Abstract
Staphylococcus aureus nasal carriage is a risk factor for infection in humans, particularly in the hospital environment. Attenuation of carriage has proven effective in reducing the prevalence of infection in some high-risk groups. To study staphylococcal factors that influence nasal colonization, a mouse model of S. aureus nasal colonization was developed. Mice were inoculated intranasally with S. aureus Reynolds, and nasal carriage was evaluated by quantitating cultures of the nasal tissues from mice sacrificed at various time points after inoculation. The majority of mice inoculated with 108 CFU of S. aureus maintained nasal carriage for at least 20 days. Nasal colonization rates were similar for inbred (BALB/c and C57BL/6) and outbred (ICR) mice. Colonization was not affected by mouse passage of strain Reynolds. Lower inoculum doses (<107 CFU) resulted in reduced colonization after 7 days. However, mice given streptomycin in their drinking water developed long-term carriage of S. aureus, and they were colonized with inocula as low as 105 CFU. Nasal colonization was also established with two other S. aureus strains (one strain each of human and murine origins). S. aureus recovered from the nares of experimentally colonized mice expressed high levels of capsule, and the ability of a capsule-defective mutant to persist in the nares was reduced in comparison to that of the parent strain. This nasal colonization model should prove useful for studies of factors that mediate S. aureus colonization and for assessment of targets for antimicrobial intervention or vaccine development.
The anterior nares are the major reservoir in humans of the opportunistic pathogen Staphylococcus aureus. Approximately 20% of humans are persistently colonized intranasally by a single strain of S. aureus. Another 60% of individuals are intermittent nasal carriers of S. aureus strains that change with varying frequency. The remaining 20% are classified as persistent noncarriers (16). Although S. aureus colonization of the nares is asymptomatic, nasal carriage is a risk factor for subsequent infection, particularly in surgical patients, intensive-care-unit patients, patients with intravascular devices, and human immunodeficiency virus-positive individuals (16). Higher colonization rates have been observed in hospitals (among both patients and health care workers) and among intravenous drug users, insulin-dependent diabetics, human immunodeficiency virus-positive or AIDS patients, patients with S. aureus skin infections, and patients undergoing continuous ambulatory peritoneal dialysis or hemodialysis (16). Since most staphylococcal infections are of endogenous origin, measures to prevent S. aureus nasal carriage may lower the prevalence of infection in these high-risk groups.
The topical application of the antibiotic mupirocin has proven effective in attenuating staphylococcal colonization of the human nares (6, 8, 12). Furthermore, mupirocin treatment of patients undergoing hemodialysis, continuous ambulatory peritoneal dialysis, or surgery has resulted in a significant decrease in the rate of staphylococcal infections (5, 17, 22). However, mupirocin-resistant strains of S. aureus have recently been reported (9). Likewise, nosocomial epidemics remain of great concern because of the emergence of strains of S. aureus resistant to multiple antibiotics, including methicillin-resistant and vancomycin-tolerant strains. Alternative techniques for the elimination of S. aureus nasal carriage are necessary to circumvent the problems created by the use and misuse of antibiotics.
Animal models of nasopharyngeal colonization by microbial pathogens, including Streptococcus pneumoniae (20, 33, 34) and Haemophilus influenzae (15, 35), have been developed for the study of bacterial colonization factors and protective mucosal immunity. Although numerous animal models of staphylococcal infection have contributed to the knowledge of virulence factors involved in disease, only a few reported studies have examined the interactions of S. aureus with the nasal mucosa in an animal model (23, 24, 27). In this paper, we describe a new mouse model for studying S. aureus nasal colonization. Future studies will employ this model to identify staphylococcal components that are critical for colonization or that can elicit protective immunity against carriage.
MATERIALS AND METHODS
Animals.
Male ICR, BALB/c, and C57BL/6 mice were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, Ind.). The mice were 6 to 8 weeks of age upon arrival and were given food and water ad libitum. The animals were housed with three to eight animals per cage in a modified barrier facility under viral-antibody-free conditions. Animal care was in accordance with the institutional guidelines set forth by Brigham and Women’s Hospital and Harvard Medical School.
S. aureus strains and growth conditions.
S. aureus Reynolds and Newman are human isolates. Strain DAK was recovered from an outbreak of murine mastitis in the Animal Resources Center at Harvard Medical School in Boston. All three isolates produce a type 5 capsule (CP5). Strain JL236 is a capsule-deficient mutant of strain Reynolds created by transposon mutagenesis (1). S. aureus strains were marked with resistance to streptomycin by selection of bacteria on tryptic soy agar (TSA) plates containing streptomycin sulfate (Sigma, St. Louis, Mo.) at 500 μg/ml. Bacteria were grown at 37°C for 24 h on Columbia medium (Difco Laboratories, Detroit, Mich.) containing 2% NaCl and 1.5% Bacto-Agar (Difco). Cells were harvested from the solid medium with a sterile loop and suspended in saline (150 mM NaCl). The total number of CFU in each inoculum was quantitated by plating duplicate serial dilutions of the inoculum on TSA. To prepare the inoculum containing mouse-passaged S. aureus, an aliquot of the pooled nasal-tissue suspension from five experimentally colonized mice (5 days after inoculation) was plated on Columbia agar with 2% NaCl and streptomycin (500 μg/ml). Bacteria were processed for inoculation as described above.
Nasal colonization model.
Mice were anesthetized by intraperitoneal injection of pentobarbital (Nembutal Sodium Solution; Abbott Laboratories, North Chicago, Ill.) at 62.5 mg/kg of body weight. The inoculum, which contained a dose of S. aureus ranging from 104 to 108 CFU in 10 μl of saline, was pipetted slowly onto the nares of the anesthetized mice without actually touching the pipette tip to the nose. Two to 27 days after inoculation, the animals were euthanized with CO2 and evaluated for nasal carriage of S. aureus. The nasal region was wiped externally with 70% ethanol, and the nasal tissue was excised and dissected with sterile scissors. The sample was then vortexed vigorously in 200 μl of saline for 15 s. The total nasal flora was evaluated by plating a 50-μl aliquot of the nasal suspension on TSA with 5% sheep blood, whereas the total number of S. aureus CFU per nose was assessed by plating aliquots of neat or diluted nasal suspensions on TSA with streptomycin (500 μg/ml). The carriage rate was defined as the number of animals with ≥1 CFU/nose divided by the total number of mice per group. The lungs from selected groups of animals (removed 5 to 27 days after inoculation) were excised, washed with saline, and homogenized in 0.5 ml of tryptic soy broth. Homogenized lung tissue was plated in triplicate on TSA with 5% sheep blood. Heparinized blood samples were obtained by tail vein puncture on day 5 or 13, and duplicate 100-μl aliquots were plated on TSA with 5% sheep blood.
Statistics.
S. aureus carriage rates of animals in each experimental group were evaluated statistically by Fisher’s exact test (two tailed). The Kruskal-Wallis test was used for comparison of quantitative cultures from different experimental groups. Pairwise comparisons were performed by Dunn’s multiple-comparisons procedure. The dose required to achieve nasal colonization in 50% of animals (CD50) at days 7 and 14 was determined by logistic regression (31).
Quantitation of CP5 produced by S. aureus nasal isolates.
S. aureus Reynolds was cultivated in vitro at 37°C for 24 h on solid or in liquid Columbia medium containing 2% NaCl. The inoculum containing bacteria cultivated on solid medium was prepared as described above. The inoculum grown in liquid medium was prepared by harvesting the cells by centrifugation, washing them once with saline, and then resuspending them in saline. Two groups of mice were inoculated intranasally (i.n.) with 108 CFU of S. aureus grown on solid or in liquid medium. After 5 days, nasal suspensions were prepared from individual animals, the nasal tissue was removed from each suspension, the samples were pooled, and bacteria were recovered from pooled samples by centrifugation. Cell-associated CP5 present on in vitro-cultivated and in vivo-isolated bacteria was measured by an enzyme-linked immunosorbent inhibition assay (1, 18).
RESULTS
Establishment of S. aureus nasal colonization in ICR mice.
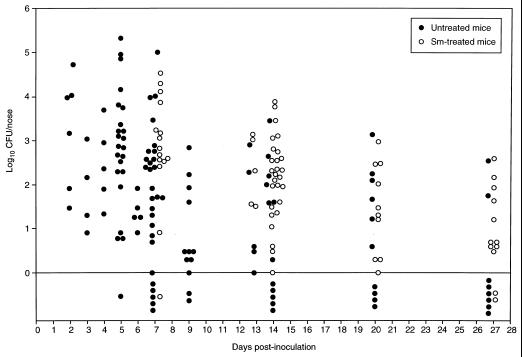
One hundred eighteen mice were inoculated i.n. with approximately 108 CFU of S. aureus Reynolds marked with resistance to streptomycin. At 2 to 27 days after inoculation, groups of 5 to 10 mice were sacrificed and the nasal tissue from each animal was cultured quantitatively. All of the animals survived until the day of sacrifice with no noticeable complications. At the time of sacrifice, 95 (81%) of the 118 mice were nasally colonized with ≥1 CFU of S. aureus; 80 (91%) of 88 mice maintained colonization between 2 and 13 days, and 15 (50%) of 30 mice remained colonized between 14 and 27 days (Fig. 1).
FIG. 1.
Nasal colonization by S. aureus Reynolds. ICR mice, given plain water or water containing streptomycin (Sm) at 1 mg/ml, were inoculated i.n. with 108 CFU of S. aureus Reynolds. The nares were cultured for S. aureus at intervals from 2 to 27 days after inoculation. Each circle represents the number of CFU recovered from the nares of an individual mouse at the time of sacrifice. The circles below the line (log10 CFU/nose = 0) represent results with animals not colonized with S. aureus (i.e., those with <1 CFU/nose).
To determine whether S. aureus had spread beyond the nasal passages, lung tissue was removed from 53 mice 5 to 27 days after inoculation. Quantitative cultures revealed that 4 of the 53 lung samples contained S. aureus; however, the total number of bacteria recovered from each of the four samples was minimal (1, 1, 3, and 6 CFU per lung sample). S. aureus was not detected in samples of blood taken from 22 mice 5 days after inoculation.
To assess whether S. aureus could be transmitted among animals, one uninoculated mouse was housed in each of eight cages with three to five mice that had been inoculated with 108 CFU of strain Reynolds. Five days later, S. aureus was recovered from six of the eight uninoculated mice (1 to 6 CFU/nose). Thus, carriage could be transferred among mice, albeit at a very low level.
The nasal flora of untreated ICR mice consisted primarily of α-hemolytic and nonhemolytic streptococci and coagulase-negative staphylococci. Occasionally, gram-negative bacteria (Escherichia coli, Stenotrophomonas spp., and Kluyvera spp.) were isolated from the nares of untreated animals. Rarely, an endogenous S. aureus strain was recovered from the nares of the ICR mice. We were always able to distinguish the endogenous staphylococcal strain from our challenge strain by its susceptibility to streptomycin, capsule type, colony morphology, and hemolysis on sheep blood agar plates. The nasal flora of mice inoculated with S. aureus did not differ from that of naive animals. Alpha-hemolytic streptococci were eradicated from the nares of nearly all streptomycin-treated mice, and, in general, the total nasal flora was reduced in these animals compared with that in untreated animals. For example, 75% of 55 untreated mice had >100 CFU of non-S. aureus, normal flora organisms recovered from the nose. In contrast, only 28% of 43 mice given streptomycin in their drinking water had >100 CFU of normal flora bacteria isolated from the nose.
To determine whether S. aureus nasal colonization was enhanced by reducing bacterial interference, a group of ICR mice was given streptomycin (1 mg/ml) in drinking water from 24 h before inoculation with 108 CFU of strain Reynolds until 24 h after inoculation. Control animals were given untreated drinking water. Groups of five or six animals were sacrificed daily at 2 to 7 days, and their nasal floras were evaluated. The majority of animals in both groups were colonized with S. aureus, but numbers of staphylococci recovered from the nares did not increase significantly when mice were given short-term treatment with streptomycin (data not shown).
In a subsequent study, mice were given streptomycin (1 mg/ml) in drinking water from 24 h before inoculation through the end of the experiment. Control animals were given untreated drinking water. Both groups were inoculated i.n. with 108 CFU of strain Reynolds. Over a 27-day time course, 66 (96%) of 69 mice given streptomycin were colonized with S. aureus, compared with 43 (68%) of 63 untreated mice (Fig. 1). A greater percentage of streptomycin-treated than of untreated mice were colonized on days 14 (P = 0.0014) and 27 (P = 0.019). The number of CFU recovered from the nares was greater for streptomycin-treated mice than for untreated mice on days 7 (P = 0.0111) and 14 (P = 0.0168). S. aureus was not recovered on days 13, 20, and 27 from the lungs (n = 26) or on day 13 from the blood (n = 5) of any streptomycin-treated mouse.
Effect of S. aureus mouse passage on nasal colonization.
ICR mice were inoculated i.n. with 3 × 108 CFU of strain Reynolds that had been cultivated in vitro or cultivated from the nares of experimentally colonized mice on day 5. Nasal carriage was assessed from groups of 5 to 15 mice 5 to 14 days after inoculation. At each time point, mice inoculated with mouse-passaged S. aureus had nasal carriage rates and total numbers of CFU of S. aureus in the nares similar to those of mice inoculated with staphylococci cultivated in vitro (data not shown).
Nasal colonization of inbred BALB/c and C57BL/6 mice.
Levels of experimental nasal colonization of inbred mouse strains BALB/c and C57BL/6 were compared with that established for the outbred mouse strain ICR. The mice were inoculated i.n. with approximately 108 CFU of mouse-passaged S. aureus Reynolds, and nasal carriage was assessed in groups of six to eight mice 3 to 14 days after inoculation. Results were compared with those for ICR mice colonized with mouse-passaged S. aureus. As shown in Fig. 2, the carriage rates for the different groups of mice were similar at each time point. However, 5 days after inoculation, the number of CFU recovered from the nares was greater for ICR mice than for the two inbred strains (P = 0.0035). All subsequent experiments were therefore performed with ICR outbred mice.
FIG. 2.
Nasal colonization of outbred (ICR) and inbred (BALB/c or C57BL/6) mouse strains with S. aureus Reynolds. Mice were inoculated i.n. with approximately 108 CFU of mouse-passaged S. aureus Reynolds, and the nares were cultured for S. aureus 3 to 14 days after inoculation. Each symbol represents the number of CFU recovered from the nares of an individual mouse at the time of sacrifice. The circles below the line (log10 CFU/nose = 0) represent results with animals not colonized with S. aureus (i.e., those with <1 CFU/nose).
Effect of bacterial dose on nasal colonization.
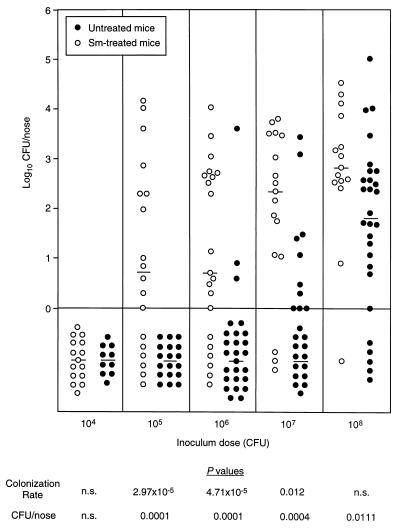
The effect of inoculum size was evaluated with untreated mice and mice given streptomycin in their drinking water. Groups of animals were inoculated i.n. with S. aureus inocula ranging from 104 to 108 CFU/mouse. Nasal carriage was quantitated 7 days later. At a dose of 108 CFU of strain Reynolds, similar colonization rates were achieved with streptomycin-treated and untreated mice (Fig. 3). Untreated mice inoculated with 108 CFU of two other S. aureus isolates, Newman (human) and DAK (murine), also had carriage rates equivalent to those obtained with strain Reynolds (data not shown).
FIG. 3.
Nasal colonization of ICR mice with S. aureus Reynolds at various inoculum doses. Animals, given plain water or water containing streptomycin (Sm) at 1 mg/ml, were inoculated i.n. with doses ranging from 104 to 108 CFU of S. aureus. Each circle represents the number of CFU recovered from the nares of an individual mouse 7 days after inoculation. The median log10 CFU/nose value for each group of animals is represented with a horizontal line. The circles below the line (log10 CFU/nose = 0) represent results with animals not colonized with S. aureus (i.e., those with <1 CFU/nose). The P values derived from the statistical comparison of colonization rates and numbers of CFU recovered from the nares of untreated or streptomycin-treated mice are shown below the graph. A P of <0.05 was considered significant. n.s., not significant.
At inocula ranging from 105 to 107 CFU/mouse, streptomycin-treated mice were colonized at a significantly higher rate than were untreated mice on day 7 (Fig. 3). Likewise, the number of CFU recovered from the nares of mice given streptomycin was greater than that recovered from the nares of untreated animals (Fig. 3). Nasal colonization was not established in either group of mice inoculated with 104 CFU of strain Reynolds. A comparison of the CD50s on day 7 revealed that the CD50 for streptomycin-treated mice (1.94 × 105 CFU) was 88-fold lower (P = 0.0001) than that for untreated mice (1.64 × 107 CFU).
To determine whether levels of nasal colonization by strains Newman and DAK were similar to that by strain Reynolds, streptomycin-treated ICR mice were inoculated i.n. with 105 to 108 CFU of each staphylococcal strain and colonization was evaluated 7 and 14 days later. Strains Newman and DAK were as effective in colonizing the nares of mice as strain Reynolds (Table 1). The CD50s on day 14 for the three S. aureus strains (Reynolds, 5.06 × 104 CFU; Newman, 2.49 × 104 CFU; and DAK, 6.43 × 103 CFU) were not significantly different.
TABLE 1.
Nasal colonization of streptomycin-treated ICR mice by the S. aureus strains Reynolds, JL236, Newman, and DAK
| Day | Dose (CFU) | Reynolds
|
JL236
|
Newman
|
DAK
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Carriage rate (%) | Median log10 CFU/nose (range) | Carriage rate (%) | Median log10 CFU/nose (range) | Carriage rate (%) | Median log10 CFU/nose (range) | Carriage rate (%) | Median log10 CFU/nose (range) | ||
| 7 | 108 | 14/15 (93) | 2.83 (<0–4.53) | NDa | ND | 5/5 (100) | 3.90 (3.56–4.26) | 5/5 (100) | 3.70 (3.29–4.19) |
| 107 | 14/17 (82) | 2.35 (<0–3.79) | 9/10 (90) | 2.52 (<0–5.28) | 5/5 (100) | 2.99 (2.86–3.75) | 5/5 (100) | 3.35 (2.87–3.96) | |
| 106 | 15/21 (71) | 0.70 (<0–4.01) | 7/10 (70) | 1.69 (<0–3.26) | 5/5 (100) | 3.34 (1.48–4.59) | 5/5 (100) | 4.14 (3.99–4.87) | |
| 105 | 12/18 (67) | 0.74 (<0–4.17) | 5/11 (45) | <0 (<0–2.57) | 2/6 (33) | <0 (<0–2.44) | 5/5 (100) | 3.32 (1.56–3.45) | |
| 104 | 0/15 (0) | <0 (<0) | ND | ND | ND | ND | ND | ND | |
| 14 | 108 | 27/27 (100) | 2.24 (0–3.94) | 5/7 (71) | 0.78 (<0–3.10) | 11/12 (92) | 2.41 (<0–4.53) | 5/5 (100) | 2.57 (1.72–4.25) |
| 107 | 16/20 (80) | 1.18 (<0–3.97) | 8/12 (67) | 0.98 (<0–3.00) | 16/20 (80) | 1.24 (<0–5.35) | 5/5 (100) | 2.57 (0.90–2.78) | |
| 106 | 13/18 (72) | 0.70 (<0–3.64) | 7/16 (44) | <0 (<0–3.35) | 18/20 (90) | 1.16 (<0–4.60) | 4/5 (80) | 0.30 (<0–1.93) | |
| 105 | 8/14 (57) | 0.54 (<0–4.34) | 4/20 (20) | <0 (<0–1.75) | 7/11 (64) | 0.48 (<0–3.46) | 2/3 (67) | 0.48 (<0–0.60) | |
ND, not determined.
Influence of CP5 expression on nasal colonization.
S. aureus capsule expression is highly influenced by environmental growth conditions (11, 13, 18, 28, 29). Although we have shown that CP8 is expressed in vivo in a rat endocarditis model (18), Herbert et al. reported that CP5 was not expressed in a rat granuloma pouch model (13). To determine whether CP5 was expressed during nasal colonization by S. aureus, two groups of mice were inoculated i.n. with strain Reynolds cultivated under conditions of high CP5 expression (solid Columbia salt medium) or low CP5 expression (liquid Columbia salt medium). After 5 days, nasal samples were obtained from individual animals and pooled in order to obtain a sufficient bacterial biomass for quantitation of CP5 expressed by S. aureus recovered (without subculture) from mouse nares.
S. aureus cultivated in Columbia salt broth produced little capsule (175 ± 99 μg of CP5/1010 CFU). These bacteria were used to inoculate the nares of untreated ICR mice, and 5 days later staphylococci were recovered from the noses of 17 of 23 mice. Nasal samples from mice carrying S. aureus were pooled and processed by the enzyme-linked immunosorbent inhibition assay, yielding 1,122 μg of CP5/1010 CFU. Strain Reynolds cultivated in vitro on Columbia salt agar produced ∼20-fold more CP5 (3,438 ± 1,167 μg of CP5/1010 CFU) than did cells cultivated in liquid medium. These bacteria were used to inoculate a separate group of 20 mice. S. aureus was recovered on day 5 from the nares of 19 of the 20 animals, and the pooled samples yielded 684 μg of CP5/1010 CFU. Although the rate of colonization was not dependent on the level of CP5 produced by the organisms in the inoculum, these experiments do confirm the expression of CP5 by S. aureus in vivo.
The role of CP5 expression during nasal colonization was assessed with the use of a capsule-deficient mutant of strain Reynolds. Strain JL236 produces CP5 at ∼9% of the parental level (30). As shown in Table 1, at 7 days after inoculation no significant differences in rates of nasal carriage were observed between streptomycin-treated mice inoculated with strain Reynolds (CD50 = 1.94 × 105 CFU) and those inoculated with strain JL236 (CD50 = 1.90 × 105 CFU). However, colonization by the capsule-deficient mutant was decreased on day 14 compared to that of the parent strain, most notably for the lowest inoculum dose (105 CFU), at which the differences in carriage rates (P = 0.0356) and CFU recovered from the nose (P = 0.0363) were significant. Furthermore, the CD50 on day 14 for strain Reynolds (5.06 × 104 CFU) was 55-fold lower (P = 0.0007) than for strain JL236 (2.81 × 106 CFU).
DISCUSSION
Many studies have addressed the role of S. aureus virulence determinants in the pathogenesis of infections. In contrast, very little is known about microbial factors that influence staphylococcal colonization. The nose is regarded as the primary site of S. aureus carriage, the source from which bacteria spread to other parts of the body. Nasal carriage of this endogenous pathogen can lead to skin colonization, which in turn poses a risk of infection if the skin is traumatized (e.g., by wounds, surgical procedures, or catheter insertion). Moreover, colonization of health care workers may contribute to the transmission of staphylococci to patients. Colonization is a multifactorial process that requires a variety of adaptive mechanisms, including nutrient acquisition, adherence to host tissues, and evasion of, or protection against, host defenses. Identifying bacterial factors that influence nasal colonization is best accomplished with an intact living animal.
We have developed a model for S. aureus nasal colonization in mice, establishing stable nasal carriage with three S. aureus strains, over a range of bacterial inocula, in inbred or outbred mouse strains over periods of 2 to 27 days. Although colonization persisted for 2 weeks in untreated animals, mice given streptomycin in their drinking water had enhanced long-term colonization (persisting for at least 27 days). Furthermore, treatment with streptomycin lowered the inoculum dose necessary to achieve persistent colonization. These observations suggest that suppression of the indigenous nasal flora results in reduced bacterial interference and creates an environment amenable to S. aureus colonization. The natural flora may inhibit experimental S. aureus nasal colonization by competing for binding sites or available nutrients. Alternatively, secreted products elaborated by members of the indigenous flora may inhibit staphylococcal growth. In untreated mice, a large inoculum dose (108 CFU) of S. aureus was capable of overcoming this apparent interference. Streptomycin treatment may be critical in comparisons of the abilities of different strains or mutants to colonize the nares, since a large inoculum may overcome the effects of a phenotypic difference or mutation in staphylococci. We considered that human S. aureus isolates (Reynolds and Newman) might not colonize the nares of mice as readily as a staphylococcal strain originally isolated from mice (DAK). However, all three strains had similar patterns of nasal colonization. Although inbred mice offer the advantage of genetic relatedness, we chose to develop the nasal colonization model in outbred animals since their makeup is more representative of the variability observed within the human population.
Our results suggest that the presence of S. aureus in the nares of inoculated mice is a result of stable colonization rather than temporary contamination. The persistence of nasal carriage of S. aureus and the comparable abilities of lower and higher inoculum doses to result in colonization support this conclusion. The paucity of S. aureus recovered from the lungs and blood of mice inoculated i.n. indicates that nasal carriage was not dependent upon or associated with infection at another site. These results are consistent with the fact that S. aureus causes infection in competent hosts only when mucosal or skin barriers are breached. Because sequential culturing (swabbing) of the nares of a single animal might traumatize the nasal mucosa and result in a wound infection rather than colonization, we thought it necessary to culture the nasal tissue of individual animals at a single time point. Nasal carriage of H. influenzae (15) and Streptococcus pneumoniae (20) had previously been assessed by sequential cultures of nasal lavage fluid from individual animals. However, this method may adversely affect colonization by reducing bacterial numbers or altering environmental conditions of the nasal mucosa.
Our model of S. aureus nasal colonization should be useful for identifying bacterial factors that influence carriage and for assessing targets for protective immunity. Animal models have been developed for studying nasopharyngeal colonization by encapsulated bacterial pathogens such as H. influenzae and Streptococcus pneumoniae. Although the role of capsular polysaccharides in nasopharyngeal carriage is unknown, capsular antibodies have been shown to reduce nasopharyngeal colonization by H. influenzae type b in infant rats (15) and to protect against transmission of Streptococcus pneumoniae from colonized infant rats to their littermates (20). In addition, intranasal immunization of adult mice with a capsular polysaccharide 6B-tetanus toxoid conjugate reduced nasopharyngeal carriage of Streptococcus pneumoniae (33). The S. aureus capsule is antiphagocytic and enhances virulence in certain animal models of infection (14, 21, 30). In the present study the levels of CP5 produced by S. aureus recovered from the nares of experimentally colonized mice were similar to the high levels of CP5 produced by staphylococci cultivated in vitro on solid medium. Likewise, high levels of CP8 were expressed by S. aureus in endocardial vegetations recovered from a rat model of endocarditis (18). Furthermore, a capsule-deficient mutant of S. aureus did not colonize the nares as effectively as the parent strain. Capsule production may influence nasal colonization by circumventing immunoglobulin A-mediated clearance from the nasal mucosa and by protecting the bacterium from desiccation in the nasal vestibule.
Most experiments addressing mechanisms of S. aureus adherence have used cultured mammalian cells or microtiter plates coated with purified host matrix proteins. Although this work has revealed a large array of staphylococcal adhesins, the role that these adhesins play in colonization can be determined only in humans or in an animal model of S. aureus carriage. Shuter et al. (26) examined the in vitro interactions between S. aureus and desquamated human nasal epithelial cells by light microscopy. They observed avid binding of the staphylococci to both cell-bound and cell-free mucus. When S. aureus was added to purified human nasal mucin bound to microtiter plates, they found that the adhesin-receptor interaction involved bacterial proteins and the carbohydrate moiety in mucin. The premise that S. aureus adherence to nasal mucin may be more important than its interaction with epithelial cells was corroborated by the results of Sanford et al. (23, 24). These investigators challenged ferrets i.n. with S. aureus and then sacrificed the animals after 60 to 90 min to examine the early interaction between the microbe and host. Most of the staphylococci were associated with the mucous gel overlaying the mucosa. Moreover, in vitro binding to nasal mucin was inhibited by pretreatment of the staphylococci with trypsin (24). Although these data support the premise that S. aureus adherence to mucin is protein mediated, other reports have indicated that lipoteichoic acid or cell wall teichoic acid may be involved in S. aureus adherence to nasal epithelial cells in vitro (2–4, 7, 32). Our model might be used to observe the possible inhibitory impact of mutations or antibodies directed against such putative adhesins on the establishment of nasal colonization. It might also serve as a model to identify genes expressed during or essential to the colonization process by methods such as in vivo expression technology or signature-tagged mutagenesis, both of which have proven to be successful in identifying virulence factors expressed during experimental S. aureus infection of animals (10, 19, 25).
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI29040 (to J. C. Lee), T32-AI07061 (to D. L. Kasper), and F32-AI09981 (to K. B. Kiser) from the National Institute of Allergy and Infectious Diseases.
We thank Derek Frederickson for his technical assistance and Andrew Onderdonk for identifying gram-negative species isolated from the nares of mice.
REFERENCES
- 1.Albus A, Arbeit R D, Lee J C. Virulence of Staphylococcus aureus mutants altered in type 5 capsule production. Infect Immun. 1991;59:1008–1014. doi: 10.1128/iai.59.3.1008-1014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aly R, Levit S. Adherence of Staphylococcus aureus to squamous epithelium: role of fibronectin and teichoic acid. Rev Infect Dis. 1987;9:S341–S350. doi: 10.1093/clinids/9.supplement_4.s341. [DOI] [PubMed] [Google Scholar]
- 3.Aly R, Shinefield H R, Litz C, Maibach H I. Role of teichoic acid in the binding of Staphylococcus aureus to nasal epithelial cells. J Infect Dis. 1980;141:463–465. doi: 10.1093/infdis/141.4.463. [DOI] [PubMed] [Google Scholar]
- 4.Aly R, Shinefield H R, Maibach H I. Adherence of Staphylococcus aureus to infant nasal mucosal cells. Am J Dis Child. 1980;134:522–523. doi: 10.1001/archpedi.1980.02130170072025. [DOI] [PubMed] [Google Scholar]
- 5.Boelaert J R, Van Landuyt H W, Godard C A, Daneels R F, Schurgers M L, Matthys E G, De Baere Y A, Gheyle D W, Gordts B Z, Herwaldt L A. Nasal mupirocin ointment decreases the incidence of Staphylococcus aureus bacteraemias in haemodialysis patients. Nephrol Dial Transplant. 1993;8:235–239. [PubMed] [Google Scholar]
- 6.Bommer J, Vergetis W, Andrassy K, Hingst V, Borneff M, Huber W. Elimination of Staphylococcus aureus in hemodialysis patients. ASAIO (Am Soc Artif Intern Organs) J. 1995;41:127–131. [PubMed] [Google Scholar]
- 7.Carruthers M, Kabat W. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect Immun. 1983;40:463–465. doi: 10.1128/iai.40.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casewell M W, Hill R L. Elimination of nasal carriage of Staphylococcus aureus with mupirocin (‘pseudomonic acid’)—a controlled trial. J Antimicrob Chemother. 1986;17:365–372. doi: 10.1093/jac/17.3.365. [DOI] [PubMed] [Google Scholar]
- 9.Cookson B D. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998;41:11–18. doi: 10.1093/jac/41.1.11. [DOI] [PubMed] [Google Scholar]
- 10.Coulter S N, Schwan W R, Ng E Y W, Langhorne M H, Ritchie H D, Westbrook-Wadman S, Hufnagle W O, Folger K R, Bayer A S, Stover C K. Staphylococcus aureus genetic loci impacting growth and survival in multiple infection environments. Mol Microbiol. 1998;30:393–404. doi: 10.1046/j.1365-2958.1998.01075.x. [DOI] [PubMed] [Google Scholar]
- 11.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in a semi-synthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 12.Doebbeling B N, Reagan D R, Pfaller M A, Houston A K, Hollis R J, Wenzel R P. Long-term efficacy of intranasal mupirocin ointment. A prospective cohort study of Staphylococcus aureus carriage. Arch Intern Med. 1994;154:1505–1508. [PubMed] [Google Scholar]
- 13.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J M, Bellon G, Dalhoff A, Doring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 14.Karakawa W W, Sutton A, Schneerson R, Karpas A, Vann W F. Capsular antibodies induce type-specific phagocytosis of capsulated Staphylococcus aureus by human polymorphonuclear leukocytes. Infect Immun. 1988;56:1090–1095. doi: 10.1128/iai.56.5.1090-1095.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kauppi M, Saarinen L, Kayhty H. Anti-capsular polysaccharide antibodies reduce nasopharyngeal colonization by Haemophilus influenzae type b in infant rats. J Infect Dis. 1993;167:365–371. doi: 10.1093/infdis/167.2.365. [DOI] [PubMed] [Google Scholar]
- 16.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10:505–520. doi: 10.1128/cmr.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kluytmans J A, Mouton J W, VandenBergh M F, Manders M J, Maat A P, Wagenvoort J H, Michel M F, Verbrugh H A. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol. 1996;17:780–785. doi: 10.1086/647236. [DOI] [PubMed] [Google Scholar]
- 18.Lee J C, Takeda S, Livolsi P J, Paoletti L C. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun. 1993;61:1853–1858. doi: 10.1128/iai.61.5.1853-1858.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lowe A M, Beattie D T, Deresiewicz R L. Identification of novel staphylococcal virulence genes by in vivo expression technology. Mol Microbiol. 1998;27:967–976. doi: 10.1046/j.1365-2958.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 20.Malley R, Stack A M, Ferretti M L, Thompson C M, Saladino R A. Anticapsular polysaccharide antibodies and nasopharyngeal colonization with Streptococcus pneumoniae in infant rats. J Infect Dis. 1998;178:878–882. doi: 10.1086/597600. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson I-M, Lee J C, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–4221. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Fontan M, Garcia-Falcon T, Rosales M, Rodriguez-Carmona A, Adeva M, Rodriguez-Lozano I, Moncalian J. Treatment of Staphylococcus aureus nasal carriers in continuous ambulatory peritoneal dialysis with mupirocin: long-term results. Am J Kidney Dis. 1993;22:708–712. doi: 10.1016/s0272-6386(12)80434-3. [DOI] [PubMed] [Google Scholar]
- 23.Sanford B A, Ramsay M A. Bacterial adherence to the upper respiratory tract of ferrets infected with influenza A virus. Proc Soc Exp Biol Med. 1987;185:120–128. doi: 10.3181/00379727-185-42525. [DOI] [PubMed] [Google Scholar]
- 24.Sanford B A, Thomas V L, Ramsay M A. Binding of staphylococci to mucus in vivo and in vitro. Infect Immun. 1989;57:3735–3742. doi: 10.1128/iai.57.12.3735-3742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwan W R, Coulter S N, Ng E Y, Langhorne M H, Ritchie H D, Brody L L, Westbrook-Wadman S, Bayer A S, Folger K R, Stover C K. Identification and characterization of the PutP proline permease that contributes to in vivo survival of Staphylococcus aureus in animal models. Infect Immun. 1998;66:567–572. doi: 10.1128/iai.66.2.567-572.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuter J, Hatcher V B, Lowy F D. Staphylococcus aureus binding to human nasal mucin. Infect Immun. 1996;64:310–318. doi: 10.1128/iai.64.1.310-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon H J. Epidemiology and pathogenesis of staphylococcal infection: I. An experimentally induced attenuated staphylococcal infection in guinea pigs and its modification by tetracycline. J Exp Med. 1963;118:149–164. doi: 10.1084/jem.118.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stringfellow W T, Dassy B, Lieb M, Fournier J M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutra L, Rainard P, Poutrel B. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type-5 capsular polysaccharide are influenced by growth in the presence of milk. J Clin Microbiol. 1990;28:2253–2258. doi: 10.1128/jcm.28.10.2253-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakker M, Park J S, Carey V, Lee J C. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–5189. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 32.Ward T T. Comparison of in vitro adherence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus to human nasal epithelial cells. J Infect Dis. 1992;166:400–404. doi: 10.1093/infdis/166.2.400. [DOI] [PubMed] [Google Scholar]
- 33.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 34.Wu H Y, Virolainen A, Matthews B, King J, Russell M W, Briles D E. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y P, Loosmore S M, Underdown B J, Klein M H. Nasopharyngeal colonization with nontypeable Haemophilus influenzae in chinchillas. Infect Immun. 1998;66:1973–1980. doi: 10.1128/iai.66.5.1973-1980.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]