Abstract
Purpose of Review
The COVID-19 pandemic has been associated with a change in alcohol consumption, resulting in an increase in alcohol-related liver disease. In this study, we reviewed the literature on (acute) alcohol-associated hepatitis (AH) in the context of the COVID-19 pandemic.
Methodology
PubMed, Ovid MEDLINE, Embase, Cochrane Library, and the pre-print servers medRxiv and bioRxiv were searched to retrieve 320 articles of which 15 abstracts, 7 full-text articles, 4 letters, 1 case report, and 1 poster were included for the final structured review.
Recent Findings
The pandemic resulted in an increase in healthcare utilization related to alcohol consumption. Admissions related to AH increased by 50% (range: 11–100%) during this time, which was disproportionally high in women, younger adults, African Americans, Hispanics, and patients living in rural areas. During this period, the number of new waiting list registrations and candidates with AH receiving liver transplantation (LT) simultaneously increased, which highlights the need for an approach to providing improvised healthcare services at the regional and individual levels.
Keywords: Pandemic, COVID-19, Coronavirus, (Acute) alcohol-associated hepatitis, Alcohol hepatitis
Introduction
Alcohol-associated liver disease (ALD) is a significant contributor to cirrhosis-related deaths and indicator for liver transplantation (LT) in the United States (US) and worldwide [1–5]. The term ALD refers to a variety of liver injuries, such as alcohol-associated hepatitis (AH) and alcoholic-associated cirrhosis (AC), caused by alcohol consumption, and is based on clinical symptoms and histological features [6, 7]. AH, which is a form of hepatic inflammation due to continuous heavy alcohol use, typically occurs in patients who have a background of daily alcohol consumption with underlying cirrhosis [7, 8]. Individuals with AH are susceptible to start drinking even more during stressful circumstances [8]. The real incidence of AH is difficult to estimate, owing to diagnostic inaccuracies of administrative coding, or its presence is missed in decompensated ALD [6]. AH is characterized by an abrupt onset of jaundice and can present asymptomatic or further progress to acute liver failure associated with a high mortality rate [9, 10].
The coronavirus disease 2019 (COVID-19) as global pandemic and public health emergency had a major impact on people’s health behavior and substance use due to stressors related to lockdown measures, travel restrictions, job loss, banned public, or social events [11]. Physical and mental problems might further be aggravated by these unhealthy behaviors, especially due to postponed health interventions [12]. Patients with ALD or alcohol use disorder (AUD) might be among those who are impacted most by this pandemic [13•]. There are several factors that contribute to this, such as higher risk of severe COVID-19 infection on patients with underlying liver disease who have a depressed immune system [14, 15], direct adverse effects of COVID-19 on the liver [16, 17], and higher risk of relapse or alcohol abuse [18, 19]. The aim of this article is to review recent data related to (acute) alcohol-associated hepatitis in the context with the COVID-19 pandemic.
Review Methodology
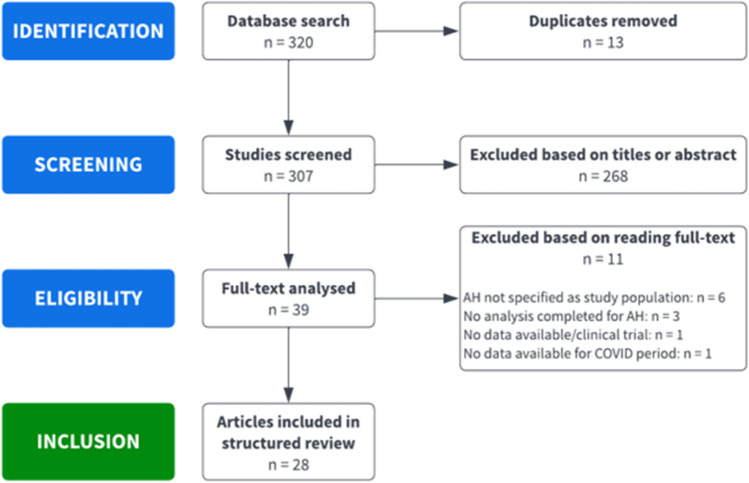
We performed a structured literature search with the assistance of an expert librarian (SR) to identify all relevant articles on acute alcohol-associated hepatitis and COVID-19. PubMed, Ovid MEDLINE, Embase, Cochrane Library, and the pre-print servers medRxiv and bioRxiv were searched for the period from March 2019 to February 2022. We conducted a search using a combination of MeSH and free-text keywords: pandemic, COVID(-19), coronavirus, (acute) alcoholic hepatitis, alcohol hepatitis, hospitalization, alcohol consumption, related alcohol abuse, alcoholism, and related liver disease. Two authors (PS, RS) reviewed independently the title and abstract of all identified studies to exclude those that did not specifically mention (acute) alcohol-associated hepatitis as their study population and did not compare data or reported changes between the pre-COVID and COVID period. The full-text review of the remaining articles was assessed to determine if it contained relevant information about the topic of interest (Fig. 1). Lastly, the reference sections of the selected articles were reviewed for additional publications about information pertinent to this topic.
Fig. 1.
Flow diagram for selection of publications included in the structured review
Recent Findings
There were twenty-eight studies (15 abstracts (20–34), 7 full-text articles (35–41), 4 letters (42–45), 1 case report (46), 1 poster (47)) that were included for the final structured review. Studies were heterogeneous regarding their study period and primary outcomes. Of included studies, 22 studies reported outcomes regarding admissions/hospitalizations, visits, or referrals (Table 1) [20, 21, 23, 25–34, 36•, 37–41, 44, 45, 47]. Thirteen studies reported data about mortality [21, 24, 25, 27, 28, 30, 32, 38, 40, 41, 45–47], 6 studies had results pertaining to liver transplantation [22, 25, 35•, 38, 42, 43], and 5 studies reported results about intensive care unit (ICU) admissions (Table 2) [24, 25, 35•, 40, 41].
Table 1.
Admissions, referrals, or visits
| Publication | Setting | Period | Age*, yr. (% male) | Admissions/referrals/visits | Miscellaneous | |
|---|---|---|---|---|---|---|
| Pre-COVID | COVID | |||||
|
Au, 2020 [20] Abstract |
Adelaide, Australia Single center |
Pre-COVID: Mar 20–May 30, 2019 COVID: Mar 20–May 30, 2020 |
Pre-COVID: 50 COVID: 47 |
AH admissions of EtOH-related admissions: 13/336 | AH admissions of EtOH-related admissions: 17/372 |
• 11% increase in EtOH-related admissions • 11% increase in AH admissions during COVID |
|
Bahar, 2021 [21] Abstract |
CA, USA Single center |
Pre-COVID: Jul 2007–Jan 2020 COVID: Feb 2020–Apr 2021 |
Pre-COVID: 47 (61) COVID: 47 (63) |
AH admissions: 411 | AH admissions: 83 |
• 1.34 additional monthly AH admission during COVID • AH admissions did not increase relatively to increasing overall admissions |
|
Cargill, 2021 [44] Letter |
London, UK Single center |
Pre-COVID: Jun 2019 COVID: Jun 2020 |
Pre-COVID: 49 (64) COVID: 50 (60) |
AH presentations of ArLD referrals: 11/28 (39%) | AH presentations of ArLD referrals: 26/67 (39%) | NR |
|
Chung, 2021 [23] Abstract |
MA, USA Single center |
Pre-COVID: Mar 23–Jul 19, 2019 COVID: Mar 23–Jul 19, 2020 |
NR | NR | NR |
• Consults for EtOH-related GI: 60% increase • Consults for AH: 127% increase during COVID |
|
Damjanovska, 2022 [36•] Full-text article |
USA Multi center (40 US health care systems) |
Pre-COVID: 1999–Jun 20, 2020 COVID: Jun 21, 2020–Jun 20, 2021 |
Pre-COVID: NR (67) COVID: NR (64) |
Prevalence AH per 100,000: 23,350 | Prevalence AH per 100,000: 8320 |
• AH OR 2.77 • AH women OR 1.14 • AH African Americans OR 2.63 |
|
Dhanda, 2020 [47] Poster |
UK Multi center (28 hospitals) |
Pre-COVID: Aug 2019 COVID: Aug 2020 |
Pre-COVID: 55 (63) COVID: 55 (63) |
ArLD admissions: 223 | ArLD admissions: 263 | 18% increase in ArLD admissions during COVID |
|
Grinspoon, 2021 [25] Abstract |
MA, USA Multi center |
Pre-COVID: Mar 10, 2016–Mar 9, 2020 COVID: Mar 10, 2020–Mar 9, 2021 |
NR | AH admissions/year: 27 | AH admissions/year: 49 | 81% increase in AH admissions for patients 40 years or younger during COVID |
|
Itoshima, 2021 [37] Full-text article |
Japan Multi center (257 hospitals) |
Pre-COVID: Jul 2018–Mar 2020 COVID: Apr–Jun 2020 |
NR | NR | NR |
• RR 1.21 increase in ArLD admissions during COVID • 14% of ArLD had AH |
|
Jain, 2021 [26] Abstract |
OH, USA Single center |
Pre-COVID: Mar 1–Aug 31, 2019 COVID: 2020 (during lockdown) |
NR | 86 | 162 | NR |
|
Moore, 2021 [27] Abstract |
NE, USA Single center |
Pre-COVID: 2016–2019 COVID: Q2–Q4 2020 |
Pre-COVID: 48 (59) COVID: 44 (62) |
Total AH hospitalizations: 162 SAH hospitalizations: 127 (78%) |
Total AH hospitalizations: 95 SAH hospitalizations: 78 (82%) |
NR |
|
Nair, 2021 [28] Abstract |
HCA Healthcare, USA Multi center (185 US hospitals) |
Pre-COVID: Feb 2019–Sept 2019 COVID: Feb 2020–Sept 2020 |
NR | AH + ALF-related admissions of all admissions: 6.5% | AH + ALF-related admissions of all admissions: 7.4% | 7% increase in cases among women during COVID |
|
Ngu, 2021 [29] Abstract |
Monash Health, Australia |
Pre-COVID: Jul 1–Oct 31, 2019 COVID: Jul 1–Oct 31, 2020 |
NR | AH admissions of ALD/pancreatitis admissions: 10/66 (15%) | AH admissions of ALD/pancreatitis admissions: 33/100 (33%) | 100% increase in AH admissions during COVID |
|
Perisetti, 2021 [45] Letter to editor |
50 healthcare organizations worldwide Multi center |
Pre-COVID: Jan 1–Dec 1, 2019 COVID: Jan 1–Dec 1, 2020 |
Pre-COVID: 50 (65) COVID: 50 (64) |
AH hospitalizations: 18,818 | AH hospitalizations: 4383 | NR |
|
Pradhan, 2021 [30] Abstract |
AZ, USA Multi center |
Pre-COVID: Mar 1–Dec 31, 2019 COVID: Mar 1-Dec 31, 2020 |
Pre-COVID: 42 (61) COVID: 40 (64) |
AH admissions in non-cirrhotic adults: 225 | AH admissions in non-cirrhotic adults: 296 | 32% increase in admissions during COVID |
|
Rutledge, 2021 [38] Full-text article |
New York City, USA Single center |
Pre-COVID: Jan 1–Mar 21, 2020 COVID: Mar 22–Aug 25, 2020 |
Pre-COVID: 49 (50) Post-COVID: 47 (63) |
AH admissions: 18 | AH admissions: 49 | NR |
|
Schimmel, 2021 [39] Full-text article |
New York City, USA Multi center (5 hospitals) |
Pre-COVID: Mar 1–May 31, 2019 COVID: Mar 1–May 31, 2020 |
Pre-COVID: 46 (77) COVID: 47 (79) |
AH visits of EtOH-related visits: 10/2790 (0.4%) | AH visits of EtOH-related visits: 7/1793 (0.4%) | NR |
|
Shaheen, 2021 [40] Full-text article |
Canada Multi center (3 databases) |
Pre-COVID: Mar 2018–Feb 2020 COVID: Mar–Sept 2020 |
Pre-COVID: 46 (62) COVID: 44 (66) |
AH admissions: 991 AH admissions per 10,000: 11.6 |
AH admissions: 417 AH admissions per 10,000: 22.1 |
• 9% monthly increase in AH hospitalization • Admitted patients for AH were younger, more likely from rural areas, lower HCV prevalence, lower comorbidity rates during COVID |
|
Silva, 2021 [31] Abstract |
Portugal Single center |
Pre-COVID: Mar 15, 2019–Mar 14, 2020 COVID: Mar 15, 2020–Mar 15, 2021 |
Pre-COVID: 63 (70) COVID: 63 (69) |
AH presentations in DLC presentation: 6% of 66 | AH presentations in DLC presentation: 14% of 74 | NR |
|
Smart, 2021 [32] Abstract |
Australia Single center |
NR | NR | NR | NR |
• 11% increase AH admissions • 48% increase in admission related to acute alcohol intoxication during COVID |
|
Sohal, 2021 [41] Full-text article |
CA, USA Multi center (3 community hospitals) |
Pre-COVID: Jan 1–Dec 15, 2019 COVID: Jan 1–Dec 15, 2020 |
Pre-COVID: 48 (82) COVID: 47 (73) |
AH admissions: 131 | AH admissions: 198 |
• 51% increase in hospitalizations • 69% increase in hospitalizations after stay-at-home orders • 94% increase in rehospitalizations • 50% increase in patients under < 40 • 125% increase in women during COVID |
|
Toy, 2021 [33] Abstract |
UT, USA Single center |
Pre-COVID: Q2 2019–Q1 2020 COVID: Q2 2020–Q1 2021 |
NR |
AH diagnoses per 1000 visits: 5.8 AH visits per 1000 visits: 4.2 |
AH diagnoses per 1000 visits: 7.5 AH visits per 1000 visits: 5.4 |
NR |
|
Wu, 2021 [34] Abstract |
MN, USA Single center |
Pre-COVID: Jan 1–Dec 31, 2019 COVID: Jan 1–Dec 31, 2020 |
Pre-COVID: 52 (62) COVID: 43 (52) |
AH admissions: 34 | AH admissions: 62 | 77% increase in annual AH admissions during COVID |
*Reported as mean or median; yr., years; COVID, coronavirus disease; AH, alcohol-associated hepatitis; EtOH, ethanol alcohol; ArLD, alcohol-related liver disease; NR, not reported; GI, gastrointestinal; US, United States; OR, odds ratio; Q, quarter; SAH, severe alcohol-associated hepatitis; ALF, acute liver failure; ALD, alcoholic liver disease; HCV, hepatitis C virus
Table 2.
ICU admissions, mortality, and liver transplantation
| Study | Setting | Study period | Age*, yr. (% males) | ICU admission | Mortality | Liver transplantation | Miscellaneous transplantation | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-COVID | COVID | Pre-COVID | COVID | Pre-COVID | COVID | |||||
|
Anderson, 2021 [42] Full-text article |
UNOS database Multi center |
Pre-COVID: Mar 2019–Jan 2020 COVID: Mar 2020-Jan 2021 |
NR | NR | NR | NR | NR |
Proportion WL registr. for AH: 1.4% Proportion DDLTs for AH: 1.6% |
Proportion WL registr. for AH: 2.4% Proportion DDLTs for AH: 3.0% |
NR |
|
Bahar, 2021 [21] Abstract |
CA, USA Single center |
Pre-COVID: Jul 2007–Jan 2020 COVID: Feb 2020–Apr 2021 |
Pre-COVID: 47 (61) COVID: 47 (63) |
NR | NR | NR | OR 1.88 | NR | NR | NR |
|
Bangaru, 2021 [22] Abstract |
California, USA Single center |
Pre-COVID: Sep 2019–Jan 2020 COVID: Feb–Aug 2020 |
NR | NR | NR | NR | NR | LT evaluation for AH: 49% | LT evaluation for AH: 82% | NR |
|
Bittermann, 2021 [43] Full-text article |
UNOS database Multi center |
Pre-COVID: Mar 1, 2018–Feb 29, 2020 COVID: Mar 1, 2020–Feb 28, 2021 |
Median: 41 (66) | NR | NR | NR | NR | NR | NR |
• Increase of 107% in additions to WL due to AH • Increase 210% in receipt of LT due to AH during COVID |
|
Cholankeril, 2021 [35•] Full-text article |
UNOS database Multi center |
Pre-COVID: Apr 1–Dec 31, 2019 COVID: Apr 1–Dec 31, 2020 |
SAH WL additions Pre-COVID: 41 (NR) COVID: 40 (NR) SAH LT recipients Pre-COVID: 41 (NR) COVID: 40 (NR) |
LT recipients with ALD: 17% | LT recipients with ALD: 17% | NR | NR |
SAH WL additions: 132 SAH LT recipients: 110 |
SAH WL additions: 207 SAH LT recipients: 168 |
• SAH WL increase 58% • SAH LT increase 53% during COVID |
|
Dhanda, 2020 [47] Poster |
UK Multi center (28 hospitals) |
Pre-COVID: Aug 2019 COVID: Aug 2020 |
Pre-COVID: 55 (63) COVID: 55 (63) |
NR | NR | 7.2% | 9% | NR | NR | NR |
|
Gorgulu, 2022 [24] Abstract |
Germany Single center |
Pre-COVID: 2017–2019 COVID: 2020 |
Median 52 (62) | AH trigger for ACLF: 24–27% | AH trigger for ACLF: 57% | AH trigger for ACLF: 69.5% | AH trigger for ACLF: 72.3% | NR | NR | NR |
|
Grinspoon, 2021 [25] Abstract |
MA, USA Multi center |
Pre-COVID: Mar 10, 2016–Mar 9, 2020 COVID: Mar 10, 2020–Mar 9, 2021 |
NR | 28% | 37% | 13% | 10% | AH admissions into LT: 1.4% | AH admissions into LT: 10.2% | NR |
|
Moore, 2021 [27] Abstract |
NE, USA Single center |
Pre-COVID: 2016–2019 COVID: Q2–Q4 2020 |
Pre-COVID: 48 (59) COVID: 44 (62) |
NR | NR | 22/162 (14%) | 12/95 (13%) | NR | NR | NR |
|
Nair, 2021 [28] Abstract |
HCA Healthcare, USA Multi center (185 US hospitals) |
Pre-COVID: Feb 2019–Sept 2019 COVID: Feb 2020–Sept 2020 |
NR | NR | NR | AH + Alcoholic liver failure: 1.4% | AH + Alcoholic liver failure: 2% | NR | NR | NR |
|
Perisetti, 2021 [45] Letter to editor |
50 healthcare organizations worldwide Multi center |
Pre-COVID: Jan 1–Dec 1, 2019 COVID: Jan 1–Dec 1, 2020 |
Pre-COVID: 51 (65) COVID: 50 (64) |
NR | NR | NR | OR 0.93 | NR | NR | NR |
|
Pradhan, 2021 [30] Abstract |
AZ, USA Multi center |
Pre-COVID: Mar 1–Dec 31, 2019 COVID: Mar 1–Dec 31, 2020 |
Pre-COVID: 42 (61) COVID: 40 (64) |
NR | NR | 3/225 (1%) | 2/296 (1%) | NR | NR | NR |
|
Rutledge, 2021 [38] Full-text article |
New York City, USA Single center |
Pre-COVID: Jan 1–Mar 21, 2020 COVID: Mar 22–Aug 25, 2020 |
Pre-COVID: 49 (50) COVID: 47 (63) |
NR | NR | 7/18 (39%) | 14/49 (29%) | Early LT per month for AH: 0.5 | Early LT per month for AH: 2 | NR |
|
Shaheen, 2021 [40] Full-text article |
Canada Multi center (3 databases) |
Pre-COVID: Mar 2018–Feb 2020 COVID: Mar–Sept 2020 |
Pre-COVID: 46 (62) COVID: 44 (66) |
111/991 (11%) | 47/417 (11%) | 76/991 (8%) | 26/417 (6%) | NR | NR | NR |
|
Smart, 2021 [32] Abstract |
Australia Single center |
NR | NR | NR | NR | NR | Patients with AH who responded to steroids: RR 0.16 | NR | NR | NR |
|
Sohal, 2021 [41] Full-text article |
CA, USA Multi center (3 community hospitals) |
Pre-COVID: Jan 1–Dec 15, 2019 COVID: Jan 1–Dec 15, 2020 |
Pre-COVID: 48 (82) COVID: 47 (73) |
33 (25%) | 55 (28%) | 20 (15%) | 26 (13%) | NR | NR | NR |
*Reported as mean or median; yr., years; ICU, intensive care unit; COVID, coronavirus disease; UNOS, United Network for Organ Sharing; NR, not reported; WL, waiting list; AH, alcohol-associated hepatitis; DDLT, deceased donor liver transplantation; LT, liver transplantation; ALD, alcoholic liver disease; SAH, severe alcohol-associated hepatitis; ACLF, acute-on-chronic liver failure; Q, quarter; RR, relative risk
Alcohol Consumption During the COVID-19 Pandemic
Consumption
The rates of high-risk alcohol consumption and AUD were already increasing over the recent decades with the pandemic further contributing to this rise as evident by boost in alcohol sales and excessive drinking reported globally [48]. At-home drinking increased in many countries around the world with a staggering 234% growth in sales in the USA [12, 49–51]. In resource-rich countries (e.g., Australia, Belgium, France, UK, and USA), women, parents of young children, middle-aged people, and people with higher income reported the largest increase in alcohol consumption [12, 52–54]. On the other hand, a reverse trend of decline in alcohol consumption was observed in some Australian and European countries where alcohol sales were restricted [55, 56].
Change in Drinking Behavior
A large survey-based study which collected 55,811 responses from 11 countries showed that 42% did not change how much they drank, but among those that did, 36% increased their consumptions [57]. More patients reported drinking spirits post- vs pre-lockdown (31% vs 22%) [47]. In some parts of the world, fear and misinformation generated a dangerous myth that consuming high-strength alcohol may kill the COVID-19 virus resulting in additional harm and about 180 deaths in Iran due to alcohol intoxication [58–60].
Reasons for Observed Drinking Behaviors
The varied global trends in alcohol consumption during the pandemic are perhaps best explained as a function of interplay of stay-at-home orders, stress levels in individuals, and availability of alcohol. The lockdown onset and offset served as an inflection point for the most remarkable changes observed in drinking habits worldwide [12, 61]. Depression, anxiety, perceived stress, boredom, lack of routine, positive urgency impulsivity, and having personal relationship with someone severely ill from COVID-19 were most commonly reported reasons for increased drinking [36•, 62, 63]. In particular, people with underlying AUD or who are socioeconomically disadvantaged were more susceptible to increased drinking during lockdown periods, owing to their lack of a support system and other coping strategies [64].
Overview of AH-Related Diagnoses, Admissions, ER Visits, and Referrals
Overall, there was an increase in healthcare utilization related to alcohol consumption (Table 1) [20, 25, 28–34, 37, 40, 41, 47]. Hospitalizations for patients with AH during the COVID-19 era increased by 50% (range: 11–100%) [20, 29, 30, 32, 34, 41]. While most studies found a decline in these numbers at the beginning of the pandemic compared with previous years, these numbers increased sharply after implementation of stay-at-home orders and subsequent quarters of 2020 [27–29, 34, 40, 41]. Rehospitalization also increased by 94% indicating there were more unresolved diseases [41]. Chung et al. found that during the lockdown, the number of general gastrointestinal (GI) consults was reduced, while the proportion of alcohol-related GI and liver disease consults significantly increased [23]. During the reopening period, the proportion of AH-related GI consults remained high and increased nearly 130% when compared with the same period in 2019. The increase was particularly seen among women [36•, 37, 41], Hispanics [38], African Americans (AA) (36), and younger adults [25, 28, 34, 40].
Population Characteristics of AH in The Pandemic
Younger Age
Mean age at presentation of AH decreased for both males and females [20, 27, 30, 35•, 38, 40, 41, 44]. A drastic 80–100% increase in AH-related hospitalizations was found in individuals of age 40 years and younger [25, 41]. Between 2019 and 2022, a 25% increase in alcohol-related deaths was observed with 40% of this increase occurring in younger adults (ages 35–44) [65]. Factors implicated in excessive alcohol consumption include college campus closures, transitions to online-only education, lack of opportunities for socializing with peers, and economic insecurity further exacerbating the stresses in an already vulnerable population [41].
Sex-Based Differences
Similar to pre-COVID times, males had the highest overall AH-related healthcare encounters [21, 27, 30, 31, 36•, 39, 40, 44, 47]. However, there was also a disproportionate increase in females presenting with AH [30, 36•, 37, 41, 44]. In addition to well-known greater harmful effects of alcohol consumption in females due to biological characteristics [66], job loss, decreased income, and social isolation are attributed to development of alcohol use disorder during the pandemic [67–69]. As an example, females had higher loss of employment globally (5.0% vs. 3.9%) in 2020 [70]. Self-reported surveys indicated experiences of motherhood in isolation and greater feelings of distress as reasons for increased drinking and consequential AH in females [71, 72].
Race and Ethnicity
There was a proportional decline in AH cases among Caucasians during the COVID era (36, 38), but a significant demographic shift in alcohol-related GI and liver disease was observed in African Americans [36•, 38] and Hispanics [30, 38]. Possible reasons for this include increased poverty, lack of housing, access to health care, lower education level, and discrimination resulting in additional psychological and economic stress which may be further perpetuated by observed healthcare disparity in the communities of color [36•, 73].
Urban Versus Rural
One study from Canada observed a greater likelihood of AH-related admissions from rural areas suggesting that AUD and ALD may expand to broader populations and differentially impact vulnerable groups [40].
Severity at Presentation
There were varied findings in terms of AH severity at presentation. Cargill et al. noted AH admissions to be sicker with 23.9% requiring high dependency unit or ICU care in June 2020 versus 10.7% in June 2019 [44]. Patients may have had a delayed presentation due to fear of seeking care during the pandemic with loss of linkage to care leading to potential relapse or increased alcohol consumption [44]. Reduction in community alcohol and drug services halted alcohol detoxification schemes significantly, reduced face-to-face meetings, and overall support for these vulnerable groups [44]. In a study reporting no observed difference in severe AH presentations (Maddrey’s discriminant function [MDF] > 32) during the COVID times compared to pre-COVID times, the authors noted that their state did not have stay-at-home mandate at any point during the pandemic [27].
Access to Health Care
Challenges with medical management that occurred during the COVID-19 pandemic are affiliated to social distancing measures (“stay-at-home orders”), which promoted harmful alcohol consumption, overfilled hospitals with limited capacities, and the fear to contract COVID-19 in a clinical setting [74]. These factors contributed to a delay in seeking medical support, interruption of routine outpatient care, either for alcohol detoxication services or chronic diseases [36•, 44]. Access to health care was exacerbated during the pandemic for people who live in rural areas due to longer distances, lack of transportation, privacy concerns, social stigma, uninsured status, lower health literacy, and greater healthcare workforce shortages [75]. In a study from Shaheen et al., patients admitted with AH during the pandemic were more likely to live in rural areas, which demonstrates a greater impact of social isolation in this group [40]. Additionally, access to alcohol-cessation counseling and specialists for recovering patients with ALD was significantly reduced; hence, it contributed to increased alcohol consumption [41]. Persetti et al. found a decrease in outpatient diagnoses of hepatocellular carcinoma (HCC) during the pandemic, which was a result of delay or discontinuity in regular surveillance [45]. Higher rates of cirrhosis in their post-COVID cohort are the consequence in patients’ altered behavior and seeking medical care with increased symptoms.
Outcomes
Mortality
Despite the increased presentation of AH in the inpatient setting, the majority of studies found that mortality rates were similar to previous years [24, 25, 27, 30, 38, 40, 41, 47]. However, one large retrospective study by Nair et al. reported a significant increase in mortality rates from 1.4 to 2% between their pre-COVID and COVID cohort [28]. An audit of a single hospital over 3 months found that the relative risk (RR) of death was significantly reduced (0.16) among patients with severe AH that responded to steroid therapy, whereas the presence of sepsis or acute kidney injury was associated with higher mortality rates [32]. Patients admitted with non-alcohol-related cirrhosis had lower mortality rates during the pandemic, irrespective of comorbidities and their demographics [40].
ICU Admissions
Although most studies found that the numbers of patients with AH treated or admitted to ICU remained stable during the pandemic, Gorgulu et al. observed a significant increase of 111–137% in those admitted to ICU due to acute-on-chronic liver failure (ACLF) precipitated by severe AH in 2020 (57%) when compared with the previous years (24–27%) [24]. There were more patients with ACLF grade 3 (54% vs 36%) and higher model for end-stage liver disease (MELD) score (27 vs 25) in the group of patients with ACLF precipitated by severe AH in comparison with patients with ACLF precipitated by other causes.
Liver Transplantation
One study described that LT evaluations sharply increased to 82.4% in contrast to 49.4% before implementation of stay-at-home orders [22].
There was a significant rise in the proportions of WL registrations (2.4% [227/9,311] vs 1.4% [138/9638]) for AH during the pandemic [42], and the number of candidates waiting for LT increased between 58 (131 vs 207) [35•] and 106.6% [43] during the COVID-19 era. No differences were observed in patients’ sociodemographic characteristics, race, or ethnicity [43] but was higher among adults younger than 50 years (2.8% absolute increase) (35). The median MELD-Na at listing (22 vs 23) for ALD and percentage of listed patients with ALD (26.6% vs 30.8%) increased in the COVID era. In addition, 40% of listings were due to ALD, exceeding listings for hepatitis C (HCV) and nonalcoholic steatohepatitis (NASH) combined [35•].
The increase of candidates with AH receiving LT ranged from 52.7 (110 vs 168) (35) to 210.2% [43] during the COVID-19 era. Patients with ALD (not limited to AH) had a 50% greater chance of getting a LT than those with other etiologies for their liver disease [35•]. The median MELD-Na at transplant (27 vs 28) significantly increased for ALD, and the percentage of ALD transplanted with MELD-Na > 30 relatively increased by 16.1 [35•]. There was a significant rise in the proportions of deceased donor liver transplants (DDLTs; 3.0% [185/6162] vs 1.6% [103/6263]) for AH during COVID [42]. This increase was particularly strong after June 2020 and may have been associated with rising alcohol sales [42]. Grinspoon et al. reported a similar trend with 10.2% of AH admissions leading to LT each year during the pandemic compared with a mean of 1.4% in previous years, which was significantly increased for patients 40 years or younger [25]. Another study found an increase in the rate of early LT (< 6 months of abstinence) from 0.5 LT/month pre-COVID to 2 LTs/month for AH [38]. Although it is not clear whether the increase in LT for AH is only due to higher alcohol misuse during the pandemic or attributable to the recent changes to the organ allocation policy, the concurrent increase in WL registrations for AH during the pandemic suggests an overall rise of AH cases [35•, 42].
Conclusions
In this structured review of alcohol-associated hepatitis in the COVID-19 pandemic, most studies reported an increased prevalence of AH-related admissions and number of liver transplantations for AH during the pandemic. During this period, especially, women, younger adults, Hispanics, African Americans, and patients living in rural areas or patients with preexisting liver conditions were at greater risk.
The outbreak of this pandemic placed an additional tremendous burden on the already strained healthcare system and caused enormous psychological stressors to people worldwide, especially during periods of social restrictions. Individuals’ health habits changed negatively as a result of these newly implemented policies, including a greater intake of alcohol, while those with ALD/AUD were most severely affected [13•]. Stay-at-home orders contributed to a rise in alcohol consumption in patients recovering with ALD/AUD who were neither able to seek professional counseling, nor attend in-person support groups, and therefore prone to relapse [18]. During the pandemic, the increased consumption of alcohol in this population was reflected in the nearly doubled frequency of AH admissions in patients with comorbid conditions like previous AH episodes [31, 41].
There are several limitations in our structural review that warrant discussion. First, several investigations were limited by methodologic quality as well as lack of complete information. Case ascertainment varied and included diagnostic coding or inconsistent definitions of AH. In addition, reports from tertiary centers may have overestimated the prevalence of AH. Second, another potential limitation is the variable study periods used in the publications pre-COVID versus COVID and different lockdown policies between US states and countries. Third, the increase of LT for ALD and AH during the pandemic may not have been related to the pandemic but rather reflective of national trends in LT for AH as well as consequence of acuity circle-based allocation and distribution implemented in February 2020 [76], which prioritize patients without HCC [35•]. Fourth, COVID-19 status in patients presenting with AH was mostly not reported in the reviewed publications, which could have been an effect modification, therefore potentially impacting the severity of AH with direct or indirect liver injury [17]. However, the COVID-19 pandemic is rather assumed to have negatively influenced unhealthy lifestyles, especially with increased alcohol consumption, and patients with decompensated chronic liver disease and ALD with COVID-19 were not actively seeking medical treatment [77].
As ALD is a leading cause of chronic liver disease worldwide and cirrhosis-related deaths in the USA [5], and because of the high mortality and morbidity associated with severe alcohol-associated hepatitis, it is crucial to address some of the gaps in the future and to tailor management to individual patients and their circumstances.
Future directions should include the following: (1) novel-efficient medical therapies for AH obtained from prospective multicenter studies to improve the management, (2) guidelines for selection of patients with AH for LT as well as monitoring of alcohol use before and after LT, (3) biomarkers in patients with ALD to assess abstinence and stabilization or improvement of liver disease, and (4) innovations in healthcare delivery through the use of technologies that can provide screening, counseling, and addiction therapy both ALD and AUD, especially during times of restricted healthcare access [6, 7, 78, 79]. Table 3 summarizes some of the relevant aspects and potential solutions from the current literature [13•].
Table 3.
Gaps, concerns, and solutions in the care of patients with ALD/AUD
| Category | Specific gaps and concerns | Potential solutions |
|---|---|---|
| Pandemic related |
• Increased risk of COVID-19 transmission • Excessive and unreliable information • Economic crisis |
• Establishment and adherence to preventative guidelines set forth by global/locoregional authorities • Creation of central and locoregional disaster management plans in advance of calamity • Public messages from authorities addressing key aspects of disaster management including trusting information from reliable sources only • Early availability of financial assistance from federal/central authorities • Incentivize companies with existing infrastructure to manufacture items of greatest needs • Increase funding in public health sector to promote research efforts for decreasing the disease burden and communal impact |
| Healthcare related |
• Restricted access to healthcare facility • Limited beds/staffing/resources • Treatment-related challenges in ALD patients with superimposed COVID-19 infection |
• Increase availability and access to telehealth/virtual visit platforms • Increase availability and utilization of home health • Ensuring prescription refills are available to patients • Redistribution of resources focusing on patients with high-risk comorbid conditions • Adherence to established hospital-driven management protocols and creation of newer ones as per the need • Multidisciplinary care approach/e-consults • Increase collaboration with researchers across the globe and sharing of evidence-based practices • Increase outreach • Availability of clinical trials |
| Patient and population related |
• Fear of going to a healthcare facility due to greater risk of being exposed to the COVID-19 virus • Vulnerable groups — young, elderly, females, minority groups, educationally and economically disadvantaged, patients with comorbid conditions, patients on steroids/immunosuppression therapy • Social isolation • Domestic violence • Economic stress |
• Utilization of telehealth platforms/virtual visits/ home health • Patient education via utilization of paper and electronic media (newspapers, magazines, TV, radio, webinars, etc.) • Consideration for participation in clinical trials • Policies should be crafted with special considerations for vulnerable groups • Change in platforms of supportive care from physical to virtual instead of eliminating altogether • Virtual support/educational programs specially targeting vulnerable sub-groups • Availability of hotline for help/guidance/counseling • Companies should consider opening up of virtual jobs rather than dissolving the positions altogether • Prioritize outreach for vulnerable groups |
| Post-pandemic related |
• Downstream effects of substance abuse (alcohol, smoking, etc.) i) Physical effects (alcohol-related disorders, cardiovascular diseases, obesity, worsening of preexisting chronic conditions) ii) Psychosocial effects of substance of abuse intake (alcohol, smoking, etc.) • Economic devastation |
• Active screening for substance-related physical and psychological problems and domestic violence at patient’s first encounter after pandemic (at all levels of care — primary or advanced care settings) • New job opportunities or conversion of on-site jobs to remote platforms to allow greater accessibility • Local support for small businesses |
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Philipp Schulz and Rehma Shabbir are co-first authors.
This article is part of the Topical Collection on Liver Transplantation
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.Guirguis J, Chhatwal J, Dasarathy J, Rivas J, McMichael D, Nagy LE, et al. Clinical impact of alcohol-related cirrhosis in the next decade: estimates based on current epidemiological trends in the United States. Alcohol Clin Exp Res. 2015;39(11):2085–2094. doi: 10.1111/acer.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179(3):340–348. doi: 10.1001/jamainternmed.2018.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong RJ, Singal AK. Trends in liver disease etiology among adults awaiting liver transplantation in the United States, 2014–2019. JAMA Netw Open. 2020;3(2):e1920294. doi: 10.1001/jamanetworkopen.2019.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018;16(8):1356–1358. doi: 10.1016/j.cgh.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon Y-H, Chen CM. Surveillance report# 105: liver cirrhosis mortality in the United States: national, state, and regional trends, 2000–2013. National Institute on Alcohol Abuse and Alcoholism (NIAAA), Bethesda, MD. 2016.
- 6.Crabb DW, Im GY, Szabo G, Mellinger JL, Lucey MR. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2020;71(1):306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 7.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113(2):175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi G, Runyon BA. Alcoholic hepatitis: a clinician's guide. Clin Liver Dis. 2012;16(2):371–385. doi: 10.1016/j.cld.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 9.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol. 2011;54(4):760–764. doi: 10.1016/j.jhep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Liangpunsakul S. Clinical characteristics and mortality of hospitalized alcoholic hepatitis patients in the United States. J Clin Gastroenterol. 2011;45(8):714–719. doi: 10.1097/MCG.0b013e3181fdef1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weerakoon SM, Jetelina KK, Knell G. Longer time spent at home during COVID-19 pandemic is associated with binge drinking among US adults. Am J Drug Alcohol Abuse. 2021;47(1):98–106. doi: 10.1080/00952990.2020.1832508. [DOI] [PubMed] [Google Scholar]
- 12.Pollard MS, Tucker JS, Green HD., Jr Changes in adult alcohol use and consequences during the COVID-19 pandemic in the US. JAMA Netw Open. 2020;3(9):e2022942. doi: 10.1001/jamanetworkopen.2020.22942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.•.Da BL, Im GY, Schiano TD. Coronavirus disease 2019 hangover: a rising tide of alcohol use disorder and alcohol-associated liver disease. Hepatol. 2020;72(3):1102–8. doi: 10.1002/hep.31307. [DOI] [PubMed] [Google Scholar]
- 14.Racanelli V, Rehermann B. The liver as an immunological organ. Hepatology. 2006;43(2 Suppl 1):S54–62. doi: 10.1002/hep.21060. [DOI] [PubMed] [Google Scholar]
- 15.Szabo G, Saha B. Alcohol's effect on host defense. Alcohol Res. 2015;37(2):159–170. doi: 10.35946/arcr.v37.2.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marjot T, Webb GJ, Barritt ASt, Moon AM, Stamataki Z, Wong VW, et al. COVID-19 and liver disease: mechanistic and clinical perspectives. Nat Rev Gastroenterol Hepatol. 2021;18(5):348–64. doi: 10.1038/s41575-021-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JU, Majid A, Judge R, Crook P, Nathwani R, Selvapatt N, et al. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5(10):886–887. doi: 10.1016/S2468-1253(20)30251-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: a modeling study. Hepatol. 2022;75(6):1480–1490. doi: 10.1002/hep.32272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au M, Chinnaratha MA, Harding D. Impact of COVID-19 on alcohol-related liver disease: an emerging public health issue in Australia. J Gastroen Hepatol. 2020;35:86. [Google Scholar]
- 21.Bahar R, Medici V, Wilson MD, Rachakonda V. Alcoholic hepatitis admissions and disease severity during the Covid-19 (Sars-Cov-2) pandemic. Hepatol. 2021;74:309a-a. [Google Scholar]
- 22.Bangaru S, Benhammou JN, Yum J, Dong TS, Patel AA, Choi G, et al. Patterns of liver transplantation evaluations for hospitalized adults with acute alcoholic hepatitis during Covid-19: a single center study. Gastroenterol. 2021;160(6):S847–S848. doi: 10.1016/S0016-5085(21)02754-2. [DOI] [Google Scholar]
- 23.Chung WH, Min M, Kothadia S, Saeed F, Scharfen J, Habr F. Increased burden of alcohol-related gastrointestinal and liver diseases during the Covid-19 pandemic: a hospital system-wide audit. Gastroenterol. 2021;160(6):S778-S. doi: 10.1016/S0016-5085(21)02570-1. [DOI] [Google Scholar]
- 24.Gorgulu E, Gu WY, Trebicka J, Mucke VT, Muecke MM, Friedrich-Rust M, et al. Acute-on-chronic liver failure (ACLF) precipitated by severe alcoholic hepatitis: another collateral damage of the COVID-19 pandemic? Gut. 2021;71:1036. doi: 10.1136/gutjnl-2021-325278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinspoon R, Gadi S, Hatipoglu D, Diaz PM, Mulligan C, Wang JY, et al. Impact of the Covid-19 pandemic on alcohol hepatitis admissions in younger patients in a large urban hospital system. Hepatol. 2021;74:28a–a29. [Google Scholar]
- 26.Jain A, Sobotka LA, Allen KD, McShane CJ, Ramsey ML, Wellner MR, et al. Alcohol-related liver disease increased in severity during the COVID-19 pandemic. Am J Gastroenterol. 2021;116:S506-S. doi: 10.14309/01.ajg.0000777800.33890.17. [DOI] [Google Scholar]
- 27.Moore NJ, Vance LM, Walters R, Chandra S. Effect of the COVID-19 pandemic on hospitalization for alcohol-associated hepatitis. Am J Gastroenterol. 2021;116:S513-S. doi: 10.14309/01.ajg.0000777872.65338.06. [DOI] [Google Scholar]
- 28.Nair GG, Jamal MM, Ayutyanont N, Heineke HS, Kubomoto S. Increase in alcoholic hepatitis cases during Covid pandemic in the US. Gastroenterol. 2021;160(6):S160-S. doi: 10.1016/S0016-5085(21)01133-1. [DOI] [Google Scholar]
- 29.Ngu NLY, Boyd DT, Morgan B, Surampudi A, Brown I, Bykersma C, et al. Increase in alcohol-related hospital admissions due to increased alcohol consumption during the COVID-19 lockdown at a Victorian tertiary health service. J Gastroen Hepatol. 2021;36:144–145. [Google Scholar]
- 30.Pradhan F, Sehmbey G, Al-Qaisi M, Rangan P, Kannadath B, Fallon M. Alcoholic hepatitis during the Covid-19 pandemic: results from a multi-center health network. Hepatol. 2021;74:309a–10a. [Google Scholar]
- 31.Azevedo Silva MLC, Ruge A, Atalaia-Martins C, Fernandes A, Vasconcelos H. Impact of covid-19 pandemic on non-covid-19 patients with decompensated liver cirrhosis. United Europ Gastroenterol J. 2021;9:886. [Google Scholar]
- 32.Smart C, Au M, Harding D. Improving inpatient management of alcoholic hepatitis: a topical and emerging public health issue in the world of COVID-19. J Gastroen Hepatol. 2021;36:42. [Google Scholar]
- 33.Toy G, Butcher R, Li HJ, Zhang Y, Gallegos-Orozco JF, Zarate EAR. A remarkable increase in alcohol-related liver disease in the setting of COVID-19. Am J Gastroenterol. 2021;116:S539-S. doi: 10.14309/01.ajg.0000778132.31801.13. [DOI] [Google Scholar]
- 34.Wu T, Rattan P, Kamath PS, Shah V, Simonetto DA. Trends in admission rates for alcohol-associated hepatitis in a single tertiary care center surrounding the Covid-19 pandemic. Hepatol. 2021;74:263a-a. [Google Scholar]
- 35.Cholankeril G, Goli K, Rana A, Hernaez R, Podboy A, Jalal P, et al. Impact of COVID-19 pandemic on liver transplantation and alcohol-associated liver disease in the USA. Hepatol. 2021;74(6):3316–29. doi: 10.1002/hep.32067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Damjanovska S, Karb DB, Cohen SM. Increasing prevalence and racial disparity of alcohol-related gastrointestinal and liver disease during the COVID-19 pandemic: A population-based national study. J Clin Gastroenterol. 2022. 10.1097/MCG.0000000000001665. Demonstrates in a large cohort study the increasing prevalence of alcohol-related diseases during the COVID-19 pandemic, and that some groups were more severely affected than others. [DOI] [PubMed]
- 37.Itoshima H, Shin JH, Takada D, Morishita T, Kunisawa S, Imanaka Y. The impact of the COVID-19 epidemic on hospital admissions for alcohol-related liver disease and pancreatitis in Japan. Sci Rep-Uk. 2021;11:1. doi: 10.1038/s41598-021-92612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutledge SM, Schiano TD, Florman S, Im GY. COVID-19 aftershocks on alcohol-associated liver disease: an early cross-sectional report from the US epicenter. Hepatol Commun. 2021;5:1151. doi: 10.1002/hep4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schimmel J, Vargas-Torres C, Genes N, Probst MA, Manini AF. Changes in alcohol-related hospital visits during COVID-19 in New York City. Addiction. 2021;116(12):3525–3530. doi: 10.1111/add.15589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaheen AA, Kong K, Ma C, Doktorchik C, Coffin CS, Swain MG, et al. Impact of the COVID-19 pandemic on hospitalizations for alcoholic hepatitis or cirrhosis in Alberta, Canada. Clin Gastroenterol Hepatol. 2021 [DOI] [PMC free article] [PubMed]
- 41.Sohal A, Khalid S, Green V, Gulati A, Roytman MM. Unprecedented rise in alcohol-related hepatitis during Covid-19 pandemic. Hepatol. 2021;74:341a-a. doi: 10.1097/MCG.0000000000001627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson MS, Valbuena VSM, Brown CS, Waits SA, Sonnenday CJ, Englesbe M, et al. Association of COVID-19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Netw Open. 2021;4(10):e2131132. doi: 10.1001/jamanetworkopen.2021.31132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bittermann T, Mahmud N, Abt P. Trends in liver transplantation for acute alcohol-associated hepatitis during the COVID-19 pandemic in the US. Jama Network Open. 2021;4:7. doi: 10.1001/jamanetworkopen.2021.18713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cargill Z, Kattiparambil S, Hansi N, Barnabas A, Shawcross DL, Williams R, et al. Severe alcohol-related liver disease admissions post-COVID-19 lockdown: canary in the coal mine? Frontline Gastroente. 2021;12(4):354–355. doi: 10.1136/flgastro-2020-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perisetti A, Kaur R, Thandassery R. Increased diagnosis of hepatocellular carcinoma in hospitalized patients with alcohol related hepatitis after the Covid-19 outbreak: a global multi-center propensity matched analysis. Clin Gastroenterol H. 2021;19(11):2450. doi: 10.1016/j.cgh.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eisa M, Kennedy R, Teferra R, Heckroth M, Eiswerth M, McClain C. SARS-COV-2 infection in patients with alcohol-associated hepatitis: metabolic similarities and treatment challenges. Am J Gastroenterol. 2021;116(4):847–848. doi: 10.14309/ajg.0000000000001002. [DOI] [PubMed] [Google Scholar]
- 47.Dhanda A, Allison M, Bodger K, Forrest E, Hood S, MacGilchrist A, et al. Increasing burden of alcohol-related liver disease in the UK associated with the coronavirus pandemic. Gut. 2021;70:A26-A. [Google Scholar]
- 48.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2002 to 2012–2013: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiat. 2017;74(9):911–923. doi: 10.1001/jamapsychiatry.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eurocare. Alcohol consumption in times of COVID-19 2020 [Available from: https://www.eurocare.org/cares.php?sp=alcohol-and-health&ssp=alcohol-consumption-in-times-of-covid-19.
- 50.IWSR. Beverage alcohol in 2020 performs better than expected 2020 [Available from: https://www.theiwsr.com/beverage-alcohol-in-2020-performs-better-than-expected/.
- 51.Nielson. Rebalancing the "COVID-19 effect" on alcohol sales 2020 [Available from: https://nielseniq.com/global/en/insights/analysis/2020/rebalancing-the-covid-19-effect-on-alcohol-sales/.
- 52.Gisle Lea. Deuxième Enquête de Santé COVID-19: Résultats préliminaires, Sciensano, Brussels, 2020 [Available from: https://www.sciensano.be/en/biblio/deuxieme-enquete-de-sante-covid-19-resultats-preliminaires.
- 53.France SP. Tabac, Alcool: Quel impact du confinement sur la consommation des Français? 200 [Available from: https://www.santepubliquefrance.fr/presse/2020/tabac-alcool-quel-impact-du-confinement-sur-la-consommation-des-francais.
- 54.Tran TD, Hammarberg K, Kirkman M, Nguyen HTM, Fisher J. Alcohol use and mental health status during the first months of COVID-19 pandemic in Australia. J Affect Disord. 2020;277:810–813. doi: 10.1016/j.jad.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Callinan S, Smit K, Mojica-Perez Y, D'Aquino S, Moore D, Kuntsche E. Shifts in alcohol consumption during the COVID-19 pandemic: early indications from Australia. Addiction. 2021;116(6):1381–1388. doi: 10.1111/add.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilian C, Rehm J, Allebeck P, Braddick F, Gual A, Bartak M, et al. Alcohol consumption during the COVID-19 pandemic in Europe: a large-scale cross-sectional study in 21 countries. Addiction. 2021;116(12):3369–3380. doi: 10.1111/add.15530. [DOI] [PubMed] [Google Scholar]
- 57.Winstock AR, Zhuparris A, Gilchrist G, Davies EL. Puljevic C, Potts L, Maier LJ, Ferris JA & Barratt MJ. Global drug survey COVID-19 special edition: Key findings report. London, United Kingdom: Global Drug Survey; 2020. http://www.globaldrugsurvey.com/downloads/GDS-CV19-exec-summary.pdf.
- 58.Winstock AR, Zhuparris A, Gilchrist G, Davies EL. Puljevic C, Potts L, Maier LJ, Ferris JA & Barratt MJ.;Drinking alcohol does not prevent or treat coronavirus infection and may impair immune function: National Institute on Alcohol Abuse and Alcoholism (NIAAA); 2020 [Available from: https://www.niaaa.nih.gov/news-events/announcement/drinking-alcohol-does-not-prevent-or-treat-coronavirus-infection-and-may-impair-immune-function.
- 59.Alcohol does not protect against COVID-19; access should be restricted during lockdown: World Health Organisation (WHO); 2020 [Available from: https://www.euro.who.int/en/health-topics/disease-prevention/alcohol-use/news/news/2020/04/alcohol-does-not-protect-against-covid-19-access-should-be-restricted-during-lockdown.
- 60.Delirrad M, Mohammadi AB. New methanol poisoning outbreaks in Iran following COVID-19 pandemic. Alcohol Alcohol. 2020;55(4):347–348. doi: 10.1093/alcalc/agaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bollen Z, Pabst A, Creupelandt C, Fontesse S, Laniepce A, Maurage P. Longitudinal assessment of alcohol consumption throughout the first COVID-19 lockdown: contribution of age and pre-pandemic drinking patterns. Eur Addict Res. 2022;28(1):48–55. doi: 10.1159/000518218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hendriksen P, Merlo A, Bruce G, Verster JC. Factors affecting alcohol use during COVID-19 lockdown: a critical review. Eur Neuropsychopharm. 2021;53:S9-S. doi: 10.1016/j.euroneuro.2021.10.019. [DOI] [Google Scholar]
- 63.Sallie SN, Ritou V, Bowden-Jones H, Voon V. Assessing international alcohol consumption patterns during isolation from the COVID-19 pandemic using an online survey: highlighting negative emotionality mechanisms. BMJ Open. 2020;10(11):e044276. doi: 10.1136/bmjopen-2020-044276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbosa C, Cowell AJ, Dowd WN. Alcohol consumption in response to the COVID-19 pandemic in the United States. J Addict Med. 2021;15(4):341–344. doi: 10.1097/ADM.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White AM, Castle IP, Powell PA, Hingson RW, Koob GF. Alcohol-related deaths during the COVID-19 pandemic. JAMA. 2022;327(17):1704–1706. doi: 10.1001/jama.2022.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, et al. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278(4):G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- 67.de Goeij MC, Suhrcke M, Toffolutti V, van de Mheen D, Schoenmakers TM, Kunst AE. How economic crises affect alcohol consumption and alcohol-related health problems: a realist systematic review. Soc Sci Med. 2015;131:131–146. doi: 10.1016/j.socscimed.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 68.Clay JM, Parker MO. Alcohol use and misuse during the COVID-19 pandemic: a potential public health crisis? Lancet Public Health. 2020;5(5):e259. doi: 10.1016/S2468-2667(20)30088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rehm J, Kilian C, Ferreira-Borges C, Jernigan D, Monteiro M, Parry CDH, et al. Alcohol use in times of the COVID 19: implications for monitoring and policy. Drug Alcohol Rev. 2020;39(4):301–304. doi: 10.1111/dar.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ILO Monitor: COVID-19 and the world of work. 7th edition. International Labour Organization; 2021. https://www.ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/documents/briefingnote/wcms_767028.pdf.
- 71.Stewart SD. COVID-19, coronavirus-related anxiety, and changes in women’s alcohol use. J Gynecol Women’s Health. 2021;21(2):556057. [Google Scholar]
- 72.Grossman ER, Benjamin-Neelon SE, Sonnenschein S. Alcohol consumption during the COVID-19 pandemic: a cross-sectional survey of US adults. Int J Environ Res Public Health. 2020;17(24):9189. doi: 10.3390/ijerph17249189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Double jeopardy: COVID-19 and behavioral health disparities for Black and Latino communities in the U.S.: Substance Abuse and Mental Health Services Administration; 2020. https://www.samhsa.gov/sites/default/files/covid19-behavioral-health-disparities-black-latino-communities.pdf.
- 74.The Lancet Gastroenterology H Drinking alone: COVID-19, lockdown, and alcohol-related harm. Lancet Gastroenterol Hepatol. 2020;5(7):625. doi: 10.1016/S2468-1253(20)30159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rural health during the pandemic: challenges and solutions to accessing care: National Institute for Health Care Management; 2022 [Available from: https://nihcm.org/publications/rural-health-during-the-pandemic.
- 76.Durkin C, Kaplan DE, Bittermann T. T2 hepatocellular carcinoma exception policies that prolong waiting time improve the use of evidence-based treatment practices. Transplant Direct. 2020;6(9):e597. doi: 10.1097/TXD.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tapper EB, Asrani SK. The COVID-19 pandemic will have a long-lasting impact on the quality of cirrhosis care. J Hepatol. 2020;73(2):441–445. doi: 10.1016/j.jhep.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Asrani SK, Trotter J, Lake J, Ahmed A, Bonagura A, Cameron A, et al. Meeting report: the Dallas consensus conference on liver transplantation for alcohol associated hepatitis. Liver Transpl. 2020;26(1):127–140. doi: 10.1002/lt.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Asrani SK, Mellinger J, Arab JP, Shah VH. Reducing the global burden of alcohol-associated liver disease: a blueprint for action. Hepatology. 2021;73(5):2039–2050. doi: 10.1002/hep.31583. [DOI] [PMC free article] [PubMed] [Google Scholar]