Abstract
Introduction
Developing effective targets, policies and services for key populations requires estimations of population sizes and HIV prevalence across countries and regions. We estimated the relative and absolute HIV prevalence among men who have sex with men (MSM), transgender women and men, and male and transgender sex workers (MSW and TGSW) in sub‐Saharan African countries using peer‐reviewed literature.
Methods
We performed a systematic review of peer‐reviewed studies assessing HIV prevalence in MSM, transgender women and men, MSW and TGSW in sub‐Saharan Africa between 2010 and 2021, following PRISMA guidelines. We searched Embase, Medline Epub, Africa Index Medicus, Africa Journal Online, Web of Science and Google Scholar. We calculated HIV prevalence ratios (PRs) between the study prevalence, and the geospatial‐, sex, time and age‐matched general population prevalence. We extrapolated results for MSM and transgender women to estimate HIV prevalence and the number living with HIV for each country in sub‐Saharan Africa using pooled review results, and regression approximations for countries with no peer‐reviewed data.
Results and discussion
We found 44 articles assessing HIV prevalence in MSM, 10 in transgender women, five in MSW and zero in transgender men and TGSW. Prevalence among MSM and transgender women was significantly higher compared to the general population: PRs of 11.3 [CI: 9.9–12.9] for MSM and 8.1 [CI: 6.9–9.6] for transgender women in Western and Central Africa, and, respectively, 1.9 [CI: 1.7–2.0] and 2.1 [CI: 1.9–2.4] in Eastern and Southern Africa. Prevalence among MSW was significantly higher in both Nigeria (PR: 12.4 [CI: 7.3–21.0]) and Kenya (PR: 8.6 [CI: 4.6–15.6]). Extrapolating our findings for MSM and transgender women resulted in an estimated HIV prevalence of 15% or higher for about 60% of all sub‐Saharan African countries for MSM, and for all but two countries for transgender women.
Conclusions
HIV prevalence among MSM and transgender women throughout sub‐Saharan Africa is alarmingly high. This high prevalence, coupled with the specific risks and vulnerabilities faced by these populations, highlights the urgent need for risk‐group‐tailored prevention and treatment interventions across the sub‐continent. There is a clear gap in knowledge on HIV prevalence among transgender men, MSW and TGSW in sub‐Saharan Africa.
Keywords: HIV, prevalence, sub‐Saharan Africa, men who have sex with men, transgender, male sex workers
1. INTRODUCTION
Sub‐Saharan Africa (SSA) is the epicentre of the HIV pandemic, with about 21 million people living with HIV [1]. Especially countries in Eastern and Southern Africa (ESA) are faced with so‐called generalized epidemics, affecting large parts of the general population, while HIV prevalence in Western and Central African (WCA) countries is mostly concentrated among people at higher risk for HIV [1]. The successful rollout of HIV treatment and prevention programmes across the subcontinent over the past decades has curbed transmission among the general population and female sex workers in many settings [2, 3, 4]. However, stigma and criminalization cause barriers to access for other key populations, such as cisgender men who have sex with men (MSM), transgender people, and cisgender male and transgender sex workers (MSW and TGSW) [5, 6, 7, 8, 9]. Currently, an estimated 54% of all new HIV infections worldwide occur among key populations and their sex partners [10], and compared to the general population, the average risk for HIV infection is about 20 times higher for sex workers and MSM, and about 10 times higher for transgender people [10]. For MSM, particular risk factors include condomless anal sex, discrimination and criminalization in many SSA settings [11, 12], while for transgender people, further HIV risks are added due to needle sharing for hormonal therapy, and transgender people are particularly vulnerable for social isolation and stigma in many countries [12, 13]. Male and TGSWs are additionally faced with the increased risks of being engaged in commercial sex, that is having many sexual partners [8, 13].
Developing effective targets, policies and interventions requires estimations of population sizes and HIV prevalence across countries and regions [14]. Furthermore, such information could improve our understanding of the relative importance of these key populations in the overall epidemic, thereby improving mathematical modelling projections on the impact of interventions for each key population and for the general epidemic. However, current population size and HIV prevalence estimates for MSM provided by the Joint United Nations Programme on HIV/AIDS (UNAIDS) largely rely on country‐reported numbers from a single survey or expert opinion—and are potentially biased [15]—while estimates for transgender people, and MSW and TGSW are mostly completely absent. Summarizing and extrapolating HIV prevalence estimates from the recent existing scientific literature to estimate country‐specific HIV prevalences for these key populations in sub‐Saharan Africa could help fill this gap in knowledge, by improving our understanding of the current HIV prevalence among these populations. While previous systematic reviews have included a limited number of studies from sub‐Saharan Africa [11, 16, 17, 18], these reviews have been conducted almost a decade ago. Several studies have been published since then, and current prevalence is likely very different from the time those reviews were conducted, as the rapid scale‐up of antiretroviral therapy (ART) and other prevention interventions across the subcontinent has substantially changed HIV epidemiology over the past decade.
The aim of this study was to estimate the recent relative and absolute HIV prevalence for MSM, transgender women and men, MSW and TGSW in sub‐Saharan Africa. We first systematically reviewed peer‐reviewed studies on the prevalence of HIV in each of these key populations, and then estimated the relative HIV prevalence by comparing prevalence estimates to geospatial‐, sex, time and age‐matched estimates of HIV prevalence in the general population. We then applied pooled estimates of relative risk and prevalence to country‐specific HIV epidemics to estimate the country‐specific HIV prevalence per risk group for each country in sub‐Saharan Africa.
2. METHODS
2.1. Search strategy and selection criteria
We followed PRISMA guidelines for systematic reviews and meta‐analyses [19]. We searched Embase, Medline Epub, Africa Index Medicus, Africa Journal Online, Web of Science and Google Scholar to identify studies that report HIV prevalence among MSM, transgender women and men, MSW and/or TGSW in sub‐Saharan African countries, in peer‐reviewed literature, reporting on data collected between 1 January 2010 and 22 October 2021. We choose this time period to strike a balance between the accuracy of our estimates of the current prevalence and relative risks in each key population versus the power to perform any meaningful meta‐analyses. We constructed search strings in collaboration with a medical librarian (see File S1 in the Appendix for the complete search strategy). We used Medical Subject Headings (MeSH) terms and “all fields” terms comprising sex work (“sex worker,” “prostitute”), LGBT people (“MSM,” “transgender,” “gay”), HIV/AIDS, prevalence (“cross‐sectional study,” “incidence,” “odds ratio”) and sub‐Saharan Africa. After an initial search in June 2018, the search has been updated in July 2020 to include the most recent publications, and again in October 2021 to include more recent publications and the regional databases Africa Index Medicus and Africa Journal Online. In both cases, no changes were made to the search terms.
We included peer‐reviewed studies that reported HIV prevalence or data from which HIV prevalence could be derived among MSM, transgender people, MSW and/or TGSW, of a site in at least one country in sub‐Saharan Africa, had a cross‐sectional or cohort study design and were published in English or French. We excluded studies that: [1] were based on self‐reported HIV status; [2] assessed subgroups of the study population (i.e. prisoners, drug‐using MSM, MSW among an MSM population), as estimates from such subgroups are likely biased towards higher HIV prevalence levels, making them not generalizable to the entire study population; [3] were a secondary analysis of previously collected data; [4] did not provide a prevalence estimate; [5] were based on data collected before 2010; and [6] failed to correctly define the different key populations (see panel S1 in Appendix). Different studies conducted at the same location were included to maximize power, except if they were based on the same dataset. In that case, we included the study presenting the greatest total number of people tested.
Two independent reviewers (MK and LvN) performed a screening of titles and abstracts of retrieved records. For those deemed eligible based on the set inclusion and exclusion criteria, full texts were examined to determine full eligibility. Any disagreements between the independent reviewers were resolved by consensus with the senior author (JACH).
2.2. Data extraction and meta‐analyses
Two authors independently extracted the following study characteristics: population studied, study location, study year, study design, recruitment method, number of participants, age distribution, type of HIV test and HIV prevalence with 95% confidence intervals (CIs). When the HIV prevalence and/or 95% CIs were not reported directly, we calculated these using the reported absolute numbers. The corresponding authors of studies were contacted if additional study information was required. The risk of bias was assessed using Joanna Briggs Institute (JBI) critical appraisal checklist (Table S2 in Appendix) [20].
For each study, we calculated a prevalence ratio (PR) of HIV prevalence in the key population of interest, compared to the HIV prevalence in the general population. We derived general population HIV prevalence data from Demographic Health Surveys (DHSs) and AIDS Indicator Surveys (AISs), which are nationally representative household surveys that often include voluntary HIV testing in adults, and have been systematically performed in many countries in sub‐Saharan Africa [21]. Typically, a DHS or AIS is performed at around 350 randomly selected sample locations in each country, and all members of about 25 households at each location are invited to participate [22]. These data were the only available general population HIV prevalence data that could be geospatially matched with locations of the studies in our review, and are generally assumed to be fairly representative of general population‐level HIV prevalence estimates [23, 24].
For each study in our review, we first geo‐located the study site, and then selected DHSs/AISs sample locations from the survey conducted closest to the year of data collection in the study, with a maximum difference of 3 years. If, after contacting corresponding authors, the year of data collection was still missing, the most reasonable DHS/AIS was selected based on the publication date of the study. We selected all sample locations within a 5‐kilometre radius from the study site, and calculated the general population HIV prevalence in the selected sample locations, standardized to the study population by age composition and gender (i.e. only males when comparing to MSM and MSW prevalence, and males and females when comparing to transgender women).
For studies without DHS/AISs data collected within 3 years before or after the study, we extracted local HIV prevalence estimates from the study by Dwyer‐Lindgren et al. [25]. They estimated yearly 5 by 5‐kilometre HIV prevalence for the whole of sub‐Saharan Africa from 2000 to 2017, for females and males (15–49 years) combined. They estimated HIV prevalence based on a variety of data sources, including local studies, antenatal care surveys and population‐based surveys, and age‐ and sex‐standardization was not possible with these data.
We calculated the PR as the ratio between the prevalence among the key population in the study and the prevalence in the general population at that location. A pooled prevalence and PR, stratified by country and region (WCA and ESA), was calculated by summing absolute numbers of all studies and calculating a combined prevalence and PR. We stratified by region to control for potential effect modification, as PRs may differ for the more concentrated epidemics in WCA versus the mixed and generalized epidemics in ESA.
For MSM and transgender women, the total number of studies identified allowed us to extrapolate HIV prevalence derived from our review to crudely estimate the country‐specific prevalence in 5 percentage point intervals (0–5%, 5–10%, 10–15%, 15–20% and >20%) for all countries in sub‐Saharan Africa. For the countries for which we had data and a sufficient number of people tested (n≥80 for MSM and n≥50 for transgender people), we divided them into the prevalence categories using the pooled country estimated prevalence. For the countries for which we had no data or an insufficient number of people tested (n<80 for MSM and n<50 for transgender people), we estimated the HIV prevalence in MSM and transgender women through a regression approximation. The cut‐off values of 80 and 50 participants, respectively, were arbitrarily chosen to ensure that studies with very small sample sizes would not dilute our regression analyses, and to ensure that countries would not be categorized based on a single study with a very small sample size.
The regression approximation was performed as follows. We first determined the relationship between the HIV prevalence in the study population and general HIV prevalence for all studies in our review by fitting a logistic Deming regression, with the HIV prevalence in the studies as the dependent variable, and corresponding HIV prevalence in the general population as the independent variable. We then applied this function to the country‐level general urban population HIV prevalence for countries for which we had insufficient peer‐reviewed data, as all peer‐reviewed studies were conducted in urban settings. The general urban population HIV prevalence for each country was estimated by multiplying general population HIV prevalence estimates derived from UNAIDS 2020 [26] with a country‐specific ratio of urban total HIV prevalence derived from DHS [21], and an average urban total HIV prevalence over all countries for countries without DHS data. The function for MSM is log odds(y) = –1.96 + 0.021x (p = 0.07), and the function for transgender women is log odds(y) = –1.64 + 0.059x (p = 0.05), where y = HIV prevalence in the key population, and x = HIV prevalence in the general population. We did not stratify our regression approximation by region. Yet, the regression models inherently capture prevalence heterogeneities across the regions, as the model is fitted using general population HIV prevalence as a predictor.
After estimating the relative HIV prevalence for each country, we roughly estimated the country‐specific absolute HIV prevalence. We first applied estimates of the proportion of MSM (1.0–4.0%) and transgender women (0.5–1.0%) within populations [27] to the United Nations population size estimates [28] to develop rough population size estimates for the key populations. We then multiplied these with prevalence estimates for the key population, assuming rural and urban HIV prevalence levels to be the same. See Appendix panel S2 for a detailed description of the applied approach.
We performed several sensitivity and validation analyses on our prevalence estimations. First, we determined the impact of preferring DHS data over data from Dwyer‐Lindgren et al. [25] as a source for HIV prevalence in the local general population by running our analyses using both DHS and Dwyer‐Lindgren et al. [25] data for studies where this was possible and compared the resulting PRs. Second, we tested the validity of our regression approximation by applying the model to countries where we had used pooled peer‐reviewed data to estimate the country‐level prevalence and compared the outcomes. Third, we tested whether the year of data collection, legal or illegal status of same‐sex relationships and an indicator for the severity of anti‐LGBT laws [30] could explain some of the observed heterogeneity in the relationship between key and general population HIV prevalence by testing them as predictors in a logistic regression model. Fourth, we determined whether UNAIDS [26] reported point estimates of prevalence, based on grey literature, fell within the prevalence category assigned to each country based on our estimates.
All analyses were done using R version 4.0.0 and ArcGIS Pro version 2.5.0.
2.3. Role of funding source, interests and registration
The study's funder had no role in study design, data collection, data analysis, data interpretation, writing or submitting of the report. Independent authors declare no competing interests. The review was not registered.
3. RESULTS AND DISCUSSION
Our search identified 9476 articles, of which 2587 were unique records (Figure 1). Based on the screening of the title and abstract, 207 full texts were retrieved, of which 48 met our inclusion criteria [12, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 82, 83, 84, 85]. Most studies were excluded because they either only collected self‐reported HIV status, re‐analysed previously collected data that was already part of the review or provided data collected pre‐2010. We did not find studies with conflated gender group definitions. A complete overview of each literature search is given in Table S1. The majority of publications (91.7%, 44/48) provided HIV prevalence data on MSM (Table S3 in Appendix), compared to 20.8% (10/48) on transgender women (Table S4 in Appendix) and 10.4% (5/48) on MSW (Table S5 in Appendix). No articles were identified with relevant data on transgender men or TGSW. The 48 articles covered 21 of the 47 sub‐Saharan African countries (44.7%); 10 in WCA and 11 in ESA (Figure 2). The five studies that assessed HIV prevalence in MSW covered only two countries: Nigeria and Kenya [40, 49, 72, 73, 74]. All studies were performed in urban settings, and all were deemed of sufficient quality based on the JBI critical appraisal checklist (see Appendix Table S2).
Figure 1.
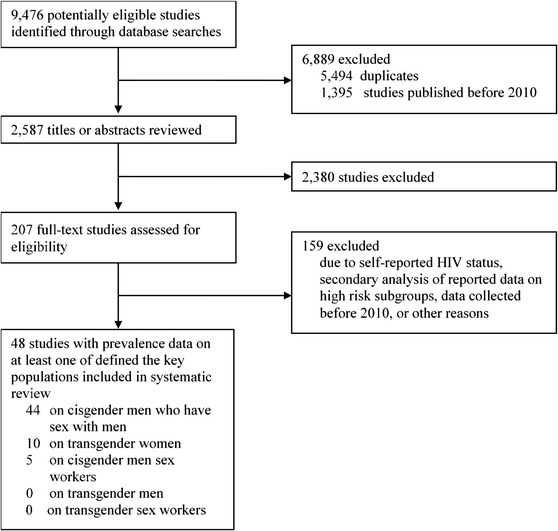
Flow chart of study selection disposition.
Figure 2.
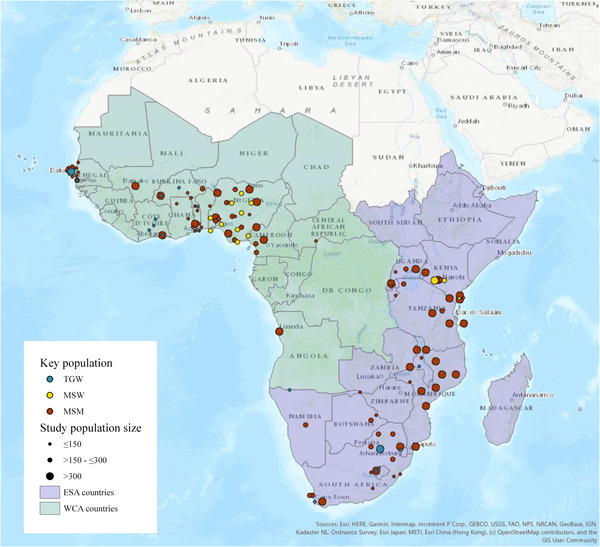
Locations of the included studies on HIV prevalence in men who have sex with men (MSM), transgender women (TGW) and male sex workers (MSW) in sub‐Saharan Africa. Abbreviations: ESA countries, Eastern and Southern African countries; WCA countries, Western and Central African countries.
Study‐, country‐ and region‐specific PRs for HIV in MSM, compared to the general male population, are shown in Figure 3 for WCA and in Figure 4 for ESA. The reported HIV prevalence among MSM in WCA ranged between 4.3% in Angola and 51.0% in Senegal. Prevalence was significantly higher in 27 of the 29 study locations, with a weighted average PR of 11.3 (95% CI: 9.9–12.9). In ESA (Figure 4), the prevalence ranged from 7.5% in Mozambique to 36.0% in South Africa. Only 24 out of the 46 study locations showed a significantly higher HIV prevalence among MSM, with a weighted average PR of 1.9 (95% CI: 1.7–2.0).
Figure 3.
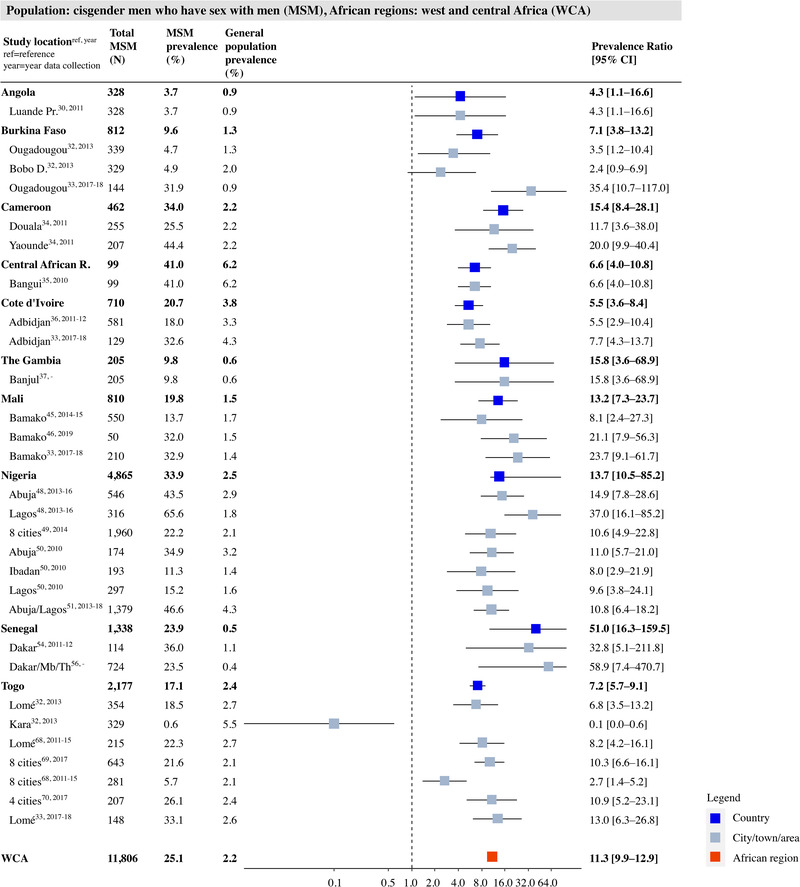
HIV prevalence and prevalence ratio (PR) for men who have sex with men (MSM) per study place, country and region in the West and Central Africa (WCA). Grey squares represent individual study locations, and weighted averages for country and region levels are in blue and red. PRs are relative risks compared to the geospatially matched general male population aged 15–49. Abbreviations: 95% CI, 95% confidence interval; MSM, men who have sex with men.
Figure 4.
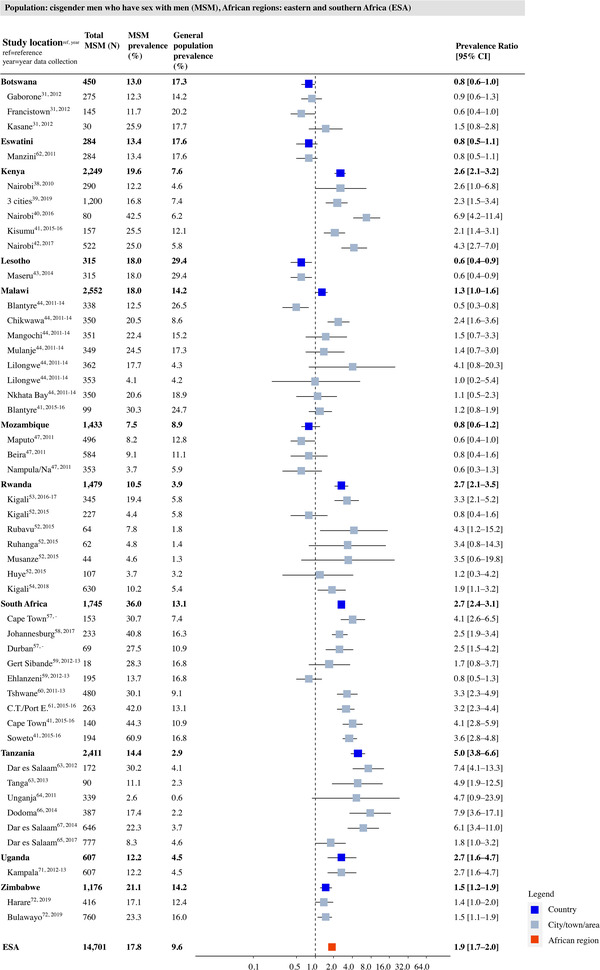
HIV prevalence and prevalence ratio (PR) for men who have sex with men (MSM) per study place, country and region in Eastern and Southern Africa (ESA). Grey squares represent individual study locations, and weighted averages for country and region levels are in blue and red. PRs are relative risks compared to the geospatially matched general male population aged 15–49. Abbreviations: 95% CI, 95% confidence interval; MSM, men who have sex with men.
Study‐, country‐ and region‐specific PRs for transgender women are shown in Figure 5. In WCA, the prevalence ranged from 4.0 in Burkina Faso to 50.0 in the Gambia. Seven out of nine study locations showed significantly higher HIV prevalence among transgender women, with PRs ranging from 1.6 to 86.7 (upper panel in Figure 5]. The weighted average PR was 8.1 (95% CI: 6.9–9.6). For ESA, the prevalence ranged from 9.4% in Rwanda to 63.5% in South Africa. Ten out of 14 study locations showed a significantly higher prevalence among transgender women, with PRs ranging from 0.5 to 4.7 and a weighted average PR of 2.1 (95% CI: 1.9–2.4; lower panel in Figure 5].
Figure 5.
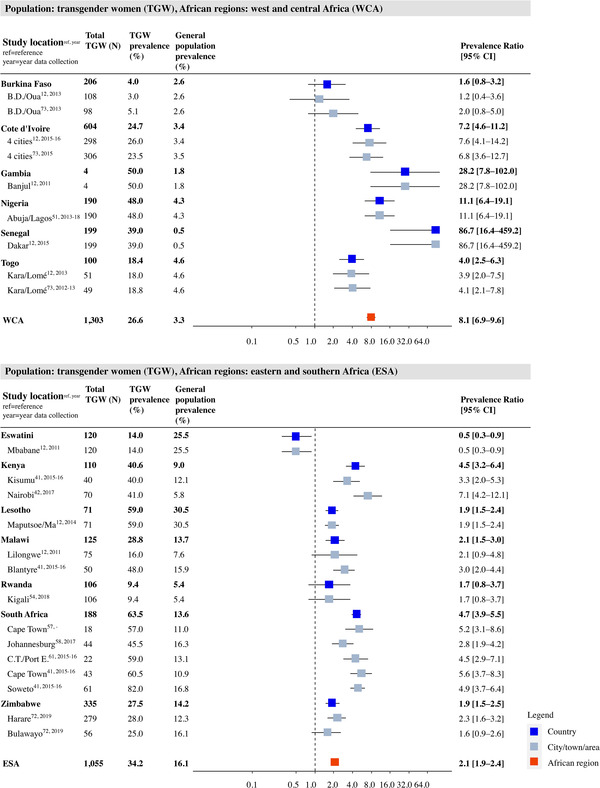
HIV prevalence and prevalence ratio (PR) for transgender women (TGW) per study place, country and region in West and Central Africa (WCA) and Eastern and Southern Africa (ESA). Grey squares represent individual study locations, and weighted averages for country and region levels are in blue and red. PRs are relative risks compared to the geospatially matched general population aged 15–49. Abbreviations: 95% CI, 95% confidence interval; ESA, Eastern and Southern Africa; TGW, transgender women; WCA, Western and Central Africa.
Study‐, country‐ and region‐specific PRs for MSW are shown in Figure 6. For MSW, all five study locations showed a significantly higher prevalence compared to the general population, with an overall PR of 8.6 (95% CI 4.6; 15.8) for Nigeria (upper panel of Figure 6] and 12.4 (95% CI: 7.3–21.0) for Kenya (lower panel of Figure 6].
Figure 6.
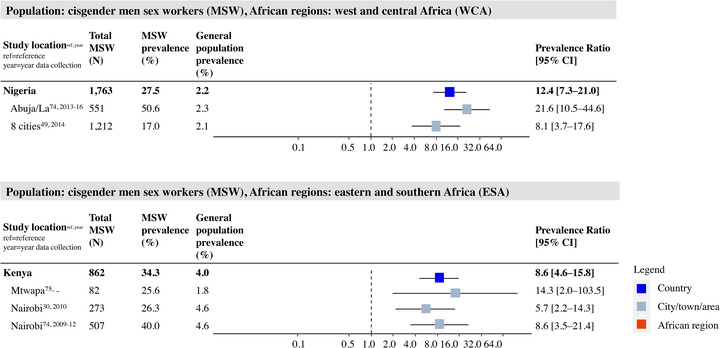
HIV prevalence and prevalence ratio (PR) for male sex workers (MSW) per study place, country and region in West and Central Africa (WCA) and Eastern and Southern Africa (ESA). Grey squares represent individual study locations, and weighted averages for country and region levels are in blue and red. PRs are relative risks compared to the geospatially matched general male population aged 15–49. Abbreviations: 95% CI, 95% confidence interval; ESA, Eastern and Southern Africa; MSW, male sex worker; WCA, Western and Central Africa.
We had sufficient data points for both MSM and transgender women to extrapolate our findings to estimate HIV prevalence for the two populations in countries in sub‐Saharan Africa for which we found no studies, using a regression approximation (Figures S1 and S2; Tables S6–S9). The resulting estimated country‐specific HIV prevalences among MSM and transgender women for all countries in sub‐Saharan Africa are presented in Figure 7c and f and are compared to general population prevalence (Figure 7a and d) and UNAIDS estimations (Figure 7b and e). For MSM, we estimated an HIV prevalence of 15–20% for 31 out of the 47 countries (66%) and a prevalence of ≥20% for seven countries (15%). For transgender women, the estimated HIV prevalence was above 15% for all but two countries and was ≥20% for 16 out of 47 countries (34%). For only 11 out of the 37 countries (30%), the UNAIDS point estimate fell within the prevalence categories assigned to those countries in our study (Figure 7 and Appendix Tables S12 and S13). For most countries (20 out of 37), our estimated HIV prevalence for MSM was higher than those published by UNAIDS. Two of the seven countries with UNAIDS reported prevalence data on transgender women. Two matched our estimates, three were higher and two were lower. Our estimations roughly translate into about 600,000–2.2 million MSM and 400,000–800,000 transgender women currently living with HIV in sub‐Saharan Africa (see Appendix Tables S10 and S11 for more details).
Figure 7.
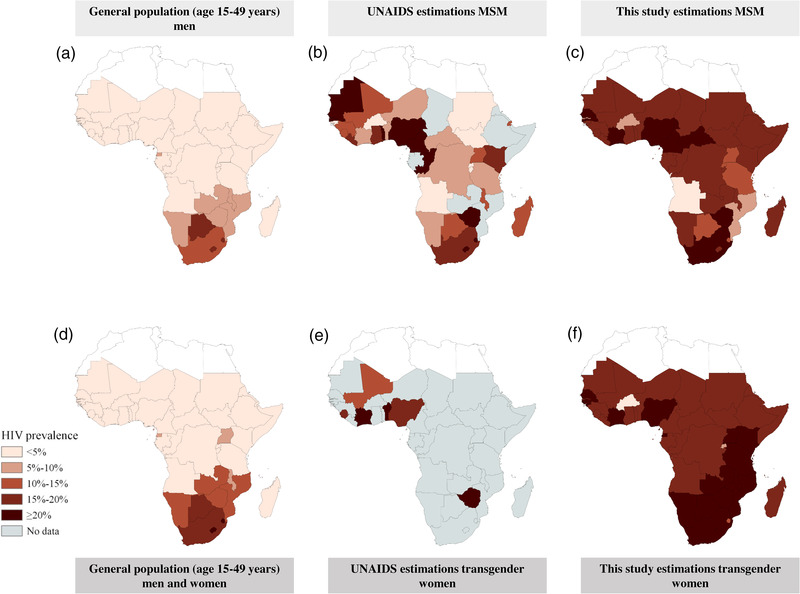
Maps of country‐level HIV prevalence levels for the general population (left column), UNAIDS reported prevalence in MSM and transgender women (middle column) and estimated HIV prevalence among MSM and transgender women based on peer‐reviewed literature (right column). The peer‐reviewed literature estimations are based on the country‐level weighted HIV prevalence derived from the included studies in the systematic literature search. For countries for which we did not find sufficient data, we estimated the country‐level prevalence using a logistic Deming regression model fitted to the relationship between the key population and general population HIV prevalence in peer‐reviewed literature.
Our sensitivity analysis showed that the impact of choosing DHS data over data from Dwyer‐Lindgren et al. had little impact on estimated PRs, as none were significantly different in settings where we could do both (see Appendix Table S16). Furthermore, the year of the survey was borderline significantly associated with a higher HIV prevalence among MSM (p = 0.04) and not significant for TGW (p = 0.12), while the legal status of same‐sex relationships and severity of anti‐LGBT laws were not significantly associated for both MSM (p = 0.9 and 0.5, respectively) and TGW (p = 0.08 and 0.3, respectively) (Appendix Tables S7 and S9). When validating regression approximations against data‐based country prevalence estimates, we found that only about 20% of countries would end up in the same prevalence category (see Appendix Tables S14 and S15).
4. DISCUSSION
Our systematic review identified 44 articles assessing HIV prevalence in MSM, 10 in transgender women, five in MSW and zero in transgender men and TGSW in sub‐Saharan Africa since 2010. Prevalence among MSM and transgender women was significantly higher than the general population, with PRs for MSM and transgender women ranging from 11.3 and 8.1, respectively, in WCA, to 1.9 and 2.1 in ESA. Furthermore, the prevalence among MSW was also significantly higher in both Nigeria (PR: 12.4 [CI: 7.3–21.0]) and Kenya (PR: 8.6 [CI: 4.6–15.6]), the only two countries with data on MSW. Extrapolating our findings to country‐ and region‐specific estimates resulted in an estimated HIV prevalence of 15–20% among MSM for roughly half of the sub‐Saharan African countries and seven countries with an estimated HIV prevalence of ≥20%. For transgender women, we estimated an HIV prevalence of 15–20% or ≥20% for all but two countries. These estimates roughly translate into about 600,000–2.2 million MSM and 400,000–800,000 transgender women currently living with HIV in sub‐Saharan Africa.
Our study is a major update of earlier reviews of studies on the HIV prevalence among MSM and transgender women in sub‐Saharan Africa [11, 20, 21, 22]. In addition, we are the first to extrapolate geospatial, age, time and sex‐matched associations with the general population HIV prevalence in each study to estimate the HIV prevalence among MSM and transgender women in all countries in sub‐Saharan Africa. It is encouraging that our findings on the PR in sub‐Saharan Africa are consistent with those from Hessou et al. [73] for MSM (a PR for Western Central Africa of 14.5 vs. 11.3 in our study, and 3.4 for Eastern Africa and 1.2 for Southern Africa vs. 1.9 for Eastern Southern Africa in our study), and in line with global estimates on HIV prevalences among transgender people [21].
Our estimates and extrapolations are important when assessing country‐level needs and targets for key population‐specific services, and estimating the required resources to meet those needs and targets. Annual HIV epidemic updates published by UNAIDS [26] provide HIV prevalence estimates for the majority of sub‐Saharan African countries on MSM, usually based on country‐reported results from a single survey that has not undergone peer review, or even based on expert opinion alone. These estimates fitted within the same prevalence categories estimated by our study for only about 30% of all countries, highlighting the need to consider incorporating peer‐reviewed evidence in the prevalence estimation exercise for these populations. UNAIDS estimates on the prevalence in transgender people are available for only a few countries, and no information exists on MSW and TGSW.
Even though it was not possible to extrapolate the findings of MSW to country‐ and region‐specific estimates, the PR for MSW compared to MSM was higher from Kenya (respectively, 8.6 vs. 2.6) and comparable for Nigeria (respectively, 12.4 vs. 13.7), suggesting similar or higher country‐ and region HIV levels for MSW. For TGSW and transgender men, we did not find any scientific literature showing how these groups are still highly underrepresented as a key population in HIV research and programming. We highly recommend more quantitative research into the population sizes, HIV prevalence, risks, service needs and uptake for MSW, transgender women and men, and TGSW throughout SSA.
While our results do not cover access to services, limited data on access to services for MSM and transgender people suggest that access to treatment is extremely poor compared to the general population. A study that tested for antiretroviral adherence in 183 HIV‐infected MSM and transgender women in several sub‐Saharan African countries found that only 34% had antiretroviral residues in their blood, and 18% of those were not virally suppressed [80]. In addition, a systematic review by Stannah et al. [27] showed that, although HIV testing among MSM had increased significantly over the past decade, pooled estimates showed only about 23% of MSM living with HIV to be on treatment. A rough back‐of‐the‐envelope calculation using these findings and our results shows that if treatment coverage for MSM is indeed only about 25%, about 500,000–1.7 million MSM in sub‐Saharan Africa require treatment but are not receiving it. Likewise, effective pre‐exposure prophylaxis (PrEP) services rollout remains challenging. While motivation to use PrEP seems high [81], Wahome et al. found low levels of PrEP adherence and the absence of an effect on HIV incidence among MSM in SSA [79]. Despite a lack of peer‐reviewed data, it seems reasonable to assume similar or even poorer access to HIV prevention and treatment for MSW, transgender people and TGSW compared to MSM [82]. Failure to provide adequate services to these key populations could result in higher rates of morbidity, mortality and onward transmission [83]. It is essential that these services are sensitive to the unique vulnerabilities and needs of each group [9], and should coincide with minimizing structural barriers against LGBT+ people and sex workers at the personal, societal and institutional levels [9, 13, 21].
Our study has several limitations. First, we only incorporated peer‐reviewed studies. We decided not to directly include grey literature in developing our estimates due to the likely heterogeneity in quality and large risk of bias in provided estimates. Our comparison between estimates derived from peer‐reviewed literature (our review) and grey literature (UNAIDS estimates) confirms the high levels of heterogeneity between the two. Nevertheless, our peer review‐based estimates should also be cautiously interpreted in light of data limitations, selection biases and small sample sizes in the individual studies. Second, the majority of the included studies used respondent‐driven sampling (RDS) as their recruitment method. RDS has been described as the preferred sampling method for populations without a readily available sampling frame, though it has potential limitations [62, 82, 84, 85]. For example, most studies did not report on additional important indicators, such as to which extent the sample was part of the same social network [84]. People in a highly interconnected social network might not be representative of the population as a whole, as people who are not part of these networks may have different underlying characteristics and risks than those within the network. However, it is difficult to determine the direction of the potential bias, as we do not have a reliable gold standard from, for example a population‐based survey. Third, for some studies, we could not use sex‐ and age‐match general population‐level prevalence to calculate a PR, as no DHS surveys [21] were conducted within a 3‐year time window around the study. We used estimated HIV prevalence in all adults aged 15–49 years as published by Dwyer‐Lindgren et al. [25] instead. However, it is reassuring that, for areas where we could use both, reverting to PRs using data from Dwyer‐Lindgren et al. [25] did not result in any major deviations in estimated PRs (Appendix Table S16). Fourth, age standardization was often based on relatively broad age ranges (e.g. interquartile ranges) reported by the individual studies. It is likely to assume that within broad standardization categories, the age distribution among the key populations was relatively younger than the general population, resulting in an underestimation of the actual PRs. Fifth, our estimates for countries where we had no data are based on a statistical model derived from the systematic review. The sole independent predictor is HIV prevalence in the general population. These estimations should be interpreted with caution, as the regression approximation correctly predicted the data‐based prevalence categories for countries with sufficient peer‐reviewed data only 20% of the time (Appendix Tables S14 and S15). However, while we place higher confidence in estimates based on peer‐reviewed directly, it should be noted that these can also be based on relatively sparse numbers of observations, making a predicted prevalence based on pooling estimates across countries in a regression approximation not necessarily less valid. Sixth, we did not control for the year of data collection in our main analysis. Yet, we observed a borderline significant trend towards higher prevalence in later years for MSM in our sensitivity analysis (p = 0.04) (Appendix Table S7). This suggests that by pooling the 10 years covered by our review, we may have slightly underestimated the current PRs and HIV prevalence among MSM in sub‐Saharan Africa. However, whether this trend in time reflects an actual divergent trend in prevalence due to increased incidence and/or survival, or is caused by improved sampling approaches by which researchers are increasingly better at finding higher‐risk individuals, is difficult to determine. Seventh, all study locations were urban settings, and our PRs and estimations are, therefore, based on HIV prevalences in urban settings. We, therefore, assumed HIV prevalence for MSM and transgender women to be the same for rural settings when extrapolating our findings to national‐level estimates.
5. CONCLUSIONS
We show that the current HIV prevalence in MSM and transgender women in sub‐Saharan Africa is alarmingly and consistently high across all regions and countries. This high prevalence, coupled with the specific risks and vulnerabilities these populations face, highlights the urgent need for risk‐group‐tailored prevention and treatment interventions across the sub‐continent. The lack of studies in multiple countries on HIV prevalences, especially among transgender people and cisgender male and TGSWs, highlights the clear need for more research.
COMPETING INTERESTS
The authors declare no competing interests.
AUTHORS’ CONTRIBUTIONS
MK and JACH conceptualized and designed the study. MK, LvN and LAH performed data collection and interpretation. MK, CAB, LvN, LAH and JACH performed all analyses. MK, CAB and JACH wrote the first draft of the manuscript. All authors contributed to writing and editing the final version of the manuscript. FMC provided overall supervision.
FUNDING
We would like to thank Aidsfonds Netherlands for funding this research project (P‐29702).
Supporting information
Figure S1: Association between the HIV prevalence in the general population and in MSM.
Figure S2: Univariate Association between the HIV prevalence in the general population and in transgender women (TGW).
Table S1: Overview of complete literature searches, including updates.
Table S2: Bias assessment.
Table S3: Overview of the prevalence of HIV infection in cisgender men who have sex with men (MSM) in the included studies and prevalence of HIV in the general male population at the study location.
Table S4: Overview of the prevalence of HIV infection in transgender women in the included studies and prevalence of HIV in the general male and female population at the study location.
Table S5: Overview of the prevalence of HIV infection in cisgender male sex workers (MSW) in the included studies and prevalence of HIV in the general male population at the study location.
Table S6: Logistic Deming regression of the relationship between HIV prevalence in the general population and in MSM (used for extrapolation).
Table S7: Univariate logistic regression on relationship between HIV prevalence in MSM and potential confounders.
Table S8: Logistic Deming regression of the relationship between HIV prevalence in the general population and in transgender women (used for extrapolation).
Table S9: Univariate logistic regression on relationship between HIV prevalence in transgender women and potential confounders.
Table S10: Review estimations for men who have sex with men (MSM).
Table S11: Review estimations for transgender women (TGW).
Table S12: Comparison of UNAIDS estimations versus estimations from this study for men who have sex with men (MSM).
Table S13: Comparison of UNAIDS estimations versus estimations from this study for transgender women.
Table S14: Comparison of estimations derived from study data to estimations derived regression model for men who have sex with men (MSM).
Table S15: Comparison of estimations derived from study data to estimations derived regression model for transgender women.
Table S16: Assesment of the impact of the use of geospatially matched Demographic Health Surveys (DHS) (52) data versus Dwyer‐Lindgren et al. (56) (DL) data.
ACKNOWLEDGEMENTS
We would like to thank Gerdien B. de Jonge, Wichor M. Bramer, Sabrina T. G. Gunput, Elise Krabbendam, Biomedical Information Specialists from the Erasmus MC Medical Library, for their assistance in formulating and performing the literature search.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. UNAIDS . Fact sheet ‐ World AIDS Day 2021. 2020. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf. Accessed 1 June 2022.
- 2. Bor J, Herbst AJ, Newell ML, Bärnighausen T. Increases in adult life expectancy in rural South Africa: valuing the scale‐up of HIV treatment. Science. 2013;339(6122):961–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McNairy ML, El‐Sadr WM. Antiretroviral therapy for the prevention of HIV transmission: what will it take? Clin Infect Dis. 2014;58(7):1003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chersich MF, Luchters S, Ntaganira I, Gerbase A, Lo YR, Scorgie F, et al. Priority interventions to reduce HIV transmission in sex work settings in sub‐Saharan Africa and delivery of these services. J Int AIDS Soc. 2013;16:17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacAllister J, Sherwood J, Galjour J, Robbins S, Zhao J, Dam K, et al. A comprehensive review of available epidemiologic and HIV service data for female sex workers, men who have sex with men, and people who inject drugs in select West and Central African countries. J Acquir Immune Defic Syndr. 2015;68:S83–90. [DOI] [PubMed] [Google Scholar]
- 6. Beyrer C, Baral SD, Collins C, Richardson ET, Sullivan PS, Sanchez J, et al. The global response to HIV in men who have sex with men. Lancet. 2016;388(10040):198–206. [DOI] [PubMed] [Google Scholar]
- 7. Beyrer C, Baral SD, Griensven FV, Goodreau SM. Global epidemiology of HIV infection in men who have sex with men. Elsevier; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baral SD, Friedman MR, Geibel S, Rebe K, Bozhinov B, Diouf D, et al. Male sex workers: practices, contexts, and vulnerabilities for HIV acquisition and transmission. Lancet. 2015;385(9964):260–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poteat T, Reisner SL, Radix A. HIV epidemics among transgender women. Curr Opin HIV AIDS. 2014;9(2):168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. UNAIDS . UNAIDS data 2019. 2019. https://www.unaids.org/sites/default/files/media_asset/2019‐UNAIDS‐data_en.pdf. Accessed 1 June 2022.
- 11. Baral SD, Grosso A, Holland C, Papworth E. The epidemiology of HIV among men who have sex with men in countries with generalized HIV epidemics. Curr Opin HIV AIDS. 2014;9(2):156–67. [DOI] [PubMed] [Google Scholar]
- 12. Poteat T, Ackerman B, Diouf D, Ceesay N, Mothopeng T, Odette KZ, et al. HIV prevalence and behavioral and psychosocial factors among transgender women and cisgender men who have sex with men in 8 African countries: a cross‐sectional analysis. PLoS Med. 2017;14(11):e1002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Poteat T, Wirtz AL, Radix A, Borquez A, Silva‐Santisteban A, Deutsch MB, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet. 2015;385(9964):274–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. UNAIDS . Confronting inequalities. Lessons for pandemic responses from 40 years of AIDS. 2021. https://www.unaids.org/sites/default/files/media_asset/2021‐global‐aids‐update_en.pdf. Accessed 1 June 2022.
- 15. UNAIDS . Estimating the size of populations at risk for HIV. 2003. https://data.unaids.org/publications/external‐documents/estimatingpopsizes_en.pdf. Accessed 1 June 2022.
- 16. Baral S, Sifakis F, Cleghorn F, Beyrer C. Elevated risk for HIV infection among men who have sex with men in low‐ and middle‐income countries 2000–2006: a systematic review. PLoS Med. 2007;4(12):e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith AD, Tapsoba P, Peshu N, Sanders EJ, Jaffe HW. Men who have sex with men and HIV/AIDS in sub‐Saharan Africa. Lancet. 2009;374(9687):416–22. [DOI] [PubMed] [Google Scholar]
- 18. Baral SD, Poteat T, Stromdahl S, Wirtz AL, Guadamuz TE, Beyrer C. Worldwide burden of HIV in transgender women: a systematic review and meta‐analysis. Lancet Infect Dis. 2013;13(3):214–22. [DOI] [PubMed] [Google Scholar]
- 19. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthcare. 2015;13(3):147–53. [DOI] [PubMed] [Google Scholar]
- 21. DHS . The DHS program STATcompiler. (cited 2022 Mar 17) http://www.statcompiler.com
- 22. DHS . Methodology. (cited 2022 Mar 17) https://www.dhsprogram.com/What‐We‐Do/Survey‐Types/DHS.cfm
- 23. Bulstra CA, Hontelez JAC, Giardina F, Steen R, Nagelkerke NJD, Bärnighausen T, et al. Mapping and characterising areas with high levels of HIV transmission in sub‐Saharan Africa: a geospatial analysis of national survey data. PLoS Med. 2020;17(3):e1003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cuadros DF, Li J, Branscum AJ, Akullian A, Jia P, Mziray EN, et al. Mapping the spatial variability of HIV infection in sub‐Saharan Africa: effective information for localized HIV prevention and control. Sci Rep. 2017;7(1):9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dwyer‐Lindgren L, Cork MA, Sligar A, Steuben KM, Wilson KF, Provost NR, et al. Mapping HIV prevalence in sub‐Saharan Africa between 2000 and 2017. Nature. 2019;570(7760):189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. UNAIDS . Countries: UNAIDS. 2020. https://www.unaids.org/en/regionscountries/countries. Accessed 1 June 2022.
- 27. Stannah J, Dale E, Elmes J, Staunton R, Beyrer C, Mitchell KM, et al. HIV testing and engagement with the HIV treatment cascade among men who have sex with men in Africa: a systematic review and meta‐analysis. Lancet HIV. 2019;6(11):e769–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. United Nations . Population: United Nations Department of Social Affairs. (cited 2022 Mar 17) https://www.un.org/en/development/desa/population/publications/database/index.asp. Accessed 1 June 2022.
- 29. Caceres CF, Konda K, Segura ER, Lyerla R. Epidemiology of male same‐sex behaviour and associated sexual health indicators in low‐ and middle‐income countries: 2003–2007 estimates. Sex Transm Infect. 2008;84(Suppl 1):i49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tafuma TA, Merrigan MB, Okui LA, Lebelonyane R, Bolebantswe J, Mine M, et al. HIV/sexually transmitted infection prevalence and sexual behavior of men who have sex with men in 3 districts of Botswana: results from the 2012 biobehavioral survey. Sex Transm Dis. 2014;41(8):480–5. [DOI] [PubMed] [Google Scholar]
- 31. Kendall C, Kerr LRFS, Mota RMS, Cavalcante S, Macena RHM, Chen S, et al. Population size, HIV, and behavior among MSM in Luanda, Angola: challenges and findings in the first ever HIV and syphilis biological and behavioral survey. J Acquir Immune Defic Syndr. 2014;66(5):544–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holland CE, Kouanda S, Lougué M, Pitche VP, Schwartz S, Anato S, et al. Using population‐size estimation and cross‐sectional survey methods to evaluate HIV service coverage among key populations in Burkina Faso and Togo. Public Health Rep. 2016;131(6):773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yaya I, Boyer V, Ehlan PA, Coulibaly A, Agboyibor MK, Traoré I, et al. Heterogeneity in the prevalence of high‐risk human papillomavirus infection in HIV‐negative and HIV‐positive men who have sex with men in West Africa. Clin Infect Dis. 2021;73(12):2184–92. [DOI] [PubMed] [Google Scholar]
- 34. Park JN, Papworth E, Kassegne S, Moukam L, Billong SC, Macauley I, et al. HIV prevalence and factors associated with HIV infection among men who have sex with men in Cameroon. J Int AIDS Soc. 2013;16(Suppl 3):18752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Marcel MS, de Dieu LJ, Magloire CPS, Grésenguet G, Ralph‐Sydney MB, Piette D, et al. Persistent high‐risk behavior and escalating HIV, syphilis and hepatitis B incidences among men who have sex with men living in Bangui, Central African Republic. Pan Afr Med J. 2018;29:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hakim AJ, Aho J, Semde G, Diarrassouba M, Ehoussou K, Vuylsteke B, et al. The epidemiology of HIV and prevention needs of men who have sex with men in Abidjan, Cote d'Ivoire. PLoS One. 2015;10(4):e0125218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mason K, Ketende S, Peitzmeier S, Ceesay N, Diouf D, Loum J, et al. A cross‐sectional analysis of population demographics, HIV knowledge and risk behaviors, and prevalence and associations of HIV among men who have sex with men in the Gambia. AIDS Res Hum Retroviruses. 2013;29(12):1547–52. [DOI] [PubMed] [Google Scholar]
- 38. Muraguri N, Tun W, Okal J, Broz D, Fisher Raymond H, Kellogg T, et al. HIV and STI prevalence and risk factors among male sex workers and other men who have sex with men in Nairobi, Kenya. J Acquir Immune Defic Syndr. 2015;68(1):91–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bhattacharjee P, Isac S, Musyoki H, Emmanuel F, Olango K, Kuria S, et al. HIV prevalence, testing and treatment among men who have sex with men through engagement in virtual sexual networks in Kenya: a cross‐sectional bio‐behavioural study. J Int AIDS Soc. 2020;23(S2):e25516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gebrebrhan H, Kambaran C, Sivro A, Adhiambo W, Siele N, Becker MG, et al. Rectal microbiota diversity in Kenyan MSM is inversely associated with frequency of receptive anal sex, independent of HIV status. AIDS. 2021;35(7):1091–101. [DOI] [PubMed] [Google Scholar]
- 41. Smith AD, Kimani J, Kabuti R, Weatherburn P, Fearon E, Bourne A. HIV burden and correlates of infection among transfeminine people and cisgender men who have sex with men in Nairobi, Kenya: an observational study. Lancet HIV. 2021;8(5):e274–83. [DOI] [PubMed] [Google Scholar]
- 42. Stahlman S, Johnston LG, Yah C, Ketende S, Maziya S, Trapence G, et al. Respondent‐driven sampling as a recruitment method for men who have sex with men in southern sub‐Saharan Africa: a cross‐sectional analysis by wave. Sex Transm Infect. 2016;92(4):292–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wirtz AL, Trapence G, Kamba D, Gama V, Chalera R, Jumbe V, et al. Geographical disparities in HIV prevalence and care among men who have sex with men in Malawi: results from a multisite cross‐sectional survey. Lancet HIV. 2017;4(6):e260–9. [DOI] [PubMed] [Google Scholar]
- 44. Koyalta D, Mboumba Bouassa RS, Maiga A, Balde A, Bagendabanga JB, Alinity AA, et al. High prevalence of anal oncogenic human papillomavirus infection in young men who have sex with men living in Bamako, Mali. Infect Agents Cancer. 2021;16(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nalá R, Cummings B, Horth R, Inguane C, Benedetti M, Chissano M, et al. Men who have sex with men in Mozambique: identifying a hidden population at high‐risk for HIV. AIDS Behav. 2015;19(2):393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keshinro B, Crowell TA, Nowak RG, Adebajo S, Peel S, Gaydos CA, et al. High prevalence of HIV, chlamydia and gonorrhoea among men who have sex with men and transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc. 2016;19(1):21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bamgboye EA, Badru T, Bamgboye A. Transactional sex between men and its implications on HIV and sexually transmitted infections in Nigeria. J Sex Transm Dis. 2017;2017:1810346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vu L, Adebajo S, Tun W, Sheehy M, Karlyn A, Njab J, et al. High HIV prevalence among men who have sex with men in Nigeria: implications for combination prevention. J Acquir Immune Defic Syndr. 2013;63(2):221–7. [DOI] [PubMed] [Google Scholar]
- 49. Ramadhani HO, Crowell TA, Nowak RG, Ndembi N, Kayode BO, Kokogho A, et al. Association of age with healthcare needs and engagement among Nigerian men who have sex with men and transgender women: cross‐sectional and longitudinal analyses from an observational cohort. J Int AIDS Soc. 2020;23(6):e25599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murenzi G, Kim HY, Munyaneza A, Tuyisenge P, Zawadi TM, Buteera AM, et al. Anogenital human papillomavirus and HIV infection in Rwandan men who have sex with men. J Acquir Immune Defic Syndr. 2020;84(5):463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Drame FM, Crawford EE, Diouf D, Beyrer C, Baral SD. A pilot cohort study to assess the feasibility of HIV prevention science research among men who have sex with men in Dakar, Senegal. J Int AIDS Soc. 2013;16(Suppl 3):18753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lyons CE, Ketende S, Diouf D, Drame FM, Liestman B, Coly K, et al. Potential impact of integrated stigma mitigation interventions in improving HIV/AIDS service delivery and uptake for key populations in Senegal. J Acquir Immune Defic Syndr. 2017;74:S52–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jobson G, Tucker A, de Swardt G, Rebe K, Struthers H, McIntyre J, et al. Gender identity and HIV risk among men who have sex with men in Cape Town, South Africa.AIDS Care. 2018. [DOI] [PubMed] [Google Scholar]
- 54. Fearon E, Tenza S, Mokoena C, Moodley K, Smith AD, Bourne A, et al. HIV testing, care and viral suppression among men who have sex with men and transgender individuals in Johannesburg, South Africa. PLoS One. 2020;15(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lane T, Osmand T, Marr A, Shade SB, Dunkle K, Sandfort T, et al. The Mpumalanga Men's Study (MPMS): results of a baseline biological and behavioral HIV surveillance survey in two MSM communities in South Africa. PLoS One. 2014;9(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sandfort TG, Lane T, Dolezal C, Reddy V. Gender expression and risk of HIV infection among Black South African men who have sex with men. AIDS Behav. 2015;19(12):2270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sullivan PS, Phaswana‐Mafuya N, Baral SD, Valencia R, Zahn R, Dominguez K, et al. HIV prevalence and incidence in a cohort of South African men and transgender women who have sex with men: the Sibanye Methods for Prevention Packages Programme (MP3) project. J Int AIDS Soc. 2020;23(S6):e25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baral SD, Ketende S, Mnisi Z, Mabuza X, Grosso A, Sithole B, et al. A cross‐sectional assessment of the burden of HIV and associated individual‐ and structural‐level characteristics among men who have sex with men in Swaziland. J Int AIDS Soc. 2013;16(Suppl 3):18768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ross MW, Nyoni J, Ahaneku HO, Mbwambo J, McClelland RS, McCurdy SA. High HIV seroprevalence, rectal STIs and risky sexual behaviour in men who have sex with men in Dar es Salaam and Tanga, Tanzania. BMJ Open. 2014;4(8):e006175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Khatib A, Haji S, Khamis M, Said C, Khalid F, Dahoma M, et al. Reproducibility of respondent‐driven sampling (RDS) in repeat surveys of men who have sex with men, Unguja, Zanzibar. AIDS Behav. 2017;21(7):2180–7. [DOI] [PubMed] [Google Scholar]
- 61. Alexander Ishungisa M, Moen K, Leyna G, Makyao N, Ramadhan A, Lange T, et al. HIV prevalence among men who have sex with men following the implementation of the HIV preventive guideline in Tanzania: respondent‐driven sampling survey. BMJ Open. 2020;10(10):e036460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mmbaga EJ, Moen K, Makyao N, Mpembeni R, Leshabari MT. HIV and STIs among men who have sex with men in Dodoma municipality, Tanzania: a cross‐sectional study. Sex Transm Infect. 2017;93(5):314–9. [DOI] [PubMed] [Google Scholar]
- 63. Mmbaga EJ, Moen K, Leyna GH, Mpembeni R, Leshabari MT. HIV prevalence and associated risk factors among men who have sex with men in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2018;77(3):243–9. [DOI] [PubMed] [Google Scholar]
- 64. Teclessou JN, Akakpo SA, Ekouevi KD, Koumagnanou G, Singo‐Tokofai A, Pitche PV. Evolution of HIV prevalence and behavioral factors among MSM in Togo between 2011 and 2015. Pan Afr Med J. 2017;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tchankoni MK, Gbeasor‐Komlanvi FA, Bitty‐Anderson AM, Sewu EK, Zida‐Compaore WIC, Alioum A, et al. Prevalence and factors associated with psychological distress among key populations in Togo, 2017. PLoS One. 2020;15(4):e0231726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferré VM, Gbeasor‐Komlanvi FA. Prevalence of human papillomavirus, human immunodeficiency virus, and other sexually transmitted infections among men who have sex with men in Togo: a national cross‐sectional survey. Clin Infect. 2019. [DOI] [PubMed] [Google Scholar]
- 67. Hladik W, Sande E, Berry M, Ganafa S, Kiyingi H, Kusiima J, et al. Men who have sex with men in Kampala, Uganda: results from a bio‐behavioral respondent driven sampling survey. AIDS Behav. 2017;21(5):1478–90. [DOI] [PubMed] [Google Scholar]
- 68. Parmley LE, Chingombe I, Wu Y, Mapingure M, Mugurungi O, Samba C, et al. High burden of active syphilis and HIV/syphilis co‐infection among men who have sex with men, transwomen, and genderqueer individuals in Zimbabwe. Sex Transm Dis. 2021:111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stahlman S, Liestman B, Ketende S, Kouanda S, Ky‐Zerbo O, Lougue M, et al. Characterizing the HIV risks and potential pathways to HIV infection among transgender women in Cote d'Ivoire, Togo and Burkina Faso. J Int AIDS Soc. 2016;19:20774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. McKinnon LR, Gakii G, Juno JA, Izulla P, Munyao J, Ireri N, et al. High HIV risk in a cohort of male sex workers from Nairobi, Kenya. Sex Transm Infect. 2014;90(3):237–42. [DOI] [PubMed] [Google Scholar]
- 71. Smith AD, Muhaari AD, Agwanda C, Kowuor D, Van Der Elst E, Davies A, et al. Heterosexual behaviours among men who sell sex to men in coastal Kenya. AIDS. 2015;29:S201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Crowell TA, Keshinro B, Baral SD, Schwartz SR, Stahlman S, Nowak RG, et al. Stigma, access to healthcare, and HIV risks among men who sell sex to men in Nigeria. J Int AIDS Soc. 2017;20(1):21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hessou PHS, Glele‐Ahanhanzo Y, Adekpedjou R, Ahouada C, Johnson RC, Boko M, et al. Comparison of the prevalence rates of HIV infection between men who have sex with men (MSM) and men in the general population in sub‐Saharan Africa: a systematic review and meta‐analysis. BMC Public Health. 2019;19(1):1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Fogel JM, Guo X, Clarke W, Breaud A, Cummings V, et al. Antiretroviral drug use and HIV drug resistance among MSM and transgender women in sub‐Saharan Africa. AIDS. 2018;32(10):1301–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wahome EW, Graham SM, Thiong'o AN, Mohamed K, Oduor T, Gichuru E, et al. PrEP uptake and adherence in relation to HIV‐1 incidence among Kenyan men who have sex with men. EClinicalMedicine. 2020;26:100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Poteat T, Malik M, Scheim A, Elliott A. HIV prevention among transgender populations: knowledge gaps and evidence for action. Curr HIV/AIDS Rep. 2017;14(4):141–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kimani M, van der Elst EM, Chiro O, Oduor C, Wahome E, Kazungu W, et al. PrEP interest and HIV‐1 incidence among MSM and transgender women in coastal Kenya. J Int AIDS Soc. 2019;22(6):e25323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Johnston LG, Khanam R, Reza M, Khan SI, Banu S, Alam MS, et al. The effectiveness of respondent driven sampling for recruiting males who have sex with males in Dhaka, Bangladesh. AIDS Behav. 2008;12(2):294–304. [DOI] [PubMed] [Google Scholar]
- 79. UNAIDS . Ending AIDS: progress towards the 90–90–90 targets. 2017. https://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf
- 80. Malekinejad M, Johnston LG, Kendall C, Kerr LR, Rifkin MR, Rutherford GW. Using respondent‐driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav. 2008;12(4 Suppl):S105–30. [DOI] [PubMed] [Google Scholar]
- 81. Yeka W, Maibani‐Michie G, Prybylski D, Colby D. Application of respondent driven sampling to collect baseline data on FSWs and MSM for HIV risk reduction interventions in two urban centres in Papua New Guinea. J Urban Health. 2006;83(6 Suppl):i60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sandfort TGM, Dominguez K, Kayange N, Ogendo A. HIV testing and the HIV care continuum among sub‐Saharan African men who have sex with men and transgender women screened for participation in HPTN 075. PLoS One. 2019;14(5):e0217501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lahuerta M, Patnaik P, Ballo T, Telly N, Knox J, Traore B, et al. HIV prevalence and related risk factors in men who have sex with men in Bamako, Mali: findings from a bio‐behavioral survey using respondent‐driven sampling. AIDS Behav. 2018;22(7):2079‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ntale RS, Rutayisire G, Mujyarugamba P, Shema E, Greatorex J, Frost SDW, et al. HIV seroprevalence, self‐reported STIs and associated risk factors among men who have sex with men: a cross‐sectional study in Rwanda, 2015. Sex Transm Infect. 2019;95(1):71‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rwema JOT, Lyons CE. HIV infection and engagement in HIV care cascade among men who have sex with men and transgender women in Kigali, Rwanda: a cross‐sectional study. J Int AIDS Soc. 2020;23(Suppl 6):e25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Association between the HIV prevalence in the general population and in MSM.
Figure S2: Univariate Association between the HIV prevalence in the general population and in transgender women (TGW).
Table S1: Overview of complete literature searches, including updates.
Table S2: Bias assessment.
Table S3: Overview of the prevalence of HIV infection in cisgender men who have sex with men (MSM) in the included studies and prevalence of HIV in the general male population at the study location.
Table S4: Overview of the prevalence of HIV infection in transgender women in the included studies and prevalence of HIV in the general male and female population at the study location.
Table S5: Overview of the prevalence of HIV infection in cisgender male sex workers (MSW) in the included studies and prevalence of HIV in the general male population at the study location.
Table S6: Logistic Deming regression of the relationship between HIV prevalence in the general population and in MSM (used for extrapolation).
Table S7: Univariate logistic regression on relationship between HIV prevalence in MSM and potential confounders.
Table S8: Logistic Deming regression of the relationship between HIV prevalence in the general population and in transgender women (used for extrapolation).
Table S9: Univariate logistic regression on relationship between HIV prevalence in transgender women and potential confounders.
Table S10: Review estimations for men who have sex with men (MSM).
Table S11: Review estimations for transgender women (TGW).
Table S12: Comparison of UNAIDS estimations versus estimations from this study for men who have sex with men (MSM).
Table S13: Comparison of UNAIDS estimations versus estimations from this study for transgender women.
Table S14: Comparison of estimations derived from study data to estimations derived regression model for men who have sex with men (MSM).
Table S15: Comparison of estimations derived from study data to estimations derived regression model for transgender women.
Table S16: Assesment of the impact of the use of geospatially matched Demographic Health Surveys (DHS) (52) data versus Dwyer‐Lindgren et al. (56) (DL) data.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


