Abstract
Purpose
The impact of body mass index (BMI) on outcomes in respiratory failure necessitating extracorporeal membrane oxygenation (ECMO) has been poorly described. We aimed to assess: (i) whether adults with class II obesity or more (BMI ≥ 35 kg/m2) have worse outcomes than lean counterparts, (ii) the form of the relationship between BMI and outcomes, (iii) whether a cutoff marking futility can be identified.
Methods
A retrospective analysis of the Extracorporeal Life Support Organization (ELSO) Registry from 1/1/2010 to 31/12/2020 was conducted. Impact of BMI ≥ 35 kg/m2 was assessed with propensity-score (PS) matching, inverse propensity-score weighted (IPSW) and multivariable models (MV), adjusting for a priori identified confounders. Primary outcome was in-hospital mortality. The form of the relationship between BMI and outcomes was studied with generalized additive models. Outcomes across World Health Organisation (WHO)-defined BMI categories were compared.
Results
Among 18,529 patients, BMI ≥ 35 kg/m2 was consistently associated with reduced in-hospital mortality [PS-matched: OR: 0.878(95%CI 0.798–0.966), p = 0.008; IPSW: OR: 0.899(95%CI 0.827–0.979), p = 0.014; MV: OR: 0.900(95%CI 0.834–0.971), p = 0.007] and shorter hospital length of stays. In patients with BMI ≥ 35 kg/m2, cardiovascular (17.3% versus 15.3%), renal (37% versus 30%) and device-related complications (25.7% versus 20.6%) increased, whereas pulmonary complications decreased (7.6% versus 9.3%). These findings were independent of confounders throughout PS-matched, IPSW and MV models. The relationship between BMI and outcomes was non-linear and no cutoff for futility was identified.
Conclusion
Patients with obesity class II or more treated with ECMO for respiratory failure have lower mortality risk and shorter stays, despite increased cardiovascular, device-related, and renal complications. No upper limit of BMI indicating futility of ECMO treatment could be identified. BMI as single parameter should not be a contra-indication for respiratory ECMO.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-022-06926-4.
Keywords: Extracorporeal membrane oxygenation, Obesity, Body mass index, Respiratory failure
Take-home message
| This large and multi-center registry study shows that patients with obesity class II or more (body mass index (BMI) ≥ 35 kg/m2) undergoing extracorporeal membrane oxygenation for respiratory failure have decreased mortality risk and decreased hospital length of stay, despite an increased risk of cardiovascular, device-related, and renal complications. The association between BMI and these outcomes is non-linear and no upper limit of BMI indicating futility was identified. |
Introduction
Obesity is prevalent in up to 20% of critically ill patients [1]. Obesity compromises long-term health and survival due to increased cardiovascular disorders, metabolic diseases, cancer, and other health problems [2]. Management of critically ill obese patients poses specific challenges [3]. Also, obese patients have an increased risk of developing acute respiratory distress syndrome (ARDS) [4] and other respiratory problems [3].
Extracorporeal membrane oxygenation (ECMO) is an established treatment for patients with severe, potentially reversible respiratory failure, not responding to conventional treatment [5]. Hence, clinicians are increasingly confronted with potential ECMO indications in obese patients. ECMO is an invasive, high resource-demanding technology associated with complications [6]. Severely obese patients may challenge the risk–benefit balance because of anticipated difficulties with cannulation and reaching sufficient blood flows.
In overweight and moderately obese critically ill patients, lower mortality was reported compared to patients with normal weight, despite increased complications. This contra-intuitive finding is labeled the ‘obesity paradox’ [1]. Data on complications and outcomes of respiratory ECMO in extreme obesity are confined to small case series with limited numbers of patients in the upper body mass index (BMI) range and variable cutoffs in analyses. Most studies did not identify obesity as risk factor for poor outcomes, though data are inconsistent [7–20]. Given the paucity of data, hesitancy to consider ECMO in daily care of these patients persists [21]. This reluctance is translated into recent ELSO guidelines, considering BMI > 40 kg/m2 as a relative contraindication for ECMO in patients affected by coronavirus disease 2019 (COVID-19) [22].
We aimed to assess whether adults with class II obesity or more (BMI ≥ 35 kg/m2) treated with ECMO for respiratory failure have worse outcomes and experience more complications than patients with BMI < 35 kg/m2. We evaluated the form of the relationship between BMI and outcomes to evaluate whether a cutoff for futility can be identified.
Methods
Data source
We queried the international ELSO registry [23], including data on primary diagnosis for ECMO and comorbidities based on the International Classification of diseases 9th and 10th (ICD9/10) revision. Patient and ECMO characteristics [24], treatments, complications, and outcomes are entered in dedicated fields. As data are de-identified, the Clinical Trial Center UZLeuven waived the need for local Ethical Committee approval.
Patients
Adults (age ≥ 18 years) receiving ECMO for respiratory failure from 1/1/2010 to 31/12/2020 were eligible. Exclusion criteria involved other than first runs, pregnancy-related runs, and runs with missing data on height or weight. Patients were dichotomized at BMI ≥ 35 kg/m2, and further stratified according to groups as defined by the World Health Organisation (WHO): underweight (BMI < 18.5 kg/m2), normal weight (BMI 18.5–25 kg/m2), overweight (BMI 25–30 kg/m2), class I (BMI 30–35 kg/m2), class II (BMI 35–40 kg/m2) or class III obesity (> 40 kg/m2); the last group was further divided into three subgroups (BMI 40–50 kg/m2, 50–60 kg/m2, > 60 kg/m2). Extreme values (BMI < 12 kg/m2 and > 70 kg/m2) were excluded [25].
Outcomes
Primary outcome was in-hospital mortality. Secondary outcomes included ECMO duration, reason for discontinuation, complications (device-related, hemorrhagic, neurological, renal, cardiovascular, pulmonary, infectious, metabolic, limb), hospital length of stay and discharge destination (Supplemental Table 1).
Confounders
Analyses were corrected for literature search-based, a priori-selected variables [26], including demographics, comorbidities, admission characteristics, and pre-ECMO variables and treatments, associated with outcomes in respiratory ECMO and recorded in the ELSO registry (Supplemental Table 2). These included gender, age, primary diagnosis, pre-ECMO variables (respiratory rate, PO2/FiO2, peak inspiratory pressure, positive end-expiratory pressure (PEEP), pH, PCO2, HCO3, lactate, mean blood pressure), pre-ECMO treatments and characteristics (renal replacement therapy, nitric oxide, prone ventilation, neuromuscular blocking agents, vasopressors/inotropes, bicarbonate, corticosteroids, cardiac arrest, bridge-to-transplantation), ECMO characteristics (cannulation type, transport on ECMO), and year of ECMO. As the number of eligible patients before 2016 was small, these were grouped. Primary diagnoses were categorized into viral pneumonia, non-viral pneumonia, trauma/burn, chemical/aspiration, asthma, pulmonary embolism/pulmonary hypertension, lung transplant complications, unspecified ARDS/acute respiratory failure and other (Supplemental Table 3) [27, 28]. Comorbidities and co-existent conditions, including associated infection, chronic respiratory and cardiac disease, and immunosuppressive condition, were identified as previously (Supplemental Table 4) [27, 28]. MP and GH independently reviewed ICD-9/10 codes and discrepancies were resolved by discussion.
Statistics
Descriptives included mean and standard deviation for continuous variables, median and interquartile range for time variables, and numbers and percentages for categorical variables. Analyses were performed using R software (4.0.3) (see online supplement). p values were considered statistically significant if two-sided p values ≤ 0.05.
Association between class II obesity or more and outcomes
To assess impact of class II obesity or more (BMI ≥ 35 kg/m2) on outcomes, we adjusted for confounders through propensity-score matching, inverse propensity-score weighted (IPSW), and multivariable models. The propensity-based models constituted the primary analyses. For ECMO duration, inverse hyperbolic sign transformation was performed because of its skewed distribution.
Propensity scores were obtained by logistic regression including the literature search-based variables. Propensity methods included: (i) IPSW calculated with the propensity-score estimations using generalized boosted models [29–31], (ii) propensity-score matching using one-to-one nearest neighbor matching without replacement with BMI ≥ 35 kg/m2 as the dependent variable within a caliper width of 0.2. Satisfactory matching was defined as an absolute value of the standardized mean difference (SMD) < 0.1 for all variables [31, 32]. For binary outcomes, matched data were analyzed using logistic mixed effects regression models. For continuous data, linear mixed effects regression models were used.
Multivariable logistic regressions were used as an alternative approach to verify the impact of BMI ≥ 35 kg/m2 on outcomes. Collinearity between confounders was checked and judged problematic in case of variation inflation factor > 5.
Exploratory analyses
Differences in complications between BMI categories were further explored to define potential drivers by comparing the incidence of the subcategories of the complications of interest in the total and propensity-matched populations.
Assessment of the shape of the relationship between BMI and outcomes and BMI subcategories
Anticipating a non-linear relationship, weighted generalized additive models (GAM) using the propensity scores were used to capture the effect of BMI as a continuous variable on outcomes. The smooth effect of BMI was calculated using smoothed splines. We explored differences across BMI categories within BMI < 35 kg/m2 and BMI ≥ 35 kg/m2 with Kruskal–Wallis rank-sum test, Chi-square, or Fisher’s exact in the propensity-matched set.
Missing data
Data were considered to be missing completely at random, except for proning. For propensity models, multivariable models and GAM analyses, multiple imputation for missing data was performed, generating five imputed datasets [33, 34]. Results were pooled by Rubin’s rule.
Sensitivity analyses
As prolonged prone ventilation was not recorded in the registry prior to December 2017, sensitivity analyses were performed limited to admissions from 2018 onward. As death is a competing risk for development of complications, sensitivity analyses for the secondary outcomes were performed on survivors only.
Results
Patient population and characteristics
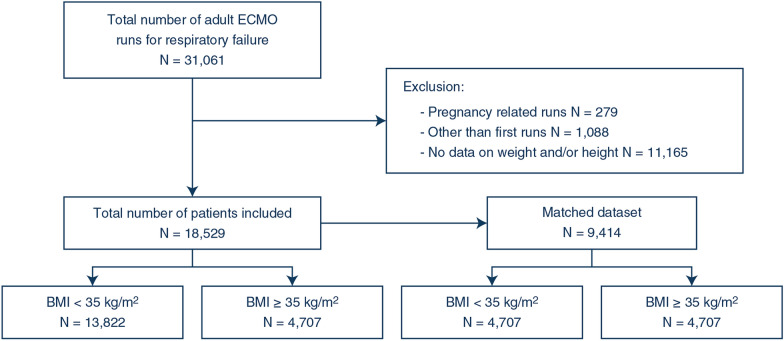
The registry included 31,061 ECMO runs. We excluded 279 pregnancy-related runs, 1088 other than first runs and 11,165 runs with missing height or weight (Fig. 1). The characteristics of the 18,529 included patients are provided in Table 1. Missingness is reported in Supplemental Table 5. The mean age was 48 ± 15 years. Most patients were male (64%). The mean BMI was 31 ± 9 kg/m2, and 25% had BMI ≥ 35 kg/m2. This proportion significantly increased over time from 21% before 2016 up to 30% in 2020.
Fig. 1.
Patient flowchart
Table 1.
Demographic, clinical, pre-ECMO, and cannulation characteristics
| Total population | Propensity matched | |||||||
|---|---|---|---|---|---|---|---|---|
| Total N = 18,529 |
BMI < 35 kg/m2 N = 13,822 |
BMI ≥ 35 kg/m2 N = 4707 |
p valuea | Total N = 9414 |
BMI < 35 kg/m2 N = 4707 |
BMI ≥ 35 kg/m2 N = 4707 |
SMD | |
| Demographics | ||||||||
| Age | 48 (15) | 49 (15) | 47 (13) | < 0.001 | 47 (14) | 47 (15) | 47 (13) | 0.016 |
| Sex, male | 11,828 (64%) | 9104 (66%) | 2724 (58%) | < 0.001 | 5453 (58%) | 2722 (58%) | 2731 (58%) | 0.020 |
| BMI (kg/m2) | 30.7 (8.6) | 26.7 (4.6) | 42.4 (6.9) | < 0.001 | 35 (10) | 27 (5) | 42 (7) | NA |
| Primary diagnosis | < 0.001 | 0.026 | ||||||
| Viral pneumonia | 3958 (22%) | 2637 (20%) | 1321 (29%) | 2686 (29%) | 1329 (28%) | 1357 (29%) | ||
| Non-viral pneumonia | 1149 (6.4%) | 947 (7.1%) | 202 (4.4%) | 407 (4.3%) | 195 (4.1%) | 212 (4.5%) | ||
| Chemical/aspiration | 265 (1.5%) | 200 (1.5%) | 65 (1.4%) | 129 (1.4%) | 63 (1.3%) | 66 (1.4%) | ||
| Trauma/burn | 595 (3.3%) | 453 (3.4%) | 142 (3.1%) | 295 (3.1%) | 151 (3.2%) | 144 (3.1%) | ||
| Asthma | 329 (1.8%) | 278 (2.1%) | 51 (1.1%) | 112 (1.2%) | 57 (1.2%) | 55 (1.2%) | ||
| Pulmonary embolism/pulmonary hypertension | 327 (1.8%) | 224 (1.7%) | 103 (2.2%) | 213 (2.3%) | 108 (2.3%) | 105 (2.2%) | ||
| Lung transplant complications | 476 (2.7%) | 466 (3.5%) | 10 (0.2%) | 25 (0.3%) | 15 (0.3%) | 10 (0.2%) | ||
| Unspecified ARDS/ARF | 7223 (40%) | 5165 (39%) | 2058 (45%) | 4248 (45%) | 2134 (45%) | 2114 (45%) | ||
| Other | 3627 (20%) | 2996 (22%) | 631 (14%) | 1299 (14%) | 655 (14%) | 644 (14%) | ||
| Comorbidities and associated conditions | ||||||||
| Chronic respiratory disease | 2864 (15%) | 2426 (18%) | 438 (9.3%) | < 0.001 | 856 (9.1%) | 418 (8.9%) | 438 (9.3%) | 0.004 |
| Chronic heart disease | 1452 (7.8%) | 1034 (7.5%) | 418 (8.9%) | 0.002 | 850 (9%) | 432 (9.2%) | 418 (8.9%) | 0.002 |
| Immunocompromised | 2053 (11%) | 1837 (13%) | 216 (4.6%) | < 0.001 | 439 (4.7%) | 223 (4.7%) | 216 (4.6%) | 0.018 |
| Associated infection | 788 (4.3%) | 592 (4.3%) | 196 (4.2%) | 0.73 | 394 (4.2%) | 198 (4.2%) | 196 (4.2%) | 0.014 |
| Pre-ECMO variables and treatments | ||||||||
| RR (/min) | 24 (7) | 24 (7) | 24 (7) | < 0.001 | 24 (7) | 24 (7) | 24 (7) | 0.008 |
| pO2/FiO2 | 0.71 (0.56, 0.99) | 0.73 (0.57, 1.03) | 0.67 (0.54, 0.88) | < 0.001 | 0.68 (0.55, 0.91) | 0.69 (0.55, 0.93) | 0.67 (0.54, 0.89) | 0.012 |
| PIP (cmH2O) | 34 (8) | 33 (8) | 35 (8) | < 0.001 | 35 (8) | 35 (9) | 35 (8) | 0.003 |
| PEEP (cmH2O) | 13 (5) | 12 (5) | 14 (5) | < 0.001 | 14 (5) | 14 (5) | 14 (5) | 0.012 |
| pH | 7.25 (0.14) | 7.25 (0.14) | 7.25 (0.13) | 0.38 | 7.25 (0.14) | 7.25 (0.14) | 7.25 (0.14) | < 0.001 |
| pCO2 (cmH2O) | 63 (22) | 63 (23) | 62 (20) | 0.74 | 62 (21) | 62 (21) | 62 (20) | 0.007 |
| HCO3 (mmol/l) | 26 (7) | 26 (7) | 26 (7) | 0.024 | 26 (7) | 26 (7) | 26 (7) | 0.008 |
| Lactate | 3.4 (3.9) | 3.5 (4) | 3.2 (3.6) | < 0.001 | 3.1 (3.5) | 3.2 (3.5) | 3.1 (3.5) | 0.004 |
| Mean BP (mmHg) | 76 (17) | 76 (17) | 77 (17) | < 0.001 | 77 (17) | 77 (17) | 77 (17) | < 0.001 |
| pre-ECMO arrest | 1659 (9%) | 1214 (9%) | 445 (9.6%) | 0.21 | 912 (9.7%) | 462 (9.8%) | 450 (9.6%) | 0.002 |
| Bridge to transplant | 1178 (6.7%) | 1126 (8.6%) | 52 (1.2%) | < 0.001 | 128 (1.4%) | 72 (1.5%) | 56 (1.2%) | < 0.001 |
| Intubation-to-time to ECMO (hours) | 33 (8–110) | 29 (8–107) | 45 (12–116) | < 0.001 | 42 (11–119) | 40 (10–120) | 45 (12–117) | 0.014 |
| pre-ECMO RRT | 1663 (9%) | 1205 (8.7%) | 458 (9.7%) | 0.036 | 935 (9.9%) | 477 (10%) | 458 (9.7%) | 0.004 |
| pre-ECMO NO | 2170 (12%) | 1600 (12%) | 570 (12%) | 0.33 | 1137 (12%) | 567 (12%) | 570 (12%) | 0.025 |
| pre-ECMO prone | 3888 (21%) | 2696 (20%) | 1192 (25%) | < 0.001 | 2351 (25%) | 1159 (25%) | 1192 (25%) | 0.015 |
| pre-ECMO NMBA | 9914 (54%) | 7018 (51%) | 2896 (62%) | < 0.001 | 5757 (61%) | 2861 (61%) | 2896 (62%) | < 0.001 |
| pre-ECMO vasopressors/inotropes | 11,132 (60%) | 8401 (61%) | 2731 (58%) | < 0.001 | 5498 (58%) | 2767 (59%) | 2731 (58%) | < 0.001 |
| pre-ECMO bicarbonate | 2131 (12%) | 1591 (12%) | 540 (11%) | 0.94 | 1080 (11%) | 540 (11%) | 540 (11%) | 0.004 |
| pre-ECMO corticosteroids | 212 (1.1%) | 181 (1.3%) | 31 (0.7%) | < 0.001 | 55 (0.6%) | 24 (0.5%) | 31 (0.7%) | 0.008 |
| ECMO | ||||||||
| Cannulation | < 0.001 | 0.020 | ||||||
| VV | 16,575 (89%) | 12,242 (89%) | 4333 (92%) | 8667 (92%) | 4334 (92%) | 4333 (92%) | ||
| Non-VV | ||||||||
| VA | 915 (4.9) | 773 (5.6) | 142 (3) | 281 (3) | 139 (3) | 142 (3) | ||
| VVA | 146 (0.8) | 118 (0.9) | 28 (0.6) | 55 (0.6) | 27 (0.6) | 28 (0.6) | ||
| Other | 892 (4.8) | 688 (5) | 204 (4.3) | 406 (4.4) | 202 (4.3) | 204 (4.3) | ||
| Year | < 0.001 | 0.025 | ||||||
| 2010–2016 | 1649 (9%) | 1305 (9%) | 344 (7%) | 716 (8%) | 372 (8%) | 344 (7%) | ||
| 2017 | 2843 (15%) | 2254 (16%) | 589 (13%) | 1190 (13%) | 601 (13%) | 589 (13%) | ||
| 2018 | 3524 (19%) | 2724 (20%) | 800 (17%) | 1597 (17%) | 797 (17%) | 800 (17%) | ||
| 2019 | 4071 (22%) | 3052 (22%) | 1019 (22%) | 2038 (22%) | 1019 (22%) | 1019 (22%) | ||
| 2020 | 6442 (35%) | 4487 (32%) | 1955 (42%) | 3873 (41%) | 1918 (41%) | 1955 (42%) | ||
| Transported on ECMO | 4150 (28%) | 2890 (26%) | 1260 (32%) | < 0.001 | 3016 (32%) | 1509 (32%) | 1507 (32%) | 0.010 |
Continuous variables are presented as mean (SD); time variables and PO2/FiO2 are presented as median (interquartile range); categorical variables are presented as numbers (proportions)
BMI body mass index; ECMO extracorporeal membrane oxygenation; ARDS acute respiratory distress syndrome; ARF acute respiratory failure; PIP peak inspiratory pressure, PEEP positive end-expiratory pressure; BP blood pressure; RRT renal replacement therapy; NO nitric oxide; NMBA neuromuscular blocking agent; RR respiratory rate; VV veno-venous; SMD absolute standardized mean difference
ap values are calculated with Wilcoxon signed-rank test, Chi-square, or Fisher exact as appropriate
Patients with class II obesity or more differed from those with BMI < 35 kg/m2, including being younger, more frequently female, and having different primary diagnoses. Less patients with BMI ≥ 35 kg/m2 suffered from chronic respiratory disease or immunocompromised state, yet chronic heart disease was more frequent. Patients with BMI ≥ 35 kg/m2 had lower PO2/FiO2 despite receiving higher PEEP and more rescue treatments with neuromuscular blocking agent (NMBAs), prone ventilation, and nitric oxyde (NO) prior to cannulation. Patients with BMI ≥ 35 kg/m2 were more frequently transported on ECMO, had longer delays between intubation and ECMO, and more frequently received renal replacement therapy prior to cannulation. Bridge-to-transplantation was performed less frequently. A subset of 4707 patients with BMI ≥ 35 kg/m2 were matched to 4707 patients with BMI < 35 kg/m2, rendering satisfactory confounder balance (Table 1) (Supplemental Fig. 1). The characteristics for the BMI subcategories are provided in Supplemental Table 6.
Association between class II obesity or more and hospital mortality
7394 (39.9%) patients died in hospital. Crude mortality was 37.9% in patients with BMI ≥ 35 kg/m2 and 40.6% for those with BMI < 35 kg/m2 (Table 2). Propensity-matched (odds ratio (OR) 0.878, 95% confidence interval (CI) 0.798–0.996, p = 0.008) and IPSW analyses (OR 0.899, 95%CI 0.827–0.979, p = 0.014) showed that patients with BMI ≥ 35 kg/m2 had a reduced mortality risk when adjusting for confounders. Multivariable logistic regression analysis (OR 0.900, 95%CI 0.834–0.971, p = 0.007) was consistent with these findings.
Table 2.
Primary and secondary outcomes for patients with class II obesity or more
| BMI < 35 kg/m2 | BMI ≥ 35 kg/m2 | Analysis | Effect size | p value | |
|---|---|---|---|---|---|
| Primary outcome | |||||
| In-hospital mortality | |||||
| Total population | 5608 (40.6%) | 1786 (37.9%) | Univariable analysis | 0.8916 (0.8333–0.9540)d | 0.001 |
| PS-matched set | 1904 (40.4%) | 1786 (37.9%) | Multivariable analysisa | 0.8999 (0.8337–0.9714)d | 0.007 |
| PS-matched analysisb | 0.8782 (0.7982–0.9662)d | 0.008 | |||
| Inverse PS weighted analysisc | 0.8994 (0.8266–0.9787)d | 0.014 | |||
| Secondary outcomes | |||||
| Cardiovascular complications | |||||
| Total population | 2117 (15.3%) | 813 (17.3%) | Univariable analysis | 1.1493 (1.0523–1.2552)d | 0.002 |
| PS-matched set | 681 (14.5%) | 813 (17.3%) | Multivariable analysisa | 1.2363 (1.1232–1.3609)d | < 0.001 |
| PS-matched analysisb | 1.2381 (1.0901–1.4061)d | 0.001 | |||
| Inverse PS weighted analysisc | 1.2101 (1.0830–1.3521)d | 0.001 | |||
| Hemorrhagic complications | |||||
| Total population | 2279 (16.5%) | 749 (15.9%) | Univariable analysis | 0.9584 (0.8758–1.0488)d | 0.356 |
| PS-matched set | 760 (16.1%) | 749 (15.9%) | Multivariable analysisa | 0.9908 (0.9000–1.0908)d | 0.851 |
| PS-matched analysisb | 0.9696 (0.8632–1.0893)d | 0.603 | |||
| Inverse PS weighted analysisc | 0.9463 (0.8458–1.0586)d | 0.335 | |||
| Limb complications | |||||
| Total population | 234 (1.7%) | 70 (1.5%) | Univariable analysis | 0.8765 (0.6697–1.1473)d | 0.337 |
| PS-matched set | 82 (1.7%) | 70 (1.5%) | Multivariable analysisa | 0.8618 (0.6482–1.1458)d | 0.306 |
| PS-matched analysisb | 0.7360 (0.3959–1.3683)d | 0.318 | |||
| Inverse PS weighted analysisc | 0.8599 (0.6222–1.1885)d | 0.361 | |||
| Infectious complications | |||||
| Total population | 541 (3.9%) | 176 (3.7%) | Univariable analysis | 0.9535 (0.8017–1.1341)d | 0.591 |
| PS-matched set | 192 (4.8%) | 176 (3.7%) | Multivariable analysisa | 1.1059 (0.9145–1.3374)d | 0.299 |
| PS-matched analysisb | 1.1472 (0.8243–1.5965) d | 0.403 | |||
| Inverse PS weighted analysisc | 1.0611 (0.8540–1.3186)d | 0.592 | |||
| Device-related complications | |||||
| Total population | 2850 (20.6%) | 1210 (25.7%) | Univariable analysis | 1.3320 (1.2330–1.4391) d | < 0.001 |
| PS-matched set | 1044 (22.2%) | 1210 (25.7%) | Multivariable analysisa | 1.2240 (1.1252–1.3314)d | < 0.001 |
| PS-matched analysisb | 1.2177 (1.0846–1.3673)d | 0.001 | |||
| Inverse PS weighted analysisc | 1.1882 (1.0763–1.3116)d | 0.001 | |||
| Renal complications | |||||
| Total population | 4142 (30%) | 1743 (37%) | Univariable analysis | 1.3615 (1.2706–1.4588)d | < 0.001 |
| PS-matched set | 1487 (31.6%) | 1743 (37) | Multivariable analysisa | 1.3241 (1.2282–1.4274)d | < 0.001 |
| PS-matched analysisb | 1.2895 (1.1755–1.4146)d | < 0.001 | |||
| Inverse PS weighted analysisc | 1.2857 (1.1802–1.4008)d | < 0.001 | |||
| Pulmonary complications | |||||
| Total population | 1283 (9.3%) | 358 (7.6%) | Univariable analysis | 0.9619 (0.8207–1.1275)d | 0.001 |
| PS-matched set | 513 (10.9%) | 358 (7.6%) | Multivariable analysisa | 0.8333 (0.7039–0.9865)d | < 0.001 |
| PS-matched analysisb | 0.8231 (0.6465–0.9654)d | < 0.001 | |||
| Inverse PS weighted analysisc | 0.8530 (0.7046–0.9678)d | < 0.001 | |||
| Metabolic complications | |||||
| Total population | 1203 (8.7%) | 477 (10.1%) | Univariable analysis | 1.1883 (1.0636–1.3277)d | 0.002 |
| PS-matched set | 422 (9%) | 477 (10.1%) | Multivariable analysisa | 1.1594 (1.0296–1.3055)d | 0.015 |
| PS-matched analysisb | 1.1361 (0.9548–1.3516)d | 0.145 | |||
| Inverse PS weighted analysisc | 1.1353(0.9903–1.3016)d | 0.069 | |||
| Neurological complications | |||||
| Total population | 917 (6.6%) | 340 (7.2%) | Univariable analysis | 1.0956 (0.9629–1.2466) d | 0.165 |
| PS-matched set | 359 (7.6%) | 340 (7.2%) | Multivariable analysisa | 0.9834 (0.8577–1.1277)d | 0.812 |
| PS-matched analysisb | 0.9981 (0.7595–1.3116)d | 0.989 | |||
| Inverse PS weighted analysisc | 0.9651 (0.8264–1.1270)d | 0.654 | |||
| Repair | |||||
| Total population | 5091 (36.8%) | 1719 (36.5%) | Univariable analysis | 0.9837 (0.9187–1.0532)d | 0.638 |
| PS-matched set | 1690 (35.9) | 1719 (36.5) | Multivariable analysisa | 1.0235 (0.9515–1.1010)d | 0.531 |
| PS-matched analysisb | 1.0183 (0.9353–1.1088)d | 0.675 | |||
| Inverse PS weighted analysisc | 1.0196 (0.9355–1.1112)d | 0.658 | |||
| Time on ECMO (hours) | |||||
| Total population | 206 (95–432) | 237 (126–433) | Univariable analysis | 0.1451 (0.0215)e,g | < 0.001 |
| PS-matched set | 230 (112-472) | 237 (126–433) | Multivariable analysisa | − 0.0161 (0.0200)e,g | 0.419 |
| PS-matched analysisb | − 0.0058 (0.0273)e,g | 0.832 | |||
| Inverse PS weighted analysisc | − 0.0026 (0.0176)e,g | 0.883 | |||
| Reason for discontinuation | |||||
| Total population | NA | NA | < 0.001 | ||
| Died or poor prognosis | 4591 (34%) | 1503(32%) | |||
| Expected recovery | 8368 (62%) | 3067 (66%) | |||
| Transplantation | 440 (3.2%) | 14 (0.3%) | |||
| Other | 179 (1.3%) | 60 (1.3%) | |||
| Matched population | NA | NA | < 0.001f | ||
| Died or poor prognosis | 1589 (34%) | 1514 (32%) | |||
| Expected recovery | 3010 (64%) | 3115 (66%) | |||
| Transplantation | 48 (1%) | 17 (0.4%) | |||
| Other | 60 (1.3%) | 61 (1.3%) | |||
| Hospital length of stay | |||||
| Total population | 27 (14–47) | 25 (14–41) | Univariable analysis | − 4.9460 (0.6266)e | < 0.001 |
| PS-matched set | 27 (14–44) | 25 (15–41) | Multivariable analysisa | − 2.9107 (0.6318)e | < 0.001 |
| PS-matched analysisb | − 2.7546 (0.6856)e | < 0.001 | |||
| Inverse PS weighted analysisc | − 2.8285 (0.4882)e | < 0.001 | |||
| Discharge destinationd | |||||
| Total population | NA | NA | < 0.001 | ||
| Home | 3329 (37%) | 874 (28%) | |||
| Other hospital | 1741 (19%) | 608 (20%) | |||
| Transfer to LTAC/rehab/hospice | 2238 (25%) | 1137 (37%) | |||
| Other | 1692 (19%) | 487 (16%) | NA | NA | < 0.001f |
| Matched population | |||||
| Home | 1201 (26%) | 985 (21%) | |||
| Other hospital | 681 (14%) | 644 (14%) | |||
| Transfer to LTAC/rehab/hospice | 667 (19%) | 1202 (26%) | |||
| Other | 1938 (41%) | 1,876 (40%) | |||
BMI body mass index; LTAC long-term acute care; PS propensity score; ECMO extracorporeal membrane oxygenation
aMultivariable model for imputed datasets (pooled by Rubin’s rule)
bLogistic mixed effects model with imputed matched datasets (pooled by Rubin’s rule)
cInverse probability weighted regression model with propensity-score estimates for imputed datasets (pooled by Rubin’s rule)
dEffect size: odds ratio (95% confidence interval)
eCoefficient (standard deviation)
fMcNemar test for paired data
gTime on ECMO was transformed with inverse hyperbolic sign transformation because of skewness
Association between class II obesity or more and secondary outcomes
Incidence of complications and duration of ECMO run and hospitalization according to BMI groups are shown in Table 2. Patients with BMI ≥ 35 kg/m2 more frequently suffered from cardiovascular (17.3% versus 15.3%), renal (37% versus 30%), and device-related complications (25.7% versus 20.6%) compared to patients with BMI < 35 kg/m2. For all complications, this increased risk was consistent across both propensity score-based and multivariable models. Exploratory analyses suggested that the increased risk for cardiovascular complications is possibly driven by an increased incidence of arrhythmia and need for cardio-pulmonary resuscitation (CPR) in patients with BMI ≥ 35 kg/m2 (Supplemental Table 7). Increased incidence of renal complications was observed across all subtypes (Supplemental Table 8). Excess device-related complications appear predominantly due to oxygenator failure (Supplemental Table 9). In contrast, 7.7% of patients with BMI ≥ 35 kg/m2 compared to 9.3% of patients with BMI < 35 kg/m2 developed pulmonary complications. This observation remained present across all adjusted analyses (Table 2) and appears due to reduced incidence of pneumothorax (Supplemental Table 10). The risk of developing other complications was not different (Table 2).
In all confounder-adjusted analyses, ECMO duration was not affected, whereas duration of hospitalization (−3 days) was significantly shorter in patients with BMI ≥ 35 kg/m2 compared to patients with BMI < 35 kg/m2. Discharge destination significantly differed with more patients being transferred to long-term acute care, rehab, or hospice and less patients being discharged home in BMI ≥ 35 kg/m2 (Table 2).
Sensitivity analyses
Sensitivity analyses in patients admitted from 2018 onward yielded similar results (Supplemental Table 11).
To assess whether the higher risk of cardiovascular, renal and device-related complications in patients with BMI ≥ 35 kg/m2 could be explained by a higher survival rate and thus a longer time exposed to the potential development of these complications—the so-called ‘immortal time bias’—additional sensitivity analyses involved survivors only. This showed that the excess risk for developing these complications persisted in survivors only (Supplemental Table 11). The effect on the duration of hospitalization further increased, implying that this is a true effect and cannot be explained by deaths occurring earlier during the disease course of patients with BMI ≥ 35 kg/m2.
Assessment of the shape of the relationship between BMI and outcomes and study of BMI subcategories
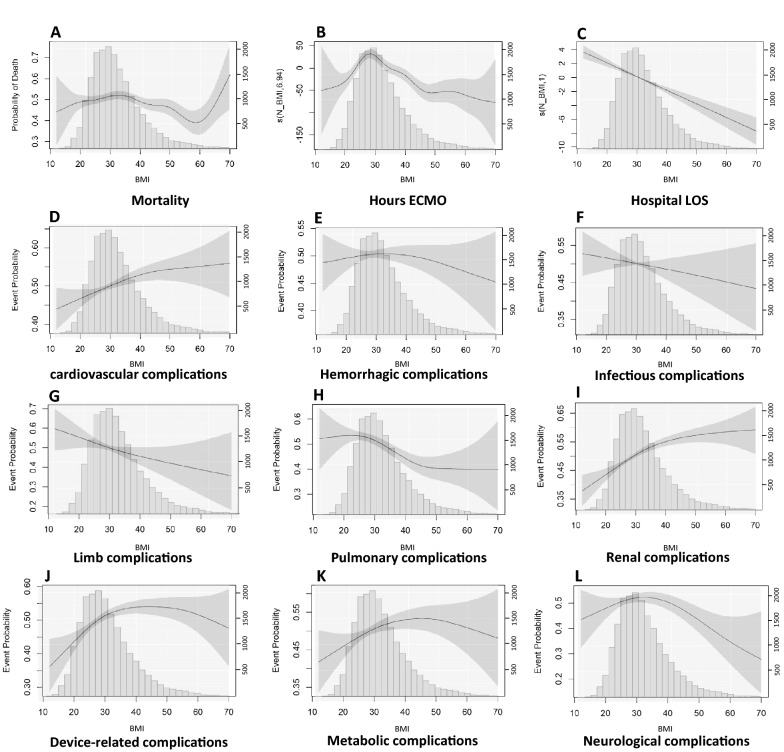
GAM analysis confirmed a non-linear relationship between BMI and mortality (Fig. 2). The shape of the relationship between BMI and mortality suggests that mortality is the highest for patients with BMI 30–35 kg/m2. Subsequently, mortality decreases until BMI values around 60 kg/m2, after which mortality appears to increase up to BMI 70 kg/m2. The confidence interval between 60 and 70 kg/m2, however, is wide because of the small number of patients. At BMI < 25 kg/m2, mortality decreases, although the margin of error is high for BMI < 20 kg/m2 due to the small sample size. Cardiovascular, renal, and device-related complications appear to increase up to a BMI of 40–50 kg/m2, after which a plateau is reached. The risk of pulmonary complications appears to gradually decrease between BMI of 25 and 45 kg/m2. For duration of ECMO, an inverse U-shape relationship was observed between the BMI and duration of ECMO and with a peak around BMI 30 kg/m2. Hospital length of stay decreased across the entire range of BMI values. Outcomes for the BMI subcategories are reported in Supplemental Table 6. Consistent with GAM analyses, data do no suggest an upper limit of BMI, above which treatment appears futile although the margin of error in BMI > 60 kg/m2 is large.
Fig. 2.
Relationship between BMI and outcomes. A Adjusted relationship between BMI and mortality risk (EDF: 6.848, p = 0.002); B adjusted relationship between BMI and duration of ECMO (EDF: 6.943, p < 0.001); C adjusted relationship between BMI and hospital length of stay (EDF: 1, p < 0.001); D adjusted relationship between BMI and cardiovascular complications (EDF:1.76, p = 0.004); E adjusted relationship between BMI and hemorrhagic complications (EDF: 1.004, p = 0.988); F adjusted relationship between BMI and infectious complications (EDF: 1.059, p = 0.311); G adjusted relationship between BMI and limb complications (EDF: 1.264, p = 0.086); H adjusted relationship between BMI and pulmonary complications (EDF: 3.114, p < 0.001); I adjusted relationship between BMI and renal complications (EDF: 2.331 p < 0.001); J adjusted relationship between BMI and device-related complications (EDF: 2.992, p < 0.001); K adjusted relationship between BMI and metabolic complications (EDF: 2.105, p = 0.047); L adjusted relationship between BMI and neurological complications (EDF: 2.449, p = 0.006). Y-axis (left) represents the probability for the binary variables, and for continuous variables the axis represents the scaled centered effect. Y-axis (right) represents the numbers of patients in the histogram. p values are calculated by generalized additive models, p values < 0.05 indicate non-linear relationship. The degree of freedom (EDF) represents the degree of the smooth relationship between the outcome and BMI. A value of 1 is considered a linear relationship, and larger values are considered as a more complex effect of BMI on the outcome
Discussion
In this large multicenter ELSO registry, we documented significantly lower mortality for adults with class II obesity or more requiring ECMO for respiratory failure, compared to those with BMI < 35 kg/m2, despite increased cardiovascular, device-related, and renal complications in the former. Hospital stay was also decreased in patients with BMI ≥ 35 kg/m2, not explained by a disproportionate number of early deaths. The findings were consistent throughout multiple confounder-adjusted statistical approaches. No BMI limit could be identified that would mark futility, albeit that the margin of error for BMI > 60 kg/m2 is large.
Obesity was considered a relative contraindication for ECMO [21] and during the COVID-19 pandemic, it was formally listed as such [22]. Previous studies on the impact of obesity on mortality in respiratory ECMO yielded inconsistent results. Al-Soufi reported no mortality difference according to weight quartiles in 1,334 adults in an earlier ELSO registry analysis, though a trend toward decreased mortality was noted among the highest quartile [18]. Small case series found no survival difference with BMI dichotomized at 30 kg/m2 [19], 35 kg/m2 [20], or 40 kg/m2 [11], as a continuous variable [8, 9, 35] or stratified by subcategories [9, 10, 12], although a trend toward improved survival was suggested for the highest BMI values [11, 12]. A systematic review and meta-analysis of mixed cardiac and respiratory ECMO patients found no association of BMI ≥ 30 kg/m2 with mortality [36]. Others observed lower mortality for patients with BMI ≥ 25 kg/m2 [13] or BMI ≥ 40 kg/m2 [14]. In a large United States nationwide readmission database including 23,876 ECMO patients, 25% of which were respiratory indications, BMI ≥ 30 kg/m2 was not associated with mortality [37]. Confounder adjustment in this study was limited to demographics and comorbidities. Paucity of data in COVID-related ECMO are contradictory [15–17, 38]. We consistently showed decreased mortality among patients with BMI ≥ 35 kg/m2 across multiple analyses extensively adjusted for confounders, associated with reduced hospital length of stay. This was not attributable to early deaths. No cutoff in the upper BMI range was identified, at which outcomes were clearly compromised.
These findings appear in line with the obesity paradox, referring to improved survival in obese critically ill patients. The obesity paradox was observed in general intensive care unit (ICU) populations [39], in pneumonia [40] and ARDS [41]. Obese COVID-19 patients have a higher risk for severe disease, but once admitted to the ICU, mortality is not higher than in non-obese patients [40]. Possible explanations include more adequate nutritional reserves, adipose tissue-released factors exerting favorable immune-modulatory effects, lower weight-based treatment dosing, and selection bias [1, 3]. Obese patients have altered pulmonary mechanics due to increased abdominal compression, promoting atelectasis and complete airway closure [42]. Increased metabolic demands and work of breathing may further predispose to the development of respiratory failure earlier in the disease course, when there is less severe parenchymal lung disease compared with non-obese patients [43]. Obese patients require higher PEEP and physicians may be hesitant to prone these patients. Optimal ventilation may necessitate transpulmonary pressure guidance, accepting airway pressures exceeding traditional safety guidelines. Hence, conventional ventilation may not be fully exploited prior to ECMO [3]. The lower incidence of pneumothorax in patients with BMI ≥ 35 kg/m2 in our cohort supports these findings and reduced length of stay might reflect lower illness severity.
Improved survival came with increased cardiovascular, device-related and renal complications. Previous small cohorts did not identify increased complications in obese patients undergoing respiratory ECMO. Studied complications include vasopressor use [14], renal replacement therapy [12, 19], bleeding or thrombotic events [11], including cannulation site bleeding [10] and cannulation-associated deep vein thrombosis [12], cerebrovascular accidents [11], and oxygenator clotting [8]. Percutaneous cannulation appeared feasible and safe [20]. The excess of cardiovascular complications in our study was explained by a higher incidence of arrhythmia and the need for CPR. Obesity is associated with increased cardiovascular morbidity, including hypertension, ventricular hypertrophy, ischemic heart disease, pulmonary hypertension, and atrial fibrillation, further promoted by co-existent diabetes, metabolic syndrome, and immune effects [1, 44]. Although the registry misses granularity on the nature of the cardiovascular events, it is conceivable that these comorbidities predispose to acute events in the critical setting. Increased renal complications in ECMO patients with BMI ≥ 35 kg/m2 is consistent with increased risk of acute kidney injury (AKI) associated with obesity in general ICU patients [45]. Vulnerability for AKI is likely multifactorial, including increased renal blood flow and hyperfiltration, possibly increasing vulnerability to acute damage, increased oxidative stress, altered immunometabolic state, challenging intravascular volume assessment, and increased abdominal pressure [46]. Finally, device-related complications were more likely to occur. Only oxygenator failure was consistently more frequent in patients with BMI ≥ 35 kg/m2. This could be linked with a procoagulant state and challenging anticoagulation management. No other clot-related problems occurred more frequently. Cannulation, theoretically, may be more challenging because of poor anatomical reference, poor visualization, and sharper entrance angle of the guidewire [10]. Strikingly, cannula problems requiring intervention for misplacement, dislodgement, clots/fibrin, mechanical failure, or inappropriate position were not more frequent in the matched population. In contrast to veno-arterial ECMO in obesity [10], we observed no excess canula-related bleeding or limb complications. Markedly, the increased complications in obese patients, consistent with other reports [1, 39, 47, 48], did not counterbalance the mortality benefit.
Our study has several strengths. This is the largest study on the relationship between BMI and outcomes in adult respiratory ECMO. Multiple statistical approaches to correct for confounding showed consistent results. Data from over 500 centers worldwide, represent real-life daily care practice. This study also has limitations. First, the ELSO registry gathers voluntarily entered information. Participating centers may be more experienced, possibly leading to selection bias. Second, the registry format is characterized by variable missingness of data (e.g., absence of delay between intubation and ECMO in 15%). We attempted to mitigate this with multiple imputation, reducing bias compared to complete case analyses. Whether low rates of proning (21%) and use of NMBA (54%) represent underreporting and/or suboptimal treatment remains unclear. Third, the registry likely suffers from underreporting of comorbidities, treatments and complications, imprecision in diagnosis (e.g., unspecified ARDS/acute respiratory failure in 40%), and lack of ventilation data during ECMO. Fourth, we cannot exclude residual confounding by unmeasured variables, or variables not released by ELSO (e.g., center information). Fifth, the decision to start ECMO in obese patients may have been scrutinized more compared to non-obese counterparts, resulting in additional selection bias. Sixth, the registry is limited to the hospital stay; longer-term patient-centered outcomes such as quality of life are not available. Seventh, the cutoff to dichotomize BMI may be considered somewhat arbitrary. However, dichotomization allowed matching and multiple other statistical approaches were performed to study the relation between BMI and outcomes. Finally, as this is a retrospective analysis, causality cannot be inferred, although we attempted to approximate a random design by different propensity-score methods.
Conclusion
Patients with obesity class II or more treated with respiratory ECMO have decreased mortality and shorter hospital length of stays, despite increased cardiovascular, device-related, and renal complications. The relationship between BMI and outcomes in non-linear. No upper limit of BMI indicating futility was identified. BMI as a single parameter should not be a contraindication for ECMO in respiratory failure.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Steffen Fieuws for his statistical advice during the design and execution of the statistical analyses. We thank all the ESLO centers for providing data to the registry.
Author contributions
GH conceived the study; GH and IGF conceived the analysis plan; GH, MP, IGF, AV, and AC designed the study; IGF led the data analyses, supported by GH; GH, MP, and IGF drafted the initial manuscript. All authors revised and approved the manuscript for submission. GH and IGF have directly accessed and verified the underlying data reported in the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This research was funded by Fonds Wetenschappelijk Onderzoek, Grant no. 1805121N.
Data availability
The ELSO registry data are available from ELSO for investigators upon research requests.
Declarations
Conflicts of interest
The authors have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schetz M, De Jong A, Deane AM, Druml W, Hemelaar P, Pelosi P, Pickkers P, Reintam-Blaser A, Roberts J, et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45:757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 2.Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson MR, Shashaty MGS. Impact of obesity in critical illness. Chest. 2021;160:2135–2145. doi: 10.1016/j.chest.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong MN, Bajwa EK, Thompson BT, Christiani DC. Body mass index is associated with the development of acute respiratory distress syndrome. Thorax. 2010;65:44–50. doi: 10.1136/thx.2009.117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, Da Silva D, Zafrani L, Tirot P, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 6.Brodie D, Slutsky AS, Combes A. Extracorporeal life support for adults with respiratory failure and related indications: a review. JAMA. 2019;322:557–568. doi: 10.1001/jama.2019.9302. [DOI] [PubMed] [Google Scholar]
- 7.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, Ko FW, Chan MC, Chan DP, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swol J, Buchwald D, Strauch JT, Schildhauer TA, Ull C. Effect of body mass index on the outcome of surgical patients receiving extracorporeal devices (VV ECMO, pECLA) for respiratory failure. Int J Artif Organs. 2017 doi: 10.5301/ijao.5000572. [DOI] [PubMed] [Google Scholar]
- 9.Merritt-Genore H, Lyden E, Ryan T, Kwapnoski Z. The effect of patient obesity on extracorporeal membrane oxygenator outcomes and ventilator dependency. J Card Surg. 2020;35:1283–1286. doi: 10.1111/jocs.14579. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez NH, O'Malley TJ, Abai B, Salvatore DM, DiMuzio PJ, Hirose H. Complications of peripheral cannulation site in obese patients on adult extracorporeal membrane oxygenation. ASAIO J. 2021;67:1294–1300. doi: 10.1097/MAT.0000000000001507. [DOI] [PubMed] [Google Scholar]
- 11.Kon ZN, Dahi S, Evans CF, Byrnes KA, Bittle GJ, Wehman B, Rector RP, McCormick BM, Herr DL, et al. Class III obesity is not a contraindication to venovenous extracorporeal membrane oxygenation support. Ann Thorac Surg. 2015;100:1855–1860. doi: 10.1016/j.athoracsur.2015.05.072. [DOI] [PubMed] [Google Scholar]
- 12.Galvagno SM, Jr, Pelekhaty S, Cornachione CR, Deatrick KB, Mazzeffi MA, Scalea TM, Menaker J. Does weight matter? Outcomes in adult patients on venovenous extracorporeal membrane oxygenation when stratified by obesity class. Anesth Analg. 2020;131:754–761. doi: 10.1213/ANE.0000000000004454. [DOI] [PubMed] [Google Scholar]
- 13.Cho WH, Oh JY, Yeo HJ, Han J, Kim J, Hong SB, Chung CR, Park SH, Park SY, et al. Obesity survival paradox in pneumonia supported with extracorporeal membrane oxygenation: analysis of the national registry. J Crit Care. 2018;48:453–457. doi: 10.1016/j.jcrc.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Lazzeri C, Bonizzoli M, Cianchi G, Batacchi S, Terenzi P, Cozzolino M, Bernardo P, Peris A. Body mass index and echocardiography in refractory ARDS treated with veno-venous extracorporeal membrane oxygenation. J Artif Organs. 2017;20:50–56. doi: 10.1007/s10047-016-0931-8. [DOI] [PubMed] [Google Scholar]
- 15.Daviet F, Guilloux P, Hraiech S, Tonon D, Velly L, Bourenne J, Porto A, Gragueb-Chatti I, Bobot M, et al. Impact of obesity on survival in COVID-19 ARDS patients receiving ECMO: results from an ambispective observational cohort. Ann Intensive Care. 2021;11:157. doi: 10.1186/s13613-021-00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trejnowska E, Drobinski D, Knapik P, Wajda-Pokrontka M, Szuldrzynski K, Staromlynski J, Nowak W, Urlik M, Ochman M, et al. Extracorporeal membrane oxygenation for severe COVID-19-associated acute respiratory distress syndrome in Poland: a multicenter cohort study. Crit Care. 2022;26:97. doi: 10.1186/s13054-022-03959-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz RA, Graf J, Zambrano JM, Ruiz C, Espinoza JA, Bravo SI, Salazar PA, Bahamondes JC, Castillo LB, et al. Extracorporeal membrane oxygenation for COVID-19-associated severe acute respiratory distress syndrome in Chile: a nationwide incidence and cohort study. Am J Respir Crit Care Med. 2021;204:34–43. doi: 10.1164/rccm.202011-4166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Soufi S, Buscher H, Nguyen ND, Rycus P, Nair P. Lack of association between body weight and mortality in patients on veno-venous extracorporeal membrane oxygenation. Intensive Care Med. 2013;39:1995–2002. doi: 10.1007/s00134-013-3028-3. [DOI] [PubMed] [Google Scholar]
- 19.Verkerk BS, Dzierba AL, Muir J, Der-Nigoghossian C, Brodie D, Bacchetta M, Rietdijk W, Bakker J. Opioid and benzodiazepine requirements in obese adult patients receiving extracorporeal membrane oxygenation. Ann Pharmacother. 2020;54:144–150. doi: 10.1177/1060028019872940. [DOI] [PubMed] [Google Scholar]
- 20.Keyser A, Philipp A, Zeman F, Lubnow M, Lunz D, Zimmermann M, Schmid C. Percutaneous cannulation for extracorporeal life support in severely and morbidly obese patients. J Intensive Care Med. 2020;35:919–926. doi: 10.1177/0885066618801547. [DOI] [PubMed] [Google Scholar]
- 21.Zakhary B, Oppenheimer BW. ECMO for all? Challenging traditional ECMO contraindications. J Crit Care. 2018;48:451–452. doi: 10.1016/j.jcrc.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Shekar K, Badulak J, Peek G, Boeken U, Dalton HJ, Arora L, Zakhary B, Ramanathan K, Starr J, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66:707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.https://www.elso.org/Registry.aspx. (2022). Accessed 20 Sep 2022
- 24.https://www.elso.org/Registry/DataDefinitions,Forms,Instructions.aspx. (2022). Accessed 20 Sep 2022
- 25.Lebel A, Subramanian SV, Hamel D, Gagnon P, Razak F. Population-level trends in the distribution of body mass index in Canada, 2000–2014. Can J Public Health. 2018;109:539–548. doi: 10.17269/s41997-018-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, Ost DE, Punjabi NM, Schatz M, et al. Control of confounding and reporting of results in causal inference studies. guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, Scheinkestel C, Cooper DJ, Brodie D, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189:1374–1382. doi: 10.1164/rccm.201311-2023OC. [DOI] [PubMed] [Google Scholar]
- 28.Nunez JI, Gosling AF, O'Gara B, Kennedy KF, Rycus P, Abrams D, Brodie D, Shaefi S, Garan AR, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. 2022;48:213–224. doi: 10.1007/s00134-021-06593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCaffrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–3414. doi: 10.1002/sim.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas L, Li F, Pencina M. Using propensity score methods to create target populations in observational clinical research. JAMA. 2020;323:466–467. doi: 10.1001/jama.2019.21558. [DOI] [PubMed] [Google Scholar]
- 31.Chesnaye NC, Stel VS, Tripepi G, Dekker FW, Fu EL, Zoccali C, Jager KJ. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15:14–20. doi: 10.1093/ckj/sfab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Austin PC, White IR, Lee DS, van Buuren S. Missing data in clinical research: a tutorial on multiple imputation. Can J Cardiol. 2021;37:1322–1331. doi: 10.1016/j.cjca.2020.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu LC, Lin SW, Chuang LP, Li HH, Liu PH, Tsai FC, Chang CH, Hung CY, Lee CS, et al. Mechanical power during extracorporeal membrane oxygenation and hospital mortality in patients with acute respiratory distress syndrome. Crit Care. 2021;25:13. doi: 10.1186/s13054-020-03428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaidi SAA, Saleem K. Obesity as a risk factor for failure to wean from ECMO: a systematic review and meta-analysis. Can Respir J. 2021;2021:9967357. doi: 10.1155/2021/9967357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian-Miller N, Hadaya J, Nakhla M, Sanaiha Y, Madrigal J, Emami S, Cale M, Sareh S, Benharash P. The impact of obesity on outcomes in patients receiving extracorporeal life support. Artif Organs. 2020;44:1184–1191. doi: 10.1111/aor.13752. [DOI] [PubMed] [Google Scholar]
- 38.Kunavarapu C, Yeramaneni S, Melo J, Sterling RK, Huskey LC, Sears L, Burch C, Rodriguez SM, Habib PJ, et al. Clinical outcomes of severe COVID-19 patients receiving early VV-ECMO and the impact of pre-ECMO ventilator use. Int J Artif Organs. 2021;44:861–867. doi: 10.1177/03913988211047604. [DOI] [PubMed] [Google Scholar]
- 39.Sakr Y, Alhussami I, Nanchal R, Wunderink RG, Pellis T, Wittebole X, Martin-Loeches I, Francois B, Leone M, et al. Being overweight is associated with greater survival in ICU patients: results from the intensive care over nations audit. Crit Care Med. 2015;43:2623–2632. doi: 10.1097/CCM.0000000000001310. [DOI] [PubMed] [Google Scholar]
- 40.Kooistra EJ, Brinkman S, van der Voort PHJ, de Keizer NF, Dongelmans DA, Kox M, Pickkers P. Body mass index and mortality in coronavirus disease 2019 and other diseases: a cohort study in 35,506 ICU patients. Crit Care Med. 2022;50:e1–e10. doi: 10.1097/CCM.0000000000005216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni YN, Luo J, Yu H, Wang YW, Hu YH, Liu D, Liang BM, Liang ZA. Can body mass index predict clinical outcomes for patients with acute lung injury/acute respiratory distress syndrome? A meta-analysis. Crit Care. 2017;21:36. doi: 10.1186/s13054-017-1615-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grieco DL, Anzellotti GM, Russo A, Bongiovanni F, Costantini B, D'Indinosante M, Varone F, Cavallaro F, Tortorella L, et al. Airway closure during surgical pneumoperitoneum in obese patients. Anesthesiology. 2019;131:58–73. doi: 10.1097/ALN.0000000000002662. [DOI] [PubMed] [Google Scholar]
- 43.Lazoura O, Parthipun AA, Roberton BJ, Downey K, Finney S, Padley S. Acute respiratory distress syndrome related to influenza A H1N1 infection: correlation of pulmonary computed tomography findings to extracorporeal membrane oxygenation treatment and clinical outcome. J Crit Care. 2012;27:602–608. doi: 10.1016/j.jcrc.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Vyas V, Lambiase P. Obesity and atrial fibrillation: epidemiology, pathophysiology and novel therapeutic opportunities. Arrhythm Electrophysiol Rev. 2019;8:28–36. doi: 10.15420/aer.2018.76.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabaz MS, Asar S, Sertcakacilar G, Sabaz N, Cukurova Z, Hergunsel GO. The effect of body mass index on the development of acute kidney injury and mortality in intensive care unit: is obesity paradox valid? Ren Fail. 2021;43:543–555. doi: 10.1080/0886022X.2021.1901738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gameiro J, Goncalves M, Pereira M, Rodrigues N, Godinho I, Neves M, Gouveia J, Silva ZCE, Jorge S, et al. Obesity, acute kidney injury and mortality in patients with sepsis: a cohort analysis. Ren Fail. 2018;40:120–126. doi: 10.1080/0886022X.2018.1430588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36:151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 48.Sakr Y, Madl C, Filipescu D, Moreno R, Groeneveld J, Artigas A, Reinhart K, Vincent JL. Obesity is associated with increased morbidity but not mortality in critically ill patients. Intensive Care Med. 2008;34:1999–2009. doi: 10.1007/s00134-008-1243-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The ELSO registry data are available from ELSO for investigators upon research requests.