Abstract
We have previously reported on a Tn4351-generated mutant of Porphyromonas gingivalis (MSM-3) which expresses enhanced arginine-specific proteinase activity and does not utilize hemin or hemoglobin for growth (C. A. Genco et al., Infect. Immun. 63:2459–2466, 1995). In the process of characterizing the genetic lesion in P. gingivalis MSM-3, we have determined that the endogenous P. gingivalis insertion sequence element IS1126 is capable of transposition within P. gingivalis. We have also determined that IS1126 transposition modulates the transcription of the genes encoding the lysine-specific proteinase, gingipain K (kgp) and the arginine-specific proteinase, gingipain R2 (rgpB). Sequence analysis of P. gingivalis MSM-3 revealed that Tn4351 had inserted 60 bp upstream of the P. gingivalis endogenous IS element IS1126. Furthermore, P. gingivalis MSM-3 exhibited two additional copies of IS1126 compared to the parental strain A7436. Examination of the first additional IS1126 element, IS11261, indicated that it has inserted into the putative promoter region of the P. gingivalis kgp gene. Analysis of total RNA extracted from P. gingivalis MSM-3 demonstrated no detectable kgp transcript; likewise, P. gingivalis MSM-3 was devoid of lysine-specific proteinase activity. The increased arginine-specific proteinase activity exhibited by P. gingivalis MSM-3 was demonstrated to correlate with an increase in the rgpA and rgpB transcripts. The second additional IS1126 element, IS11262, was found to have inserted upstream of a newly identified gene, hmuR, which exhibits homology to a number of TonB-dependent genes involved in hemin and iron acquisition. Analysis of total RNA from P. gingivalis MSM-3 demonstrated that hmuR is transcribed, indicating that the insertion of IS1126 had not produced a polar effect on hmuR transcription. The hemin-hemoglobin defect in P. gingivalis MSM-3 is proposed to result from the inactivation of Kgp, which has recently been demonstrated to function in hemoglobin binding. Taken together, the results presented here demonstrate that the introduction of Tn4351 into the P. gingivalis chromosome has resulted in two previously undocumented phenomena in P. gingivalis: (i) the transposition of the endogenous insertion sequence element IS1126 and (ii) the modulation of gingipain transcription and translation as a result of IS1126 transposition.
The gram-negative anaerobe Porphyromonas gingivalis has been implicated as a major pathogen associated with the induction and/or progression of adult periodontal disease (5). This organism is armed with a number of putative virulence factors; of these, the cysteine proteinases have received considerable attention due to their ability to degrade and inactivate host defense proteins (iron binding proteins, immunoglobulins, and complement components), structural proteins (collagen, fibronectin, and fibrinogen), and plasma protein inhibitors (10, 35). The majority of the P. gingivalis proteinase activity is due to the production of cysteine proteinases referred to as gingipains, which cleave synthetic and natural substrates after arginine and lysine residues.
The genes encoding arginine specific gingipains (rgpA and rgpB) have been characterized (26, 33, 35, 36). The translated portion of rgpA encodes a prepropeptide, catalytic, and hemagglutinin domain, and the initial polyprotein is apparently subject to posttranslational processing. Although the rgpA and rgpB genes share a strong degree of similarity, the rgpB gene does not possess the hemagglutinin domain present in the C-terminal region of the rgpA gene. Nakayama et al. (27) have suggested that rgpA and rgpB may have been generated through the duplication of an ancestral rgp gene, with insertion of the hemagglutinin domain into one copy of the two resulting genes and homologous recombination between the proteinase domains of rgpA and rgpB. P. gingivalis has been demonstrated to undergo nonreciprocal recombination, further supporting this scenario (27).
The gene encoding the lysine-specific gingipain (kgp) has also been characterized from a number of different P. gingivalis strains (2, 29, 32). Like rgpA, the initial translation product of kgp is composed of four functional regions: the signal peptide, the NH2-terminal prosequence, the mature proteinase domain, and the COOH-terminal hemagglutinin domain (29). Sequence comparison reveals that kgp is nearly identical to rgpA at the C terminus and suggests that a recombinational rearrangement event (i.e., transposition or gene conversion) may have occurred in this region.
Transposition of IS elements can lead to inactivation of genes, to the transcriptional activation of dormant genes, or to genomic rearrangement, all of which can contribute to the genetic diversity of bacterial populations (8, 31, 34, 44). To date, three endogenous insertion sequence elements have been characterized in P. gingivalis. PGIS2 was recently identified by our laboratory and has been demonstrated to be capable of transposition within P. gingivalis (44). IS195 is an insertion sequence-like element recently reported by Lewis and Macrina (20) that is associated with protease genes in P. gingivalis. IS195 was found flanking the kgp genes in P. gingivalis strains HG66 and 381 and within a prtP gene (kgp homolog) from P. gingivalis W83. The P. gingivalis insertion sequence IS1126 was originally described by Maley et al. (24); however, transposition within the P. gingivalis genome was not demonstrated by these investigators. Barkocy-Gallagher et al. (2) have demonstrated that an incomplete copy of IS1126 is found directly 3′ of the prtP gene in P. gingivalis W12. Aduse-Opoku et al. (1) have recently reported that located in the 3′ end of the tla gene (which is homologous to the 3′ portion of the rgpA gene), is a copy of a vestigial IS1126 in which an essential region of the transposase gene is deleted. These observations suggest that recombination within the gene locus encoding the arginine- and lysine-specific proteinases may have occurred via an IS1126-mediated transposition event. In this study, we demonstrate for the first time the transposition of IS1126 within P. gingivalis. We also show that IS1126 transposition modulates the transcription of the genes encoding gingipain K (kgp), gingipain R1, and gingipain R2 (rgpB).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. gingivalis A7436, W50, HG66, ATCC 33277 (12), and MSM-3 (11), and Escherichia coli XL1-Blue MR and JM109 were used in these studies. P. gingivalis A7436, W50, HG66, and 33277 were maintained on anaerobic blood agar (ABA) plates (Remel, Lenexa, Kans.). P. gingivalis MSM-3 was maintained on ABA plates supplemented with 1 μg of erythromycin per ml. All P. gingivalis cultures were incubated at 37°C in an anaerobic chamber (Coy Laboratory Products, Inc.) with 85% N2, 5% H2, and 10% CO2 for 3 to 5 days. After incubation at 37°C, cultures were inoculated in Anaerobe Broth MIC (Difco) or TSB (see below) and then incubated at 37°C (under anaerobic conditions) for 24 h. E. coli strains were typically maintained in Luria-Bertani media and incubated aerobically with shaking.
P. gingivalis MSM-3 is a hemin-hemoglobin utilization mutant isolated after transpositional mutagenesis of P. gingivalis A7436 with the Bacteroides fragilis transposon Tn4351 (11). P. gingivalis MSM-3 cultures grown by continuous passage and those recovered from subcutaneous chambers implanted in BALB/c mice (11) maintain their nonpigmented phenotype and erythromycin resistance, indicating that there is no apparent reversion of the mutation. Cultures passaged continuously also maintain increased levels of arginine-specific proteinase activity, as well as a decreased lysine-specific proteinase activity.
Enzyme activity assay.
The amidolytic activity of whole cultures was determined with either N-benzoyl-l-arginine-p-nitroanilide (BApNA) or N-carbobenzoxy-l-lysine-p-nitroanilide (z-KPNA). Samples were preincubated in 0.2 M Tris-HCl–0.1 M NaCl–5 mM CaCl2–10 mM cysteine (pH 7.6) for 5 min at 37°C and assayed for amidase activity with 2 mM substrate. The formation of p-nitroaniline was monitored spectrophotometrically at 405 nm.
Isolation of genomic DNA.
P. gingivalis cells were pelleted and suspended in 15 ml of 10 mM NaCl–20 mM Tris-HCl (pH 8.0)–100 μg proteinase K per ml–0.5% (wt/vol) sodium dodecyl sulfate (SDS). Cells were gently mixed and incubated for 6 h or overnight at 50°C. Genomic DNA was extracted by gentle inversion with an equal volume of phenol-chloroform for 10 min at room temperature. The mixture was centrifuged at 4,000 rpm and at 10 to 12°C for 20 min, and the upper aqueous layer was removed. The DNA sample was precipitated with 3.0 M sodium acetate (pH 5.5), and two volumes of ethanol were added to the aqueous phase. The DNA was spooled out at the aqueous ethanol interphase by using a sterile glass rod. The DNA was washed with 70% (wt/vol) ethanol, touched to the side of a sterile tube to drain the ethanol, air dried, and dissolved in 5 ml of TE buffer. P. gingivalis A7436 and MSM-3 genomic DNA were partially digested, ligated, and packaged by using the SuperCos1 Cosmid Vector Kit, as described by the manufacturer (Stratagene, Inc.).
Southern blot analysis.
Agarose gels were blotted against nylon membranes as described by Sambrook et al. (38). After blotting, nylon membranes were prehybridized for 30 min at 65°C and then hybridized for 2 h (65°C) in Rapid hybridization buffer (Amersham Life Sciences) containing the appropriate probe (see Table 1 and Results). Probes were labeled by using 32P as described by the Prime-a-Gene labeling system (Promega). After hybridization, membranes were washed twice with 2× SSC–0.1% SDS (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 15 min at 65°C and exposed to X-ray film.
TABLE 1.
Probes and oligonucleotides used for Southern blot, Northern blot, and RT-PCR analyses
| Probe designation | Descriptiona |
|---|---|
| rgpA/rgpB | PCR fragment corresponding to bp 649 to 1,177 of rgpB; recognizes both rgpA and rgpB (528 bp) |
| hmuR | PCR fragment corresponding to bp 8 to 493 of hmuR (505 bp: forward and reverse primers used each contain 10 nonspecific bases) |
| prtT | PCR fragment corresponding to bp 914 to 1,200 of prtT (286 bp) |
| 11261 | 17-bp oligonucleotide derived from IS1121 flanking sequences; 5′-CTCATGCTCGACTGACT-3′ |
| 11262 | 18-bp oligonucleotide derived from IS11262 flanking sequences; 5′-CCTACAAATTGGGATTGC-3′ |
| kgp | 33-bp oligonucleotide probe 5′-CATACGAACCGGCGTATTATACAAGTCGCCATG-3′ |
The length of the DNA fragment is indicated in parentheses.
Northern blot analysis.
For total RNA isolation, P. gingivalis strains were grown in 125 ml of TSB broth (30 g of Trypticase soy broth, 5 g of yeast extract, 0.5 g of cysteine, and 1 mg of menadione per liter supplemented with 1.5 μM hemin). Total RNA was prepared by using the Purescript kit (Gentra).
Northern blot analysis was conducted by electrophoresis of RNA samples in a 1% agarose gel containing 2.2 M formaldehyde, followed by capillary transfer to a Hybond-N membrane. Filters were hybridized with probes specific for rgpA and rgpB (rgpA/B) (labeled with [32P]dCTP by using the High Prime labeling system [Boehringer Mannheim]) and a kgp oligonucleotide probe 5′ labeled with [32P]ATP and polynucleotide kinase (Table 1). Hybridization was conducted in a mixture containing 1 M NaCl, 1% SDS, and 10% dextran sulfate at 65°C for the rgpA/B probe and at 55°C for the kgp probe. Nonspecific radioactivity was removed by two washes at room temperature in 30 mM NaCl–3.0 mM sodium citrate and two washes at 65°C or 55°C (kgp probe) in a mixture containing 30 mM NaCl, 3 mM sodium citrate, and 0.1% SDS. Membranes were exposed to X-ray films, and autoradiographs were scanned by using the Eagle Eye II still video system (Stratagene).
RT-PCR.
P. gingivalis cultures were grown to the mid-logarithmic phase in basal medium (BM) or BM supplemented with hemin (1.5 μM) (11). Total RNA was isolated by using the RNagents kit (Promega). Samples were initially treated with DNase prior to reverse transcriptase PCR (RT-PCR). To 1.0 μg of total RNA was added 1 μl of 10× DNase I (Promega) and 1 U of DNase I in diethyl pyrocarbonate (DEPC)-treated water (final volume, 10 μl). Samples were incubated at room temperature for 15 min. DNase I was inactivated by the addition of 1 μl of 25 mM EDTA to the reaction mixture. The samples were then heated to 65°C for 10 min and placed on ice. To this was added 25 μl of 2× reaction mix, 100 ng of each primer, 1 μl of RT-Taq mix, and DEPC-treated water to a final volume of 50 μl. The samples were overlaid with mineral oil and placed in a Thermacycler. cDNA synthesis was performed at 50°C for 30 min, followed by predenaturation at 94°C for 2 min. PCR amplification was carried out by using the following parameters: denaturation at 94°C for 1 min, annealing at 50°C for 2 min, and elongation at 72°C for 2 min for 35 cycles. Primers were designed to amplify a 505-bp fragment for the hmuR gene and a 286-bp fragment for prtT gene (Table 1).
DNA sequencing and computer analysis.
DNA sequencing was performed by using the PRISMTM Ready Reaction DyeDeoxy Terminator Cycle Sequencing Kit (Perkin-Elmer, Foster City, Calif.) and 373A DNA sequencer (Applied Biosystems). Computer analysis was performed as outlined by the Intelligenetics Suite and Blast programs.
GenBank accession number.
The partial sequence of hmuR was deposited into GenBank under accession number U87395 (hmuR was previously designated hemB). The remainder of A7436 hmuR was sequenced as described above.
RESULTS
Characterization of the insertion site of Tn4351 in P. gingivalis MSM-3.
We previously reported on the initial characterization of a P. gingivalis hemin uptake mutant, P. gingivalis MSM-3, isolated after transpositional mutagenesis of P. gingivalis A7436 with the B. fragilis transposon Tn4351 (11). Southern blot analysis of HindIII-digested P. gingivalis MSM-3 genomic DNA with a Tn4351-specific probe revealed a 5-kb fragment (data not shown) containing the partial ermF gene, the entire tetracycline-resistance gene, and IS4351 attached to the chromosomal junction fragment (data not shown). Using the tetracycline gene as a selective marker, this fragment was cloned from P. gingivalis MSM-3 into plasmid pGEM3Zf(−). An AvaI-AvaI fragment which contains a portion of the IS4351 sequence (Fig. 1) attached to the P. gingivalis MSM-3 chromosomal junction fragment, and the multiple cloning site of pGEM3Zf(−) was purified and used as a probe to screen a P. gingivalis A7436 cosmid library for wild-type sequences containing the insertion site. Cosmid DNA from positive colonies was digested with HindIII and analyzed by Southern blot hybridization by using the previous AvaI-AvaI restriction fragment as a probe. A 5.3-kb DNA fragment was identified and subjected to nucleotide sequence determination.
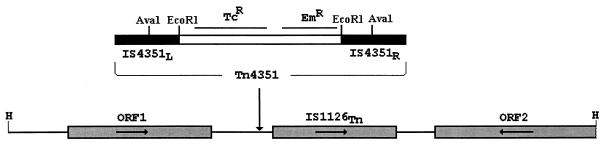
FIG. 1.
Genetic organization of DNA flanking Tn4351 target sequence in P. gingivalis MSM-3 genome. Tn4351 contains a tetracycline resistance gene (Tcr) and an erythromycin-clindamycin resistance gene (Emr) flanked by direct repeat insertion sequence IS4351. The Tn4351 insertion was located 60 bp upstream from an IS1126 element (designated IS1126Tn). Arrows in ORF1, IS1126Tn, and ORF2 indicate the direction of transcription. H, HindIII.
Computer analysis of the nucleotide sequence of the 5.3-kb fragment demonstrated that, in MSM-3, Tn4351 had inserted in a noncoding region 60 bp upstream from the P. gingivalis IS1126 element (Fig. 1). The nucleotide sequence of the element in P. gingivalis MSM-3 was 98% identical to the IS1126 purified from P. gingivalis W83, as previously reported by Maley and Roberts (23). The IS1126 element in MSM-3 (designated IS1126Tn) was 1,334 bp in length with 12-bp imperfect repeats at either end. When compared to the previously reported sequence of IS1126 (23), a 4-bp deletion in the major open reading frame (ORF), presumably representing the IS1126 transposase, was noted. Also identified in this 5.3-kb region were two long ORFs (Fig. 1). ORF1 contained 1,347 bp coding for a putative 449-amino-acid protein. The protein encoded by ORF2 exhibited 45% identity to the polynucleotide phosphorylase genes of both E. coli and Photohabdus spp. (4, 37).
P. gingivalis MSM-3 contains two additional copies of IS1126.
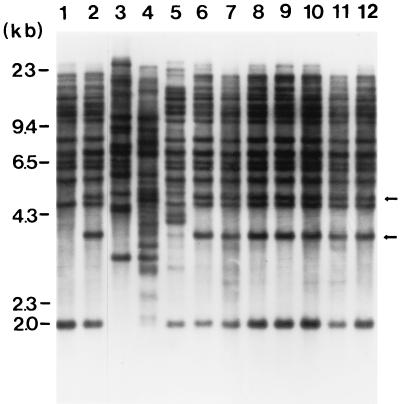
The insertion of Tn4351 upstream of the P. gingivalis IS1126 element led us to postulate that IS1126 could transpose and that this could be responsible for the mutation in MSM-3. To explore this possibility, Southern blot analysis was performed with P. gingivalis A7436 and MSM-3 genomic DNA digested with BamHI and probed with a fragment isolated from IS1126. Since IS1126 does not contain a BamHI site, a single hybridizing fragment was assumed to represent a single copy of the element. However, it is possible that there may be two or more comigrating fragments which hybridize with the IS1126 probe. Likewise, it is possible that the hybridizing bands may represent vestigial copies of IS1126. As shown in Fig. 2, two additional bands of 4 and 5 kb were observed in P. gingivalis MSM-3 compared to the wild-type strain P. gingivalis A7436. Seven additional independently isolated Tn4351-generated transconjugants were examined and exhibited an IS1126 banding pattern identical to that of P. gingivalis MSM-3 (Fig. 2). These Tn4351-generated transconjugants were also nonpigmented on ABA plates. These observations suggest that the insertion of Tn4351 may be site specific. In addition, these results suggest that introduction of Tn4351 into P. gingivalis may have resulted in the duplication and transposition of the endogenous IS element IS1126. We also examined genomic DNA from three other P. gingivalis strains to determine the number of IS1126 elements present. The hybridization patterns indicate that strains HG66, ATCC 33277, and W50 were different from A7436 and from each other. The variation in number and size of IS1126-bearing restriction fragments among different strains is in agreement with previous studies (22) and suggests the mobile nature of IS1126 within the P. gingivalis chromosome.
FIG. 2.
Southern blot hybridization analysis of P. gingivalis chromosomal DNA probed with insertion sequence IS1126. Marker DNAs are indicated at left in kilobases. Chromosomal DNA from P. gingivalis A7436 (lane 1), MSM-3 (lane 2), HG66 (lane 3), 33277 (lane 4), W50 (lane 5), and seven independently isolated Tn4351-generated transconjugants (lanes 6 to 12) were isolated, digested with BamHI, and electrophoretically separated on a 0.8% agarose gel. Arrows on the right indicate two additional copies of IS1126. The Southern blot was probed with a [32P]dCTP-labeled, 526-bp SacI-HincII fragment purified from IS1126Tn.
Examination of the IS1126 insertion sites in P. gingivalis MSM-3.
To examine the insertion site of the first additional IS1126 element (designated IS11261), DNA from P. gingivalis MSM-3 was digested with BamHI, and a 5-kb fragment was isolated, cloned into pGEM3Zf, and transformed into E. coli JM109. Sequence analysis revealed that IS11261 had inserted 185 bp upstream of the start codon of the signal peptide of the P. gingivalis kgp gene (29) (Fig. 3A). DNA sequence analysis revealed that the entire kgp gene was intact in P. gingivalis MSM-3. The absence of IS11261 in the corresponding region of the parental strain was verified by using an oligonucleotide constructed from MSM-3 genomic DNA which flanks IS11261 (Table 1). A P. gingivalis A7436 cosmid library was screened with this probe, and DNA sequence analysis of positive clones revealed that IS11261 was not present in the corresponding region of the A7436 genome (data not shown).
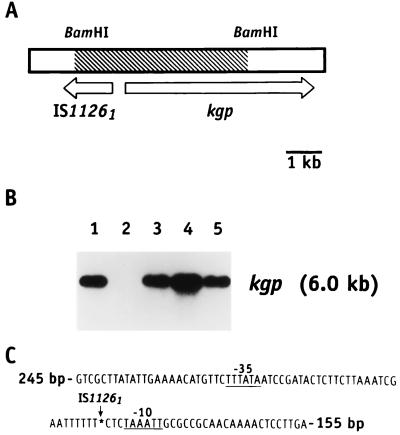
FIG. 3.
Localization of IS11261 upstream of kgp and Northern blot analysis of kgp transcript in P. gingivalis. To identify the insertion site of IS11261, P. gingivalis MSM-3 chromosomal DNA was digested with BamHI and a 5-kb fragment was isolated, cloned, and sequenced (represented by hatched area). (A) The insertion of IS1126 was located 185 bp upstream of the start codon of kgp. Arrows represent the size and direction of transcription of IS11261 and kgp. (B) Northern blot analysis of total RNA from P. gingivalis A7436 (lane 1), MSM-3 (lane 2), W50 (lane 3), 33277 (lane 4), and HG66 (lane 5). Equal amounts of total RNA were loaded and confirmed by equal staining intensity of the rRNA bands stained with ethidium bromide (data not shown). (C) Nucleotide sequence of IS11261 insertion site. Putative −35 and −10 promoter boxes are denoted and underlined. Actual site of insertion of IS11261 within the sequence is indicated by an arrow. Numbers represent the position of the bases in relation to the kgp start codon.
IS11261 insertion shuts down kgp transcription and corresponding Lys-specific cysteine proteinase activity.
To examine the consequence of the insertion of IS11261 5′ to the kgp gene, we examined RNA from cultures of P. gingivalis for the presence of a kgp transcript. Total RNA from P. gingivalis A7436 and MSM-3, as well as two additional P. gingivalis laboratory strains (W50 and 33277), was isolated and probed with a kgp-specific oligonucleotide (Table 1). The kgp transcript (6 kb) was detected in the P. gingivalis parental strain A7436, as well as in additional P. gingivalis laboratory strains W50 and 33277 (Fig. 3B). However, we did not detect a kgp transcript in RNA obtained from P. gingivalis MSM-3 (Fig. 3B).
Promoter sequences for kgp have not been previously identified; however, the insertion site of IS11261 proximal to the kgp start codon and the absence of a kgp transcript in P. gingivalis MSM-3 suggested that the site of insertion may represent the putative kgp promoter. We thus examined the IS11261 insertion site for putative −35 and −10 sequences. Interestingly, a region located 220 bp upstream of the kgp start codon (TTTATA) was found to exhibit 67% homology with the E. coli consensus −35 sequence, while a region located 182 bp upstream of the kgp start codon (TAAATT) exhibited 83% homology to the −10 sequence (Fig. 3C). The IS11261 insertion was located 3 bp upstream of the putative −10 sequence (Fig. 3C). These findings suggest that IS11261 has inserted into the kgp promoter region, resulting in disruption of kgp transcription.
To confirm that the absence of kgp transcription resulted in translational effects, P. gingivalis MSM-3 and A7436 were assayed for the presence of lysine-specific proteinase activity. In agreement with our previous studies (11), we found that P. gingivalis MSM-3 exhibited enhanced arginine-specific proteinase activity compared with A7436. However, in agreement with the transcriptional studies, MSM-3 was found to possess virtually no lysine-specific proteinase activity when compared to the parental strain A7436. Lysine- and arginine-specific proteinase activities of P. gingivalis were as follows. For strain MSM-3 the BApNA and z-Lys-pNA activities were 121.7 and 1.085 U, respectively, while for strain A7436 the BApNA and z-Lys-pNA activities were and 34.8 and 26.120 U, respectively. These activities are defined as the amount which gives an optical density of 1.0/min and are derived from the results of three separate experiments.
Enhanced rgpA and rgpB transcription in P. gingivalis MSM-3.
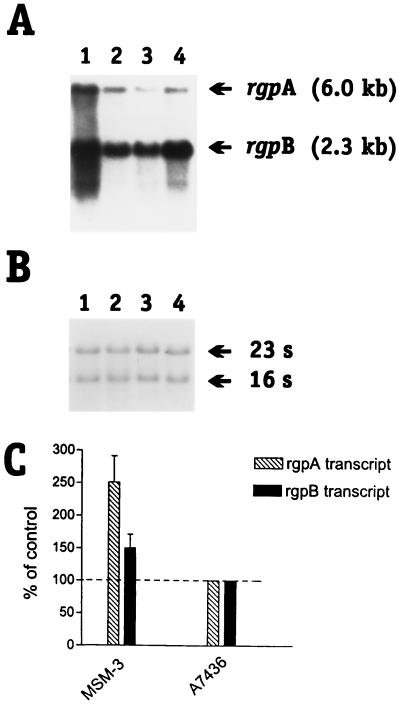
To determine if the enhanced arginine-specific proteinase activity correlated with increased transcription of rgpA and/or rgpB, Northern blot analysis of P. gingivalis A7436 and MSM-3 total RNA was performed. Northern blot analysis with a probe which recognizes sequences present in both rgpA and rgpB (Table 1) revealed two transcripts representing rgpA and rgpB in both P. gingivalis A7436 and MSM-3 (Fig. 4). Densitometry scans of the Northern blot depicted in Fig. 4 indicated that the levels of the rgpA and rgpB transcripts detected in P. gingivalis MSM-3 were increased compared to the parental strain A7436 (Fig. 4C). Densitometry scans of the Northern blot depicted in Fig. 4A revealed that the relative band intensity representing the rgpA transcript in P. gingivalis MSM-3 was approximately 2.5-fold of that observed in P. gingivalis A7436. The relative band intensity representing the rgpB transcript was also higher in P. gingivalis MSM-3 compared to the rgpB transcript in strain A7436. Thus, the increased arginine-specific proteinase activity in P. gingivalis MSM-3 results from increased transcription of the rgpA and rgpB genes. We also observed both rgpA and rgpB transcripts in two additional P. gingivalis laboratory strains (W50 and 33277).
FIG. 4.
Northern blot analysis of rgpA and rgpB transcripts in P. gingivalis. (A) Total RNA from P. gingivalis was hybridized with a rgpA-rgpB-specific probe (a 528-bp DNA fragment corresponding to bp 649 to 1,177 from the rgpB gene). Upper bands correspond to rgpA, and the lower bands correspond to rgpB. Lanes 1 to 4 correspond to P. gingivalis MSM-3, A7436, W50, and 33277, respectively. (B) Equal amounts of total RNA were loaded and confirmed by equal staining intensity of the rRNA bands stained with ethidium bromide. 23S and 16S refer to the rRNA bands. (C) Densitometry scan of the hybridizing bands. Data represents the mean ± the standard deviation of three separate experiments and are expressed as the percentage of the control value, arbitrarily set at 100%.
Examination of the second additional IS1126 element.
In some instances, insertion of an IS element can transcriptionally activate expression of an adjacent gene by virtue of readthrough transcription from a promoter within the element (34). To determine if the second additional IS1126 element (designated IS11262) had inserted proximal to the rgpA or rgpB genes and had resulted in the increased transcription of these genes, we examined the site of insertion of IS11262. A 4-kb BamHI restriction fragment was cloned from P. gingivalis MSM-3, and the nucleotide sequence of IS11262 and its junction fragments were analyzed. Analysis of IS11262 indicated that it was identical to the IS1126 element isolated from P. gingivalis W83 (23) with the restoration of the 4-bp 5′-GAAG-3′ deletion observed in IS1126Tn (Fig. 5A). Examination of the DNA flanking IS11262 revealed that IS11262 was located 322 bp downstream from the P. gingivalis prtT gene (Fig. 5B). The prtT gene encodes for a streptopain-related cysteine proteinase which was originally cloned from P. gingivalis ATCC 53977 but does not share homology with kgp, rgpA, or rgpB (22, 30). Northern blot analysis of P. gingivalis MSM-3 and A7436 with a probe specific for prtT (Table 1) showed that similar transcript levels of prtT were present in both strains, thus indicating that the insertion of IS11262 did not alter the transcription of this proximal gene (data not shown).
FIG. 5.
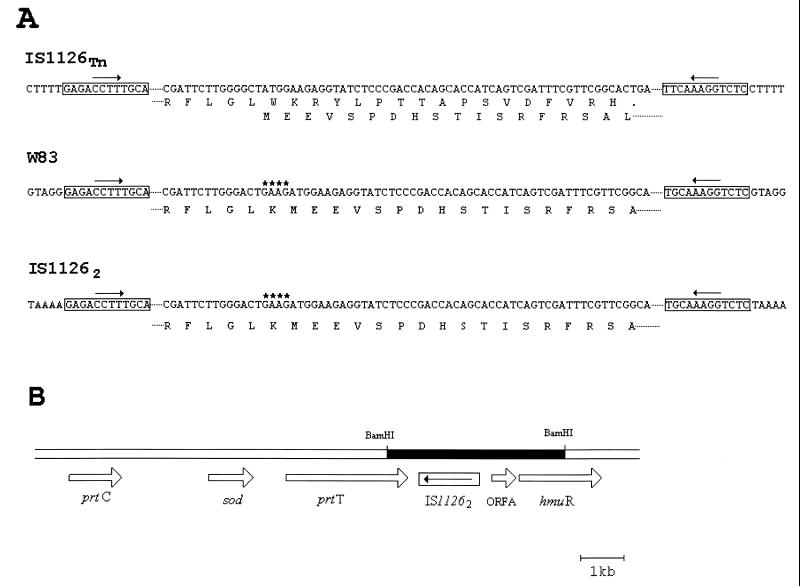
Nucleotide sequence of the IS1126 elements isolated in this study and the transposition site of IS11262. (A) Comparison of the nucleotide sequences and the deduced amino acid sequences of different IS1126 elements. Partial nucleotide and amino acid sequences of IS1126Tn were compared with those of IS1126 from P. gingivalis W83 and IS11262. Boxes represent the 12-bp terminal inverted repeats of IS1126. The 5-bp nucleotide sequences flanking inverted repeats are duplicated target sequences generated after IS1126 transposition. Asterisks denote the 4-bp 5′-GAAG-3′ deletion found in IS1126Tn which resulted in premature termination of IS1126 transposase synthesis. (B) Location of the duplicated copy of IS11262 found in P. gingivalis MSM-3 genome. A 4-kb BamHI restriction fragment (shaded) was cloned directly from MSM-3 chromosomal DNA into pGEM3Zf, and the nucleotide sequence was determined. Large arrows represent the size and orientation of the prtT, sod, prtC, and hmuR genes and of orfA. Small arrow in IS11262 indicates the direction of IS1126 transposase gene. prtT, cysteine protease gene; sod, superoxide dismutase gene; prtC, putative collagenase gene.
To confirm that IS11262 was not present in the corresponding region of the parental strain A7436, a radiolabeled oligonucleotide corresponding to the MSM-3 genomic DNA sequences which flank IS11262 (Table 1) was used to screen a P. gingivalis A7436 cosmid library. A HindIII-generated fragment of approximately 7 kb from two independent clones was subcloned, and nucleotide sequence analysis confirmed that IS11262 was not present in the corresponding region of the wild-type genome (data not shown).
Located 677 bp downstream of the IS11262 insertion site in P. gingivalis MSM-3, a small ORF (orfA) of 428 bp was identified. This ORF was identical to an ORF recently identified by Karunakaran et al. (18) in P. gingivalis ATCC 53977. Further downstream of orfA, a 1.9-kb ORF was fortuitously identified (Fig. 5). This ORF exhibited homology to the Yersinia enterocolitica hemR gene, which is a member of the hemin uptake operon of Y. enterocolitica (39), and to several genes whose products have been shown to be TonB-dependent outer membrane receptors involved in the acquisition of iron. These include the E. coli fepA, fhuA, cirA, btuB, and fhuE genes (7, 13, 16, 21, 39), the V. cholerae irgA gene (13), and the Pseudomonas aeruginosa pfeA gene (6). Furthermore, we found that a region of the translated ORF exhibited extensive homology to TonB box IV, which has been postulated to be the domain of the TonB-dependent receptors that physically interact with the TonB protein (40). Based upon this homology, we postulated that this gene may be a TonB-dependent outer membrane receptor which functions in the acquisition of hemin and hemoglobin in P. gingivalis, and thus we designated this ORF hmuR.
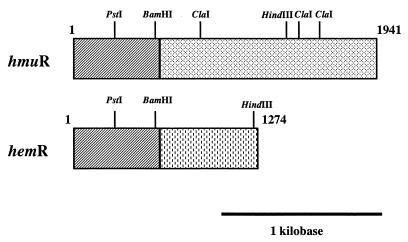
Karunakaran et al. (18) also recently reported upon the identification of the hemR gene from P. gingivalis 53977. The amino-terminal region of hmuR exhibited extensive homology to the initial 516 bases of the P. gingivalis hemR gene, suggesting that hmuR may be a hemR homolog (Fig. 6). The carboxy terminus of hmuR exhibits identity to genes involved in hemoglobin binding and utilization, while the carboxy terminus of hemR exhibits extensive identity with the prtT gene of P. gingivalis (41). P. gingivalis hemR also exhibits homology to genes involved in hemin and iron acquisition from a number of microorganisms and has been postulated to encode for a TonB-dependent outer membrane receptor (18).
FIG. 6.
Comparison of the genetic organization of the hmuR and hemR genes of P. gingivalis. The hmuR gene was cloned and sequenced from P. gingivalis A7436, while the hemR gene was cloned and sequenced from P. gingivalis 53977 (18). Hatched boxes denote the regions of identity between the two genes. Restriction sites are noted.
Transcription of hmuR is not altered in P. gingivalis MSM-3.
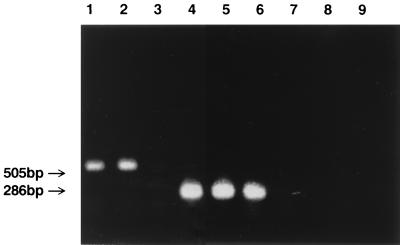
To determine if the transcription of hmuR in P. gingivalis MSM-3 was altered by the insertion of IS11262, total RNA from P. gingivalis A7436 and MSM-3 were examined by both Northern blot analysis and RT-PCR. Northern blot and RT-PCR analysis with a probe specific for an 505-bp internal fragment of hmuR (Table 1) revealed that similar levels of the hmuR transcript were detected in P. gingivalis A7436 and MSM-3 (Fig. 7 and data not shown). Since transcription of prtT was shown to be unaffected by the insertion of IS11262 (data not shown), amplification of the prtT transcript was used as a positive control for these experiments. As anticipated, a prtT transcript was detected in P. gingivalis A7436 and MSM-3 (Fig. 7). These results indicate that the insertion of IS11262 upstream of hmuR did not produce a polar effect on hmuR transcription. Thus, the hemin utilization defect observed in P. gingivalis MSM-3 is not attributed to transcriptional inactivation of hmuR.
FIG. 7.
Transcription of hmuR in P. gingivalis strains. Cultures were grown in BM without hemin for 16 h. Lanes: hmuR amplified from A7436 (lane 1), hmuR amplified from MSM-3 (lane 2), hmuR amplified from W50 (lane 3), prtT amplified from A7436 (lane 4), prtT amplified from MSM-3 (lane 5), prtT amplified from W50 (lane 6). Negative controls included hmuR amplified from A7436 RNA by using Taq polymerase (lane 7), hmuR amplified from MSM-3 RNA by using Taq polymerase (lane 8), and hmuR amplified from W50 RNA by using Taq polymerase (lane 9).
DISCUSSION
Transposition of IS1126.
P. gingivalis IS1126 was originally described by Maley and Roberts (24). During experiments involving the transfer of the Bacteroides-E. coli shuttle vector pNJR12 into P. gingivalis W83, these investigators found that IS1126 had transposed into pNJR12 (24). However, transposition of IS1126 in P. gingivalis was not demonstrated in this study. We have demonstrated for the first time the transposition of IS1126 within P. gingivalis. We also demonstrated that IS1126 transposition modulates the transcription of the genes encoding gingipain K (kgp) and gingipains R (rgpA and rgpB). Transposition of IS1126 in P. gingivalis was observed after introduction of the Bacteroides transposon Tn4351, suggesting that the introduction of Tn4351 into P. gingivalis may have resulted in IS1126 duplication and transposition. This was observed in several independently isolated Tn4351-generated transconjugants, suggesting that IS1126 transposition in P. gingivalis may be site specific. It is also possible that transposition of IS1126 may have occurred spontaneously during laboratory passage. However, Southern blot hybridization analysis of genomic DNA from 15 independent passages of P. gingivalis MSM-3 demonstrated that laboratory passage did not result in the transposition of IS1126 (data not shown). Thus, these results lead us to conclude that IS1126 transposition was mediated by Tn4351; however, this needs to be definitively proven. Recently, we identified a new IS element in P. gingivalis designated PGIS2 and reported its transposition following the introduction of Tn4351 (44). Though the precise mechanism of IS transposition within P. gingivalis has not yet been elucidated, our results indicate that the transposition of endogenous IS elements is associated with the introduction of Tn4351 into the P. gingivalis genome. The complexity of P. gingivalis genomic rearrangements after Tn4351 transposition and the apparent site specificity of insertion will thus restrict its use for further transpositional mutagenesis for P. gingivalis.
Lewis and Macrina (20) recently described a new P. gingivalis insertion sequence, IS195. These investigators identified a naturally occurring variant of P. gingivalis W83 carrying IS195 within the coding region of prtP gene (kgp homolog). IS195 was also present downstream of the prtP gene in P. gingivalis HG66 and 381. Comparison of the nucleotide sequences of rgpA and kgp indicates that a majority of the C-terminal sequences of these genes are identical. It has been suggested that recombinational rearrangement, such as transposition or gene conversion, may have occurred in this nucleotide region between kgp and rgpA. At least two other DNA regions on the P. gingivalis chromosome that may encode for other hemagglutinins share homology with this region (14), and this suggests that these DNA regions may have also taken part in this recombinational event. It is also possible that these DNA regions may have been supplied from the chromosomal DNA of other P. gingivalis cells (horizontal gene transfer). Gene conversion type recombination has been observed in P. gingivalis (26), and thus it is reasonable to postulate that recombination between P. gingivalis rgpA and kgp could occur by such a mechanism. Our results suggest that, in addition to gene conversion, the transposition of endogenous IS elements may facilitate recombinational rearrangements in P. gingivalis and that recombination within kgp and rgpA genes could have occurred via a transposition event mediated by P. gingivalis IS1126.
IS1126 transposition modulates gingipain expression.
Although it is well established that transposition of IS elements can inactivate a targeted gene, in this study we report for the first time that IS1126 transposition can modulate gingipain expression in P. gingivalis. The location of the IS11261 insertion in P. gingivalis MSM-3 indicates that IS11261 has inserted into a putative kgp promoter region. Directly associated with and flanking the area of IS11261 insertion are regions which exhibit extensive homology to consensus bacterial −35 and −10 sequences, suggesting that this area corresponds to the putative kgp promoter. Insertion into the putative promoter or ribosomal binding site would disrupt the transcription of kgp with concomitant disruption of lysine-specific cysteine proteinase activity.
The increased transcription of the rgpA and rgpB genes may be due to the absence of kgp in the P. gingivalis proteinase population. The Kgp protease appears to be the major lysine-specific protease expressed in P. gingivalis, and its absence could serve as an intracellular stress signal, signaling the organism to upregulate the transcription of other gingipains, such as rgpA and rgpB. This scenario is supported by recent studies by Tokuda et al. (43), which suggest that kgp and rgp transcription may be coordinately linked. Alternatively, the increased rgpA and rgpB transcription may result from additional but uncharacterized IS1126 elements which may have transposed to different chromosomal loci but whose movements have not led to the generation of novel hybridizing bands due to preexisting IS1126 elements in this region.
Recent studies by Kuboniwa et al. (18) have demonstrated that Kgp can bind human hemoglobin and that binding is mediated through Kgp domains which are distinct from the proteinase domain. We have also demonstrated that Kgp can bind human hemoglobin and that binding is to the hemagglutinin domains of the protein (9). Okamoto et al. (28) recently reported that P. gingivalis kgp-deficient mutants are nonpigmented and are markedly decreased in their ability to bind hemoglobin. Although these mutants could not bind hemoglobin, these investigators failed to demonstrate if the kgp-deficient mutants were capable of growing with hemin and/or hemoglobin as sole iron sources. The phenotype of the kgp mutants described by these investigators is similar to the phenotype of P. gingivalis MSM-3, the mutant we describe in this study which resulted from IS1126 insertional inactivation of the kgp gene. In previous studies, we determined that P. gingivalis MSM-3 grew poorly with hemin or hemoglobin as the sole iron sources (10). Hemoglobin binding assays demonstrated that P. gingivalis MSM-3 bound less hemoglobin compared to the parental strain (41). Thus, the decreased ability of P. gingivalis MSM-3 to utilize hemin and hemoglobin as sole iron sources may result from disruption of the kgp gene. The observation that P. gingivalis MSM-3 did not exhibit a total decrease in hemoglobin binding may be due the presence of multiple hemoglobin receptors in P. gingivalis, including HmuR, and as has been described for other gram-negative organisms (3). We should stress that the exact role of Kgp in hemin-hemoglobin transport in P. gingivalis remains to be defined. Aduse-Opoku et al. (1) have reported on the identification of the tla gene which is required by P. gingivalis for growth with low levels of hemin. These investigators found that a P. gingivalis tla mutant produced significantly lower arginine- and lysine-specific protease activities and, on the basis of these results, suggested that a regulatory link exists between tla and other members of this gene family. Taken together, the results reported in the present study, as well as those of other investigators (1, 19, 27, 28), indicate that the gingipains may function in hemin-hemoglobin utilization and that expression of the genes encoding these proteins may be coordinately regulated by hemin.
Identification of hmuR.
In this study we have also identified a novel P. gingivalis gene, hmuR, which exhibits a high degree of homology to genes encoding TonB-dependent outer membrane receptors. In most organisms, the energy for the transport of ligands across the outer membrane is provided by the TonB protein. The transport of hemin in Shigella dysenteriae (25), Haemophilus influenzae (17), and Yersinia enterocolitica (42) requires the TonB protein. The TonB protein interacts with respective ligands at several unique sites termed TonB boxes. The protein encoded by hmuR exhibits extensive homology to other TonB-dependent ligands at TonB box IV, the domain of the receptor believed to physically interact with the TonB protein (40). Although we have previously demonstrated that hemin transport in P. gingivalis occurs via an energy-dependent process (12) and have postulated the existence of a TonB homolog in P. gingivalis, a P. gingivalis TonB homolog has not yet been identified. Our results also indicate that the defect in the ability of P. gingivalis MSM-3 to utilize hemin for growth is not a result of transcriptional inactivation of hmuR by IS1126 insertion. However, whether or not HmuR is translated in MSM-3 remains to be determined. Nonetheless, a P. gingivalis hmuR mutant was demonstrated to grow poorly with hemin or hemoglobin as the sole sources of iron (41) and the identification of the hmuR gene in this study was fortuitous.
Interestingly, we found that hmuR was nearly identical at the 5′ end with P. gingivalis hemR (18). Our studies demonstrate that the 3′ end of hmuR exhibits identity to genes involved in hemoglobin binding and/or utilization. Karunakaran et al. (18) have shown that the 3′ region of hemR exhibits identity to the prtT gene of P. gingivalis. The differences in the 3′ regions of hmuR and hemR may have resulted from (i) a rearrangement event that mediated the insertion of a portion of prtT into hemR via homologous recombination, and hmuR is representative of the ancestral gene, or (ii) a rearrangement event in which the region homologous to prtT was deleted from hmuR. Either scenario is reminiscent of the proposed genomic rearrangements in the P. gingivalis proteinase and hemagglutinin genes (26).
Conclusions.
There is increasing evidence that gingipains are major virulence factors of P. gingivalis and may be directly responsible for the clinical features of adult periodontal disease such as gingival crevicular fluid production, neutrophil accumulation, and bleeding (10). Since the majority of P. gingivalis strains examined in one study appear to produce gingipains R and gingipain K (32), it has been postulated that the involvement of these proteinases in virulence may be due to differential regulation and enhanced expression in virulent strains. The results presented here indicate that transposition of P. gingivalis IS elements can modulate the expression of gingipain K and, indirectly, gingipains R. Taken together, these results suggest that transposition of IS elements (those which have been described and those remaining to be identified) within the P. gingivalis genome and that the subsequent modulation of gingipain expression may be common events which serve to alter the virulence potential of P. gingivalis.
ACKNOWLEDGMENTS
This study was supported by Public Health Service grants DE09161 from the National Institute of Dental Research and G12-RR03034 from the National Center for Research Resources, (C.A.G.) and grant 6P04A 034 14 from the State Committee of Scientific Research (KBN, Warsaw, Poland) (J.P.).
REFERENCES
- 1.Aduse-Opoku J, Slaney J, Rangarajan M, Muir J, Young K, Curtis M A. The Tla protein of Porphyromonas gingivalis W50: a Homolog of the R1 protease precursor (PrpR1) is an outer membrane receptor required for growth on low levels of hemin. J Bacteriol. 1997;179:4778–4788. doi: 10.1128/jb.179.15.4778-4788.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkocy-Gallagher G A, Han N, Patti J M, Whitlock J, Progulske-Fox A, Lantz S M. Analysis of the prtP gene encoding porphypain, a cysteine proteinase of Porphyromonas gingivalis. J Bacteriol. 1996;178:2734–2741. doi: 10.1128/jb.178.10.2734-2741.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C-J, Sparling P F, Lewis L A, Dyer D W, Elkins C. Identification and purification of a hemoglobin-binding outer membrane protein from Neisseria gonorrhoeae. Infect Immun. 1996;64:5008–5014. doi: 10.1128/iai.64.12.5008-5014.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke D J, Dowds C A. The gene coding for polynucleotide phosphorylase in Photorhabdus sp. strain K122 is induced at low temperatures. J Bacteriol. 1994;176:3775–3784. doi: 10.1128/jb.176.12.3775-3784.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler C W, Kalmar J R, Genco C A. Pathogenic strategies of the oral anaerobe Porphyromonas gingivalis. Trends Microbiol. 1995;3:45–51. doi: 10.1016/s0966-842x(00)88874-5. [DOI] [PubMed] [Google Scholar]
- 6.Dean C R, Poole K. Cloning and characterization of the ferric enterobactin receptor gene (pfeA) of Pseudomonas aeruginosa. J Bacteriol. 1993;175:317–324. doi: 10.1128/jb.175.2.317-324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fecker L, Braun V. Cloning and expression of the fhu genes involved in iron (III)hydoxamate uptake by Escherichia coli. J Bacteriol. 1983;156:1301–1314. doi: 10.1128/jb.156.3.1301-1314.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 9.Genco, C. A., and J. Potmepa. Unpublished data.
- 10.Genco C A, Potempa J, Mikolajczyk-Pawlinska J, Travis J. Role of gingipains R in Porphyromonas gingivalis pathogenesis. Clin Infect Dis. 1999;28:456–465. doi: 10.1086/515156. [DOI] [PubMed] [Google Scholar]
- 11.Genco C A, Simpson W, Forng R-Y, Egal M, Odusanya B M B. Characterization of a Tn4351-generated hemin uptake mutant of Porphyromonas gingivalis: evidence for the coordinate regulation of virulence factors by hemin. Infect Immun. 1995;63:2459–2466. doi: 10.1128/iai.63.7.2459-2466.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genco C A, Odusanya B M, Brown G. Binding and accumulation of hemin in Porphyromonas gingivalis are induced by hemin. Infect Immun. 1994;62:2885–2892. doi: 10.1128/iai.62.7.2885-2892.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg M B, Bayko S A, Calderwood S B. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB dependent proteins. Mol Microbiol. 1992;6:2407–2418. doi: 10.1111/j.1365-2958.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 14.Griggs D W, Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of Fur protein to promoters. J Bacteriol. 1989;171:1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han N, Whitlock J, Progulske-Fox A. The hemagglutinin gene (hagA) of Porphyromonas gingivalis 381 contains four large, contiguous, direct repeats. Infect Immun. 1996;64:4000–4007. doi: 10.1128/iai.64.10.4000-4007.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller K, Kadner R J. Nucleotide sequence of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol. 1985;161:904–908. doi: 10.1128/jb.161.3.904-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarosik G P, Sanders J D, Cope L D, Muller-Egberhard U, Hansen E J. A functional TonB gene is required for both utilization of heme and virulence expression by Haemophilus influenzae type b. Infect Immun. 1994;62:2470–2477. doi: 10.1128/iai.62.6.2470-2477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karunakaran T, Madden T, Kuramitsu K. Isolation and characterization of a hemin-regulated gene, hemR, from Porphyromonas gingivalis. J Bacteriol. 1997;179:1898–1908. doi: 10.1128/jb.179.6.1898-1908.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuboniwa M, Amano A, Shizukuishi S. Hemoglobin-binding protein purified from Porphyromonas gingivalis is identical to lysine-specific cysteine proteinase (lys gingipain) Biochem Biophys Res Commun. 1998;249:38–43. doi: 10.1006/bbrc.1998.8958. [DOI] [PubMed] [Google Scholar]
- 20.Lewis J P, Macrina F L. IS195, an insertion sequence-like element associated with protease genes in Porphyromonas gingivalis. Infect Immun. 1998;66:3035–3042. doi: 10.1128/iai.66.7.3035-3042.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundrigan M D, Kadner R J. Nucleotide sequence of the gene for ferric enterochelin receptor FepA in Escherichia coli. J Biol Chem. 1986;261:10797–10801. [PubMed] [Google Scholar]
- 22.Madden T E, Clark V L, Kuramitsu J. Revised sequence of the Porphyromonas gingivalis prtT cysteine protease/hemagglutinin gene: homology with the streptococcal pyrogenic exotoxin B/streptococcal proteinase. Infect Immun. 1995;63:238–247. doi: 10.1128/iai.63.1.238-247.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maley J, Roberts I S. Characterization of IS1126 from Porphyromonas gingivalis W83: a new member of the IS4 family of insertion sequence elements. FEMS Microbiol Lett. 1994;123:219–224. doi: 10.1111/j.1574-6968.1994.tb07225.x. [DOI] [PubMed] [Google Scholar]
- 24.Maley J, Shoemaker N B, Roberts I S. The introduction of colonic-Bacteroides shuttle plasmids into Porphyromonas gingivalis: identification of a putative P. gingivalis insertion-sequence element. FEMS Microbiol Lett. 1992;93:75–81. doi: 10.1016/0378-1097(92)90492-7. [DOI] [PubMed] [Google Scholar]
- 25.Mills M, Payne S. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of a analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama K. Domain-specific rearrangement between the two Arg-gingipain encoding genes in Porphyromonas gingivalis: possible involvement of nonreciprocal recombination. Microbiol Immunol. 1997;41:185–196. doi: 10.1111/j.1348-0421.1997.tb01189.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama K, Ratnayake D B, Tsukuba T, Kadowaki T, Yamamoto K, Fujimura S. Hemoglobin receptor protein is intragenically encoded by the cysteine proteinase encoding genes and the haemagglutinin-encoding gene of Porphyromonas gingivalis. Mol Microbiol. 1998;27:51–61. doi: 10.1046/j.1365-2958.1998.00656.x. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto K, Nakayama K, Kadowaki T, Abe N, Ratnayake D B, Yamamoto K. Involvement of a lysine-specific cysteine proteinase in hemoglobin adsorption and heme accumulation by Porphyromonas gingivalis. J Biol Chem. 1998;273:21225–21231. doi: 10.1074/jbc.273.33.21225. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto K, Kadowaki T, Nakayama K, Yamamoto K. Cloning and sequencing of the gene encoding a novel lysine-specific cysteine proteinase (Lys-gingipain) in Porphyromonas gingivalis: structural relationship with the arginine specific cysteine proteinase (Arg-gingipain) J Biochem. 1996;120:398–406. doi: 10.1093/oxfordjournals.jbchem.a021426. [DOI] [PubMed] [Google Scholar]
- 30.Otogoto J-I, Kuramitsu H K. Isolation and characterization of the Porphyromonas gingivalis prtT gene, coding for protease activity. Infect Immun. 1993;61:117–123. doi: 10.1128/iai.61.1.117-123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou J T, Baron L S, Rubin F A, Kopecko D J. Specific insertion and deletion of insertion sequence 1-like DNA-element causes the reversible expression of the virulence capsular antigen Vi of Citrobacter freundii in Escherichia coli. Proc Natl Acad Sci USA. 1988;85:4402–4405. doi: 10.1073/pnas.85.12.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavloff N, Pemberton P A, Potempa J, Chen W-C, Pike R, Prochazka V, Kiefer M C, Travis J, Barr P J. Molecular cloning and characterization of Porphyromonas gingivalis lysine-specific gingipain. J Biol Chem. 1997;272:1595–1600. doi: 10.1074/jbc.272.3.1595. [DOI] [PubMed] [Google Scholar]
- 33.Pike R, McGraw W, Potempa J, Travis J. Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization and evidence for the existence of complexes with hemagglutinins. J Biol Chem. 1994;269:406–411. [PubMed] [Google Scholar]
- 34.Podglajen I, Breuil J, Collatz E. Insertion of a novel DNA sequence, IS1186, upstream of the silent carbapenemase gene cfiA, promotes expression of carbapeneem resistance in clinical isolates of Bacteroides fragilis. Mol Microbiol. 1994;12:105–114. doi: 10.1111/j.1365-2958.1994.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 35.Potempa J, Pike R, Travis J. Host and Porphyromonas gingivalis proteinases (gingipains) in periodontitis: a biochemical model of infection and tissue destruction. Prospect Drug Discover Design. 1995;2:225–448. [Google Scholar]
- 36.Potempa J, Pike R, Travis J. The multiple forms of trypsin-like activity present in various strains of Porphyromonas gingivalis are due to the presence of either Arg-gingipain or Lys-gingipain. Infect Immun. 1995;63:1176–1182. doi: 10.1128/iai.63.4.1176-1182.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regnier P, Grunberg-Manago M, Portier C. Nucleotide sequence of the pnp gene of Escherichia coli encoding polynucleotide phosphorylase. J Biol Chem. 1987;262:63–68. [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sauer M, Hantke K, Braun V. Sequence of the fhuE outer membrane receptor gene of Escherichia coli K-12 and properties of mutants. Mol Microbiol. 1990;4:427–437. doi: 10.1111/j.1365-2958.1990.tb00609.x. [DOI] [PubMed] [Google Scholar]
- 40.Schramm E, Mende J, Braun V, Kamp R M. Nucleotide sequence of the colicin B activity gene cba: consensus pentapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987;169:3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson, W., T. Karunakaran, and C. A. Genco. 1999. Unpublished data.
- 42.Stojiljkovic I, Hantke K. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in gram-negative bacteria. EMBO J. 1992;11:4359–4367. doi: 10.1002/j.1460-2075.1992.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokuda M, Karunakaran T, Kuramitsu H K. Regulation of protease expression in Porphyromonas gingivalis. Infect Immun. 1998;66:5232–5237. doi: 10.1128/iai.66.11.5232-5237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang C-Y, Bond V C, Genco C A. Identification of a second endogenous Porphyromonas gingivalis insertion sequence. J Bacteriol. 1997;179:3808–3812. doi: 10.1128/jb.179.11.3808-3812.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]