DS, Mohamed AA. Variations in fatty acid composition, glucosinolate profile and some phytochemical contents in selected oil seed rape (Brassica napus L.) cultivars. Grasas Aceites. 2010;61:143–150. doi: 10.3989/gya.087009. [DOI] [Google Scholar]
- 48.Khan S, et al. Optimization of nitrogen rate and planting density for improving yield, nitrogen use efficiency, and lodging resistance in oilseed rape. Front. Plant Sci. 2017;8:532. doi: 10.3389/fpls.2017.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirel B, Tétu T, Lea PJ, Dubois F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability. 2011;3:1452–1485. doi: 10.3390/su3091452. [DOI] [Google Scholar]
- 50.Laufer D, Nielsen O, Wilting P, Koch H-J, Märländer B. Yield and nitrogen use efficiency of fodder and sugar beet (Beta vulgaris L.) in contrasting environments of northwestern Europe. Eur. J. Agron. 2016;73:124–132. doi: 10.1016/j.eja.2015.11.008. [DOI] [Google Scholar]
- 51.Chapman HD, Pratt PF. Methods of analysis for soils, plants and waters. Soil Sci. 1962;93:68. doi: 10.1097/00010694-196201000-00015. [DOI] [Google Scholar]
- 52.Spencer, J. F., Spencer, D. M. & Smith, A. Yeast Genetics: Fundamental and Applied Aspects. (Springer Science & Business Media, 2012).
- 53.Hiscox J, Israelstam G. A method for the extraction of chlorophyll from leaf tissue without maceration. Can. J. Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- 54.Dolatabadian A, Sanavy SM, Chashmi N. The effects of foliar application of ascorbic acid (vitamin C) on antioxidant enzymes activities, lipid peroxidation and proline accumulation of canola (Brassica napus L.) under conditions of salt stress. J. Agron. Crop Sci. 2008;194:206–213. doi: 10.1111/j.1439-037X.2008.00301.x. [DOI] [Google Scholar]
- 55.Stahl WR. Scaling of respiratory variables in mammals. J. Appl. Physiol. 1967;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- 56.Nelson J, Milun A, Fisher H. Gas chromatographic determination of tocopherols and sterols in soya sludges and residues—An improved method. J. Am. Oil. Chem. Soc. 1970;47:259–261. doi: 10.1007/BF02609487. [DOI] [PubMed] [Google Scholar]
- 57.Timsina J, Singh U, Badaruddin M, Meisner C, Amin M. Cultivar, nitrogen, and water effects on productivity, and nitrogen-use efficiency and balance for rice–wheat sequences of Bangladesh. Field Crop Res. 2001;72:143–161. doi: 10.1016/S0378-4290(01)00171-X. [DOI] [Google Scholar]
- 58.Fageria, N. & Barbosa Filho, M. Screening rice cultivars for higher efficiency of phosphorus absorption [Oryza sativa]. Pesquisa Agropecuaria Brasileira (1981).
- 59.Snedecor, G. & Cochran, W. Statistical Methods, 6th ed. 507 (Iowa State Univ Press, 1990).
- 60.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. doi: 10.2307/3001478. [DOI] [Google Scholar]
- 61.Afiah S, Ghoneim E. Correlation, stepwise and path coefficient analysis in Egyptian cotton under saline conditions. Arab. Univ. J. Agric. Sci. 2000;8:607–618. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data of the current work are included in the submitted article. For further data, please contact the corresponding author (M.E.) via mohamedebaid979@gmail.com.