Abstract
The use of nanotechnological products is increasing steadily. In this scenario, the application of nanotechnology in food science and as a technological platform is a reality. Among the several applications, the main use of this technology is for the development of foods and nutraceuticals with higher bioavailability, lower toxicity, and better sustainability. In the health field, nano-nutraceuticals are being used as supplementary products to treat an increasing number of diseases. This review summarizes the main concepts and applications of nano-nutraceuticals for health, with special focus on treating cancer and inflammation.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s43450-022-00338-7.
Keywords: Cancer, Drug, Food science, Inflammation, Nanoparticles, Nanotechnology
Introduction
Nanotechnology is a branch of science dedicated to studying structures at atomic and molecular scales. It is currently attracting significant investments focused on developing new nanostructured materials. Nanotechnology can be applied in several areas of study. In particular, the application of nanotechnology for therapeutic and diagnostic purposes in oncology, cardiology, and neurology has been increasing (Bhardwaj and Kaushik 2017). The success of using nanotechnology is due to the unique characteristics that nanoparticles can have, such as biodegradability, biocompatibility, and industrial scalability (Prasad et al. 2018). Several nanomaterials have shown promising results in clinical trials as alternative therapies for various diseases. The most striking features of these nanomaterials are the reduction of adverse reactions and the higher efficacy in comparison with traditional drugs (Chen et al. 2017; Wen et al. 2017).
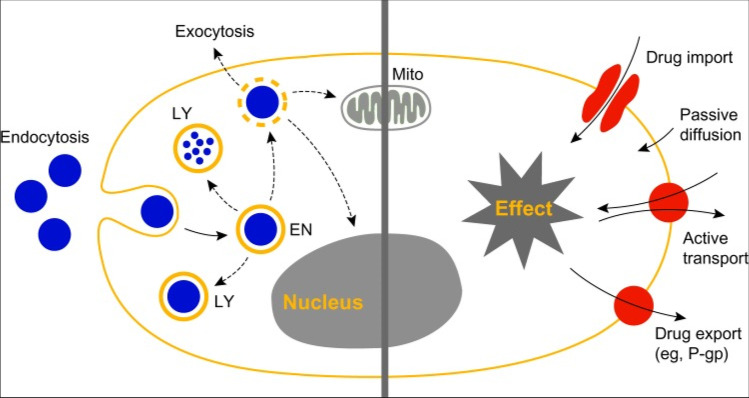
The internalization of nanoparticles in cells is influenced by their physicochemical properties and the cells’ microenvironment (Fig. 1) (Li and Monteiro-Riviere 2016; Brkić Ahmed et al. 2017; Zhou et al. 2017; Dreifuss et al. 2018). The main characteristics involved are size, shape, surface charge, surface functional groups, and hydrophilicity. The effect of the core composition is generally not taken into account because surface characteristics are more prominent (Li and Monteiro-Riviere 2016; Zhang et al. 2016; Reifarth et al. 2018).
Fig. 1.
Elements of nanoparticle interactions with cells as determined by experimental conditions. Figure adapted Kettiger et al. (2013)
Nanoparticles (NPs) can be uptaken across the plasma membrane by various mechanisms, from passive diffusion to active transport. The endocytic and non-endocytic pathways are the most common among these mechanisms (Kettler et al. 2014; Zhang et al. 2016; Brkić Ahmed 2017; Zhou et al. 2017; Dreifuss et al. 2018). Diffusion is the movement of molecules from more to less concentrated gradients, and usually occurs for small, hydrophobic, and uncharged molecules. Fat-soluble NPs are also capable of diffusing across the cell membrane. Water-soluble molecules passively cross the membrane using the facilitated diffusion promoted mainly by the cell’s pores. This diffusion is dependent on size and electrical charge. For very large NPs, transporter proteins act against the concentration gradient to promote membrane crossing (Kettler et al. 2014; Li and Monteiro-Riviere 2016; Belli et al. 2017; Brkić Ahmed 2017; Zhou et al. 2017; Dreifuss et al. 2018; Reifarth et al. 2018).
Geiser et al. (2005) demonstrated that NPs with different sizes (78 nm, 200 nm, and 1000 nm) cross the cell membrane indicating that the transport occurs through pores or diffusion pathways (Kettler et al. 2014). On the other hand, charged NPs are unable to cross the plasma membrane using diffusion pathways. In this case, just NPs with size varying from 10 to 30 nm, the same size as the channel pore, are able to move. Furthermore, the transport of nanoparticles by carriers is considered unlikely due to the high specificity of the carrier structure (Li and Monteiro-Riviere 2016; Zhang et al. 2016; Belli et al. 2017; Dreifuss et al. 2018).
The endocytic pathway depends on particle size and surface modifications, as schematically shown in Fig. 1. Micrometer-sized particles can enter cells mainly by phagocytosis or macropinocytosis. Phagocytosis (Fig. 2) consists of the formation of membrane bulges that gradually envelop the particles and carry them to the intracellular medium. The shape and size of the phagosomes are proportional to the nanoparticles, but normally the size does not exceed a few micrometers (Cooper et al. 2014; Reifarth et al. 2018).
Fig. 2.
Pathways of nanoparticle internalization. Reproduced from Zhang et al. (2015)
Macropinocytosis (Fig. 2) is an actin-regulated process that involves the engulfment of large amounts of extracellular fluid and particles through the wrinkling of the plasma membrane. The membrane villi, which are vascular projections that increase the surface area of a membrane, assume diverse shapes, and when close together, form large organelles called macropinosomes. Actin plays a pivotal role in the uptake process, projecting the membrane with the rearrangement of microfilaments of “arm” protrusions, capturing the extracellular fluid with nanoparticles (Cooper et al. 2014; Kettler et al. 2014; Zhang et al. 2015; Li and Monteiro-Riviere 2016).
Clathrin-mediated endocytosis (Fig. 2) is the most common endocytic route used by nanoparticles. In this pathway, the receptor-ligand binding promotes the clathrin recruitment with plasma membrane formation on the cytosolic side. The cavities are assembled into closed polygonal structures that facilitate endocytosis (Cooper et al. 2014; Kettler et al. 2014; Zhang et al. 2015; Li and Monteiro-Riviere 2016).
Caveolin-dependent endocytosis (Fig. 2) involves the assembly of hook-shaped caveolin layers on the cytosolic side of the plasma membrane, with complex biochemical signaling cascades, forming cavities varying from 50 to 80 nm in diameter. However, the extent to which the entry of modified NPs is regulated by biochemical signaling remains poorly understood. Although clathrin and caveolin can promote endocytosis, clathrin and caveolin endocytosis (Fig. 2) can also occur by receptor-binding forms (Cooper et al. 2014; Zhang et al. 2015; Belli et al. 2017; Reifarth et al. 2018).
NPs without conjugates can be endocytosed through non-specific interactions (Fig. 3) (Cooper et al. 2014; Kettler et al. 2014; Zhang et al. 2015; Li and Monteiro-Riviere 2016). The nanosystems administered in the bloodstream are rapidly accumulated in the tumor site by the enhanced permeation and retention (EPR) effect due the architecture of the blood vessels formed by the tumor. Routes of accumulation of NPs in tumor tissue can be observed by the EPR effect, which is attributed to the tumor microenvironment, the low clearance rate, and poor lymphatic drainage (Fig. 3). The effectiveness of the EPR effect is related to the particle size, surface charge, and/or hydrophobicity (Cooper et al. 2014; Daraee et al. 2016; Hill and Mohs 2016; Karaman et al. 2018; Portilho et al. 2018).
Fig. 3.
Retention and permeation of nanoparticles in tumor tissues. Reproduced from Drude et al. (2017)
Search Strategy
Search terms related to studies of nutraceuticals as nanoplatforms for cancer and inflammation treatment were compiled as follows: cancer, inflammation, oxidative stress, antioxidant, nanoformulations, nutraceuticals, nanonutraceuticals, and immunomodulation. Data for this article were collected using the Pub-Med, Scopus, Science Direct, Web of Science, Google Scholar, Springer, and Elsevier databases. Articles published in the last 10 years (2011 to 2021) in selected and internationally recognized journals were chosen. The exclusion criteria applied during the searches were publications not relevant to the area of interest, publication before 2011, articles with repeated data, and inconclusive outcomes.
Discussion
Nanotechnology in Food Science
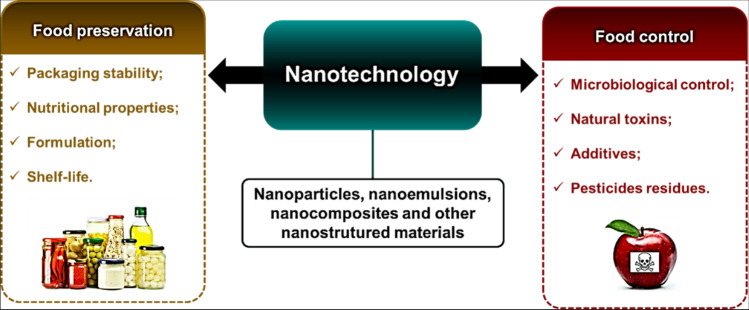
Globally, large companies seek to improve the quality of processed foods, and to achieve this goal, a series of efforts are needed to ensure the organoleptic and nutritional properties of the food products (Neme et al. 2021; Perera et al. 2021). Nanotechnology offers tools to increase the shelf-life of food products, since it is possible, for example, to minimize microbial proliferation using nanomaterials (Singh et al. 2017; Dey et al. 2021). Nanotechnology can act in food science in one of the following aspects: (i) improving or enriching the quality of manufactured products; and (ii) enhancing quality control to prevent microbial proliferation and detect substances harmful to human health (Pushparaj et al. 2022). For these purposes, nanomaterials with unique properties can be developed, which enable food production on a large scale with better quality and safety. These applications of nanotechnology in food science are illustrated in Fig. 4.
Fig. 4.
Some nanotechnology applications in food science, including preservation and quality control segments
Nanotechnology includes encouraging nano-protocols for the fabrication of environmentally friendly processes and smart nano-packing, producing low-calorie beverages and products with improved texture and taste (Yu et al. 2018; Neme et al. 2021). Nanomaterial-based physically enhanced packaging and active and smart/intelligent packaging have been studied and developed on a laboratory scale (Alfei et al. 2020). Bio-based packaging uses biodegradable or biocompatible nanomaterials, which are sustainable alternatives to conventional packaging. Biodegradable plastics, including ethylene terephthalate (bio-poly), polylactic acid (PLA), polyester amide, polybutylene succinate, polyvinyl alcohol (PVA), cellulose, and starch thermoplastics have been employed in recent years in place of conventional plastics (Chausali et al. 2021). Furthermore, certain inorganic materials have been investigated, such as TiO2, ZnO, and MgO, which also provide antimicrobial characteristics to food packaging (Dani et al. 2021).
Abutalib and Rajeh (2021) synthesized Ag and TiO2 nanoparticles with excellent dielectric properties and structural and electrical conductivity. To improve the antibacterial effect and mechanical properties of the polymer blend (Cs/PEO), hydrogels and aerogels were combined with metal oxide nanoparticles. The potential use of these materials in the food packaging industry was confirmed. Furthermore, Liu and collaborators (2022) obtained soluble soybean polysaccharide (SSPS)-based nanocomposite films with in situ synthesized AgNPs. These nanocomposite films exhibited excellent antibacterial activity, and heat-sealed pouches made from the nanocomposite films extended the shelf-life of green grapes (Liu et al. 2022).
Nanotechnology has also contributed to important advances in food science by improving nutrient delivery systems. These nanomaterials can prevent the digestion of nutrients in the mouth and stomach. One of the ways to ensure efficient delivery of nutrients is encapsulation, so selection of nanomaterials as encapsulants to fabricate edible nanoparticles has to be carefully done with full consideration of their individual physicochemical and structural properties as well as the molecular interactions with those of co-existing molecules in the nanoparticles (Razavi et al. 2020). Some works have described the encapsulation of therapeutic bioactive substances such as nucleic acids, peptides, and proteins with hydrophilic nature (Smaoui et al. 2021).
Additionally, the toxicity level of synthetic biopolymers must also be considered for their application in the food sector. Thus, advanced hydrophilic biopolymer compounds have been explored on a large scale. PLA is one of the most common biopolymers used to encapsulate bioactive ingredients and for drug delivery (Ogawara et al. 2016; Tyler et al. 2016). For example, Di Martino et al. (2015) used chitosan and chitosan-grafted PLA as a matrix for bovine serum albumin (BSA) encapsulation in a nanoparticle structure, which was prepared through a polyelectrolyte complexation method with dextran sulfate.
PLA is also often used in food packaging to prolong the shelf life of the final product. Zhang and collaborators prepared antibacterial polylactic acid film with high barrier properties and tensile strength. Cinnamaldehyde inclusion was used as reinforcement in the antibacterial polylactic acid film. The results showed that the tensile strength of the antibacterial film increased by 20%, and the antibacterial activity against Escherichia coli and Listeria monocytogenes was 100%. The release rate of cinnamaldehyde from the antibacterial film was slow and varied smoothly for 20 days (Zhang et al. 2020).
In a study by Karkhanis et al. (2021), a nanocomposite of PLA and cellulose nanocrystals (CNCs) was used to extend the shelf life of dry foods. The addition of CNC in PLA film improved its barrier performance, and shelf-life models predicted longer shelf-life for crackers within PLA/CNC films compared to PLA. The shelf-life of crackers packaged in films predicted from various mathematical simulations supported the experimental data that the crackers packaged in the PLA/CNC films had longer shelf-life than those packaged in pure PLA films, clearly demonstrating the potential of CNC-based films to extend the shelf-life of dry foods.
Another range of nanotechnology applications in food science is the use of nanoparticles and nanocomposites for quality control and consumer health safety. Some works have reported the use of nanomaterials for the microbiological control of harmful species present in food. Liu et al. (2022) developed a fluorescence sensing platform based on the fluorescence resonance energy transfer between upconversion nanoparticles combined with aptamers and black hole quencher 1 (BHQ-1) ligated with cDNA for the detection of pathogenic bacteria. Using this sensing platform, the authors successfully detected one strain of Staphylococcus aureus with a limit of detection (LOD) of 6 CFU/ml in the linear range of 36–3.6 × 107 CFU/ml. This method was tested in relevant food samples with good recoveries.
Another area for application of nanotechnology in food science is the analysis of potentially toxic substances present in manufactured products. In this regard, chemical sensors and other sensing strategies can be developed using nanomaterials, such as nanoparticles and nanocomposites. Some examples of nanomaterials explored in this field and some methods used are summarized in Fig. 5. This shows that the same nanomaterials can have their chemical structure optimized and adapted to act more adequately as sensors. For example, carbon quantum dots (CQDs) are versatile fluorescent nanoparticles discovered about 20 years ago, whose surface is rich in functional groups (Xu et al. 2004; Dsouza et al. 2021; Liu et al. 2022). Some authors have reported that this surface, rich in amines, carboxyls, or other functional groups, favors the interaction with analytes and allows the development of sensors (Pan et al. 2017; Sistani and Shekarchizadeh 2021; Guan et al. 2022).
Fig. 5.
Nanomaterials are employed in the food safety field to develop chemical sensors and other sensing strategies
The sensors most commonly found in food analysis are optical sensors based on the change of fluorescent properties of nanomaterials through a turn-on/turn-off sensor (Petdum et al. 2022; Rong et al. 2021). This term is used in the literature to indicate that the intensity of the fluorescence spectrum of the nanomaterial increases or decreases in the presence of the analyte, i.e., fluorescence enhancement or fluorescence quenching, respectively (Marimuthu et al. 2021). For example, Zhang et al. (2021) synthesized Se, N-doped carbon dots that emitted strong blue and yellow fluorescence, and applied them to determinate F– in tap water and milk samples. The authors observed an F-fluorescence turn-on sensor in the carbon dots/curcumin system. The detection and quantitation limits were measured as 0.39 and 1.30 μmol/l, respectively. The F-values in tap water and milk samples had relative standard deviations below 7.9% in real usage.
Vijeata et al. (2022) synthesized carbon dots from different parts of the bael patra fruit, Aegle marmelos (L.) Corrêa, Rutaceae, for fluorometric detection of the azo dye allura red. Under the optimized conditions, the decreased fluorescence intensity exhibited a good linear relationship with the increasing concentration of allura red, and the sensor turn-off was robustly developed. The sensor acted well in the presence of amino acids in different food samples. Nair et al. (2021) synthesized a sulfur-doped graphene quantum dot-based fluorescence turn-on sensor to detect omethoate, a systemic organophosphorus insecticide used in horticulture. The fluorescence “turn-on” process includes aggregation-induced quenching of S-GQD with an aptamer via S-GQD-aptamer complex formation and its subsequent fluorescence recovery with the addition of omethoate. The reported turn-on sensor exhibited a low detection limit (0.001 ppm) with high selectivity for omethoate in relation to other pesticides.
Colorimetric sensors are applied to detect both unwanted chemical substances in foods and their freshness. Hoang et al. (2021) investigated the potential of silver nanoparticles (AgNPs) for the recognition of acrylamide (AA) in food samples. AA is produced by Maillard reactions and has been classified as a probable carcinogen since 1994 by the International Cancer Agency (Pan et al. 2020). In this study, the authors used thiourea (TU) as a supporting agent for directly recognizing AA based on the crosslinking aggregation of electrochemically synthesized colloidal AgNPs. This replacement helped AA to easily adsorb and interact with the e-AgNPs via the amine group, resulting in a change in color and absorbance of the e-AgNPs-TU solution. Under optimized conditions, the analysis of the dispersion state variation of e-AgNPs showed excellent performance in sensing AA, with a good linear relation in the range of 0.1–1000 μΜ (R2 = 0.99) and a LOD of 0.024 μmol/l.
The residues of gentamicin in food pose a serious threat to human health. Given this scenario, Zhang et al. (2020) developed a colorimetric sensor that showed high sensitivity, selectivity, and stability for gentamicin detection based on gold nanoparticles (AuNPs). The authors proposed a lysine-functionalized AuNP colorimetric sensor to detect gentamicin quickly, according to the following mechanism: The interaction between lysine and AuNPs favors the adsorption of the amino acid on the surface of AuNPs. After adding gentamicin, the strong hydrogen bond between the analyte and lysine promotes the aggregation of AuNPs. The color of AuNPs changes from wine red to blue, an obvious color change. The results showed that the colorimetric sensor had high selectivity and sensitivity for detecting gentamicin, and the linear range and limit of detection were 5–60 nmol/l and 1.22 nmol/l, respectively.
Another type of sensor is made from polymer-based nanocomposites. Kwan and collaborators (2019) dispersed CQDs in thin solid-state films for the development of low-cost, biocompatible, and transparent PVA and polyethylene glycol nanocomposites. The composite films showed photoluminescent properties with the addition of CQDs. A tartrazine sensor was applied for food quality control since it could detect tartrazine at a concentration as low as 10 μmol/l. Ng et al. (2021) immobilized N-doped carbon dots in thin PVA films for tartrazine sensing. The quenching of carbon dots was affected by surface functional groups, so the effects of film properties on their photoluminescence should be investigated. The linear relation between change in the fluorescence intensity and tartrazine concentration indicated dynamic quenching at low tartrazine concentration (< 10 μmol/l), attaining a detection limit of 4.66 μmol/l.
Carneiro et al. (2019) explored strategies for sensing food contaminants. The authors synthesized CQDs from riboflavin and applied them for the sensing of pesticides commonly found in Brazil in rice, oranges, carrots, and bell peppers. The linear discriminant analysis (LDA) algorithm was used as a chemometric tool to discriminate analytes with a sensitivity as low as 250 ng/ml. Additionally, this group later applied the same strategy to differentiate food additives (Carneiro et al. 2021). Their results proved that the sensing strategy involving CQDs and LDA can be applied in the quality control of additives used in olive preservation formulations. Furthermore, according to the proposed methodology, additives were classified with 100% accuracy, since two canonical factors correctly discriminated the analytes in the real sample.
Nanotechnology has advanced substantially recent years, including in the area of aquaculture nutrition. Studies have been conducted on the possibility of using nanotechnology to deliver nutraceuticals and minerals to aquatic animals. Although the results of some of these studies have shown positive effects of nanotechnology on aquaculture nutrition, in some cases dietary toxicity and other negative effects have been observed.
Saffari et al. (2016, 2018) showed that selenium nanoparticles act more efficiently on growth performance of feed, as demonstrated by the hemato-immunological and antioxidant defense system of the Eurasian common carp (Cyprinus carpio), when effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth were evaluated. Kohshahi et al. (2019) showed that combining curcumin and selenium nanoparticles promoted the innate immune responses of rainbow trout (Oncorhynchus mykiss). Shahpar and Johari (2019) observed no differences in the growth performance of rainbow trout (Oncorhynchus mykiss) larvae caused by different dietary sources (organic, inorganic, and nanoparticulate) of zinc. However, the zinc oxide nanoparticles caused the lowest survival rates.
Dekani et al. (2019) showed that high doses of all three organic, inorganic, and nanoparticulate zinc sources caused obvious toxicity to the common carp. However, ZnO nanoparticles displayed higher accumulation in fish’s digestive, muscular, and integumentary systems. The activity of superoxide dismutase and alkaline phosphatase increased in the plasma of the fish that received 500 mg/kg of all zinc sources, especially ZnO nanoparticles.
Johari et al. (2020) showed that the addition of copper nanoparticles to the diet of common carp caused signs of toxicity (including reduction of survival and growth), which was dose-dependent. Kazemi et al. (2019) compared the effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of male rainbow trout, and showed that zinc oxide nanoparticles were not a good substitute for mineral zinc in the feed of male breeders.
Ziaei-Nejad et al. (2021) showed that the dietary interaction of iron nanoparticles and a Lactobacillus probiotic could improve some of the biochemical factors of common carp. Pirani et al. (2021) used nanomicelles containing curcumin to deliver this phytochemical to common carp and Pacific white shrimp (Penaeus vannamei), respectively. Veisi et al. (2021) showed that nanoencapsulated silymarin could counteract silver nanoparticle toxicity in Nile tilapia (Oreochromis niloticus). Afshari et al. (2021) reported that the mixture of iron and copper nanoparticles improved the growth and immunity responses of snow trout (Schizothorax zarudnyi).
Nano-Nutraceuticals for Cancer Prevention and Treatment
Chemoprevention is defined as the use of natural, synthetic, or biological agents to reverse, suppress, or prevent a variety of steps in carcinogenesis, including initiation, promotion, and progression of premalignant cells to invasive cancer (Siddiqui and Sanna 2016). Over the past few decades, research has demonstrated the efficacy of dietary products for chemoprevention in cell cultures and preclinical animal model systems. However, many in vitro and in vivo effects could not be applied in clinical practice (Surh 2003; Gao and Hu 2010; Adhami and Mukhtar 2013). Several factors play a role in limiting the bioavailability and systemic delivery of key components of bioactive agents, such as compound solubility, stability due to gastric and colonic pH, metabolism by the gut microbiota, absorption through the intestinal wall, active efflux mechanism, and first-pass metabolic reactions associated with the liver, which were the likely reasons for clinical failure of drug bioavailability after oral administration (Adhami and Mukhtar 2013; Aqil et al. 2013).
New technologies have generated great interest in developing innovative delivery systems capable of overcoming physicochemical and pharmacokinetic limitations and associating controlled release and protection of phytochemicals. In this context, nanotechnology has proved to be a powerful strategy for preventing and treating tumors (Huang et al. 2010; Wang et al. 2014). Rapid cancer diagnosis and accurate delivery of compounds targeted to the site of neoplasms with reduced adverse effects in healthy tissues are antitumor therapies’ goals. Through nanoformulations, antitumor drugs can be optimized to maximize their performance by adapting their particle size, shape, and surface properties to increase treatment efficacy, reduce side effects, and overcome drug resistance (Jain and Stylianopoulos 2010; Markman et al. 2013). The main research in nano-nutraceuticals for cancer therapy is summarized in Table S1.
Polyphenolic compounds are among the most diverse dietary compounds, with various health-promoting properties, including antioxidant, anti-inflammatory, and antineoplastic activities (Rasouli et al. 2016). Nanoformulations of natural polyphenols as bioactive agents, including resveratrol, curcumin, quercetin, epigallocatechin-3-gallate, chrysin, baicalein, luteolin, honokiol, silibinin, and coumarin derivatives, resulted in improved efficacy of cancer prevention and treatment. The impact of nanoformulation methods of these agents on tumor cells has gained greater attention due to enhanced target therapy and bioavailability, and increased stability (Jain et al. 2018; Nassir et al. 2018; Velavan et al. 2018; Sufi et al. 2020).
Resveratrol, 3,5,4′-trihydroxystilbene (1), a naturally occurring polyphenol and a phytoalexin produced by several plants in response to injury or attack by pathogens, has attracted considerable attention in the last decade associated with its wide use in a large variety of therapeutic applications, including chemopreventive and chemotherapeutic purposes. Sources of resveratrol in food include the skin of grapes, blueberries, raspberries, mulberries, and peanuts. Resveratrol acts through several cell signaling pathways, such as cell cycle arrest, cell proliferation suppression, apoptosis induction, inflammation reduction, and inhibition of adhesion, invasion, and metastasis (Berretta et al. 2020; Rauf et al. 2018; Vervandier-Fasseur and Latruffe 2019; Talebi et al. 2022). Despite promising preclinical results, resveratrol has had limited success in clinical settings, mostly due to its low bioavailability and poor pharmacokinetics (Annaji et al. 2021).
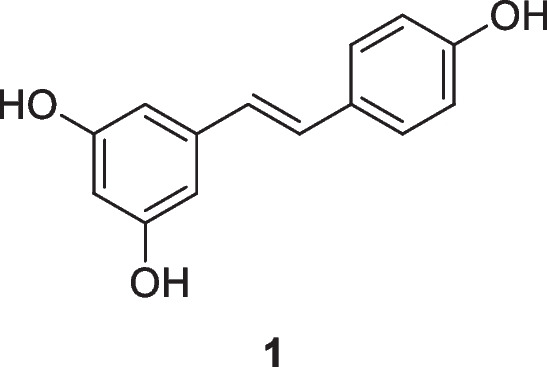
The first resveratrol nanoformulation was developed with chitosan NPs, and the study suggested that these nanoformulations have sustained release in vitro (Zu et al. 2016). Wu and colleagues(2017a, b) observed that chitosan nanoparticles with sodium tripolyphosphate (CS-TPP nanoparticles) loaded with resveratrol can be efficiently delivered to tumor cells by the EPR effect, with the cumulative release of nanoparticles in mimetic tumor tissue conditions (pH 6.5) higher than in physiological conditions (pH 7.4), as well as having lower cytotoxicity.
In a study by Carletto et al. (2016), resveratrol-loaded nanocapsules inhibited murine melanoma tumor growth. Decreased tumor volume, increased necrotic area and inflammatory infiltrate, and prevention of lung metastasis and hemorrhage were observed when resveratrol-loaded nanocapsules were compared to free resveratrol in treated mice. Antitumor effects of the resveratrol nanoformulation were also observed in breast cancer cells. Gold nanoparticles coated with resveratrol showed anti-invasive properties in human breast cancer cells in response to stimulation with 12-O-tetradecanoylphorbol-13-acetate. Such effects were mediated by suppressing matrix metallopeptidase 9 (MMP-9), cyclooxygenase-2 (COX-2), nuclear factor kappa-light-chain-enhancer of activated B cells, activator protein 1, phosphatidylinositol 3-kinase/protein kinase B, and extracellular signal–regulated kinase and/or by activating HO-1 signaling cascades (Park et al. 2016).
Resveratrol-encapsulating polymeric nanoparticles and their cytotoxic effects on prostate cancer cells (LNCaP) were studied by Nassir et al. (2018). These resveratrol NPs significantly reduced cell viability, inducing cell cycle arrest in the G1-S transition phase, externalization of phosphatidylserine, DNA cutting, loss of mitochondrial membrane potential, and generation of reactive oxygen species in LNCaP cells
Curcumin (2), a polyphenol first extracted from Curcuma longa L., Zingiberaceae, in 1815, has long been known in traditional Ayurvedic medicine and has recently been shown to have promising antineoplastic activity (Hassanalilou et al. 2019; Tajuddin et al. 2019; Pricci et al. 2020; Termini et al. 2020). Curcumin exhibits an array of biological activities, especially associated with its high redox activity. Curcumin has been reported to modulate growth factors, enzymes, transcription factors, kinase, inflammatory cytokines, and pro-apoptotic (by upregulation) and anti-apoptotic (by downregulation) proteins (Giordano and Tommonaro 2019). This polyphenolic compound, alone or combined with other agents, may be effective for cancer therapy. Despite sustained and intense focus as a potential anticancer agent, including progression to clinical trials, it has failed to deliver a useful drug due to its low aqueous solubility, poor stability, low bioavailability, rapid metabolism, and systemic elimination (Feng et al. 2017; Nelson et al. 2017).
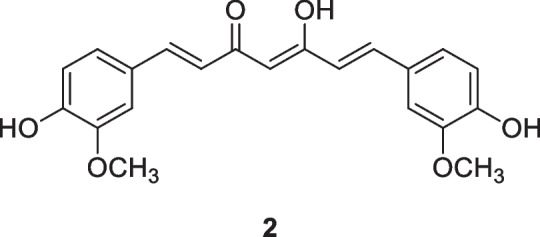
Curcumin nanoparticle formulations are being studied to improve these clinically relevant parameters. Bisht et al. (2016) developed a liposomal nanoformulation of EF24 (Lipo-EF24) that showed potent antineoplastic activity in vitro and in vivo in clinically relevant pancreatic cancer models. The experimental results demonstrated that Lipo-EF24 has therapeutic efficacy against pancreatic cancer tumors by suppressing NF-kB activation, mainly by inhibiting the phosphorylation and subsequent degradation of its inhibitor protein IkB-α. Furthermore, due to its very favorable toxicity profile, Lipo-EF24 is also a promising candidate for further evaluation in combinatorial regimens alongside gemcitabine or other targeted agents.
Poly(lactic-co-glycolic acid) (PLGA) nanoparticle formulations encapsulated with curcumin were shown to be effective in enhancing the therapeutic effects in metastatic tumor cells (Yallapu et al. 2010). More recently, Zaman et al. (2016) evaluated the antitumor effects of curcumin-encapsulated PLGA nanoparticles (nano-curcumin) on cervical cancer cell lines (Caski and SiHa). Compared to free curcumin, Nano-CUR effectively inhibited the growth of Caski and SiHa cells, induced apoptosis, and arrested the cell cycle in the G1-S transition phase, modulating miRNAs, transcription factors, and proteins associated with carcinogenesis. Furthermore, the Nano-CUR formulation effectively reduced tumor burden in an orthotopic model of cervical cancer in NSG mice, suggesting Nano-CUR is a chemopreventive and therapeutic modality for the general management of cervical cancer.
The antitumor effects of nanoformulated curcumin were also evaluated in breast cancer cells (MCF7) and compared with the chemotherapeutic agents cyclophosphamide, adriamycin, and 5-fluorouracil. Nanocurcumin had a relatively high cytotoxic effect on MCF7 breast cancer cells, suppressing the expression of cyclin D1, a critical proto-oncogene in the development and metastasis of breast cancer, acting as an important regulator of G1 to S phase progression in many different cell types (Alao 2007). Nano-curcumin decreased cell proliferation by 83.6%, which was more than achieved by cyclophosphamide (63.31%), adriamycin (70.75%), and 5-fluorouracil (75.04 %) (Hosseini et al. 2019).
Sufi and collaborators (2020) demonstrated that nanoencapsulation of curcumin analogs incorporated with indole and PLGA nanoparticles loaded with curcumin increased its bioavailability and maximized its antitumor potential. The physicochemical characterization and the antitumor potential of the nanoparticles were evaluated in the SW480 human colon cancer cell line. The nanoformulation preserved the drug from degradation in wide pH ranges (4.75, 7.40, and 9.25). The treatment of SW480 cells with nanoparticles triggered nuclear fragmentation, cell cycle block, and inhibition of apoptosis and metastatic biomarkers. Thus, these nanoparticles could be potent nanoformulations against colon cancer due to their ability to tolerate extreme pH environments.
Among the dietary compounds highlighted as possible antitumor therapeutic agents is lycopene (3), a carotenoid pigment responsible for the orange-red color of some foods (Song et al. 2021). Tomato and tomato-based products are the main dietary sources of lycopene, accounting for approximately 80% of lycopene consumption in Western countries. Due to its chemical structure, lycopene exhibits unique and distinct biological properties. Its antioxidant function is the most important and is related to its anti-inflammatory and antitumor properties. These properties act by inhibiting several endogenous interleukins and activating various antitumor mechanisms, like inhibiting the production of nitric oxide and inflammatory cytokines (Magne et al. 2022). However, one of the main obstacles when considering the biological effects of lycopene is its variable and significantly low bioavailability (Papaioannou et al. 2016). The antitumor efficacy of lycopene-loaded nanoparticles (LYC-SLNs) was investigated by Jain et al. (2018) in MCF-7 human breast adenocarcinoma cells. The results obtained by the group revealed a considerably higher cellular uptake of LYC-SLNs in MCF-7 cells, with significantly reduced concentration and cell survival time, compared to free lycopene.

Jain et al. (2018) also investigated a new strategy for formulating lycopene loaded with protein nanoparticles (LYC-WPI-NPs) in MCF-7 cells. The authors observed that LYC-WPI-NPs increased the oral bioavailability of lycopene, controlling its release from the nanoformulation and facilitating its absorption through lymphatic pathways. Furthermore, the prophylactic efficacy of LYC-WPI-NPs was evaluated in an animal model of experimentally induced breast cancer. LYC-WPI-NPs decreased tumor proliferation and increased the survival rate of treated animals.
Epigallocatechin-3-gallate (4, EGCG) is the most abundant catechin in green tea, with health benefits. Research evidence demonstrates that EGCG induced apoptotic cell death in various forms of cancer, such as colon, breast, kidney, lung, and brain. The main limitations of this catechin are its short half-life, low bioavailability, and instability in alkaline and neutral conditions (Singh et al. 2011; Granja et al. 2016). To achieve the maximum therapeutic response of EGCG, magnetite nanoparticles loaded with EGCG (nano EGCG) were developed, and the antitumor activity was evaluated in A549 lung adenocarcinoma cells. Nano EGCG had chemotherapeutic efficacy via modulation of reactive oxygen species, with consequent activation of Nrf2/Keap1 signaling and apoptosis of A549 cells (Velavan et al. 2018).

The antitumor therapeutic efficacy of EGCG nanoformulations was also investigated in a human lung cancer cell line. Chen et al. (2020) developed an EGCG nanoemulsion (nano-EGCG) and evaluated its stability, reduction of side effects, and antitumor effects. Nano-EGCG inhibited proliferation, colony formation, migration, and invasion of H1299 cells through the activation of AMPK signaling pathways. Nano-EGCG was found to inhibit tumor cell invasion through matrix metalloproteinase (MMP)-2 and MMP-9-independent mechanisms. These mechanisms of action of nano-EGCG suggest its application in preventing and treating lung cancer.
Recently, the applicability of EGCG as a drug carrier or as a dispersant to enhance the effects of other drugs has been explored (Hajipour et al. 2018). Fang et al. (2019) developed a new class of EGCG-encapsulated nano-realgar nanoparticles (EGCG-RNPs). They evaluated, in vitro and in vivo, the antitumor effects in a model of acute promyelocytic leukemia. EGCG-RNPs inhibited the proliferation of APL HL-60 leukemia cells. In a subcutaneous solid tumor model of HL-60 cells, EGCG-RNPs significantly inhibited tumor growth. This antitumor mechanism may be correlated with increased absorption and prolongation of realgar retention time in HL-60 cells due to use of EGCG as a carrier. However, the specific mechanisms of HL-60 cell uptake and efflux of EGCG-RNPs require further study.
Quercetin (5) is a widely distributed antioxidant flavonoid contained in many fruits and vegetables. Quercetin supplements are used for their cancer-preventive effects. Quercetin nanoformulated as phytosomes had antitumor effects on MCF-7 human breast cancer cells, increasing apoptosis and decreasing mRNA expression of the Nrf2 downstream genes NQO1 and MRP1 (Minaei et al. 2016). Mohammed et al. (2021) demonstrated that quercetin nanoparticles encapsulated with poly(d,l)-lactic-co-glycolic acid (Q-PLGA-NPs) had apoptotic activity against MCF-7 and CAL51 human breast cancer cell lines. Furthermore, in vivo studies did not show significant liver and kidney functional biomarker changes. No abnormalities or tissue damage were observed via histological images of liver, spleen, lung, heart, and kidney tissues.

Nano-Nutraceuticals for Reduction of Inflammation and Oxidative Stress
Various dietary compounds have well-defined anti-inflammatory and antioxidant properties, capable of specifically activating or inhibiting several cell signaling pathways. Nanotechnology has been widely used to circumvent the limitations related to the stability and pharmacokinetic properties of these compounds. A body of evidence indicates that various drug-delivery nanosystems loaded with plant-based bioactive compounds are effective in modulating oxidative stress and chronic inflammation related to aging-associated disorders (Siu et al. 2018; El-Naggar et al. 2019; Jaguezeski et al. 2019; Singh et al. 2019; Tahmasebi et al. 2021).
The therapeutic potential of resveratrol (1) has been reported for various pathological conditions associated with aging, such as type 2 diabetes, cardiovascular disease, hypertension, stroke, chronic and inflammatory kidney disease, and dementia (Berman et al. 2017; Rarmírez-Garza et al. 2018; Singh et al. 2019). Interestingly, several studies have demonstrated the antioxidant properties of nanoencapsulated resveratrol. Fan et al. (2018) observed that resveratrol loaded in zein nanoparticles with bovine serum albumin-caffeic acid conjugate exhibited greater stability and exerted significantly superior cellular antioxidant activity in comparison with free resveratrol. The bioavailability of folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles was also shown to be 5.95-fold higher compared to free resveratrol after intravenous administration, presenting better targeting to the site of action and sustained release without toxic effects on organs (Lian et al. 2019).
Anti-inflammatory properties of resveratrol-loaded nanoparticles, such as galactosylated poly (lactic-co-glycolic acid) nanoparticles (RES-GNPs), have also been described. The results indicate that RES-GNPs can effectively promote intestinal absorption of resveratrol and strengthen its bioactivity, acting as a promising system for treating inflammatory diseases (Siu et al. 2018). Liu and collaborators (2020) observed that resveratrol-loaded biopolymer core-shell nanoparticles were a highly effective delivery system for resveratrol, significantly increasing its bioavailability and anti-inflammatory activity. These oral delivery systems may be particularly suitable for functional foods or pharmaceuticals.
Over the past decade, the anti-inflammatory and antioxidant properties of curcumin (2) in preventing pathological conditions associated with aging, such as atherosclerosis, cardiovascular and neurodegenerative diseases, osteoporosis, and rheumatoid arthritis, have been repeatedly reported (Kumar et al. 2018; Sarker and Franks 2018; Salehi et al. 2019). Both in vitro and in vivo evidence suggests that nanocurcumin formulations have better health-promoting properties than free curcumin, while its water solubility and bioavailability were significantly improved by nanoencapsulation with lipid or polymeric nanoparticles, nanogels, and dendrimers, and by conjugation with metallic oxide nanoparticles (Kakkar et al. 2013; Shome et al. 2016).
Fan and collaborators (2018) observed, in vitro, that bovine serum dextran albumin nanoparticles loaded with curcumin (BSA-dextran-curcumin) showed better bioavailability and exerted significantly greater antioxidant activity in Caco-2 cells than free curcumin. Furthermore, since the BSA-dextran-curcumin nanoparticles are resistant to pH change and the encapsulated curcumin has high stability, its use as a nutritional supplement in mildly acidic beverages such as dairy drinks and fruit juices has become a promising tool (Fan et al. 2018). Interestingly, the addition of curcumin-loaded nanocapsules produced from the polymer Eudragit L-100 to the diets of dairy ewes resulted in higher antioxidant capacity and lower lipid peroxidation in their milk. In practice, nanotechnology enhances the beneficial effects of curcumin in milk, possibly leading to the development of a desirable nutraceutical food for consumers (Jaguezeski et al. 2019).
Anti-inflammatory activities of different curcumin-loaded nanoformulations have been repeatedly reported (Ameruoso et al. 2017; El-Naggar et al. 2019). Recently, the anti-inflammatory and therapeutic effects of curcumin-loaded nanoformulations have been investigated in patients with mild and severe cases of COVID-19. SinaCurcumin®, a new and potent nanocurcumin product, effectively reduced inflammation and improves COVID-19 patients’ health in both mild and severe stages by upregulating Treg cell activity and modulating inflammatory mediators such as the inflammatory mediators FoxP3, IL-10, IL-35, and TGF-β. In addition, the reduction in mortality in treated patients was one of the promising results of the treatment, highlighting the possibility of using SinaCurcumin® as a therapeutic agent (Tahmasebi et al. 2021).
Quercetin (5) has antioxidant properties, including ROS reduction and modulation of transduction pathways and antioxidant enzyme activities. It has especially demonstrated the ability to prevent the oxidation of low-density lipoproteins, scavenging free radicals, and chelation of transition metal ions. In recent years, innovative nanotechnology-based approaches have been developed to increase the bioavailability of quercetin. Antioxidant effects of quercetin-loaded nanoformulations were evaluated in an alloxan-induced diabetic mouse model. This in vivo study showed a significant reduction in fasting glucose, oxidative stress, lipid peroxidation, and protein carbonylation in the group supplemented with quercetin nanoparticles. It has become clear that small physicochemical differences have significant biological implications for the cellular uptake of nanoparticles and their actions within biological systems (Alam et al. 2016).
Recently, Hyunjin et al. (2021) investigated the physicochemical property of quercetin encapsulated in soluble soy polysaccharides (SSPS) with chitosan. The results obtained indicated that quercetin with chitosan and SSPS had better solubility and stability in an aqueous solution, resulting in improved antitumor, antioxidant, and anti-inflammatory activities compared to free quercetin.
Epigallocatechin-3-gallate (4) has shown potent antioxidant, anti-inflammatory, anti-atherogenic, and antitumor activity (Granja et al. 2016; Shi et al. 2018). In an attempt to improve the bioavailability of EGCG, innovative nanodelivery systems have been widely used (Granja et al. 2017). EGCG-loaded nanoparticles exert antioxidant and anti-inflammatory properties in in vitro models. Avadhani et al. (2017) synthesized nanotransfersomes containing EGCG and hyaluronic acid to synergize these compounds’ UV radiation protection capabilities. This optimized nanotransfersomal formulation reduced lipid peroxidation and intracellular ROS levels and increased the viability of human keratinocytes in vitro. The anti-inflammatory effects of EGCG encapsulated in PLGA particles were evaluated in an in vitro model of inflammation. EGCG-loaded microparticles inhibited inflammation in human dermal fibroblasts by suppressing the expression of TNF-α, IL-1β, and IL-6. Interestingly, the inhibitory effects persisted even after drug withdrawal (Wu et al. 2017a, b).
In vivo studies also point to the anti-inflammatory effects of EGCG nanoformulations. Shariare et al. (2020) developed a novel nanophytosomal preparation of EGCG and evaluated its anti-inflammatory properties. The results demonstrated that nanophytosomal EGCG had anti-inflammatory activity that significantly inhibited carrageenan-induced rat paw edema. Furthermore, studies showed that the phytosomal preparation was stable when stored under ambient conditions for 1 month.
Selenium is an indispensable trace element that plays an important role in antioxidant defense and redox state regulation, by modulating specific metabolic pathways (Hariharan and Dharmaraj 2020; Lin et al. 2021). With the development of new nanotechnologies in recent decades, selenium nanoparticles have emerged as promising agents for clinical application due to their low toxicity, degradability, and high bioavailability (Hosnedlova et al. 2018; Lin et al. 2021). The therapeutic potential of selenium nanoparticles (SeNP), derived from Foeniculum vulgare Mill. Apiaceae (popularly known as fennel), was investigated in arthritic Balb/c mice. SeNPs at a dose of 10 mg/kg were effective in treating rheumatoid arthritis, showing significant anti-arthritic and antioxidant activity, with reduced paw edema and normal biochemical parameters (Arif et al. 2019).
Furthermore, SeNPs can also serve as drug delivery systems to eliminate intracellular pathogens. Liu and collaborators (2019) introduced ciprofloxacin-engineered selenium lipid nanocarriers as an effective drug delivery system to prevent lung infections from interstitial lung disease. Furthermore, the use of SeNPs in a targeted delivery system proved to be effective in an in vivo model of tuberculosis. The data obtained showed that SeNPs preferentially target macrophages and accumulate in lysosomes, releasing the antibiotic isoniazid and increasing the efficiency of the intracellular elimination of Mycobacterium tuberculosis (Pi et al. 2020).
Perspectives and Future Directions
Nano-nutraceuticals can bring new perspectives to the use for traditional nutraceuticals, increasing their permeability, targeting, and bioavailability as well as improving their pharmacokinetics. The increasing number of studies in this field demonstrates their feasibility and applicability. In economic terms, the use of nutraceuticals as nanodevices represents an important saving tool, reducing the amount of active ingredient due to better targeting. Nano-nutraceuticals represent a new frontier in the development of novel drugs using nutraceutical matrixes.
Conclusion
The findings summarized here indicate that the biogenesis of nanomaterials to develop formulations has considerable potential for producing nutraceutical foods and effective therapeutic agents. Nanoformulations increase the stability and bioavailability of dietary compounds and enable the design of intelligent drug delivery platforms applicable against various pathologies, showing significant antitumor, anti-inflammatory, and antioxidant effects.
Supplementary Information
(PDF 145 kb)
Authors Contribution
TMM, LMRA, SVC, LMUDF, PBAF, PFNS, FLP, AOSB, SAJ, ER-J: conceptualization; formal analysis; supervision; writing—original draft; writing—review and editing. RS-O: conceptualization; formal analysis; supervision; writing—original draft; writing—review and editing; supervision; funding. All authors have read the final manuscript and approved its submission.
Funding
This study was funded by Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro Carlos Chagas Filho (FAPERJ) (Cientista do Nosso Estado: E-26/200.815/2021; Rede NanoSaude: E-26/010.000981/2019, Pesquisa na UEZO: E-26/010.002362/2019; Temáticos: E-26/211.269/2021, Infraestrutura e Pesquisa na UEZO e UERJ: E-26//211.207/2021, Bolsa de Pós Doutorado Senior (PDS): E-26/202.320/2021) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Bolsa de Produtividade 1B: 301069/2018-2) to Ralph Santos-Oliveira.
References
- Abutalib M, Rajeh A. Enhanced structural, electrical, mechanical properties and antibacterial activity of Cs/PEO doped mixed nanoparticles (Ag/TiO2) forS food packaging applications. Polym Test. 2021;93:107013. doi: 10.1016/j.polymertesting.2020.107013. [DOI] [Google Scholar]
- Adhami VM, Mukhtar H. Human cancer chemoprevention: hurdles and challenges. Top Curr Chem. 2013;329:203–220. doi: 10.1007/128_2012_342. [DOI] [PubMed] [Google Scholar]
- Afshari A, Sourinejad I, Gharaei A, Johari SA, Ghasemi Z. The effects of diet supplementation with inorganic and nanoparticulate iron and copper on growth performance, blood biochemical parameters, antioxidant response and immune function of snow trout Schizothorax zarudnyi (Nikolskii, 1897) Aquaculture. 2021;539:7366. doi: 10.1016/j.aquaculture.2021.736638. [DOI] [Google Scholar]
- Alam MM, Abdullah K, Braj S, Alim N, Imrana N. Ameliorative effect of quercetin nanorods on diabetic mice: mechanistic and therapeutic strategies. RSC Adv. 2016;6:55092–55103. doi: 10.1039/C6RA04821H. [DOI] [Google Scholar]
- Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfei S, Marengo B, Zuccari G. Nanotechnology application in food packaging: a plethora of opportunities versus pending risks assessment and public concerns. Food Res Int. 2020;137:109664. doi: 10.1016/j.foodres.2020.109664. [DOI] [PubMed] [Google Scholar]
- Ameruoso A, Palomba R, Palange AL, Cervadoro A, Lee A, Di Mascolo D, Decuzzi P. Ameliorating amyloid-β fibrils triggered inflammation via curcumin-loaded polymeric nanoconstructs. Front Immunol. 2017;8:1411. doi: 10.3389/fimmu.2017.01411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaji M, Poudel I, Boddu SHS, Arnold RD, Tiwari AK, Babu RJ. Resveratrol-loaded nanomedicines for cancer applications. Cancer Rep. 2021;4:e1353. doi: 10.1002/cnr2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–141. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif A, Bhatti A, John P. Therapeutic potential of Foeniculum vulgare Mill. derived selenium nanoparticles in arthritic Balb/c Mice. Int J Nanomedicine. 2019;14:8561–8572. doi: 10.2147/IJN.S226674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avadhani KS, Manikkath J, Tiwari M, Chandrasekhar M, Godavarthi A, Vidya SM, Hariharapura RC, Kalthur G, Udupa N, Mutalik S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017;24:61–74. doi: 10.1080/10717544.2016.1228718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli V, Guarnieri D, Biondi M, Della Sala F, Netti PA. Dynamics of nanoparticle diffusion and uptake in three-dimensional cell cultures. Colloids Surface B. 2017;149:7–15. doi: 10.1016/j.colsurfb.2016.09.046. [DOI] [PubMed] [Google Scholar]
- Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. NPJ Precis Oncol. 2017;1:35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta M, Bignucolo A, Di Francia R, Comello F, Facchini G, Ceccarelli M, Iaffaioli RV, Quagliariello V, Maurea N. Resveratrol in cancer patients: from bench to bedside. Int J Mol Sci. 2020;21:2945. doi: 10.3390/ijms21082945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj V, Kaushik A. Biomedical applications of nanotechnology and nanomaterials. Micromachines. 2017;8:298. doi: 10.3390/mi8100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht S, Schlesinger M, Rupp A, Schubert R, Nolting J, Wenzel J, Holdenrieder S, Brossart P, Bendas G, Feldmann G. A liposomal formulation of the synthetic curcumin analog EF24 (Lipo-EF24) inhibits pancreatic cancer progression: towards future combination therapies. J Nanobiotechnol. 2016;14:57. doi: 10.1186/s12951-016-0209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkić Ahmed L, Milić M, Pongrac IM, Marjanović AM, Mlinarić H, Pavičić I, Gajović S, Vinković Vrček I. Impact of surface functionalization on the uptake mechanism and toxicity effects of silver nanoparticles in HepG2 cells. Food Chem Toxicol. 2017;107:349–361. doi: 10.1016/j.fct.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Carletto B, Berton J, Ferreira TN, Dalmolin LF, Paludo KS, Mainardes RM, Farago PV, Favero GM. Resveratrol-loaded nanocapsules inhibit murine melanoma tumor growth. Colloids Surface B. 2016;144:65–72. doi: 10.1016/j.colsurfb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Carneiro SV, de Queiroz VHR, Cruz AAC, Fechine LMUD, Denardin JC, Freire RM, do Nascimento RF, Fechine PB. Sensing strategy based on Carbon Quantum Dots obtained from riboflavin for the identification of pesticides. Sensors Actuat B-Chem. 2019;301:127149. doi: 10.1016/J.SNB.2019.127149. [DOI] [Google Scholar]
- Carneiro SV, Holanda M, Cunha H, Oliveira J, Pontes SM, Cruz A, Fechine LM, Moura T, Paschoal AR, Zambelli RA, Freire RM, Fechine PB. Highly sensitive sensing of food additives based on fluorescent carbon quantum dots. J Photoch Photobio A. 2021;411:113198. doi: 10.1016/j.jphotochem.2021.113198. [DOI] [Google Scholar]
- Chausali N, Prasad R, Saxena J. Recent trends in nanotechnology applications of bio-based packaging. J Agr Food Res. 2021;7:100257. doi: 10.1016/j.jafr.2021.100257. [DOI] [Google Scholar]
- Chen BH, Hsieh CH, Tsai SY, Wang CY, Wang CC. Anticancer effects of epigallocatechin-3-gallate nanoemulsion on lung cancer cells through the activation of AMP-activated protein kinase signaling pathway. Sci Rep-UK. 2020;10:5163. doi: 10.1038/s41598-020-62136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhou S, Cai Y, Tang F. Nucleic acid aptamer application in diagnosis and therapy of colorectal cancer based on cell-SELEX technology. NPJ Precis Oncol. 2017;1:37. doi: 10.1038/s41698-017-0041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DL, Conder CM, Harirforoosh S. Nanoparticles in drug delivery: mechanism of action, formulation and clinical application towards reduction in drug-associated nephrotoxicity. Expert Opin Drug Deliv. 2014;11:1661–1680. doi: 10.1517/17425247.2014.938046. [DOI] [PubMed] [Google Scholar]
- Dani R, Rawal Y, Murdia M, Bagchi P. A review on applications of nanomaterials in hotel industry: prospects for food processing, packaging, and safety. Mater Today-Proc. 2021;46:11247–11249. doi: 10.1016/j.matpr.2021.02.633. [DOI] [Google Scholar]
- Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:410–422. doi: 10.3109/21691401.2014.955107. [DOI] [PubMed] [Google Scholar]
- Dekani L, Johari SA, Joo HS. Comparative toxicity of organic, inorganic and nanoparticulate zinc following dietary exposure to common carp (Cyprinus carpio) Sci Total Environ. 2019;656:1191–1198. doi: 10.1016/j.scitotenv.2018.11.474. [DOI] [PubMed] [Google Scholar]
- Dey A, Pandey G, Rawtani D. Functionalized nanomaterials driven antimicrobial food packaging: a technological advancement in food science. Food Control. 2021;131:108469. doi: 10.1016/j.foodcont.2021.108469. [DOI] [Google Scholar]
- Di Martino A, Kucharczyk P, Zednik J, Sedlarik V. Chitosan grafted low molecular weight polylactic acid for protein encapsulation and burst effect reduction. Int J Pharm. 2015;496:912–921. doi: 10.1016/j.ijpharm.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Dreifuss T, Ben-Gal TS, Shamalov K, Weiss A, Jacob A, Sadan T, Motiei M, Popovtzer R. Uptake mechanism of metabolic-targeted gold nanoparticles. Nanomedicine-UK. 2018;13:1535–1549. doi: 10.2217/nnm-2018-0022. [DOI] [PubMed] [Google Scholar]
- Drude N, Singh S, Winz OH, Möller M, Mottaghy FM, Morgenroth A. Multistage Passive and Active Delivery of Radiolabeled Nanogels for Superior Tumor Penetration Efficiency. Biomacromolecules. 2017;18:2489–2498. doi: 10.1021/acs.biomac.7b00629. [DOI] [PubMed] [Google Scholar]
- Dsouza SD, Buerkle M, Brunet P, Maddi C, Padmanaban DB, Morelli A, Payam AF, Maguire P, Mariotti D, Svrce V. The importance of surface states in N-doped carbon quantum dots. Carbon. 2021;183:1–11. doi: 10.1016/J.CARBON.2021.06.088. [DOI] [Google Scholar]
- El-Naggar ME, Al-Joufi F, Anwar M, Attia MF, El-Bana MA. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surfaces B. 2019;177:389–398. doi: 10.1016/j.colsurfb.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Fan Y, Liu Y, Gao L, Zhang Y, Yi J. Improved chemical stability and cellular antioxidant activity of resveratrol in zein nanoparticle with bovine serum albumin-caffeic acid conjugate. Food Chem. 2018;261:283–291. doi: 10.1016/j.foodchem.2018.04.055. [DOI] [PubMed] [Google Scholar]
- Fang W, Peng ZL, Dai YJ, Wang DL, Huang P, Huang HP. (-)-Epigallocatechin-3-gallate encapsulated realgar nanoparticles exhibit enhanced anticancer therapeutic efficacy against acute promyelocytic leukemia. Drug Deliv. 2019;26:1058–1067. doi: 10.1080/10717544.2019.1672830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T, Wei Y, Lee RJ, Zhao L. Liposomal curcumin and its application in cancer. Int J Nanomedicine. 2017;12:6027–6044. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini-Rev Med Chem. 2010;10:550–567. doi: 10.2174/138955710791384081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiser M, Rothen-Rutishauser B, Kapp N, Schürch S, Kreyling W, Schulz H, Semmler M, Im Hof V, Heyder J, Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Tommonaro G. Curcumin and cancer. Nutrients. 2019;11:2376. doi: 10.3390/nu11102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A, Frias I, Neves AR, Pinheiro M, Reis S. Therapeutic potential of epigallocatechin gallate nanodelivery systems. Biomed Res Int. 2017;2017:5813793. doi: 10.1155/2017/5813793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granja A, Pinheiro M, Reis S. Epigallocatechin gallate nanodelivery systems for cancer therapy. Nutrients. 2016;8:307. doi: 10.3390/nu8050307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H, Wang D, Sun B. Dual-mode colorimetric/fluorometric sensor for the detection of glutathione based on the peroxidase-like activity of carbon quantum dots. Inorg Chem Commun. 2022;136:109147. doi: 10.1016/J.INOCHE.2021.109147. [DOI] [Google Scholar]
- Hajipour H, Hamishehkar H, Soltan N, Ahmad S, Barghi S, Maroufi NF, Taheri RA. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif Cells Nanomed B. 2018;46:283–292. doi: 10.1080/21691401.2017.1423493. [DOI] [PubMed] [Google Scholar]
- Hariharan S, Dharmaraj S. Selenium and selenoproteins: it's role in regulation of inflammation. Inflammopharmacology. 2020;28:667–695. doi: 10.1007/s10787-020-00690-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanalilou T, Ghavamzadeh S, Khalili L. Curcumin and gastric cancer: a review on mechanisms of action. J Gastroint Cancer. 2019;50:185–192. doi: 10.1007/s12029-018-00186-6. [DOI] [PubMed] [Google Scholar]
- Hill TK, Mohs AM. Image-guided tumor surgery: will there be a role for fluorescent nanoparticles? Wires Nanomed Nanobio. 2016;8:498–511. doi: 10.1002/wnan.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang V-T, Dinh N, Nh02/wN. Nga D, Khi N, Trang V, Lam V, Le A-T. Highly selective recognition of acrylamide in food samples using colorimetric sensor based on electrochemically synthesized colloidal silver nanoparticles: role of supporting agent on cross-linking aggregation. Colloid Surface A. 2021;636:128165. doi: 10.1016/j.colsurfa.2021.128165. [DOI] [Google Scholar]
- Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Peng Q, Baron M, Melcova M, Opatrilova R, Zidkova J, Bjørklund G, Sochor J, Kizek R. Nano-selenium and its nanomedicine applications: a critical review. Int J Nanomedicine. 2018;13:2107–2128. doi: 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini S, Chamani J, Hadipanah MR, Ebadpour N, Hojjati AS, Mohammadzadeh MH, Rahimi HR. Nano-curcumin's suppression of breast cancer cells (MCF7) through the inhibition of cyclinD1 expression. Breast cancer (Dove Medical Press) 2019;11:137–142. doi: 10.2147/BCTT.S195800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Yu H, Ru Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J Food Sci. 2010;75:50–57. doi: 10.1111/j.1750-3841.2009.01457.x. [DOI] [PubMed] [Google Scholar]
- Hyunjin M, Lertpatipanpong P, Hong Y, Kim C-T, Baek S. Nano-encapsulated quercetin by soluble soybean polysaccharide/chitosan enhances anti-cancer, anti-inflammation, and anti-oxidant activities. J Funct Foods. 2021;87:104756. doi: 10.1016/j.jff.2021.104756. [DOI] [Google Scholar]
- Jaguezeski AM, Gündel SS, Favarin FR, Gündel A, Souza CF, Baldissera MD, Cazarotto CC, Volpato A, Fortuoso BF, Ourique AF, Da Silva AS. Low-dose curcumin-loaded Eudragit L-100-nanocapsules in the diet of dairy sheep increases antioxidant levels and reduces lipid peroxidation in milk. J Food Biochem. 2019;43:e12942. doi: 10.1111/jfbc.12942. [DOI] [PubMed] [Google Scholar]
- Jain A, Sharma G, Ghoshal G, Kesharwani P, Singh B, Shivhare US, Katare OP. Lycopene loaded whey protein isolate nanoparticles: an innovative endeavor for enhanced bioavailability of lycopene and anti-cancer activity. Int J Pharm. 2018;546:97–105. doi: 10.1016/j.ijpharm.2018.04.061. [DOI] [PubMed] [Google Scholar]
- Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johari SA, Sarkheil M, Asghari S, Haghighat F, Dekani L, Keyvanshokooh S. Comparative toxicity of nanoparticulate and ionic copper following dietary exposure to common carp (Cyprinus carpio) Comp Biochem Physiol C Toxicol Pharmacol. 2020;229:108680. doi: 10.1016/j.cbpc.2019.108680. [DOI] [PubMed] [Google Scholar]
- Kakkar V, Muppu SK, Chopra K, Kaur IP. Curcumin loaded solid lipid nanoparticles: an efficient formulation approach for cerebral ischemic reperfusion injury in rats. Eur J Pharm Biopharm. 2013;85:339–345. doi: 10.1016/j.ejpb.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Karaman DŞ, Sarparanta MP, Rosenholm JM, Airaksinen AJ. Multimodality imaging of silica and silicon materials in vivo. Adv Mater. 2018;30:e1703651. doi: 10.1002/adma.201703651. [DOI] [PubMed] [Google Scholar]
- Karkhanis SS, Stark NM, Sabo RC, Matuana LM. Potential of extrusion-blown poly (lactic acid)/cellulose nanocrystals nanocomposite films for improving the shelf life of a dry food product. Food Packag Shelf Life. 2021;29:100689. doi: 10.1016/j.fpsl.2021.100689. [DOI] [Google Scholar]
- Kazemi E, Sourinejad I, Ghaedi A, Johari SA, Ghasemi Z. Effect of different dietary zinc sources (mineral, nanoparticulate, and organic) on quantitative and qualitative semen attributes of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2019;515:734529. doi: 10.1016/j.aquaculture.2019.734529. [DOI] [Google Scholar]
- Kettiger H, Schipanski A, Wick P, Huwyler J. Engineered nanomaterial uptake and tissue distribution: from cell to organism. Int J Nanomedicine. 2013;8:3255–3269. doi: 10.2147/IJN.S49770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettler K, Veltman K, van de Meent D, van Wezel A, Hendriks AJ (2014) Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ Toxicol Chem 33:481–492. 10.1002/etc.2470 [DOI] [PubMed]
- Kohshahi AJ, Sourinejad I, Sarkheil M, Johari SA. Dietary cosupplementation with curcumin and different selenium sources (nanoparticulate, organic, and inorganic selenium): influence on growth performance, body composition, immune responses, and glutathione peroxidase activity of rainbow trout (Oncorhynchus mykiss) Fish Physiol Biochem. 2019;45:793–804. doi: 10.1007/s10695-018-0585. [DOI] [PubMed] [Google Scholar]
- Kumar SSD, Houreld NN, Abrahamse H. Therapeutic potential and recent advances of curcumin in the treatment of aging-associated diseases. Molecules. 2018;23:835. doi: 10.3390/molecules2304083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan M, Leo CP, Senanayake SMN, Lim G, Tan MK. Carbon-dot dispersal in PVA thin film for food colorant sensing. J Environ Chem Eng. 2019;8:103187. doi: 10.1016/j.jece.2019.103187. [DOI] [Google Scholar]
- Li Y, Monteiro-Riviere NA. Mechanisms of cell uptake, inflammatory potential and protein corona effects with gold nanoparticles. Nanomedicine. 2016;11:3185–3203. doi: 10.2217/nnm-2016-0303. [DOI] [PubMed] [Google Scholar]
- Lian B, Wu M, Feng Z, Deng Y, Zhong C, Zhao X. Folate-conjugated human serum albumin-encapsulated resveratrol nanoparticles: preparation, characterization, bioavailability and targeting of liver tumors. Artf Cells Nanomed B. 2019;47:154–165. doi: 10.1080/21691401.2018.1548468. [DOI] [PubMed] [Google Scholar]
- Lin W, Zhang J, Xu JF, Pi J. The advancing of selenium nanoparticles against infectious diseases. Front Pharmacol. 2021;12:682284. doi: 10.3389/fphar.2021.682284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Meng J, Cao L, Li Y, Deng P, Pan P, Hu C, Yang H. Synthesis and investigations of ciprofloxacin loaded engineered selenium lipid nanocarriers for effective drug delivery system for preventing lung infections of interstitial lung disease. J Photochem Photobiol B. 2019;197:111510. doi: 10.1016/j.jphotobiol.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang X, Zou Y, Peng Y, McClements DJ, Hu K. Resveratrol-loaded biopolymer core-shell nanoparticles: bioavailability and anti-inflammatory effects. Food Funct. 2020;11:4014–4025. doi: 10.1039/d0fo00195c. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang S, Jin S, Feng X, Bai Y, Han X, Fu G. Synthesis of surface protein-imprinted nanoparticles based on metal coordination and anchored carbon dots for enhanced fluorescence detection. Talanta. 2022;238:123070. doi: 10.1016/J.TALANTA.2021.123070. [DOI] [PubMed] [Google Scholar]
- Magne TM, Barros AOS, Fechine PBA, Alencar LMR, Ricci-Junior E, Santos-Oliveira R. Lycopene as a multifunctional olatform for the treatment of cancer and inflammation. Rev Bras Farmacogn. 2022;32:321–330. doi: 10.1007/s43450-022-00250-0. [DOI] [Google Scholar]
- Marimuthu M, Arumugam SS, JiaoT SD, Li H, Chen Q. Metal organic framework based fluorescence sensor for detection of antibiotics. Trends Food Sci Technol. 2021;116:1002–1028. doi: 10.1016/J.TIFS.2021.08.02. [DOI] [Google Scholar]
- Markman JL, Rekechenetskiy A, Holler E, Ljubimova JY. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv Drug Deliv Rev. 2013;65:1866–1879. doi: 10.1016/j.addr.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minaei A, Sabzichi M, Ramezani F, Hamishehkar H, Samadi N. Co-delivery with nano-quercetin enhances doxorubicin-mediated cytotoxicity against MCF-7 cells. Mol Biol Rep. 2016;43:99–105. doi: 10.1007/s11033-016-3942-x. [DOI] [PubMed] [Google Scholar]
- Mohammed HA, Sulaiman GM, Anwar SS, Tawfeeq AT, Khan RA, Mohammed S, Al-Omar MS, Alsharidah M, Rugaie OA, Al-Amiery AA. Quercetin against MCF7 and CAL51 breast cancer cell lines: apoptosis, gene expression and cytotoxicity of nano-quercetin. Nanomedicine (London, England) 2021;16:1937–1961. doi: 10.2217/nnm-2021-0070. [DOI] [PubMed] [Google Scholar]
- Nair RV, Chandran PR, Mohamed AP, Pilla S. Sulphur-doped graphene quantum dot based fluorescent turn-on aptasensor for selective and ultrasensitive detection of omethoate. Anal Chim Acta. 2021;1181:338893. doi: 10.1016/J.ACA.2021.338893. [DOI] [PubMed] [Google Scholar]
- Nassir AM, Shahzad N, Ibrahim IAA, Ahmad I, Md S, Ain MR. Resveratrol-loaded PLGA nanoparticles mediated programmed cell death in prostate cancer cells. Saudi Pharm J. 2018;26:876–885. doi: 10.1016/j.jsps.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA. The essential medicinal chemistry of curcumin: miniperspective. J Med Chem. 2017;60:1620–1637. doi: 10.1021/acs.jmedchem.6b00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neme K, Nafady A, Uddin S, Tola Y. Application of nanotechnology in agriculture, postharvest loss reduction and food processing: food security implication and challenges. Heliyon. 2021;7:e08539. doi: 10.1016/j.heliyon.2021.e08539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng HK, Gin L, Leo CP. The effects of DMAEMA polyelectrolyte and TiO2 photocatalyst on the tartrazine quenching of N-doped carbon dot immobilized in PVA/microfibrillated cellulose film. J Environ Chem Eng. 2021;9:104850. doi: 10.1016/j.jece.2020.104850. [DOI] [Google Scholar]
- Ogawara K, Shiraishi T, Araki T, Watanabe T, Ono T, Higaki K. Efficient anti-tumor effect of photodynamic treatment with polymeric nanoparticles composed of polyethylene glycol and polylactic acid block copolymer encapsulating hydrophobic porphyrin derivative. Eur J Pharm Sci. 2016;82:154–160. doi: 10.1016/j.ejps.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Pan J, Zheng Z, Yang J, Wu Y, Lu F, Chen Y, Gao W. A novel and sensitive fluorescence sensor for glutathione detection by controlling the surface passivation degree of carbon quantum dots. Talanta. 2017;166:1–7. doi: 10.1016/j.talanta.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Pan M, Liu K, Yang J, Hong L, Xie X, Wang S. Review of research into the determination of acrylamide in foods. Foods. 2020;9:524. doi: 10.3390/foods9040524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou EH, Liakopoulou-Kyriakides M, Karabelas AJ. Natural origin lycopene and its “green” downstream Processing. Crit Rev Food Sci. 2016;56:686–709. doi: 10.1080/10408398.2013.817381. [DOI] [PubMed] [Google Scholar]
- Park SY, Chae SY, Park JO, Lee KJ, Park G. Gold-conjugated resveratrol nanoparticles attenuate the invasion and MMP-9 and COX-2 expression in breast cancer cells. Oncol Rep. 2016;35:3248–3256. doi: 10.3892/or.2016.4716. [DOI] [PubMed] [Google Scholar]
- Perera KY, Jaiswal S, Jaiswal AK. A review on nanomaterials and nanohybrids based bio-nanocomposites for food packaging. Food Chem. 2021;376:131912. doi: 10.1016/j.foodchem.2021.131912. [DOI] [PubMed] [Google Scholar]
- Petdum A, Kaewnok N, Panchan W, Charoenpanich A, Sirirak J, Sahasithiwat S, Sooksimuang T, Wanichacheva N. Novel rapid “turn on” tetrahydro-[5]helicene-based fluorescence sensor for selective detection of Cd2+ with a remarkable large stokes shift and its applications in food samples and living Cell. J Photochem Photobiol A Chem. 2022;423:113578. doi: 10.1016/j.jphotochem.2021.113578. [DOI] [Google Scholar]
- Pi J, Shen L, Yang E, Shen H, Huang D, Wang R, Hu C, Jin H, Cai H, Cai J, Zeng G, Chen ZW. Macrophage-targeted isoniazid-selenium nanoparticles promote antimicrobial immunity and synergize bactericidal destruction of Tuberculosis Bacilli. Angew Chem Int Edit. 2020;59:3226–3234. doi: 10.1002/anie.201912122. [DOI] [PubMed] [Google Scholar]
- Pirani F, Moradi S, Ashouri S, Johari SA, Ghaderi E, Kim HP, Yu IJ. Dietary supplementation with curcumin nanomicelles, curcumin, and turmeric affects growth performance and silver nanoparticle toxicity in Cyprinus carpio. Environ Sci Pollut Res Int. 2021;28:64706–64718. doi: 10.1007/s11356-021-15538-2. [DOI] [PubMed] [Google Scholar]
- Portilho FL, Helal-Neto E, Cabezas SS, Pinto SR, Dos Santos SN, Pozzo L, Sancenón F, Martínez-Máñez R, Santos-Oliveira R. Magnetic core mesoporous silica nanoparticles doped with dacarbazine and labelled with 99mTc for early and differential detection of metastatic melanoma by single photon emission computed tomography. Artif Cells Nanomed Biotechnol. 2018;46(sup1):1080–1087. doi: 10.1080/21691401.2018.1443941. [DOI] [PubMed] [Google Scholar]
- Prasad M, Lambe UP, Brar B, Shah I, Manimegalai J, Ranjan K, Rao R, Kumar S, Mahant S, Khurana SK, Iqbal HMN, Dhama K, Misri J, Prasad G. Nanotherapeutics: an insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed Pharmacother. 2018;97:1521–1537. doi: 10.1016/j.biopha.2017.11.026. [DOI] [PubMed] [Google Scholar]
- Pricci M, Girardi B, Giorgio F, Losurdo G, Ierardi E, Di Leo A. Curcumin and colorectal cancer: from basic to clinical evidences. Int J Mol Sci. 2020;21:2364. doi: 10.3390/ijms21072364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushparaj K, Liu W-C, Meyyazhagan A, Orlacchio A, Pappusamy M, Vadivalagan C, Robert AA, Arumugam VA, Kamyab H, Klemeš JJ, Khademi T, Mesbah M, Chelliapan S, Balasubramanian B. Nano- from nature to nurture: a comprehensive review on facets, trends, perspectives and sustainability of nanotechnology in the food sector. Energy. 2022;240:122732. doi: 10.1016/j.energy.2021.122732. [DOI] [Google Scholar]
- Ramírez-Garza SL, Laveriano-Santos EP, Marhuenda-Muñoz M, Storniolo CE, Tresserra-Rimbau A, Vallverdú-Queralt A, Lamuela-Raventós RM. Health effects of resveratrol: results from human intervention trials. Nutrients. 2018;10:1892. doi: 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasouli H, Farzaei MH, Mansouri K, Mohammadzadeh S, Khodarahmi R. Plant cell cancer: may natural phenolic compounds prevent onset and development of plant cell malignancy? A Lit review. Molecules. 2016;21:1104. doi: 10.3390/molecules21091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A, Imran M, Butt MS, Nadeem M, Peters DG, Mubarak MS. Resveratrol as an anti-cancer agent: a review. Crit Rev Food Sci Nutr. 2018;58:1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- Razavi R, Kenari RE, Farmani J, Jahanshahi M. Fabrication of zein/alginate delivery system for nanofood model based on pumpkin. Int J Biol Macromol. 2020;165(Pt B):3123–3134. doi: 10.1016/j.ijbiomac.2020.10.176. [DOI] [PubMed] [Google Scholar]
- Reifarth M, Hoeppener S, Schubert US. Uptake and intracellular fate of engineered nanoparticles in mammalian cells: capabilities and limitations of transmission electron microscopy-polymer-based nanoparticles. Adv Mater. 2018;30:1703704. doi: 10.1002/adma.201703704. [DOI] [PubMed] [Google Scholar]
- Rong YH, Ouyang M, Wang Q, Li Jiao T, Chen Q. Ratiometric upconversion fluorometric turn-off nanosensor for quantification of furfural in foods. Sensors Actuators B Chem. 2021;350:130843. doi: 10.1016/j.snb.2021.130843. [DOI] [Google Scholar]
- Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio) Aquac Nutr. 2016;23:611–617. doi: 10.1111/anu.12428. [DOI] [Google Scholar]
- Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT. Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio) Fish Physiol Biochem. 2018;44:1087–1097. doi: 10.1007/s10695-018-0496-y. [DOI] [PubMed] [Google Scholar]
- Salehi B, Stojanović-Radić Z, Matejić J, Sharifi-Rad M, Anil Kumar NV, Martins N, Sharifi-Rad J. The therapeutic potential of curcumin: a review of clinical trials. Eur J Med Chem. 2019;163:527–545. doi: 10.1016/j.ejmech.2018.12.01. [DOI] [PubMed] [Google Scholar]
- Sarker MR, Franks SF. Efficacy of curcumin for age-associated cognitive decline: a narrative review of preclinical and clinical studies. GeroScience. 2018;40:73–95. doi: 10.1007/s11357-018-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahpar Z, Johari SA. Effects of dietary organic, inorganic, and nanoparticulate zinc on rainbow trout, Oncorhynchus mykiss larvae. Biol Trace Elem Res. 2019;190:535–540. doi: 10.1007/s12011-018-1563-z. [DOI] [PubMed] [Google Scholar]
- Shariare MH, Afnan K, Iqbal F, Altamimi MA, Ahamad SR, Aldughaim MS, Alanazi FK, Kazi M. Development and optimization of epigallocatechin-3-gallate (EGCG) nano phytosome using design of experiment (DoE) and their in vivo anti-inflammatory studies. Molecules. 2020;25:5453. doi: 10.3390/molecules25225453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Shi YL, Li XM, Yang R, Cai ZY, Li QS, Ma SC, Ye JH, Lu JL, Liang YR, Zheng XQ. Food-grade encapsulation aystems for (-)-epigallocatechin gallate. Molecules. 2018;23:445. doi: 10.3390/molecules23020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shome S, Talukdar AD, Choudhury MD, Bhattacharya MK, Upadhyaya H. Curcumin as potential therapeutic natural product: a nanobiotechnological perspective. J Pharm Pharmacol. 2016;68:1481–1500. doi: 10.1111/jphp.1261. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Sanna V. Impact of nanotechnology on the delivery of natural products for cancer prevention and therapy. Mol Nutr Food Res. 2016;60:1330–1341. doi: 10.1002/mnfr.201600035. [DOI] [PubMed] [Google Scholar]
- Singh AP, Singh R, Verma SS, Rai V, Kaschula CH, Maiti P, Gupta SC. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev. 2019;39:1851–1891. doi: 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK. Application of nanotechnology in food science: perception and overview. Front Microbiol. 2017;8:1501. doi: 10.3389/fmicb.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistani S, Shekarchizadeh H. Fabrication of fluorescence sensor based on molecularly imprinted polymer on amine-modified carbon quantum dots for fast and highly sensitive and selective detection of tannic acid in food samples. Anal Chim Acta. 2021;1186:339122. doi: 10.1016/J.ACA.2021.339122. [DOI] [PubMed] [Google Scholar]
- Siu FY, Ye S, Lin H, Li S. Galactosylated PLGA nanoparticles for the oral delivery of resveratrol: enhanced bioavailability and in vitro anti-inflammatory activity. Int J Nanomedicine. 2018;13:4133–4144. doi: 10.2147/IJN.S164235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaoui S, Ben Hlima H, Ben Braïek O, Ennouri K, Mellouli L, Mousavi Khaneghah A. Recent advancements in encapsulation of bioactive compounds as a promising technique for meat preservation. Meat Sci. 2021;181:108585. doi: 10.1016/j.meatsci.2021.108585. [DOI] [PubMed] [Google Scholar]
- Song X, Luo Y, Ma L, Hu X, Simal-Gandara J, Wang LS, Bajpai VK, Xiao J, Chen F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent. Semin Cancer Biol. 2021;73:331–346. doi: 10.1016/j.semcancer.2021.03.028. [DOI] [PubMed] [Google Scholar]
- Sufi SA, Hoda M, Pajaniradje S, Mukherjee V, Coumar SM, Rajagopalan R. Enhanced drug retention, sustained release, and anti-cancer potential of curcumin and indole-curcumin analog-loaded polysorbate 80-stabilizied PLGA nanoparticles in colon cancer cell line SW480. Int J Pharm. 2020;588:119738. doi: 10.1016/j.ijpharm.2020.119738. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Tahmasebi S, Saeed BQ, Temirgalieva E, Yumashev AV, El-Esawi MA, Navashenaq JG, Valizadeh H, Sadeghi A, Aslani S, Yousefi M, Jadidi-Niaragh F, Adigozalou J, Ahmadi M, Roshangar L. Nanocurcumin improves Treg cell responses in patients with mild and severe SARS-CoV2. Life Sci. 2021;276:119437. doi: 10.1016/j.lfs.2021.119437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajuddin WNBWM, Lajis NH, Abas F, Othman I, Naidu R. Mechanistic understanding of curcumin's therapeutic effects in lung cancer. Nutrients. 2019;11:2989. doi: 10.3390/nu11122989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi M, Talebi M, Farkhondeh T, Samarghandian S. Therapeutic effects of resveratrol in inflammatory bowel diseases: shedding light on the role of cellular and molecular pathways. Rev Bras Farmacogn. 2022;32:160–173. doi: 10.1007/s43450-022-00247-9. [DOI] [Google Scholar]
- Termini D, Den Hartogh DJ, Jaglanian A, Tsiani E. Curcumin against prostate cancer: current evidence. Biomolecules. 2020;10:1536. doi: 10.3390/biom10111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B, Gullotti D, Mangraviti A, Utsuki T, Brem H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv Drug Deliv Rev. 2016;107:163–175. doi: 10.1016/j.addr.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Veisi S, Johari SA, Tyler CR, Mansouri B, Esmaeilbeigi M. Antioxidant properties of dietary supplements of free and nanoencapsulated silymarin and their ameliorative effects on silver nanoparticles induced oxidative stress in Nile tilapia (Oreochromis niloticus) Environ Sci Pollut Res Int. 2021;28:26055–26063. doi: 10.1007/s11356-021-12568-8. [DOI] [PubMed] [Google Scholar]
- Velavan B, Divya T, Sureshkumar A, Sudhandiran G. Nano-chemotherapeutic efficacy of (-)-epigallocatechin 3-gallate mediating apoptosis in A549 cells: involvement of reactive oxygen species mediated Nrf2/Keap1signaling. Biochem Biophys Res Commun. 2018;503:1723–1731. doi: 10.1016/j.bbrc.2018.07.105. [DOI] [PubMed] [Google Scholar]
- Vervandier-Fasseur D, Latruffe N. The potential use of resveratrol for cancer prevention. Molecules. 2019;24:4506. doi: 10.3390/molecules24244506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijeata A, Chaudhary S, Chaudhary GR. Fluorescent carbon dots from Indian Bael patra as effective sensing tool to detect perilous food colorant. Food Chem. 2022;373:131492. doi: 10.1016/j.foodchem.2021.131492. [DOI] [PubMed] [Google Scholar]
- Wang S, Su R, Nie S, Sun M, Zhang J, Wu D, Moustaid-Moussa N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25:363–376. doi: 10.1016/j.jnutbio.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CY, Bi JH, Wu LL, Zeng JB. Aptamer-functionalized magnetic and fluorescent nanospheres for one-step sensitive detection of thrombin. Mikrochim Acta. 2017;185:77. doi: 10.1007/s00604-017-2621-5. [DOI] [PubMed] [Google Scholar]
- Wu J, Wang Y, Yang H, Liu X, Lu Z. Preparation and biological activity studies of resveratrol loaded ionically cross-linked chitosan-TPP nanoparticles. Carbohydr Polym. 2017;175:170–177. doi: 10.1016/j.carbpol.2017.07.058. [DOI] [PubMed] [Google Scholar]
- Wu YR, Choi HJ, Kang YG, Kim JK, Shin JW. In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int J Nanomedicine. 2017;12:7007–7013. doi: 10.2147/IJN.S146296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ray R, Gu Y, Ploehn HJ, Gearheart L, Raker K, Scrivens WA. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J Am Chem Soc. 2004;126:12736–12737. doi: 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- Yallapu MM, Gupta BK, Jaggi M, Chauhan SC. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Yu H, Park J, Kwon C, Hong S-C, Park K-M, Chang P-S. An overview of nanotechnology in food science: preparative methods, practical applications, and safety. J Chem. 2018;2018:5427978. doi: 10.1155/2018/5427978. [DOI] [Google Scholar]
- Zaman MS, Chauhan N, Yallapu MM, Gara RK, Maher DM, Kumari S, Sikander M, Khan S, Zafar N, Jaggi M, Chauhan SC. Curcumin nanoformulation for cervical cancer treatment. Sci Rep-UK. 2016;6:20051. doi: 10.1038/srep20051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Gao H, Bao G. Physical principles of nanoparticle cellular endocytosis. ACS Nano. 2015;9:8655–8671. doi: 10.1021/acsnano.5b03184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Geng Y, Ye N, Xiang Y. A simple and sensitive colorimetric sensor for determination of gentamicin in milk based on lysine functionalized gold nanoparticles. Microchem J. 2020;158:105190. doi: 10.1016/j.microc.2020.105190. [DOI] [Google Scholar]
- Zhang X, Tan X, Hu Y. Blue/yellow emissive carbon dots coupled with curcumin: a hybrid sensor toward fluorescence turn-on detection of fluoride ion. J Hazard Mater. 2021;411:125184. doi: 10.1016/J.JHAZMAT.2021.125184. [DOI] [PubMed] [Google Scholar]
- Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle-liver interactions: cellular uptake and hepatobiliary elimination. J Control Release. 2016;240:332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Zhou G, Zhang J, Pan C, Liu N, Wang Z, Zhang J. Enhanced uptake of Fe3O4 nanoparticles by intestinal epithelial cells in a state of inflammation. Molecules. 2017;22:1240. doi: 10.3390/molecules22081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei-nejad S, Abaei NK, Doost BN, Johari SA. Effects of supplemental feeding of common carp (Cyprinus carpio) with iron nanoparticles and probiotic Lactobacillus on blood biochemical factors. Biol Bull Russ Acad Sci. 2021;48:177–184. doi: 10.1134/S1062359021020163. [DOI] [Google Scholar]
- Zu Y, Zhang Y, Wang W, Zhao X, Han X, Wang K, Ge Y. Preparation and in vitro/in vivo evaluation of resveratrol-loaded carboxymethyl chitosan nanoparticles. Drug Deliv. 2016;23:981–991. doi: 10.3109/10717544.2014.924167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 145 kb)