Abstract
Optimal immune responses against an intracellular bacterial pathogen, such as Salmonella, involve the production of gamma interferon (IFN-γ), which activates macrophages. It has recently been suggested that, interleukin-18 (IL-18), in addition to IL-12, contributes to the induction of IFN-γ following infection. Given this hypothesis, an optimal host immune response against intracellular bacterial pathogens would include the induction of IL-18 secretion by macrophages due to Salmonella infection. We questioned whether Salmonella could induce macrophages to upregulate their expression of IL-18 mRNA and secretion of IL-18. With cultures of murine macrophages, we were surprised to find that infection by wild-type Salmonella dublin resulted in decreased expression of IL-18 mRNA and IL-18 secretion rather than an increase. Reduction of macrophage-derived IL-18 expression by wild-type Salmonella occurred early in the response, suggesting a direct effect. Furthermore, mice orally inoculated with wild-type Salmonella were shown to have reduced IL-18 mRNA expression at mucosal sites within hours postinoculation. Together these studies demonstrate Salmonella-induced reductions in IL-18 expression, suggesting that this intracellular pathogen may be capable of limiting a potentially protective immune response.
Salmonella spp. efficiently invade the gut mucosa and survive as intracellular pathogens of macrophages (15). It is this ability to survive intracellularly that limits the effectiveness of antibody- and neutrophil-mediated damage and thus allows the pathogen to disseminate systemically from mucosal sites. Because of the intracellular nature of Salmonella infections, cell-mediated immunity is required for an optimal protective host response.
Cytokines can play an important role in the protective host response against Salmonella. In particular, interleukin-12 (IL-12)-induced gamma interferon (IFN-γ) production has been shown to be an important component of immune responses against Salmonella (19, 22) and other intracellular bacterial infections (1, 10, 16, 17, 27, 37). However there have also been reports of IL-12-independent mechanisms for IFN-γ production which may contribute to a protective host response (24, 33, 34, 39). While the relative importance of IL-12-dependent and IL-12-independent mechanisms is not altogether clear with respect to the development of salmonellosis, it is clear that the elaboration of IFN-γ and the establishment of a TH1 response is required for limiting Salmonella infections (2, 26, 28).
A newly discovered cytokine (29), IL-18, may also contribute to IFN-γ production during Salmonella infection. IL-18 has also been shown to synergize with IL-12 for optimal IFN-γ production, and the synergy may be the result of IL-18-induced upregulation of IL-12 receptor expression (40, 43). In fact, one recent publication suggests that IL-18 is required for optimal immunity against Salmonella infection (21). Curiously, however, it is not known whether this cytokine is directly induced in macrophages following infection or whether upregulation at mucosal sites occurs to initiate a host response against Salmonella. Since IL-18 may contribute to the development of an IFN-γ-mediated TH1 response, it might be expected that intracellular infection with Salmonella would cause increased expression of this cytokine.
Here we investigate the induction of IL-18 by Salmonella in infected murine macrophages and in vivo at mucosal sites following oral inoculation. Unexpectedly, we found that wild-type Salmonella did not induce IL-18 expression. Rather, wild-type Salmonella actually limited expression of IL-18 mRNA and protein following infection. These results suggest a possible mechanism through which Salmonella might limit a host response and thereby limit an optimal IFN-γ-mediated TH1 response.
MATERIALS AND METHODS
Isolation of peritoneal macrophages and in vitro infection with Salmonella.
Elicited peritoneal macrophages were isolated as previously described (6). Briefly, BALB/c or C57BL/6 mice (Charles Rivers, Wilmington, Mass.) weighing 20 to 24 g were injected intraperitoneally with 250 μl of incomplete Freund’s adjuvant (Sigma Chemical Co., St. Louis, Mo.). Three days later, the peritoneal cavities were lavaged with RPMI 1640 (Cellgro, Washington, D.C.) containing 2% fetal calf serum (Atlanta Biologics, Norcross, Ga.) to remove the elicited peritoneal macrophages. After two washes, the cells were allowed to adhere to plastic culture flasks (Costar, Cambridge, Mass.) for 30 to 45 min in RPMI 1640 containing 2% fetal calf serum. The nonadherent cells were washed off, and adherent cells were cultured with varying numbers of viable Salmonella dublin organisms (wild-type strain SL1363, an isogenic aroA mutant, strain SL1438 (35), or UV-killed SL1363) or Escherichia coli (strain O:157) (ratios of Salmonella to macrophages), 30:1, 10:1, 3:1, and 1:1) or Salmonella-derived lipopolysaccharide (LPS) (500 ng/ml; Sigma) at 37°C in medium with no antibiotics present. After 60 min, the extracellular bacteria were killed by washing the plates in medium containing gentamicin. Subsequent cultures were also performed in the presence of gentamicin to eliminate the growth of extracellular bacteria. Gentamicin was selected as an antibiotic due to its limited uptake by eukaryotic cells (13). Therefore, the addition of gentamicin should have had little effect on the viability of Salmonella organisms which had entered macrophages. The viability of infected macrophages was assessed by trypan blue exclusion at the end of culture.
Cloning and expression of murine IL-18.
The gene encoding murine IL-18 was cloned into the pFLAG expression plasmid, and recombinant murine IL-18 was isolated as previously described (12).
Semiquantitative amplification of IL-18 mRNA expression by RT-PCR.
Prior to performing analyses on experimental samples, it was necessary to determine the specificity, sensitivity, and range of linearity for amplification of IL-18 mRNA by reverse transcription (RT)-PCR with methodologies similar to that previously reported in our laboratory (4). To this end, limiting dilutions of cloned IL-18 DNA were added to individual tubes, each containing 20 ng of control cDNA derived from murine muscle. PCR was performed with the IL-18-specific positive- and negative-strand primers AACTTTGGCCGACTTCACTGTACAA and CTATTGATGTAAGTTAGTGAGAGTG, respectively. A total of 25 cycles was used for amplification at 95°C for denaturation, 60°C for annealing, and 72°C for extension. Following PCR, 30% of the total amplified product was electrophoresed on ethidium bromide-stained agarose gels and visualized under UV fluorescence. Densitometric analysis of PCR-amplified bands was performed with NIH Image software. Each gel image was imported into NIH Image with Photoshop (Adobe Systems, San Jose, Calif.), a gel-plotting macro was used to outline the bands, and the intensity was calculated on the uncalibrated optical density setting.
For experimental analyses, at the appropriate times, total RNA was isolated from cultured cells or from mucosal tissues, as previously described (3, 4, 6), with Trizol reagent (Gibco-BRL, Gaithersburg, Md.). Two micrograms of total RNA was reverse transcribed with SuperScript II reverse transcriptase (Gibco-BRL). A portion of the total cDNA was then used to amplify IL-18, IL-12p40, and/or glyceraldehyde-3-phosphate dehydrogenase (G3PDH) genes. The amplified products were visualized under UV illumination following electrophoresis on ethidium bromide-stained agarose gels. Amplification of the appropriate gene fragments was assured by comparison with molecular weight markers run on the same gel and by direct DNA sequencing of selected amplified fragments as previously described (3, 4, 6).
Preparation and characterization of an anti-IL-18 polyclonal antibody.
A polyclonal antibody against murine IL-18 was produced as previously described (12). Briefly, rabbits were immunized with a synthetic peptide (Research Genetics, Huntsville, Ala.) representing the carboxy-terminal portion of murine IL-18 (amino acid sequence, KLILLKKKDENGDKSVMFTLTNLHQS) which had been coupled to the carrier protein, keyhole limpet hemocyanin, as previously described (30). Sera from hyperimmunized rabbits were collected, and enzyme-linked immunosorbent assays (ELISAs) were used to determine the specificity and titer. For ELISAs, microtiter plates were coated with peptides or recombinant murine IL-18 at 1 mg/ml in carbonate buffer (pH 8.0) overnight, followed by blocking the plates with 2% bovine serum albumin in phosphate-buffered saline. Limiting dilutions of antisera were then placed on the plates for 2 h, followed by washing and incubation for 2 h with an alkaline phosphatase-conjugated anti-rabbit antibody (Southern Biotechnology, Birmingham, Ala.). After unbound antibody was washed off, nitrophenol phosphate was added to each well and absorbancies were determined at 405 nm.
To generate a control antibody, an irrelevant peptide (amino acid sequence, KPDTKIEVAHFITKLLSYTKQLFRHGPF) was also conjugated to keyhole limpet hemocyanin and used to immunize rabbits in a manner identical to that described above. Furthermore, for the ELISA analyses, a variety of irrelevant control peptides were used to demonstrate specificity of binding, including peptides with the amino acid sequences YKAGVGTTSAFL, YEPKKVVGFGA, RVGHFVEAPALRHALLPARHLHVG, and HKNMGGPKGHHCQAHDQI.
Finally, for the in vitro analyses, total immunoglobulin was isolated from control and anti-IL-18 antisera with protein A affinity matrix (Sigma) as previously described (30).
Detection and quantification of IL-18 protein secretion.
Supernatants from uninfected or Salmonella-infected murine macrophages were collected and concentrated fivefold (Centriplus 50kD concentrator; Amicon, Beverly, Mass.). The supernatants were then slot blotted onto nitrocellulose (S&S, Keene, N.H.). The filter was then washed with phosphate-buffered saline and blocked in 5% dry milk. After being washed, the filter was probed with the polyclonal rabbit anti-murine IL-18 antibody described above. Horseradish peroxidase-conjugated anti-rabbit antibody (Jackson Labs, West Grove, Pa.) was then added for 1 h, and the unbound antibody was washed off. The bound antibody was detected by enhanced chemiluminescence (ECL) (Amersham, Arlington Heights, Ill.) followed by exposure of the filters to X-ray film.
IFN-γ ELISA.
Supernatants from splenocytes cocultured with uninfected or Salmonella-infected macrophages were collected, and the amount of IFN-γ present was quantified by capture ELISA (Genzyme, Cambridge, Mass.). The amount of IFN-γ in culture supernatants was determined by extrapolation of absorbancies from a standard curve generated by limiting dilutions of recombinant IFN-γ.
RESULTS
IL-18 mRNA expression is reduced in Salmonella-infected macrophages.
To address whether macrophages could respond to Salmonella infection by increasing IL-18 mRNA expression, a semiquantitative RT-PCR analysis was developed. Using methodologies previously described in our laboratory (4), we determined that the level of sensitivity was approximately 0.3 pg of IL-18 DNA in a background of 20 ng of irrelevant cDNA. A linear range of amplification was observed over at least 4 log units of input IL-18 DNA.
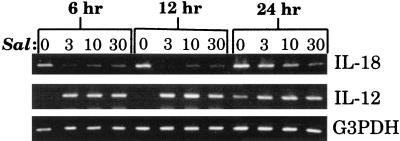
Using conditions for linear amplification, the expression of IL-18 mRNA in Salmonella-infected macrophages was investigated. Peritoneal macrophages were isolated from BALB/c mice and infected with varying numbers of wild-type S. dublin organisms (strain SL1363). At various times postinfection, RNA was isolated and reverse transcribed, and PCR was performed to quantify IL-18 mRNA expression. As shown in Fig. 1, infected macrophages expressed less IL-18 mRNA than did uninfected macrophages at each of the times evaluated. The reduction in IL-18 mRNA expression occurred as early as 6 h postinfection and persisted until 24 h postinfection. This reduction in IL-18 mRNA expression was not due to reduced macrophage viability, which was always greater than 95% during these periods of culture with these doses of Salmonella (5). Furthermore, while IL-18 mRNA expression decreased, there were significant increases in IL-12p40 mRNA expression in Salmonella-infected macrophages (Fig. 1), which is consistent with a previous report (9). The differences in gene amplification could not be attributed to differences in RNA loading or efficiency of RT, as evidenced by amplification of the housekeeping gene, G3PDH, from the same cDNA samples.
FIG. 1.
IL-18 mRNA expression following infection of macrophages with S. dublin. Peritoneal macrophages were uninfected (lanes 0) or infected with ratios of Salmonella to macrophages of 3:1 (lanes 3), 10:1 (lanes 10), and 30:1 (lanes 30) for the indicated times. Following infection, RNA was extracted, reverse transcribed, and subjected to PCR to quantify the expression of IL-18 or IL-12p40 mRNA. The results are shown as amplified products electrophoresed on ethidium bromide-stained agarose gels. To control for RNA loading and efficiency of RT, amplification of the housekeeping gene, G3PDH, was performed on the same cDNA samples. This experiment was performed four times with similar results.
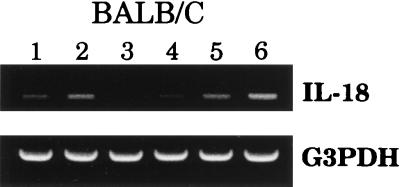
While it was clear from the results shown in Fig. 1 that wild-type S. dublin SL1363 could limit IL-18 mRNA expression, we questioned whether this result was common to LPS-expressing bacteria. BALB/c macrophages were cultured in the presence of Salmonella-derived LPS, UV-killed Salmonella SL1363, an isogenic aroA Salmonella mutant (strain SL1438), or E. coli O:157. After 12 h, RNA was isolated and IL-18 mRNA expression was quantified by RT-PCR. Figure 2 shows increased IL-18 mRNA expression in macrophages exposed to LPS, E. coli O:157, or the isogenic aroA S. dublin mutant (strain SL1438). Exposure of macrophages to UV-killed wild-type S. dublin SL1363 had little effect, while viable wild-type S. dublin SL1363 again suppressed IL-18 mRNA expression (Fig. 2). Taken together, these results demonstrate that Salmonella-induced downregulation of IL-18 mRNA required viable, wild-type S. dublin and was not merely an LPS-mediated phenomenon.
FIG. 2.
Wild-type Salmonella reduces IL-18 mRNA expression in BALB/c macrophages. Peritoneal macrophages were uninfected (lane 1) or exposed to LPS (500 ng/ml) (lane 2), S. dublin SL1363 (lane 3), UV-killed S. dublin SL1363 (lane 4), an isogenic aroA mutant (lane 5), or E. coli (lane 6). Twelve hours after exposure, RNA was extracted and RT-PCR was performed. The results are shown as amplified products electrophoresed on ethidium bromide-stained agarose gels. To control for RNA loading and efficiency of RT, amplification of the housekeeping gene, G3PDH, was performed on the same cDNA samples. This experiment was performed twice with similar results.
Similar studies were conducted with macrophages isolated from C57BL/6 mice, since this strain has been shown to express more of a TH1 phenotype than BALB/c mice (7, 8, 14). Figure 3 shows that wild-type S. dublin SL1363 could also downregulate IL-18 mRNA expression in macrophages derived from C57BL/6 mice; however, this effect was not as pronounced as that observed with macrophages from BALB/c mice (compare Fig. 1 and 3). As with BALB/c macrophages, LPS increased IL-18 mRNA expression in C57BL/6 macrophages (Fig. 3).
FIG. 3.
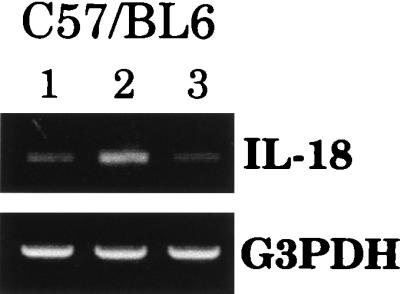
Reduction in IL-18 mRNA expression induced by wild-type Salmonella is not strain specific. Peritoneal macrophages isolated from C57BL/6 mice were uninfected (lane 1) or exposed to LPS (500 ng/ml) (lane 2) or S. dublin SL1363 (lane 3). Twelve hours after exposure, RNA was extracted and RT-PCR was performed. The results are shown as amplified products electrophoresed on ethidium bromide-stained agarose gels. To control for RNA loading and efficiency of RT, amplification of the housekeeping gene, G3PDH, was performed on the same cDNA samples. This experiment was performed twice with similar results.
Characterization of a polyclonal anti-murine IL-18 antiserum.
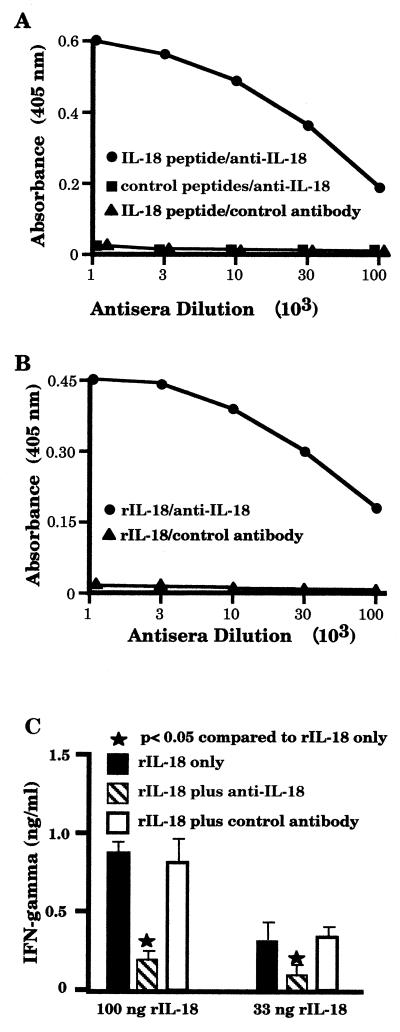
Salmonella-induced reduction in IL-18 mRNA expression suggested that IL-18 secretion might also be diminished. Therefore, to address this possibility, it was necessary to produce an anti-murine IL-18 antibody, since no such reagent was commercially available at that time. Rabbits were immunized with a peptide which represented the carboxy-terminal end of murine IL-18. An ELISA was used to demonstrate that this antiserum had a titer of greater than 1:100,000 against the immunizing IL-18 peptide but did not react significantly with several irrelevant control peptides (Fig. 4A). Control antibodies were unable to recognize the IL-18 peptide, demonstrating the specificity of the antiserum. Not only could this antiserum react against the IL-18 peptide used as an immunogen but it could also recognize recombinant murine IL-18 (Fig. 4B), whereas a control antiserum could not.
FIG. 4.
Characterization of a polyclonal anti-IL-18 antiserum. Rabbits were immunized with a peptide representing the carboxy-terminal end of murine IL-18 (IL-18 peptide) conjugated to keyhole limpet hemacyanin. Following immunization, antiserum was collected and assayed for reactivity against various peptides (A) or rIL-18 (B). To demonstrate that the anti-IL-18 antibody could reduce IL-18-induced IFN-γ production, BALB/c splenic leukocytes were activated with 500 ng of concanavalin A/ml in the presence of various concentrations of recombinant murine IL-18 (100 or 33 ng/ml) with or without the addition of anti-IL-18 or control antibodies (1 μg/ml). Following 72 h of culture, the supernatants were taken and the quantity of IFN-γ was determined by capture ELISA (C). The results of each ELISA are presented as mean absorbance values of triplicate determinations, with the standard deviations always being less than 11% of the mean values. These experiments were performed twice with similar results.
Finally, a bioassay was performed to examine whether the anti-IL-18 antibody could limit the ability of recombinant IL-18 (rIL-18) to induce IFN-γ production. Murine splenic leukocytes were cultured in the presence of a suboptimal concentration of concanavalin A plus recombinant murine IL-18 to induce IFN-γ production. To inhibit this production, protein A-isolated antibody from control or anti-IL-18 antiserum was also added. As shown in Fig. 4C, the anti-IL-18 antibody was effective in blocking IL-18-induced IFN-γ production, whereas the control antibody was not.
Salmonella-induced reduction in IL-18 secretion by macrophages.
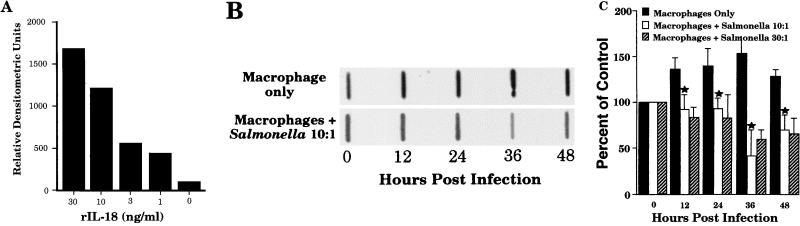
To investigate the ability of wild-type Salmonella to limit secretion of macrophage-derived IL-18, a protein slot blot analysis was performed. This assay was used because at the time there was no commercially available ELISA for murine IL-18 and because we found the slot blot to be much more sensitive than Western blot analyses (data not shown). Dilutions of rIL-18 were blotted onto nitrocellulose membranes, followed by probing the blots with the anti-IL-18 antiserum and subsequent detection by ECL. Densitometric scans of slot blots to detect rIL-18 showed that the sensitivity of this assay was up to 1 ng/ml (Fig. 5A). Using this slot blot analysis (Fig. 5B), we showed that culture supernatants of uninfected macrophages expressed some constitutive IL-18 secretion, and this level increased slightly over time in culture. However, when infected with varying doses of Salmonella for 60 min followed by removal of extracellular bacteria, supernatants from these macrophage cultures contained significantly less IL-18 reactivity (Fig. 5B). Densitometric scans of slot blots were performed in an effort to quantify the differences in secretion (Fig. 5C). These results clearly demonstrate decreased IL-18 secretion by BALB/c macrophages infected with wild-type S. dublin SL1363.
FIG. 5.
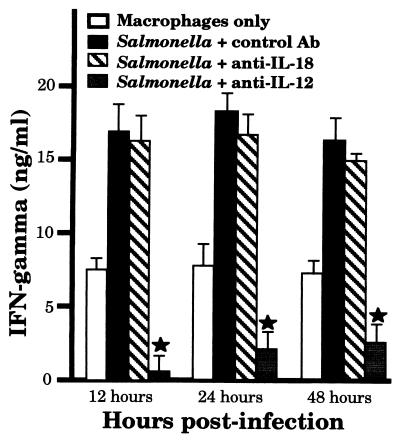
Salmonella-infected macrophages secrete reduced amounts of IL-18. Dilutions of rIL-18 were slot blotted onto nitrocellulose and probed with anti-IL-18, and bound antibody was detected by ECL. The results are presented as densitometric scans of one representative slot blot analysis (A). (B) Representative results of one slot blot analysis with supernatants from untreated- or wild-type-Salmonella-infected macrophages (ratio, 10:1) at various times postinfection. (C) Relative densitometric units of reactivity from slot blots with supernatants from untreated or Salmonella-infected macrophages (ratios, 10:1 and 30:1) taken at various times postinfection. The reactivity at time 0 was selected as a reference point to which all other values were normalized. This graph is shown as an average of three experiments. The error bars indicate standard deviations. ★, P of <0.05 compared to results for macrophages only.
IFN-γ production in Salmonella-infected leukocyte cultures is not due to the presence of IL-18.
IFN-γ production by splenic leukocyte cultures has been reported following Salmonella infection (32), and it has been suggested that IL-12-dependent and IL-12-independent mechanisms might be responsible (19, 22, 24, 33, 34, 39). The results presented in Fig. 1, 2, 3, and 5 suggest that IL-18 would not be involved in Salmonella-induced IFN-γ secretion in such leukocyte cultures. To address this question, macrophages were left uninfected or infected with Salmonella for 1 h, and then extracellular bacteria were removed. At various times postinfection (12, 24, or 48 h), concanavalin A-activated splenic leukocytes were added to the macrophages along with medium, a control antibody, anti-IL-18, or anti-IL-12. Seventy-two hours after the addition of leukocytes, the supernatants were taken and assayed for IFN-γ production. As shown in Fig. 6, treatment with anti-IL-18 antibody had no significant effect on IFN-γ secretion compared to that of cultures containing a control antibody. In contrast, treatment with anti-IL-12 dramatically decreased the presence of IFN-γ in these cocultures. These results are consistent with the notion that during Salmonella infection, IL-18 is not a significant contributor to IFN-γ production in response to intracellular infection.
FIG. 6.
Induction of leukocyte-derived IFN-γ secretion by Salmonella-infected macrophages does not involve IL-18 secretion. Cultured peritoneal macrophages were uninfected or infected with wild-type Salmonella (ratio, 10:1 [bacteria to cells]) for 1 h, followed by removal of extracellular bacteria. At 12, 24, or 48 h postinfection, concanavalin A-activated splenic leukocytes were added to each well of cultured macrophages in medium or in medium containing 1 μg of control, anti-IL-18, or anti-IL-12 antibody. Following 3 days of coculture, the supernatants were taken and the quantity of IFN-γ secretion was determined by ELISA. The results are presented as means of triplicate determinations, with standard deviations always being less than 9% of the mean values. This experiment was performed three times with similar results. ★, P of <0.05 compared to results for Salmonella + control Ab.
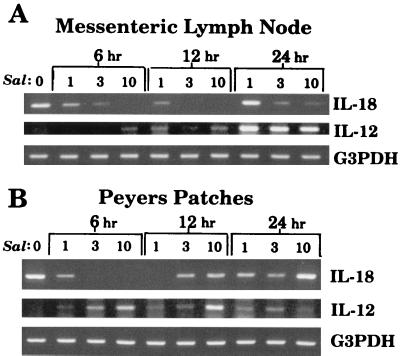
IL-18 mRNA expression is diminished at mucosal sites following oral inoculation with Salmonella.
The ability of Salmonella to limit IL-18 expression in vivo should be consistent with the results from in vitro cultures (Fig. 1, 2, 3, 5, and 6). To address this possibility, mice were orally inoculated with various doses of wild-type S. dublin, and at various times postinfection, the Peyer’s patches and mesenteric lymph nodes were removed. RNA was extracted, and RT-PCR was performed to quantify IL-18 mRNA expression at these mucosal sites. As shown in Fig. 7, there was a time- and dose-dependent decrease in IL-18 mRNA expression following oral inoculation with wild-type S. dublin. Reduced expression was observed in both the Peyer’s patches and mesenteric lymph nodes by 6 h, and the highest dose of Salmonella was most effective at inducing this inhibition. In contrast, mRNA expression for IL-12p40 was significantly increased at mucosal sites following oral inoculation (Fig. 7), which is consistent with a previous report (4).
FIG. 7.
Salmonella induces a reduction in IL-18 mRNA expression at mucosal sites following oral inoculation. BALB/c mice were orally inoculated with 1 × 107 3 × 107, or 10 × 107 S. dublin organisms (Sal) and euthanized 6, 12, or 24 h postinfection. RNA from the mesenteric lymph nodes (A) or the Peyer’s patches (B) was extracted, reverse transcribed, and PCR amplified to quantify IL-18 mRNA expression. The results are shown as amplified products electrophoresed on ethidium bromide-stained agarose gels. For comparison, IL-12p40 mRNA expression was also determined for each sample. To control for RNA loading and efficiency of RT, amplification of the housekeeping gene, G3PDH, was performed on the same cDNA samples. This experiment was performed four times with similar results.
DISCUSSION
Taken together, our results demonstrate that intracellular infection of cultured macrophages, and oral inoculation with viable wild-type S. dublin, had the effect of limiting IL-18 expression. Presently, the mechanisms responsible for this unexpected reduction in IL-18 expression following infection by wild-type Salmonella are not clear. UV-killed wild-type Salmonella, an isogenic aroA mutant, or E. coli could not limit IL-18 expression (Fig. 2). Furthermore, IL-18 reduction induced by wild-type Salmonella is not an LPS-mediated phenomenon, since this bacterial product can upregulate IL-18 mRNA expression (Fig. 2 and 3) (20, 42). These findings suggest that some event during the invasion of macrophages by wild-type Salmonella is responsible for decreased IL-18 expression. It is especially significant that the isogenic aroA mutant of S. dublin does not reduce IL-18 expression (Fig. 2), since this attenuated strain can effectively invade macrophages but has a limited ability for intracellular replication (35).
The importance of IFN-γ in the protective host response against intracellular bacterial infections is well established (2). Therefore, those factors which can induce IFN-γ production have a significant role in the control of such pathogens by the host. IL-18, like IL-12, has been assigned the role of IFN-γ induction. Although IL-18 is suggested to have the same physiologic endpoint as IL-12, there are significant differences in the activities and functions of these two cytokines. First, IL-12 can directly initiate a TH1 response via phosphorylation of STAT 4 following interaction with the IL-12 receptor complex on T lymphocytes. This is different from the mechanism used by IL-18, which stimulates IFN-γ production via an IL-1 receptor-activating kinase. In fact, IL-18 alone does not stimulate STAT 4 phosphorylation, which is a hallmark of an optimal TH1 response. Furthermore, it has been suggested that IL-18 cannot independently induce TH1 cell differentiation (36). Second, the potencies with which these two cytokines stimulate IFN-γ secretion are markedly different. For example, we have found that 10 to 20 pg of rIL-12 can induce approximately 1 ng of IFN-γ from 106 unstimulated splenic leukocytes (5). In contrast, we have found that 10 to 100 ng of IL-18 is required to induce 1 ng of IFN-γ from splenic leukocytes, which have to be costimulated with a suboptimal concentration of mitogen (Fig. 6). In fact, IL-18 does not directly induce IFN-γ production in unactivated T cells (38, 41). Finally, IL-18 has been shown to be an inducer of IL-12 receptor expression. This activity likely plays an important and indirect role in augmenting IFN-γ production. Taken together, these considerations suggest that IL-18 is most accurately described as a costimulatory factor for IFN-γ production.
There are also significant differences in the expressions of IL-12 and IL-18. We (4, 9) and others (20) have demonstrated that IL-12 is not constitutively expressed but is induced in professional antigen-presenting cells following appropriate stimulation. Conversely, IL-18 is constitutively expressed at both the mRNA and protein levels by a variety of cell populations. Further, IL-18 can be secreted as an inactive precursor form (31) which cannot induce IFN-γ production. Therefore, quantification of IL-18 secretion must take into account the fact that the inactive form might be present.
Exogenous administration of rIL-18 can clearly augment the protective host response against intracellular pathogens. Such a result has been observed for murine models of Salmonella (21) and Cryptococcus (18) infection when pharmacological levels of rIL-18 have been administered prior to infection. However, the relative importance of endogenously produced IL-18 in the protective host response against intracellular pathogens is less certain for several reasons. First, it is not clear if the levels of IL-18 being administered exogenously for a protective response are physiologically relevant to the amount produced in response to pathogens. Several investigations have demonstrated hundreds of picograms of IL-18 secretion per milliliter following stimulation (21, 25, 31, 36); however, this level seems several orders of magnitude less than that necessary to induce significant IFN-γ production. The quantification of secreted IL-18 is further complicated by the possibility that this cytokine is secreted in precursor form, which has no IFN-γ-inducing activity (31). Second, the ability of endogenously produced IL-18 to directly and significantly augment IFN-γ production in vivo is not clear. One study demonstrated that administration of anti-IL-18 antibodies did enhance the pathogenesis of Yersinia infection in a murine model but did not alter IFN-γ production. In attempting to explain such results, it should not be forgotten that IL-18 can also induce synthesis of tumor necrosis factor, IL-1, and several other chemokines (11) which may have positive effects on the protective host response. Third, it is difficult to assess the relative importance of endogenous IL-18 production in the protective host response. For example, treatment of mice with anti-IL-18 antibodies reduced bacterial counts in the spleen and liver by less than 1 log unit at day 7 postinfection in a murine model (21). However there were no data in this report demonstrating a difference in survival. These results are in contrast to studies which limited endogenous IFN-γ (26, 28) or IL-12 (19, 23) production. Such investigations demonstrated substantial differences in survival and microbial burden following infection with Salmonella. It is again tempting to speculate that the limited importance of endogenous IL-18 production in salmonellosis is due in part to the ability of intracellular Salmonella to restrict expression of this cytokine.
The original hypothesis when this work began was that infection with wild-type Salmonella would augment the expression of IL-18 both in vitro and in vivo. The work presented here clearly demonstrates that this was not a correct hypothesis. Surprisingly, wild-type S. dublin reduced IL-18 mRNA and protein expression in infected macrophages (Fig. 1, 2, 3, and 5) and also decreased IL-18 mRNA expression in vivo (Fig. 7). Taken together, these results suggest that wild-type Salmonella infection may limit a potentially protective host response.
ACKNOWLEDGMENT
This work was supported by Public Health Service grant AI32976 from the National Institutes of Health.
REFERENCES
- 1.Afonso L C, Scharton T M, Vieira L Q, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 2.Billiau A. Interferon-gamma: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 3.Bost K L, Bieligk S C, Jaffe B M. Lymphokine mRNA expression by transplantable murine B lymphocytic malignancies. Tumor-derived IL-10 as a possible mechanism for modulating the anti-tumor response. J Immunol. 1995;154:718–729. [PubMed] [Google Scholar]
- 4.Bost K L, Clements J D. In vivo induction of interleukin-12 mRNA expression after oral immunization with Salmonella dublin or the B subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1995;63:1076–1083. doi: 10.1128/iai.63.3.1076-1083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bost K L, Clements J D. Intracellular Salmonella dublin induces substantial secretion of the 40-kilodalton subunit of interleukin-12 (IL-12) but minimal secretion of IL-12 as a 70-kilodalton protein in murine macrophages. Infect Immun. 1997;65:3186–3192. doi: 10.1128/iai.65.8.3186-3192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bost K L, Mason M J. Thapsigargin and cyclopiazonic acid initiate rapid and dramatic increases of IL-6 mRNA expression and IL-6 secretion in murine peritoneal macrophages. J Immunol. 1995;155:285–296. [PubMed] [Google Scholar]
- 7.Brown D R, Green J M, Moskowitz N H, Davis M, Thompson C B, Reiner S L. Limited role of CD28-mediated signals in T helper subset differentiation. J Exp Med. 1996;184:803–810. doi: 10.1084/jem.184.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown D R, Swier K, Moskowitz N H, Naujokas M F, Locksley R M, Reiner S L. T helper subset differentiation in the absence of invariant chain. J Exp Med. 1997;185:31–41. doi: 10.1084/jem.185.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong C, Bost K L, Clements J D. Differential production of interleukin-12 mRNA by murine macrophages in response to viable or killed Salmonella spp. Infect Immun. 1996;64:1154–1160. doi: 10.1128/iai.64.4.1154-1160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello C A. IL-18: a TH1-inducing, proinflammatory cytokine and new member of the IL-1 family. J Allergy Clin Immunol. 1999;103(Pt. 1):11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 12.Elhofy A, Bost K L. Limitations for purification of murine interleukin-18 when expressed as a fusion protein containing the FLAG peptide. BioTechniques. 1998;25:426–433. [PubMed] [Google Scholar]
- 13.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 14.Falcone M, Rajan A J, Bloom B R, Brosnan C F. A critical role for IL-4 in regulating disease severity in experimental allergic encephalomyelitis as demonstrated in IL-4-deficient C57BL/6 mice and BALB/c mice. J Immunol. 1998;160:4822–4830. [PubMed] [Google Scholar]
- 15.Finlay B B, Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989;3:1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 16.Flynn J L, Goldstein M M, Triebold K J, Sypek J, Wolf S, Bloom B R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 17.Heinzel F P, Schoenhaut D S, Rerko R M, Rosser L E, Gately M K. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawakami K, Qureshi M H, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-gamma production. J Immunol. 1997;159:5528–5534. [PubMed] [Google Scholar]
- 19.Kincy-Cain T, Clements J D, Bost K L. Endogenous and exogenous interleukin-12 augment the protective immune response in mice orally challenged with Salmonella dublin. Infect Immun. 1996;64:1437–1440. doi: 10.1128/iai.64.4.1437-1440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marshall J D, Aste-Amezaga M, Chehimi S S, Murphy M, Olsen H, Trinchieri G. Regulation of human IL-18 mRNA expression. Clin Immunol. 1999;90:15–21. doi: 10.1006/clim.1998.4633. [DOI] [PubMed] [Google Scholar]
- 21.Mastroeni P, Clare S, Khan S, Harrison J A, Hormaeche C E, Okamura H, Kurimoto M, Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999;67:478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mastroeni P, Harrison J A, Chabalgoity J A, Hormaeche C E. Effect of interleukin 12 neutralization on host resistance and gamma interferon production in mouse typhoid. Infect Immun. 1996;64:189–196. doi: 10.1128/iai.64.1.189-196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mastroeni P, Harrison J A, Robinson J H, Clare S, Khan S, Maskell D J, Dougan G, Hormaeche C E. Interleukin-12 is required for control of the growth of attenuated aromatic-compound-dependent salmonellae in BALB/c mice: role of gamma interferon and macrophage activation. Infect Immun. 1998;66:4767–4776. doi: 10.1128/iai.66.10.4767-4776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDyer J F, Goletz T J, Thomas E, June C H, Seder R A. CD40 ligand/CD40 stimulation regulates the production of IFN-gamma from human peripheral blood mononuclear cells in an IL-12- and/or CD28-dependent manner. J Immunol. 1998;160:1701–1707. [PubMed] [Google Scholar]
- 25.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muotiala A, Makela P H. The role of IFN-gamma in murine Salmonella typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 27.Murray H W, Hariprashad J. Interleukin 12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nauciel C, Espinasse-Maes F. Role of gamma interferon and tumor necrosis factor alpha in resistance to Salmonella typhimurium infection. Infect Immun. 1992;60:450–454. doi: 10.1128/iai.60.2.450-454.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 30.Pascual D W, Bost K L. Anti-peptide antibodies recognize anti-substance P antibodies in an idiotypic fashion. Pept Res. 1989;2:207–212. [PubMed] [Google Scholar]
- 31.Puren A J, Fantuzzi G, Dinarello C A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96:2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 33.Reiner S L, Zheng S, Wang Z E, Stowring L, Locksley R M. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scharton-Kersten T, Caspar P, Sher A, Denkers E Y. Toxoplasma gondii: evidence for interleukin-12-dependent and -independent pathways of interferon-gamma production induced by an attenuated parasite strain. Exp Parasitol. 1996;84:102–114. doi: 10.1006/expr.1996.0096. [DOI] [PubMed] [Google Scholar]
- 35.Smith B P, Reina-Guerra M, Stocker B A, Hoiseth S K, Johnson E. Aromatic-dependent Salmonella dublin as a parenteral modified live vaccine for calves. Am J Vet Res. 1984;45:2231–2235. [PubMed] [Google Scholar]
- 36.Stoll S, Jonuleit H, Schmitt E, Muller G, Yamauchi H, Kurimoto M, Knop J, Enk A H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998;28:3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 37.Sypek J P, Chung C L, Mayor S E, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomura M, Maruo S, Mu J, Zhou X Y, Ahn H J, Hamaoka T, Okamura H, Nakanishi K, Clark S, Kurimoto M, Fujiwara H. Differential capacities of CD4+, CD8+, and CD4−CD8− T cell subsets to express IL-18 receptor and produce IFN-gamma in response to IL-18. J Immunol. 1998;160:3759–3765. [PubMed] [Google Scholar]
- 39.Tripp C S, Kanagawa O, Unanue E R. Secondary response to Listeria infection requires IFN-gamma but is partially independent of IL-12. J Immunol. 1995;155:3427–3432. [PubMed] [Google Scholar]
- 40.Xu D, Chan W L, Leung B P, Hunter D, Schulz K, Carter R W, McInnes I B, Robinson J H, Liew F Y. Selective expression and functions of interleukin 18 receptor on T helper (Th) type 1 but not Th2 cells. J Exp Med. 1998;188:1485–1492. doi: 10.1084/jem.188.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang J, Murphy T L, Ouyang W, Murphy K M. Induction of interferon-gamma production in Th1 CD4+ T cells: evidence for two distinct pathways for promoter activation. Eur J Immunol. 1999;29:548–555. doi: 10.1002/(SICI)1521-4141(199902)29:02<548::AID-IMMU548>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimoto T, Nagai N, Ohkusu K, Ueda H, Okamura H, Nakanishi K. LPS-stimulated SJL macrophages produce IL-12 and IL-18 that inhibit IgE production in vitro by induction of IFN-gamma production from CD3intIL-2Rbeta+ T cells. J Immunol. 1998;161:1483–1492. [PubMed] [Google Scholar]
- 43.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]