Abstract
Kinectin 1 antisense RNA 1 (KTN1-AS1), a long non-coding RNA (lncRNA), has been proved to have tumor-promoting properties and its expression is enhanced in several human tumors. However, the role of KTN1-AS1 in the pathogenesis of esophageal squamous cell carcinoma (ESCC) remains unknown. This study aimed to investigate the expression status, functional roles, and molecular mechanisms of KTN1-AS1 in the development of ESCC. Considerable upregulation of KTN1-AS1 was confirmed in esophageal cancer cells and ESCC tissues and its expression was associated with TNM stage, pathological differentiation, and lymph node metastasis. SOX2 directly activated transcription of KTN1-AS1, and overexpression of KTN1-AS1 facilitated ESCC cells proliferation and invasion in vitro and in vivo. Furthermore, KTN1-AS1 could bind to retinoblastoma binding protein 4 (RBBP4) in the nucleus and enhanced its binding with histone deacetylase 1 (HDAC1), thereby activating the epithelial–mesenchymal transition (EMT) process through downregulating E-cadherin expression at the epigenetic level. In conclusion, KTN1-AS1, induced by SOX2, acts as a tumor-promoting gene and may serve as a potential therapeutic and prognostic biomarker for ESCC.
Subject terms: Cancer, Oncology
Introduction
Esophageal cancer is one of the most common malignant tumors worldwide, ranking sixth and seventh in mortality and morbidity, respectively, among all tumors. Esophageal squamous cell carcinoma (ESCC) is one of the predominant histological types of esophageal cancer1,2. The technology for diagnosis and treatment of esophageal cancer has constantly improved3. However, owing to the lack of specific symptoms and sensitive screening methods at an early stage, most patients with esophageal cancer are diagnosed at an advanced stage. Moreover, the 5-year survival rate after comprehensive treatment remains below 20% due to recurrence and distant metastasis. Therefore, identification of novel diagnostic and prognostic biomarkers, in addition to sensitive mediators of metastasis and recurrence, is highly desirable for improving the prognosis and survival of ESCC patients.
LncRNAs, with lengths > 200 nucleotides, represent diverse types of RNA molecules with limited or no protein-coding capability, and different biological functions depending on their subcellular location. Most nuclear lncRNAs participate in transcriptional regulation by recruiting DNA methyltransferase or histone acetylation and deacetylation enzymes to specific genomic sites or by interacting with RNA-binding proteins to affect activity of transcription complex4,5. Cytoplasmic lncRNAs, excluding their mRNA regulatory function6, can affect target gene expression by modulating microRNAs (miRNAs)7,8. KTN1-AS1 is located on human chromosome 14q22.3, and is one of three lncRNA signatures derived from the Atlas of ncRNA in cancer (TANRIC) database for predicting survival of patients with head and neck squamous cell carcinoma9. Subsequent studies have shown that KTN1-AS1 played an important role in the development and progression of non-small cell lung cancer, hepatocellular carcinoma, glioma, pancreatic cancer, bladder cancer, and ovarian cancer10–16. However, the role of KTN1-AS1 in ESCC has not yet been elucidated.
In this study, we detected the expression of KTN1-AS1 in ESCC tissues and cells, and analyzed the relationship of KTN1-AS1 with clinical characteristics of ESCC patients. Further studies focused on its functions on ESCC cells in vitro and in vivo, and explored the potential mechanisms of KTN1-AS1 in the carcinogenesis of ESCC.
Results
Upregulated expression of KTN1-AS1 in ESCC
The expression levels of KTN1-AS1 were higher in many tumors including ESCC according to the Gene Expression Profiling Interactive Analysis (GEPIA) database (Fig. 1A,B). The elevated expression of KTN1-AS1 was also detected in ESCC tissues and human esophageal cancer cell lines (Fig. 1C,D). Furthermore, based on the median expression value of KTN1-AS1, patients with ESCC were divided into high and low expression groups (n = 56 and n = 55, respectively); and KTN1-AS1 expression was found to be related to TNM stage, pathological differentiation, and lymph node metastasis (Table 1). Moreover, KTN1-AS1 expression was associated with ESCC patients’ survival (Fig. 1E). Results of univariate and multivariate Cox regression analysis suggested that depth of invasion, lymph node metastasis, and expression level of KTN1-AS1 were independent prognostic indicators for ESCC patients (Table 2).
Figure 1.
KTN1-AS1 is upregulate in esophageal squamous cell carcinoma (ESCC) tissues and is associated with poor prognosis. (A) The KTN1-AS1 expression profile across all tumor samples and corresponding normal tissues obtained from the Gene Expression Profiling Interactive Analysis (GEPIA). (B) The relative expression of KTN1-AS1 in 182 tumor samples compared with 286 normal samples obtained from the GEPIA database. (C) The expression levels of KTN1-AS1 in ESCC and the corresponding normal tissues were detected by qRT-PCR method. (D) The expression levels of KTN1-AS1 in human ESCC cell lines [Kyse150, Kyse170, Eca109, TE1, and Pools (the normal control comes from average expression of 10 normal tissues)]. (E) The overall survival of 111 patients with ESCC with high or low KTN1-AS1 expression was assessed using Kaplan Meier analysis; log-rank P = 0.0005. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
Table 1.
Relative expression level of KTN1-AS1 in ESCC patients.
| Characteristics | N | KTN1-AS1 expression | P value | |
|---|---|---|---|---|
| Low n (%) | High n (%) | |||
| Age (years) | 0.397 | |||
| < 60 | 46 | 25 (54.3%) | 21 (45.7%) | |
| ≥ 60 | 65 | 30 (46.2%) | 35 (53.8%) | |
| Gender | 0.138 | |||
| Male | 69 | 38 (55.1%) | 31 (44.9%) | |
| Female | 42 | 17 (40.5%) | 25 (59.5%) | |
| Smoking | 0.782 | |||
| Negative | 51 | 26 (51.0%) | 25 (49.0%) | |
| Positive | 60 | 29 (48.3%) | 31 (51.7%) | |
| Family history of UGIC | 0.442 | |||
| Negative | 90 | 43 (47.8%) | 47 (52.2%) | |
| Positive | 21 | 12 (57.1%) | 9 (42.9%) | |
| TNM stage | < 0.001 | |||
| I + II | 39 | 33 (84.6%) | 6 (15.4%) | |
| III + IV | 72 | 22 (30.6%) | 50 (69.4%) | |
| Depth of invasion | 0.633 | |||
| T1/2 | 58 | 30 (51.7%) | 28 (48.3%) | |
| T3/4 | 53 | 25 (47.2%) | 28 (52.8%) | |
| Pathological differentiation | < 0.001 | |||
| Well/moderate | 64 | 49 (76.6%) | 15 (23.4%) | |
| Poor | 47 | 6 (12.8%) | 41 (87.2%) | |
| LN metastasis | < 0.001 | |||
| Negative (N0) | 32 | 25 (78.1%) | 7 (21.9%) | |
| Positive (N1/2/3) | 79 | 30 (38.0%) | 49 (62.0%) | |
Table 2.
Univariate and multivariate Cox regression analysis for clinicopathological features with prognosis of patients with ESCC.
| Variables | Univariate analysis | P | Multivariate analysis | P |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age (< 60 vs. ≥ 60) | 1.407 (0.865–2.287) | 0.168 | 0.794 (0.472–1.334) | 0.383 |
| Gender (male vs. female) | 0.812 (0.493–1.338) | 0.415 | 0.831 (0.446–1.550) | 0.561 |
| Smoking (negative vs. positive) | 1.086 (0.676–1.745) | 0.732 | 0.883 (0.486–1.606) | 0.683 |
| Family history of UGIC (negative vs. positive) | 0.620 (0.317–1.212) | 0.162 | 0.876 (0.423–1.816) | 0.722 |
| TNM stage (I/II vs. III/IV) | 3.086 (1.737–5.484) | < 0.001 | 0.800 (0.308–2.076) | 0.646 |
| Depth of invasion (T1/2 vs. T3/4) | 2.895 (1.781–4.706) | < 0.001 | 2.928 (1.798–4.769) | < 0.001 |
| LN metastasis (N0 vs. N1/2/3) | 3.434 (1.798–6.557) | < 0.001 | 2.703 (1.381–5.291) | 0.004 |
| Pathological differentiation (well/moderate vs. poor) | 2.232 (1.391–3.580) | 0.001 | 1.046 (0.526–2.083) | 0.897 |
| KTN1-AS1 (low vs. high) | 2.354 (1.449–3.823) | 0.001 | 1.893 (1.145–3.131) | 0.013 |
SOX2 induces KTN1-AS1 expression in ESCC cells
Given the high expression of KTN1-AS1 in ESCC, the mechanism leading to its upregulation attracted our attention. To study the potential transcription factors regulating KTN1-AS1, we searched two online databases, hTFtarget and animalTFDB3, and predicted that there were 127 transcription factors (Supplementary Fig. S1A). We further analyzed the correlation between the 127 transcription factors and the expression of KTN1-AS1 through the GEPIA database, and found that there were 36 transcription factors with an R value greater than 0.3, including SOX2 (Supplementary Fig. S1B). A positive correlation was found between SOX2 and KTN1-AS1 (Fig. 2A,B) and the mRNA expression of SOX2 was also significantly upregulated in ESCC tissues and cell lines (Fig. 2C,D). After transfection of pcDNA3.1-SOX2 and si-SOX2 into Kyse150 and Kyse170 cells (Fig. 2E), the expression level of KTN1-AS1 was substantially increased in SOX2 overexpressing cells and remarkably decreased in SOX2 knockdown cells (Fig. 2F). So we chose SOX2 for the following study.
Figure 2.
SOX2 induces KTN1-AS1 expression in esophageal squamous cell carcinoma (ESCC) cells. (A) The relevance of expression between KTN1-AS1 and SOX2 was analyzed by the Gene Expression Profiling Interactive Analysis (GEPIA) database. (B) The correlation between the expression of KTN1-AS1 and SOX2 was analyzed by qRT-PCR method. (C) The expression levels of SOX2 in ESCC and the corresponding normal tissues were detected by qRT-PCR method. (D) The expression levels of SOX2 in human ESCC cell lines. (E) The overexpression and knockdown efficiency of SOX2 in Kyse150 and Kyse170 cells were detected by qRT-PCR method. (F) The regulation effect of SOX2 on KTN1-AS1 expression in Kyse150 and Kyse170 cells were detected using qRT-PCR method. (G) Luciferase activity assay was conducted in Kyse150 and Kyse170 cells to verify the direct binding of SOX2 to the KTN1-AS1 promoter. (H) Chromatin Immunoprecipitation (ChIP) assay was used to confirm the binding site of SOX2 on the promoter of KTN1-AS1 in Kyse150 and Kyse170 cells. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
As shown in Fig. 2G, according to the location of the three possible binding sites (site 1: − 137 bp to − 129 bp, site 2: − 408 bp to − 400 bp, and site 3: − 1113 to − 1105 bp), we constructed a luciferase reporter gene plasmid containing the − 1239 bp to + 56 bp region of KTN1-AS1 promoter, and co-transfected it with pcDNA3.1-SOX2 in Kyse150 and Kyse170 cells, respectively. The luciferase activity was found to be substantially increased. To further clarify the specific site of action, a truncated plasmid containing the fragment of − 594 bp to + 56 bp was constructed, and its luciferase activity was also considerably upregulated. The elevated luciferase activity was significantly decreased when site 2 was mutated, while no obvious change was observed accompanied with site 1 or site 3 mutation, suggesting the key role of site 2. The binding effect of SOX2 on site 2 of the KTN1-AS1 promoter was further confirmed by ChIP assay (Fig. 2H). These results collectively suggest that the elevated expression of KTN1-AS1 may be regulated by SOX2 in ESCC.
KTN1-AS1 facilitates proliferation, migration, and invasion of ESCC cells
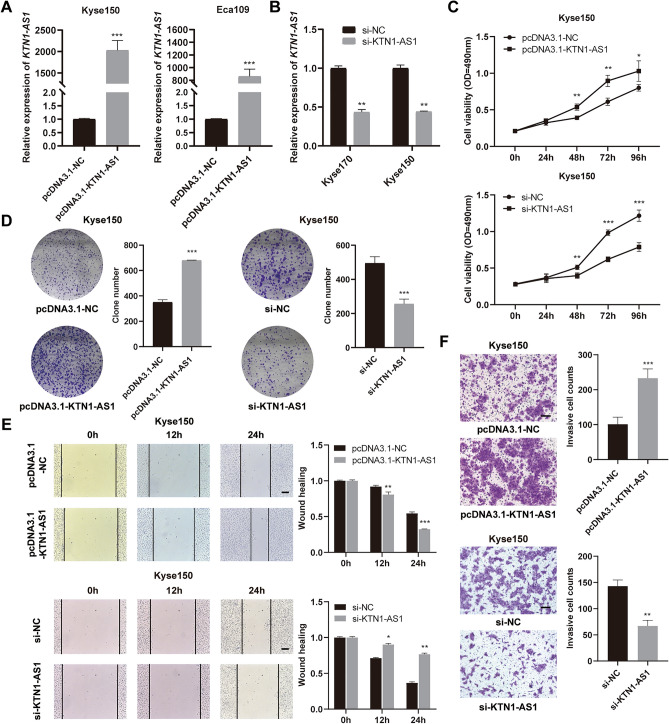
To investigate the biological function of KTN1-AS1 in ESCC cells, the overexpression plasmid of KTN1-AS1 was transfected into Kyse150 and Eca109 cells, and a striking upregulation of KTN1-AS1 was detected in the transfected cells (Fig. 3A). Furthermore, si-KTN1-AS1 was used to knockdown the expression of KTN1-AS1 in Kyse170 and Kyse150 cells (Fig. 3B). Overexpression of KTN1-AS1 stimulated the proliferation, migration, and invasion capability of Kyse150 cells, similar results were obtained in KTN1-AS1 overexpressed Eca109 cells (Supplementary Fig. S2A–D). While knockdown of KTN1-AS1 alleviated the growth, migration, and invasion ability of Kyse150 (Fig. 3C–F) and Kyse170 cells (Supplementary Fig. S2E–H). These results suggest that KTN1-AS1 may play an oncogenic role in ESCC.
Figure 3.
KTN1-AS1 facilitates esophageal squamous cell carcinoma (ESCC) cells biological functions. (A,B) The overexpression efficiency of KTN1-AS1 in Kyse150 and Eca109 cells, and the knockdown efficiency of KTN1-AS1 in Kyse170 and Kyse150 cells. (C,D) MTS and clone formation assays were used to demonstrate the cell proliferation ability in Kyse150 cells in response to upregulation and downregulation of KTN1-AS1. (E,F) Cell migration and invasion ability were verified through wound healing and transwell assays in Kyse150 cells by overexpressing and downregulating KTN1-AS1 (magnification: ×100). Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
KTN1-AS1 interacts with RBBP4 in the nucleus
The subcellular localization analysis in ESCC cells showed that KTN1-AS1 was distributed in both nucleus and cytoplasm (Fig. 4A). RNA pull-down assay followed by mass spectrometry analysis was then performed to detect the RNA binding proteins, and RBBP4 was proved to be one of the differentially expressed proteins (Fig. 4B). The interaction between KTN1-AS1 and RBBP4 was further verified by Western blot and RIP assay (Fig. 4C,D). However, there were no significant differences in RBBP4 mRNA and protein expression levels in KTN1-AS1 overexpressed or knockdown cells (Fig. 4E), which was consistent with the poor correlation predicted by the GEPIA database (Fig. 4F). The expression level of RBBP4 was upregulated in esophageal carcinoma according to GEPIA database (Fig. 4G). Our results also showed an upregulation of RBBP4 in ESCC tissues and cell lines (Fig. 4H,I).
Figure 4.
KTN1-AS1 interacts with RBBP4 in the nucleus. (A) The subcellular location of KTN1-AS1 in esophageal squamous cell carcinoma (ESCC) cell lines. (B) RNA pull-down assay was performed in Kyse150 and Kyse170 cells, and the RNA-related proteins were determined with SDS-PAGE gel and coomassie brilliant blue staining. Original gel image was presented in Supplementary Fig. S5A. (C) Western blot assay was performed to detect the specific association between RBBP4 and KTN1-AS1 in Kyse150 and Kyse170 cells. Original western blots were presented in Supplementary Fig. S5B, with blots cut prior to hybridization with antibodies. (D) RIP assay showed the interaction between KTN1-AS1 and RBBP4 in Kyse150 and Kyse170 cells. (E) The regulation effect of KTN1-AS1 on RBBP4 expression was detected by qRT-PCR and western blot. Original western blots were presented in Supplementary Fig. S5C, with blots cut prior to hybridization with antibodies. (F) The relevance between KTN1-AS1 and RBBP4 expression was predicted by the GEPIA database. (G) The relative expression of RBBP4 in 182 tumor samples compared with 286 normal samples obtained from the Gene Expression Profiling Interactive Analysis (GEPIA) database. (H) The expression levels of RBBP4 in ESCC and the corresponding normal tissues. (I) The expression levels of RBBP4 in ESCC cell lines. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
KTN1-AS1 relates to the epithelial-to-mesenchymal transition (EMT) process by inhibiting the expression of E-cadherin at the epigenetic level
Considering that SOX2 could influence the migration and invasion capability of esophageal cancer cells and up-regulate the expression of KTN1-AS1 at transcriptional level, we then detected the influence of KTN1-AS1 on EMT related markers. As shown in Fig. 5A,B, overexpression of KTN1-AS1 in Kyse150 cells considerably decreased the mRNA and protein expression levels of E-cadherin, and increased those of N-cadherin, vimentin, and MMP2; while downregulation of KTN1-AS1 in Kyse170 cells demonstrated the opposite tendency, suggesting the possible role of KTN1-AS1 in EMT process. Subsequently, we explored the expression changes of some EMT related markers, including E-cadherin, N-cadherin, Vimentin, MMP2, Snail1, and Twist1, after knocking down RBBP4. With the downregulation of RBBP4 in Kyse170 cells, the mRNA expression level of MMP2 was correspondingly downregulated, while the expression of E-cadherin was upregulated (Supplementary Fig. S3).
Figure 5.
KTN1-AS1 relates to epithelial-to-mesenchymal transition (EMT) process by interacting with RBBP4 and HDAC1 to silence E-cadherin expression. (A) The mRNA expression levels of E-cadherin, N-cadherin, Vimentin, and MMP2 after KTN1-AS1 overexpression and knockdown. (B) The regulatory effect of KTN1-AS1 on protein levels of E-cadherin, N-cadherin, Vimentin, and MMP2 was detected by western blot. Original western blots were presented in Supplementary Fig. S5D, with blots cut prior to hybridization with antibodies. (C) Inhibition of RBBP4 increased the expression level of E-cadherin and partially reversed the regulation effect of KTN1-AS1 on the expression level of E-cadherin in Kyse150 and Kyse170 cells. (D) Co-IP assay was performed to examine the RBBP4-HDAC1 interaction in the groups with KTN1-AS1 overexpression and inhibition in Kyse150 and Kyse170 cells. Original western blots were presented in Supplementary Fig. S5E, with blots cut prior to hybridization with antibodies. (E) RIP assay showed the interaction between KTN1-AS1 and HDAC1 in Kyse150 and Kyse170 cells. (F) After transfection of pcDNA3.1-NC or pcDNA3.1-KTN1-AS1 for 12–24 h in Kyse150 and Kyse170 cells, then cells with pcDNA3.1-KTN1-AS1 were treated with or without 300 nM Trichostatin A (TSA) for additional 48 h, the mRNA expression of E-cadherin was detected by qRT-PCR method. (G) ChIP-qPCR was performed to detect the enrichment of ac-H3 in the promoter region of E-cadherin after overexpression and knockdown of KTN1-AS1 in Kyse150 cells. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
As previous studies have demonstrated the involvement of HDAC1 in the transcriptional regulation of E-cadherin17,18, and RBBP4 and HDAC1/2 have been proved to form a core deacetylase complex in both the NuRD and Sin3 complex19,20, we proposed that KTN1-AS1 might regulate E-cadherin expression at the epigenetic level in the nucleus. The expression of E-cadherin was increased after knocking down of RBBP4, and the inhibition of E-cadherin due to overexpression of KTN1-AS1 could be alleviated by the inhibition of RBBP4 (Fig. 5C). The interaction between RBBP4 and HDAC1 was noticeably strengthened in KTN1-AS1 overexpressed cells, while impaired in KTN1-AS1 knockdown cells detected by Co-IP assay (Fig. 5D), furthermore, KTN1-AS1 was also verified to bind with HDAC1 (Fig. 5E), suggesting that KTN1-AS1 could combine with RBBP4 and HDAC1 to form a complex and simultaneously enhanced the binding effect of RBBP4 and HDAC1. In addition, a rescue experiment was conducted using the HDAC1 inhibitor TSA. As shown in Fig. 5F, TSA treatment alleviated the downregulation of E-cadherin caused by overexpression of KTN1-AS1. Moreover, as shown in Fig. 5G, overexpression of KTN1-AS1 weakened the enrichment of acetylation of histone H3 (ac-H3) at the promoter region of E-cadherin, whereas knockdown of KTN1-AS1 enhanced the enrichment of ac-H3, indicating that the binding action of KTN1-AS1 with RBBP4 and HDAC1 could finally influence the expression of E-cadherin via regulating the level of histone acetylation.
Inhibition of RBBP4 partially reverses the promoting effect of KTN1-AS1 on the biological behavior of ESCC cells
For the molecular mechanism by which KTN1-AS1 affects the expression of E-cadherin, we further verified in terms of cellular phenotype. The overexpression plasmid of KTN1-AS1 was co-transfected with si-RBBP4 into Kyse150 and Kyse170 cells to investigate their effects on cell function. Reduced RBBP4 expression partially reversed the enhanced proliferation, migration, and invasion ability induced by overexpression of KTN1-AS1 (Fig. 6A–D and Supplementary Fig. S4A–D), indicating that the effect of KTN1-AS1 on the malignant behavior of esophageal cancer cells is affected by RBBP4.
Figure 6.
RBBP4 partially reverses the biological function of KTN1-AS1 on esophageal squamous cell carcinoma (ESCC) cells. (A,B) MTS and clone formation assays were performed to analyze the cell proliferation ability after co-transfected with pcDNA3.1-KTN1-AS1 and si-RBBP4 in Kyse150 cells. (C,D) Wound healing and transwell invasion assays were conducted to explore the migration and invasion ability after co-transfected with pcDNA3.1-KTN1-AS1 and si-RBBP4 in Kyse150 cells. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
KTN1-AS1 promotes ESCC cell growth in vivo
To further verify the carcinogenic effect of KTN1-AS1 on ESCC, the tumor xenograft experiments were conducted to investigate the effects of KTN1-AS1 on tumor growth in vivo. Compared with control group, the tumor size, tumor volume and weight in the KTN1-AS1 upregulated group were significantly increased (Fig. 7A,B). Furthermore, the expression of KTN1-AS1 in the xenograft tissues of the KTN1-AS1 overexpression group was increased, accompanied by the increased mRNA expression level of N-cadherin, Vimentin, and MMP2, while the expression of E-cadherin was decreased (Fig. 7C). All these findings indicated that KTN1-AS1 could promote ESCC tumor growth in vivo.
Figure 7.
KTN1-AS1 promotes esophageal squamous cell carcinoma (ESCC) cell growth in vivo. (A) Xenograft tumor images dissected from nude mice after stable overexpression of KTN1-AS1. (B) The tumor volume and weight of different groups (n = 7). (C) The relative expression of KTN1-AS1, E-cadherin, N-cadherin, Vimentin, and MMP2 in xenograft tissues detected by qRT-PCR method. (D) The possible mechanism of KTN1-AS1 regulates ESCC progression. Error bars are shown as mean ± SD from three replicate experiments (n = 3) (*P < 0.05, **P < 0.01, ***P < 0.001).
In conclusion, all these findings suggested that KTN1-AS1 might promote ESCC progression by activating EMT process via RBBP4/HDAC1/E-cadherin axis (Fig. 7D).
Discussion
It is well known that lncRNAs take up a significant proportion acting as either tumor suppressors or oncogenes in the tumor carcinogenesis. In this study, KTN1-AS1 was proved to be upregulated in esophageal cancer tissues and cells, and exhibited as an oncogene in facilitating ESCC cells proliferation, migration, and invasion. The expression of KTN1-AS1 maybe induced by SOX2, and has a relationship with the EMT process.
In non-small cell lung cancer (NSCLC), signal transducer and activator of transcription 1 (STAT1) was proved to bind to the promoter region of KTN1-AS1 and activated its transcription11. Since the promoter region of KTN1-AS1 is riched in transcriptional regulatory elements, we speculated that there should be other transcriptional factors involved in the regulation of abnormal KTN1-AS1 expression in ESCC. Amplification of SOX2 is one of the gene characteristics of ESCC and the expression of SOX2 is specifically highly in ESCC21. In the present study SOX2 was proved to activate transcription of KTN1-AS1. In addition, SOX2 acts as a potential EMT-inducing transcriptional factor in promoting cancer cells invasion and metastasis, and SOX2 induced lncRNAs have been demonstrated to play pivotal roles in tumorigenesis22–24, so there may be potential promoting role for KTN1-AS1 on ESCC cells progression.
Nuclear-localized lncRNAs can recruit chromatin modification and remodeling complexes to specific genomic sites to change the chromosome structure and modification status, and DNA/RNA methylation status, and further control the related genes expression25. Accumulating evidence has demonstrated that lncRNAs participate in these processes by binding to specific proteins26. Our present study found that KTN1-AS1 could bind with RBBP4 in the nucleus. As a chaperone protein, RBBP4 exerts its oncogenic function by participating in the formation of gene regulatory complexes, such as polycomb repressive complex 2 (PRC2)27, nucleosome remodeling factor (NURF)28, NuRD29, Sin3 complex19, and the deacetylase module (HDAC1/2, MTA1/2/3, RBBP4/7) complex30,31. Previous studies of KTN1-AS1 have primarily focused on the mechanism of competing endogenous RNA (ceRNA). In this study, we first discussed the underlying mechanism between KTN1-AS1 and RNA-binding protein in ESCC.
EMT is a cellular program known to be critical for malignant progression of tumors32. Since KTN1-AS1 could regulate the expression of EMT-related genes, we paid more attention to whether KTN1-AS1 regulates the expression of EMT-related genes through RBBP4 and HDAC1. E-cadherin, a typical epithelial cell marker, is a Ca2+ dependent transmembrane glycoprotein closely related to intercellular adhesion. The dysregulation of E-cadherin expression that leads to carcinogenesis happens mostly at the epigenetic level33,34. HDACs are enzymes mediating the removal of acetyl from lysine residues in either histones or other proteins, causing the repression of gene transcription and subsequent changes in signaling events35, and HDAC1 was demonstrated to be involved in the transcriptional regulation of E-cadherin expression17,18. Our subsequent studies found that KTN1-AS1 could also bind to HDAC1 and affect the binding ability of RBBP4 to HDAC1. KTN1-AS1 may silence the expression of E-cadherin by forming a complex with RBBP4 and HDAC1 and enhancing its deacetylation effect on the promoter of E-cadherin in the nucleus, thereby promoting the EMT process in ESCC.
Conclusions
In summary, this study identifies the novel oncogenic role of lncRNA KTN1-AS1 in ESCC. Transcriptionally activated KTN1-AS1 by SOX2 may silence the expression of E-cadherin at epigenetic level by binding with RBBP4 and HDAC1 in the nucleus. KTN1-AS1 may act as a potential therapeutic and prognostic biomarker for ESCC.
Methods
Clinical specimens
One hundred and eleven ESCC patients were included in this study. The patients didn’t undergo any radiotherapy or chemotherapy before operation between the years of 2013 to 2015 in the Fourth Hospital of Hebei Medical University. The clinical information was collected from the hospital records. The study was reported in accordance with ARRIVE guidelines and informed consent was obtained from all patients. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University.
Cell culture and treatment
Human esophageal cancer cell lines (Kyse150, Kyse170, Eca109, and TE1) were purchased from American Type Culture Collection (ATCC). Cells were cultured in RPMI-1640 (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine serum (Invitrogen) in an atmosphere containing 5% CO2 at 37 °C. For Trichostatin A (TSA) (CAS No. 58880-19-6, Sigma) treatment, Kyse 150 and Kyse170 cells were transfected for 12–24 h, then treated with 300 nM TSA for additional 48 h.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) assay
The TRIzol reagent (Solarbio, Beijing, China) was used to extract RNA from tissues and cells. The cDNA was synthesized using the Transcriptor First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland). GoTap®qPCR Master Mix (Promega, Madison, WI, USA) was used to perform the quantitative real time PCR in the StepOne plus Real-Time PCR System (Applied Biosystems). The relative expression levels of mRNA and lncRNA were normalized to GAPDH as endogenous control respectively by using the 2−△△Ct method. Primer sequences were listed in Supplementary Table S1.
Cell transfection
The pcDNA3.1-KTN1-AS1 was purchased from GenScript (Nanjing, China). For overexpression of SOX2, the cDNA encoding SOX2 was amplified and was inserted into pcDNA3.1 vector (Invitrogen), named pcDNA3.1-SOX2. The SOX2 siRNA, KTN1-AS1 siRNA, RBBP4 siRNA, and si-NC were purchased from GenePharma (Shanghai, China), and the sequences were listed in Supplementary Table S2. Lipofectamine 2000 (Invitrogen) was used to perform the transfection. The transfection efficiency was detected by qRT-PCR method.
Cell proliferation assay
The cellular proliferation capability was detected using MTS and clone formation assays. For MTS assay, 1 × 103 cells after 24 h of transfection were seeded into 96-well plate. The CellTiter 96®AQueous One Solution Reagent (Promega) was added after incubation of 0 h, 24 h, 48 h, 72 h, and 96 h. Then the optical density for each well was measured after incubation for 2 h. As to clone formation assay, 3 × 103/5 × 103 cells after 24 h of transfection were seeded into a 6-well plate and were routine cultured for 1 week. The 4% paraformaldehyde was used to fix the cells and stained with crystal violet solution.
Cell migration and invasion assay
Wound healing and transwell assays were performed to detect the migration and invasion ability. For wound healing assay, transfected cells were inoculated in a 6-well plate. A straight scratch was made in each well when the cell density was close to overgrown, and pictures were taken at the same position at 0 h, 12 h, and 24 h using a microscope. For transwell assay, 1 × 105 cells after 24 h of transfection were seeded onto the upper compartment of the matrigel-coated chamber (Corning Costar, Corning, NY, USA) with 200 μL of serum-free RPMI-1640 medium; in the lower chamber, 600 μL of the medium containing 10% fetal bovine serum were added. Cells on the upper surface were wiped off after 24 h of incubation; the 4% paraformaldehyde was used to fix the invasive cells on the lower surface of the chamber and stained with crystal violet solution.
Western blot assay
The RIPA lysis buffer and PMSF (Solarbio) were used to extract proteins from cells. BCA Protein Assay Kit (Multi Sciences, Hangzhou, China) was used to detect the protein concentration. The protein lysates were transferred to PVDF membranes (Millipore, Sigma, Burlington, MA, USA) after separated by 10% polyacrylamide gel electrophoresis. The enhanced chemiluminescence detection reagent (Multi Sciences) was used to detect the protein bands by Chemi XT 4 (Syngene). The main antibodies were listed as follows: anti-β-actin (ZenBioScience, Cat# 380624), anti-E-cadherin (ZenBioScience, Cat# R22490), anti-N-cadherin (ZenBioScience, Cat# 380671), anti-Vimentin (ZenBioScience, Cat# R22775), anti-MMP2 (ZenBioScience, Cat# 380817), anti-RBBP4 (ZenBioScience, Cat# 385565), anti-HDAC1 (Proteintech, Cat# 10197-1-AP).
Subcellular fractionation assay
The nuclear and cytoplasm fractions of esophageal cancer cell lines were isolated by PARIS™ Kit Protein and RNA Isolation System (Invitrogen). The subcellular localization of KTN1-AS1 was detected by qRT-PCR method. GAPDH and U6 were used as control genes expressed in cytoplasm and nucleus, respectively.
Vector construction
The fragments of KTN1-AS1 containing the predicted binding sites of SOX2 were amplified and inserted into the pGL3-basic vector (Promega). The point mutations of the binding sites were performed using Q5® Site-Directed Mutagenesis Kit (New England Biolabs). Mutation primers were designed in NEBase changer (NEBaseChanger). Primers used for fragment amplification were listed in Supplementary Table S3. All recombinant plasmids were sequenced correctly.
RNA pull-down assay
Full-length sense and antisense KTN1-AS1 sequences were obtained using RiboMAX™ Large Scale RNA Production System-T7 (Promega). The Pierce™ RNA 3′ End Desthiobiotinylation Kit (Thermo Fisher Scientific) was then applied to biotin-label the obtained RNA sequence. The purified biotin-labeled RNA was incubated with magnetic beads and protein lysate using the Pierce™ Magnetic RNA–Protein Pull-Down Kit (Thermo Fisher Scientific). Proteins were separated using polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue (Beyotime, Jiangsu, China). The different bands between sense and antisense KTN1-AS1 were verified using mass spectrometry and detected through western blot analysis.
Luciferase reporter assay
In Kyse150 and Kyse170 cells, the promoter-reporter gene plasmids were separately co-transfected with pcDNA3.1-SOX2 or pcDNA3.1 empty plasmid. After 48 h of transfection, the luciferase activity was detected using the Dual-Luciferase Reporter Assay System (Promega) and normalized to Renilla activity.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was carried out using the EZ-ChIP™ kit (Millipore). After sonicating the cross-linked chromatin DNA, the antibodies against SOX2 (ZenBioScience, Cat# 864316) and acetyl histones H3 (Active motif, Ca# 61937) were used for precipitating the DNA fragments overnight at 4 °C, and protein A/G agarose beads were added to collect the precipitated complexes. The precipitated DNA fragment was purified and subjected to PCR detection. Primers used for the ChIP sequence were listed in Supplementary Table S4.
RNA immunoprecipitation (RIP) assay
Cells were re-suspended in NP-40 lysis buffer. RNA was immunoprecipitated with antibodies against RBBP4 (ZenBioScience, Cat# 385565) and HDAC1 (Proteintech, Cat# 10197-1-AP). The qRT-PCR method was performed to detect the expression of KTN1-AS1.
Co-immunoprecipitation (Co-IP) assay
Cells were lysed with NP-40 lysis buffer and the lysates were precleared with protein A/G agarose beads (Santa Cruz Biotechnology, Dallas, Texas, USA). The supernatant was incubated with an antibody against RBBP4 overnight at 4 ℃ and protein A/G agarose beads were then added for further incubation. The precipitates were washed with lysis buffer and then suspended in 5 × SDS-PAGE sample loading buffer. After boiling for 10 min, the samples were analyzed by western blot and detected by the relevant antibodies.
Tumor xenograft model
G-418 bioreagent (Merck, Rahway, NJ, USA) was used to generate KTN1-AS1 stable expression Kyse150 cells. A total of 5 × 106 cells were subcutaneously injected into one side of male BALB/c-nude mice purchased from Beijing HFK Bioscience CO., Ltd. Tumor volume was measured and calculated every 4 days. The mice were dissected on the 28 days, and tumors weight were measured. The animal experiments were conducted at the Experimental Animal Center of the Fourth Hospital of Hebei Medical University under the guidelines of NIH, and this study was approved by the Committee on the Ethics of Animal Experiments of the Fourth Hospital of Hebei Medical University.
Statistical analysis
SPSS 22.0 software package and GraphPad Prism 8.0 were used to perform data analysis and graphing. The statistical differences analysis between the two groups was conducted using Student's t-test. For overall survival analysis, Kaplan–Meier method and log-rank test were used. Univariate and multivariate Cox regression analysis was used to investigate the independent prognostic parameters. All experiments data came from three independent experiments performed in duplicate and presented as mean ± SD. P < 0.05 was considered statistically significant.
Ethics statement
The study involving the usage of patients tissues was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University. The study was reported in accordance with ARRIVE guidelines.
Supplementary Information
Author contributions
C.L.Y. performed the experiment and was a major contributor in writing the manuscript. L.J.T., G.Y.L., and D.Z.M. contributed to the design of the work. X.T.X. and Y.Z.Y. performed the analysis of data. G.W. contributed to the conception and revision of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (No. 81772612); Natural Science Foundation of Hebei Province (Nos. H2021206259, H2020206368, H2020206363).
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-24743-z.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Soerjomataram I, Ferlay J, Forman D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64:381–387. doi: 10.1136/gutjnl-2014-308124. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg. Today. 2020;50:12–20. doi: 10.1007/s00595-019-01878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao G, Fan X, Zhang P, Liu X, Huang L, Ji S. Methylation-dependent MCM6 repression induced by LINC00472 inhibits triple-negative breast cancer metastasis by disturbing the MEK/ERK signaling pathway. Aging. 2021;13:4962–4975. doi: 10.18632/aging.103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pistoni M, Rossi T, Donati B, Torricelli F, Polano M, Ciarrocchi A. Long noncoding RNA NEAT1 acts as a molecular switch for BRD4 transcriptional activity and mediates repression of BRD4/WDR5 target genes. Mol. Cancer Res. 2021;19:799–811. doi: 10.1158/1541-7786.MCR-20-0324. [DOI] [PubMed] [Google Scholar]
- 6.Kretz M, Siprashvili Z, Chu C, Webster DE, Zehnder A, Qu K, et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 2013;493:231–235. doi: 10.1038/nature11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu WW, Zheng CC, Zuo Q, Li JQ, Hong P, Qin YR, et al. Genome-wide identification of key regulatory lncRNAs in esophageal cancer metastasis. Signal Transduct. Target. Ther. 2021;6:88. doi: 10.1038/s41392-021-00476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ, Tao QF, et al. A long noncoding RNA activated by TGF-β promotes the invasion-metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666–681. doi: 10.1016/j.ccr.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Cao W, Liu JN, Liu Z, Wang X, Han ZG, Ji T, et al. A three-lncRNA signature derived from the Atlas of ncRNA in cancer (TANRIC) database predicts the survival of patients with head and neck squamous cell carcinoma. Oral Oncol. 2017;65:94–101. doi: 10.1016/j.oraloncology.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Jiang Y, Wu K, Cao W, Xu Q, Wang X, Qin X, et al. Long noncoding RNA KTN1-AS1 promotes head and neck squamous cell carcinoma cell epithelial–mesenchymal transition by targeting miR-153-3p. Epigenomics. 2020;12:487–505. doi: 10.2217/epi-2019-0173. [DOI] [PubMed] [Google Scholar]
- 11.Liu C, Li X, Hao Y, Wang F, Cheng Z, Geng H, et al. STAT1-induced upregulation of lncRNA KTN1-AS1 predicts poor prognosis and facilitates non-small cell lung cancer progression via miR-23b/DEPDC1 axis. Aging. 2020;12:8680–8701. doi: 10.18632/aging.103191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu Y, Tang Q, Feng H, Zhu L, Wang Y. lncRNA KTN1-AS1 promotes glioma cell proliferation and invasion by negatively regulating miR-505-3p. Oncol. Rep. 2020;44:2645–2655. doi: 10.3892/or.2020.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Wang L, Wang Y, Chen T, Liu R, Yang W, et al. LncRNA KTN1-AS1 promotes tumor growth of hepatocellular carcinoma by targeting miR-23c/ERBB2IP axis. Biomed. Pharmacother. 2019;109:1140–1147. doi: 10.1016/j.biopha.2018.10.105. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZB, Liu N. Long non-coding RNA KTN1-AS1 promotes progression in pancreatic cancer through regulating microRNA-23b-3p/high-mobility group box 2 axis. Aging. 2021;13:20820–20835. doi: 10.18632/aging.203481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Xiang L, He D, Zhu R, Fang J, Wang Z, et al. The long noncoding RNA KTN1-AS1 promotes bladder cancer tumorigenesis via KTN1 cis-activation and the consequent initiation of Rho GTPase-mediated signaling. Clin. Sci. 2021;135:555–574. doi: 10.1042/CS20200908. [DOI] [PubMed] [Google Scholar]
- 16.Xie X, Wen Q, Yang X, Chen W, Liu Y, Liu W, et al. H3K27ac-activated lncRNA KTN1-AS1 aggravates tumor progression by miR-505-3p/ZNF326 axis in ovarian cancer. Ann. Transl. Med. 2022;10:599. doi: 10.21037/atm-22-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Burstin, J. et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 137, 361–371, 371.e361–365 (2009). [DOI] [PubMed]
- 18.Aghdassi A, Sendler M, Guenther A, Mayerle J, Behn CO, Heidecke CD, et al. Recruitment of histone deacetylases HDAC1 and HDAC2 by the transcriptional repressor ZEB1 downregulates E-cadherin expression in pancreatic cancer. Gut. 2012;61:439–448. doi: 10.1136/gutjnl-2011-300060. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Iratni R, Erdjument-Bromage H, Tempst P, Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/S0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Ng HH, Erdjument-Bromage H, Tempst P, Bird A, Reinberg D. Analysis of the NuRD subunits reveals a histone deacetylase core complex and a connection with DNA methylation. Genes Dev. 1999;13:1924–1935. doi: 10.1101/gad.13.15.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Huang S, Chen S, Chen J, Wang Z, Wang Y, et al. SOX2 promotes chemoresistance, cancer stem cells properties, and epithelial-mesenchymal transition by β-catenin and Beclin1/autophagy signaling in colorectal cancer. Cell Death Dis. 2021;12:449. doi: 10.1038/s41419-021-03733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Zhou J, Wang Z, Wang P, Li S. Upregulation of SOX2 activated LncRNA PVT1 expression promotes breast cancer cell growth and invasion. Biochem. Biophys. Res. Commun. 2017;493:429–436. doi: 10.1016/j.bbrc.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Wu JH, Tang JM, Li J, Li XW. Upregulation of SOX2-activated lncRNA ANRIL promotes nasopharyngeal carcinoma cell growth. Sci. Rep. 2018;8:3333. doi: 10.1038/s41598-018-21708-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q, Hao Q, Prasanth KV. Nuclear long noncoding RNAs: Key regulators of gene expression. Trends Genet. 2018;34:142–157. doi: 10.1016/j.tig.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrè F, Colantoni A, Helmer-Citterich M. Revealing protein-lncRNA interaction. Brief. Bioinform. 2016;17:106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Jiao L, Liu X, Yang X, Liu X. A dimeric structural scaffold for PRC2-PCL targeting to CpG island chromatin. Mol. Cell. 2020;77:1265–1278.e1267. doi: 10.1016/j.molcel.2019.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu P, Wang Y, He L, Huang G, Du Y, Zhang G, et al. ZIC2-dependent OCT4 activation drives self-renewal of human liver cancer stem cells. J. Clin. Investig. 2015;125:3795–3808. doi: 10.1172/JCI81979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivanochko D, Halabelian L, Henderson E, Savitsky P, Jain H, Marcon E, et al. Direct interaction between the PRDM3 and PRDM16 tumor suppressors and the NuRD chromatin remodeling complex. Nucleic Acids Res. 2019;47:1225–1238. doi: 10.1093/nar/gky1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millard CJ, Varma N, Saleh A, Morris K, Watson PJ, Bottrill AR, et al. The structure of the core NuRD repression complex provides insights into its interaction with chromatin. Elife. 2016;5:e13941. doi: 10.7554/eLife.13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millard CJ, Fairall L, Ragan TJ, Savva CG, Schwabe JWR. The topology of chromatin-binding domains in the NuRD deacetylase complex. Nucleic Acids Res. 2020;48:12972–12982. doi: 10.1093/nar/gkaa1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat. Rev. Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 33.Wong SHM, Fang CM, Chuah LH, Leong CO, Ngai SC. E-cadherin: Its dysregulation in carcinogenesis and clinical implications. Crit. Rev. Oncol. Hematol. 2018;121:11–22. doi: 10.1016/j.critrevonc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Yang FL, Wei YX, Liao BY, Wei GJ, Qin HM, Pang XX, et al. LncRNA HOTAIR regulates the expression of E-cadherin to affect nasopharyngeal carcinoma progression by recruiting histone methylase EZH2 to mediate H3K27 trimethylation. Genomics. 2021;113:2276–2289. doi: 10.1016/j.ygeno.2021.03.036. [DOI] [PubMed] [Google Scholar]
- 35.Lei W, Zhang K, Pan X, Hu Y, Wang D, Yuan X, et al. Histone deacetylase 1 is required for transforming growth factor-beta1-induced epithelial-mesenchymal transition. Int. J. Biochem. Cell Biol. 2010;42:1489–1497. doi: 10.1016/j.biocel.2010.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available from the corresponding author on reasonable request.