Abstract
Adherence of type 1-piliated Escherichia coli to carbohydrate structures of vaginal mucosa plays a major role in the pathogenesis of ascending urinary tract infections in women. Colonization of the vaginal introitus is influenced by interactions between pathogens, vaginal fluid, and vaginal epithelium. In this study, the type and amount of carbohydrates and glycoproteins present in vaginal fluid were determined. Free and protein-bound oligosaccharides in vaginal fluid specimens were analyzed by fluorophore-assisted carbohydrate electrophoresis (FACE) and high-pressure liquid chromatography (HPLC). Two-dimensional electrophoretic separations of vaginal fluid glycoproteins were performed together with bacterial overlay assays. The results of FACE showed that the majority of the oligosaccharides are in the free state and the bound oligosaccharides are undetectable. HPLC analysis of free sugars revealed glucose as the major sugar (3.3 ± 0.3 mM), and the concentrations of mannose and glucosamine were 0.065 ± 0.04 and 0.02 ± 0.001 mM, respectively. Radiolabeled E. coli bound three vaginal fluid glycoproteins with the following molecular masses and pIs: 82 kDa and pI 5.5, 55 kDa and pI 4.5, and 55 kDa and pI 6.5. The binding was inhibited by mannose and by deglycosylation of the proteins prior to the overlay assay. One of these putative receptors was identified to be the heavy chain of secretory IgA (S-IgA). These data suggest that the free mannose in the fluid is less than that required to affect E. coli-epithelial cell binding interactions and that S-IgA may bind E. coli in the vaginal introitus.
Urinary tract infections (UTIs) are among the most common bacterial diseases ranging from asymptomatic bacteriuria to kidney infection and renal failure (6, 15, 20, 28). Bacterial adherence to the vaginal surface is considered an important prerequisite for colonization and subsequent infection. Numerous studies suggest that differences in the composition of the mucosal surface affect bacterial adherence (19, 21, 24, 26, 30, 32). It has also been established that the most common uropathogen isolated from urine and vaginal fluid from infected or colonized women is type 1-piliated Escherichia coli (2, 17, 27). The vaginal surface is itself devoid of glands but is lubricated with cervical mucus and a fluid transudate from the rich vascular network of the lamina propia (38). Glycoproteins and oligosaccharides of the vaginal fluid which affect E. coli adherence can be derived from the cervical mucus or plasma filtrate or from shedding of surface components from the epithelial cells. The host receptors for type 1 fimbriae are limited to oligosaccharides carrying terminal mannose (Man) residues with Man-α-1,3-Man and Man-α-1,6-Man structures (5). Animal studies have shown that E. coli expressing type 1 pili can cause UTIs, whereas mutant E. coli or wild-type E. coli grown under conditions that suppress type 1 pili expression do not cause infection (14). It has been shown that systemic administration of immune sera to purified FimH (the adhesin that confers mannose-specific binding activity to type 1 pili) resulted in reduced bladder colonization in mice by uropathogenic E. coli (18). Also, immunization with FimH and specific domains of FimH reduced in vivo colonization of the bladder mucosa in an experimental mouse model (14, 18, 34). These results clearly establish the functional importance of mannose-sensitive type 1 pili in UTIs.
Previous work from our laboratory demonstrated that every vaginal fluid sample tested bound type 1-piliated bacteria and this binding was inhibited by mannose (37). In another study, it was shown that vaginal fluid influenced the adherence of type 1-piliated E. coli to epithelial cells in vitro (7). Vaginal fluid components may bind bacteria competitively and thus either inhibit adherence to epithelial cells or promote adherence by binding and aggregating the bacteria. These studies suggest that the composition of vaginal fluid in terms of glycoproteins and free carbohydrates is important in vaginal colonization by bacteria.
In this study, the amounts of total sugar versus free sugar and the types and amounts of specific sugars in vaginal fluid were determined by fluorophore-assisted carbohydrate electrophoresis (FACE) and high-pressure liquid chromatography (HPLC) analyses. We also compared the protein profiles of vaginal fluid with vaginal cells and serum by two-dimensional (2D) electrophoresis and identified putative receptors for type 1-piliated E. coli in the vaginal fluid by bacterial overlay assay. We show here that vaginal fluid contains relatively high concentrations of free oligosaccharides compared to those of glycoprotein-derived oligosaccharides and the free oligosaccharides consisted mainly of glucose plus small amounts of mannose and glucosamine. We also show that vaginal fluid is in part derived from plasma transudate and that it contains at least three putative bacterial receptors that show specific binding of radiolabeled type 1-piliated E. coli in a mannose-inhibitable manner. One of these three glycoproteins was identified as the heavy chain of secretory immunoglobulin A (S-IgA).
MATERIALS AND METHODS
Sample collection and preparation.
Vaginal fluid specimens were collected from pre- and postmenopausal women (age range, 23 to 80 years) visiting the urology clinics at Northwestern University. The samples were from women with and without UTIs. Some of the postmenopausal women were receiving hormone replacement therapy. The fluids were collected and processed as described earlier (7, 37). Briefly, the specimens were collected by gently scraping the vaginal mucosa with sterile Dacaron-tipped applicators (Hardwood Products Company, Guilford, Mass.) and immersing the applicators in 1-ml portions of sterile phosphate-buffered saline (PBS) (pH 7.3). This method of vaginal fluid collection was estimated to have diluted the actual in situ concentration of the vaginal fluid approximately 20- to 40-fold, and all concentrations of saccharides reported in this study refer to this diluted vaginal fluid solution. The specimens were cultured for bacterial flora and were labeled “colonized” if there were >10 E. coli present. If no E. coli were present, then the samples were labeled “noncolonized.” The sample was immediately centrifuged and filtered (0.2-μm pore size; Costar, Cambridge, Mass.) free of vaginal cells and bacteria and stored at −70°C. Total protein concentration was determined by bicinchoninic acid protein assay (Pierce Chemical Co., Rockford, Ill.). Proteins in the vaginal fluid were precipitated by the addition of 3 volumes of ice-cold 100% ethanol. The samples were kept on ice for 10 min and then centrifuged for 5 min at 14,000 × g. The supernatant containing the free oligosaccharides was dried by centrifugal vacuum evaporation (Savant Speed Vac; Forma Scientific Inc., Marietta, Ohio), and the protein pellet was saved for other analyses. To recover the total oligosaccharides, the vaginal fluid was treated with deglycosylating enzymes (see below) prior to ethanol precipitation.
Treatment with glycosidases.
Vaginal fluid (100-μl) samples were treated with 0.04 U of N-glycosidase (Boehringer Mannheim, Indianapolis, Ind.) to remove N-linked oligosaccharides and a combination of 0.04 U of neuraminidase and O-glycosidase (Boehringer Mannheim) to remove O-linked oligosaccharides in 0.1 M sodium phosphate, pH 7.2, for 18 h at 37°C. The released oligosaccharides were separated from the protein by precipitation with ethanol and processed for FACE analysis.
FACE analysis.
The free oligosaccharides as well as oligosaccharides released from glycoproteins were labeled with the fluorescent tag aminonaphthalene-1,3,6-trisulfonic acid (ANTS) by reductive amination with sodium cyanoborohydride. The reagents, gels, and equipment were purchased from Glyko Inc. (Novato, Calif.), and the protocols described in the Glyko manual were followed. Labeling was performed at 37°C for 18 h, the samples were dried in the Speed Vac and diluted with gel loading buffer, and 1/10 of the total sample was analyzed by precast N-linked profiling gels (Glyko) electrophoresed at 15 mA for 1.5 h. The glucose tetramer (G4) in the glucose (Glc) standard ladder had a lower intensity and therefore could be easily identified and compared with oligosaccharides from the samples. The gels were visualized on a transilluminator (320 nm) and photographed immediately.
Monosaccharide composition analysis by HPLC.
Neutral (glucose and mannose) and amino sugars (glucosamine and galactosamine) from the vaginal fluid samples were released with 2 N trifluoroacetic acid (TFA) at 100°C for 5 h. d-Rhamnose was used as an internal control to account for recovery losses during hydrolysis. The monosaccharides were separated and quantitated on a Dionex Carbopack PA-1 column by high-pH anion-exchange chromatography as detailed elsewhere (12, 22).
Electrophoresis and protein transfer.
For 2D electrophoresis, aliquots of samples containing 25 μg of protein were separated by isoelectric focusing 10-in. rod gels using the ISO-DALT system as described earlier (11). Briefly, the first dimension which separates proteins according to their isoelectric points was carried out at 14,000 V h in 5% polyacrylamide containing 9 M urea and 2% total ampholytes with a pH range of 3 to 10. At the end of isoelectric focusing, each gel was extruded from the elongated glass tube and equilibrated. The tube gels were layered over slab gels consisting of a linear polyacrylamide gradient (9 to 18%) containing 1% sodium dodecyl sulfate. Electrophoresis was performed at a constant voltage of 100 to 150 V for 16 h. The proteins from the gel were transferred to a nitrocellulose membrane (Bio-Rad) (0.45 μM) at 120 V for 2 h with a transfer buffer consisting of 25 mM Tris, 192 mM glycine, and 20% methanol (pH 8.3). Blots were washed in distilled water and incubated with blocking buffer (1% bovine serum albumin in PBS) overnight at 4°C. Fifty micrograms of protein was used for all polyvinylidene difluoride (PVDF) membrane transfers. Proteins marked in figures by letters were identified by comparison to the reference maps of plasma protein and urine (10). Molecular mass was estimated by comparison to known masses of serum proteins resolved on a companion 2D gel. Isoelectric points were estimated by comparison to a standard carbamylation charge train of rabbit creatine kinase, which comigrated with the serum protein reference gel (11).
Bacterial labeling.
Bacterial overlay assays were done with 35S-labeled E. coli HB101 transformed with the recombinant plasmid pWRS1-17, which encodes type 1 pili. E. coli HB101 transformed with the vector alone (pHSS22), which does not express type 1 pili, was used as a negative control. Both piliated and nonpiliated bacteria were grown overnight at 37°C in shaking liquid Luria-Bertani medium (Gibco, Grand Island, N.Y.) supplemented with 40 μg of kanamycin (Sigma) per ml, containing 5 μCi of [35S]methionine (New England Nuclear, Boston, Mass.) per ml. The bacteria were then collected by centrifugation at 3,500 × g for 10 min at 4°C, washed three times, and resuspended in PBS. Concentration of bacteria was estimated by determining optical density values at 540 nm on a Coleman spectrophotometer. Viable counts were carried out to determine the labeling efficiency. The bacteria were tested for d-mannose-inhibitable agglutination of a 1% solution of guinea pig erythrocytes in PBS to confirm the expression of type 1-piliated bacteria (4).
Western blotting.
The membrane to which vaginal proteins were transferred was subjected to Western blotting with rabbit polyclonal α-chain-specific anti-S-IgA antibody (catalog no. I 9889; Sigma).
Bacterial binding to vaginal proteins and S-IgA.
The membranes to which proteins were transferred were blocked with PBS containing 1% bovine serum albumin overnight at 4°C and washed with PBS (containing 0.3% Tween 20) three times for 10 min each. The membranes were then incubated with approximately 108 bacteria (70 to 160 bacteria per cpm) per ml of either 35S-labeled type 1-piliated E. coli HB101/pWRS1-17. To serve as a negative control, parallel membranes were incubated with 35S-labeled wild-type HB101 with no type 1 pili expression (HB101/pHSS22) for 2 h at 37°C with gentle shaking. Membranes were then washed three times with PBS containing 0.05% Tween 20, dried, and subjected to autoradiography.
RESULTS
Free and bound sugars in vaginal fluid.
The total and free sugars from individual samples of vaginal fluid were analyzed qualitatively by the FACE technique, and the results from three representative samples are shown in Fig. 1. Equal amounts of vaginal fluid were treated either with deglycosylating enzymes or buffer alone, and the released oligosaccharides were separated from the glycoproteins by ethanol precipitation and subsequently labeled with ANTS. The oligosaccharides derived before (free) and after treatment with deglycosylating enzymes (total) were electrophoresed in adjacent lanes as shown in Fig. 1. The leftmost lane shows the Oligo Ladder Standard, which consists of a mixture of glucose polymers ranging from G1 to G20. The intensity of the G4 band in the standard mixture is less than the adjacent bands, and this allows its easy identification even if lower bands run off the gel. It can be seen that the total amount of carbohydrates generated from the vaginal fluid is comparable to the amount of free carbohydrates and that the vaginal fluid consists primarily of mono-, di-, tri-, and tetrasaccharides. Low-intensity bands are observed corresponding to G5 to G9 which could represent higher polymers of sugars (see Discussion). Faint bands smaller than G1 in size were observed in samples and were not identified. This experiment shows that the majority of the oligosaccharides are in the free state and the bound oligosaccharides are undetectable. In a separate experiment, 100-μl vaginal fluid samples were dialyzed to remove free oligosaccharides and the remaining protein was treated with deglycosylating enzymes. When the released oligosaccharides were labeled with ANTS and subjected to FACE analysis, no fluorescent bands were visible (data not shown). Since the detection limit of the FACE technique is in the range of 1 to 10 nmol of any single oligosaccharide (Glyko instruction manual), we can assume that less than 100 μM oligosaccharides is present in the vaginal fluid in the bound state. This observation confirms the findings shown in Fig. 1 that the majority of oligosaccharides in the vaginal fluid are free.
FIG. 1.
FACE analysis of sugars from vaginal fluid samples. Lane 1, glucose polymer standard; lanes 2, 4, and 6, free oligosaccharides generated from three different samples (I, II, and III) by precipitation of vaginal fluid with ethanol. Total oligosaccharides (generated by treatment of vaginal fluid with N- and O-glycosidases) prior to ethanol precipitation from the same three samples are shown in the adjacent lanes (lanes 3, 5, and 7). See text for details.
Monosaccharides in vaginal fluid.
In order to address the potential role of vaginal fluid carbohydrates in bacterial binding, it was important to determine the composition of the different free saccharides and their relative amounts in the fluid. Monosaccharide composition analysis from individual vaginal fluid samples and pooled samples were analyzed by HPLC. The acid treatment with trifluoroacetic acid breaks down all oligosaccharides into monosaccharides, and the individual monosaccharides were separated and quantitated by comparison of vaginal fluid HPLC profiles with standard HPLC profiles of glucose, mannose, and glucosamine. It was found that glucose was the major component, whereas mannose and glucosamine were identified as minor components, accounting for less than 3% of the free monosaccharides. The mean (n = 6) values of glucose, mannose, and glucosamine were 3.3 ± 0.3, 0.065 ± 0.04, and 0.02 ± 0.001 mM, respectively.
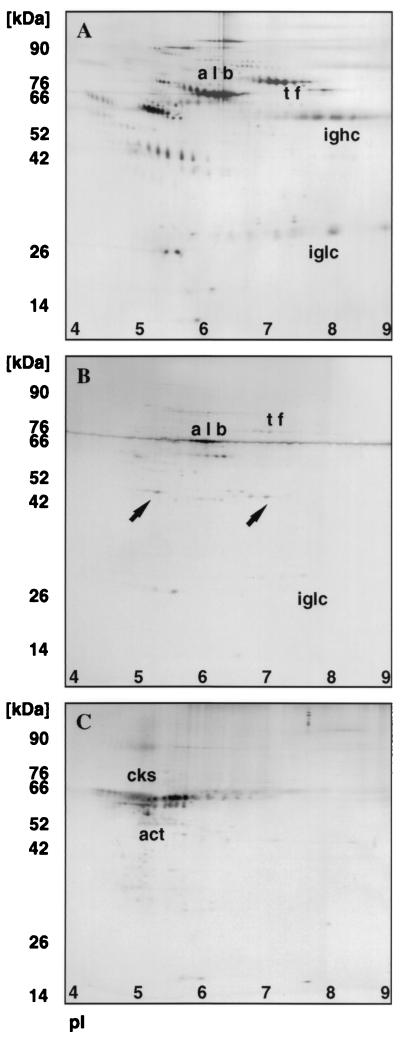
2D protein profiles of human serum, vaginal fluid, and vaginal epithelial cells.
Figure 2 shows representative 2D protein profiles of serum (Fig. 2A), vaginal fluid (Fig. 2B), and vaginal cells (Fig. 2C). The pIs of the vaginal proteins range from 4.5 to 8.0, and there are at least two sets of unique proteins distinctly different from that observed in serum. A number of common proteins were observed between serum and vaginal fluid, allowing reliable pattern alignment and matching. Albumin, transferrin, and immunoglobulin chains were found in all vaginal fluid samples tested. Comparison with a published 2D urine map (10) with vaginal fluid revealed no additional overlapping proteins between the samples, suggesting that the vaginal fluid samples were unlikely to be contaminated with urinary proteins during sample collection. Thus, vaginal fluid contained both a unique family of proteins and also contained proteins with migration patterns identical to those of known plasma proteins. These findings suggest that vaginal fluid may be composed in part of a plasma transudate. Vaginal cells show the presence of actin and cytokeratins typically expressed by epithelial cells.
FIG. 2.
2D electrophoresis pattern of serum (A), vaginal fluid (B), and vaginal epithelial cells (C). The positions of known proteins albumin (alb), transferrin (tf), immunoglobulin heavy chain (ighc), immunoglobulin light chain (iglc), cytokeratins (cks) and actin (act) were designated by comparison to master protein profiles in the Swiss 2D protein data bank from the Internet. In panel B, two proteins in vaginal fluid distinctly different from that observed in serum (panel A) are indicated by arrows.
Identification of bacterial binding proteins in vaginal fluid.
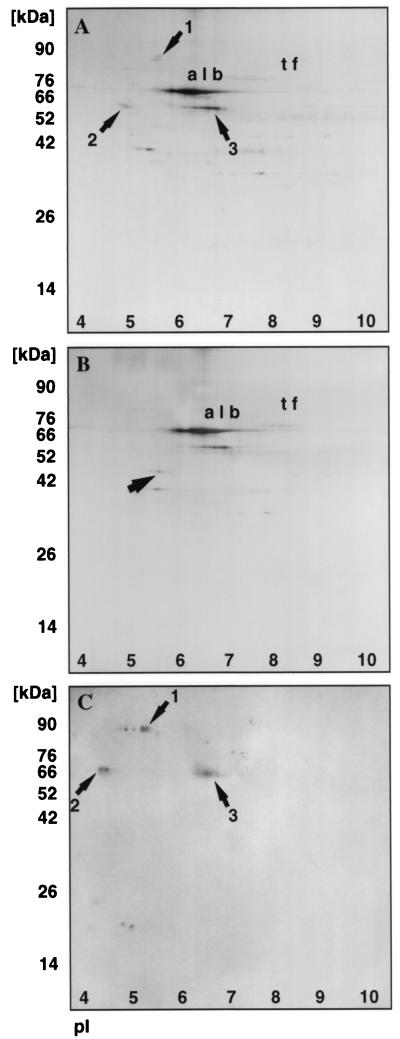
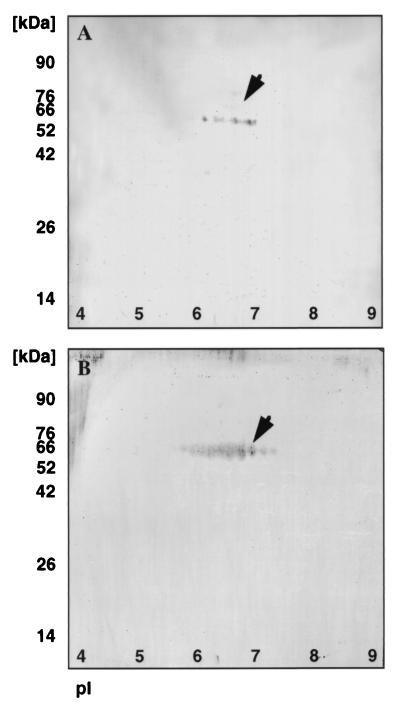
In order to identify specific glycoproteins that might be involved in bacterial binding, we performed a bacterial overlay assay on vaginal fluid before and after deglycosylation and the results are shown in Fig. 3. Vaginal protein samples were subjected to 2D electrophoresis before and after deglycosylation. The gels were run in duplicate; one set was stained with silver nitrate, and the other was transferred to a PVDF membrane prior to overlay with 35S-labeled type 1-piliated bacteria. Comparison of the silver-stained gel in Fig. 3A and the autoradiogram in Fig. 3C clearly indicates that vaginal fluid contains at least three proteins that bind radiolabeled bacteria. Protein 1 had an apparent molecular mass of 82 kDa (pI ≈ 5.5), and proteins 2 and 3 had a molecular mass of 55 kDa (pIs ≈ 4.5 and 6.5). Proteins 1 and 2 appear glycosylated as seen by comparing Fig. 3A and B. It appears that protein 2 is comprised of at least two different glycosylated forms that resolve as one new spot with an altered molecular mass upon deglycosylation. Protein 1 depicted by a band at 82 kDa is stained weakly by silver nitrate, and upon deglycosylation, the corresponding protein in Fig. 3B could not be detected. It should be noted that intensity of staining of glycoproteins is dependent on the amount and type of attached carbohydrates. Several glycosylated proteins can comigrate as one spot which can be resolved upon deglycosylation; in contrast, large carbohydrates can inhibit staining of glycoproteins and can be visualized only upon deglycosylation. When deglycosylated vaginal fluid was subjected to bacterial overlay assay, as expected, no bacterial binding proteins were detected. Comparison of these putative receptors for type 1-piliated bacteria with 2D protein profiles of proteins from the database identified protein 3 as the heavy (alpha) chain of S-IgA, whereas proteins 1 and 2 could not be matched to any known protein. To confirm this, Western blotting was performed on vaginal fluid with alpha-chain-specific anti-S-IgA antibody. This antibody exhibited no reactivity towards commercially available secretory component (data not shown), ruling out any cross-reactivity with secretory component present in vaginal fluid. The weak positive staining in Fig. 4A corresponds to band 3 in Fig. 3A and C. For further confirmation, bacterial overlay assay with commercially available S-IgA was performed. A PVDF membrane containing S-IgA was incubated with labeled E. coli, and the results are shown in Fig. 4B. The S-IgA band could be exactly matched with the protein band 3 in Fig. 3A and C.
FIG. 3.
Bacterial overlay assay using 35S-labeled type 1-piliated E. coli (HB101/pWRS1-17). Silver-stained gels of vaginal fluid before (A) and after (B) deglycosylation. The proteins albumin (alb) and transferrin (tf) are indicated. (C) Autoradiogram of bacterial overlay assay performed on vaginal fluid proteins. Proteins 1, 2, and 3 bind radiolabeled bacteria. Protein 2 in panel A (arrow) resolves as a single spot with an altered molecular mass in panel B (arrow).
FIG. 4.
Detection of S-IgA and bacterial overlay assay of S-IgA in vaginal fluid. (A) A 50-μg sample of vaginal fluid was subjected to Western blotting with polyclonal anti-S-IgA antibody. (B) Autoradiograph of bacterial overlay assay performed on 20 μg of S-IgA, using 35S-labeled type 1-piliated E. coli (HB101/pWRS1-17).
DISCUSSION
Oligosaccharides carrying terminal mannose (Man) in Manα1→3Man and Manα1→6Man structures are known to be important host receptors for type 1-piliated bacteria (2, 5). A number of receptors for type 1-piliated E. coli, such as polymorphonuclear leukocyte membrane glycoproteins gp70, gp80, and gp150 (25), leukocyte integrins CD11 and CD18 (8), and human neutrophil lysosome-associated membrane glycoprotein (Lamp-1) (16), have been identified. Wu et al. have identified uroplakins Ia and Ib on bladder epithelium as receptors for type 1-piliated E. coli (41). In addition, there are reports describing the binding of Helicobacter pylori and Yersinia enterocolitica to purified intestinal mucins (19, 29) and of Pseudomonas aeruginosa and Pseudomonas cepacia to respiratory mucins (23, 26).
Previous studies from our laboratory have shown that vaginal fluid binds type 1-piliated bacteria in a mannose-inhibitable manner. We have also shown that the vaginal fluid significantly alters bacterial adherence in vitro and may either inhibit or stimulate bacterial adherence to epithelial cells (7). These studies strongly suggest that vaginal fluid is an important factor in colonization of uropathogens in ascending UTI; therefore, characterization of vaginal fluid in terms of its receptors for type 1-piliated bacteria seems a compelling extension of our previous studies.
In this study, relatively large amounts of free carbohydrates were found to be present in vaginal fluids compared to bound carbohydrates. These carbohydrates were characterized by FACE and HPLC. By HPLC analysis, glucose was the major component of the vaginal fluid, mannose and glucosamine were found in much smaller quantities, and galactose and galactosamine were not detected. Di- to hexasaccharides were evident by FACE analysis and accounted for the majority of the total carbohydrates. It is presumed that the oligosaccharides are of the malto series derived from glycogen, since the nonkeratinized squamous epithelial cells lining the vagina are rich in glycogen (9). The in situ concentration of the glucose oligosaccharides in the vaginal fluid based on the glucose content was calculated to be between 80 and 160 mM. The in situ concentration of mannose based on the HPLC analysis was calculated to be between 1 and 2 mM, which is at least an order of magnitude below the concentration that could potentially inhibit the type 1-piliated E. coli adhesin receptor binding (27). When vaginal fluid was treated with N-glycosidase under conditions determined to yield oligosaccharides from glycoproteins, no new oligosaccharide bands were observed by FACE analysis. N- and O-glycosidase-treated vaginal fluid samples were electrophoresed and compared with untreated vaginal fluid samples by 1D and 2D electrophoresis. The electrophoretic mobilities (1D) and protein profiles (2D) were remarkably similar between O-glycosidase-treated and untreated vaginal fluid samples (data not shown), whereas minor but specific differences were observed between N-glycosidase-treated and untreated vaginal fluid samples (Fig. 3A and B). These results indicate that almost all the detectable oligosaccharides in the vaginal fluids were free and not protein bound. However, the small fraction of the total sugar that is bound is likely to play a major and key role in bacterial binding.
The source of the vaginal fluid components was investigated by 2D electrophoresis, and the protein profiles show that vaginal fluid is in part a transudate from plasma. There were no overlapping proteins between the vaginal cells and vaginal fluid, so the protein components from cells apparently do not contribute any substantial portion of the vaginal fluid.
The 2D results also revealed three glycoproteins in the vaginal fluid that bind type 1-piliated bacteria presumably via mannose receptors: protein 1 with an apparent molecular mass of 82 kDa and proteins 2 and 3 both with apparent molecular masses of 55 kDa but with different pIs. Proteins 1 and 2 could not be matched or aligned with a known protein from the database. Attempts to identify the two bands by N-terminal sequencing were unsuccessful due to the low levels of these two proteins. Protein 3 was identified as the heavy chain of S-IgA by comparison with 2D profile of human milk (1). It was observed that bacterial binding to all three proteins required type 1 pili expression and was mannose inhibitable. Also, when oligosaccharides from the glycoproteins were enzymatically removed, the resultant proteins did not exhibit bacterial binding.
Vaginal fluid is considered to be a mixture of cervical mucus and plasma transudate. Antibodies in fluid secretions are thought to provide the immune defense against mucosal pathogens (3, 13, 39). It is well established that S-IgA is a product of local synthesis and is a major component of the antibodies (64% of the total immunoglobulins) present on mucosal surfaces. S-IgA is a heavily glycosylated molecule and is known to have receptors for type 1-piliated adhesins (39). In secretions, the S-IgA is present as dimers in two isotypic forms, IgA1 and IgA2, in association with a secretory component produced by local epithelial cells. IgA2, which is more heavily glycosylated than IgA1, reacts more strongly with mannose-specific lectins, whereas IgA1 reacted more strongly with galactose-specific lectins (40). Wold et al. (39) have shown that the interaction between mannose-specific lectins such as type 1-fimbriated E. coli and S-IgA resulted in the inhibition of bacterial adherence to host cells. This antibacterial property of S-IgA has been attributed to the presence of high-mannose oligosaccharide chains on S-IgA especially on the IgA2 subclass. Several investigators have measured S-IgA in the vaginal fluid, and it was found to range from 5 to 20 μg per ml (35, 36). Individual differences in the quantities of S-IgA in a given woman’s vaginal fluid sample may be related to physiological factors such as menstrual cycle and menopausal status. Based on our present findings together with previous results reported by Svanborg-Eden and Svennerholm (33), Gaffney et al. (7), and Stamey and Howell (31), we suggest that S-IgA in vaginal fluid could have important effects on vaginal colonization by type 1-piliated E. coli.
In summary, we provide information on the type and amounts of carbohydrates and glycoproteins present in the vaginal fluids. Free mannose is present in very small amounts and below the concentration levels that could affect the binding of type 1-piliated E. coli to epithelial cells (27). The bound mannose, even though it is in small amounts, could play an important role in the bacterial adherence to vaginal fluid and may alter susceptibility to UTIs. There are at least three glycoproteins in vaginal fluid that bind type 1-piliated E. coli. One of these, S-IgA, binds type 1-piliated bacteria in a mannose-sensitive manner. The levels of the antibody present in the fluid could affect colonization by type 1-piliated bacteria of the vaginal mucosa. The amount and type of S-IgA present in any individual vaginal fluid sample could be an indicator of susceptibility to bacterial adherence and subsequent colonization. Passive administration of S-IgA could provide protection against pathogens. We have recently developed a cell culture model system by immortalization of primary vaginal cells, and this cell line will be used to further explore the effects of S-IgA on bacterial adherence and colonization.
ACKNOWLEDGMENTS
We thank Yi Qian for help with 2D electrophoresis.
This work was supported in part by NIH grant DK 42648.
REFERENCES
- 1.Anderson N G, Powers M T, Tollaksen S L. Proteins of human milk. I. Identification of major components. Clin Chem. 1982;28:1045–1055. [PubMed] [Google Scholar]
- 2.Bloch C A, Stocker B A D, Orndorff P E. A key role for type 1 pili in enterobacterial communicability. Mol Microbiol. 1992;6:697–701. doi: 10.1111/j.1365-2958.1992.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 4.Duguid J P, Glegg S, Wilson M I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979;12:213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- 5.Firon N, Ofek I, Sharon N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem Biophys Res Commun. 1982;105:1426. doi: 10.1016/0006-291x(82)90947-0. [DOI] [PubMed] [Google Scholar]
- 6.Fowler J E, Stamey T A. Studies of introital colonization in women with recurrent urinary tract infections: role of bacterial adherence. J Urol. 1977;117:472–476. doi: 10.1016/s0022-5347(17)58501-8. [DOI] [PubMed] [Google Scholar]
- 7.Gaffney R A, Venegas M F, Kanerva C, Navas E L, Anderson B E, Duncan J L, Schaeffer A J. Effect of vaginal fluid on adherence of type 1 piliated Escherichia coli to epithelial cells. J Infect Dis. 1995;172:1528–1535. doi: 10.1093/infdis/172.6.1528. [DOI] [PubMed] [Google Scholar]
- 8.Gbarah A, Gahmberg C G, Ofek I, Jacobi U, Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991;59:4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregoire A T, Ledger W D, Morgan M J. The glycogen content of the human female genital tract in cycling, menopausal, and women with endometrial and cervical carcinoma. Fertil Steril. 1973;24:198–201. doi: 10.1016/s0015-0282(16)39553-x. [DOI] [PubMed] [Google Scholar]
- 10.Grover P K, Resnick M I. Two-dimensional analysis of proteins in unprocessed human urine using double stain. J Urol. 1993;150:1069–1072. doi: 10.1016/s0022-5347(17)35688-4. [DOI] [PubMed] [Google Scholar]
- 11.Guevera J, Johnston D A, Ramagali L S, Martin B A, Capatillo S, Rodriquez L V. Qualitative aspects of silver deposition in proteins resolved by complex polyacrylamide gels. Electrophoresis. 1982;3:197–202. [Google Scholar]
- 12.Hardy M R, Townsend R R, Lee Y C. Monosaccharide analysis of glycoconjugates by anion exchange chromatography with pulsed amperometric detection. Anal Biochem. 1988;170:54–62. doi: 10.1016/0003-2697(88)90089-9. [DOI] [PubMed] [Google Scholar]
- 13.Hocini H, Barra A, Belec L, Iscaki S, Preud’homme J L, Pillot J, Bouvet J P. Systemic and secretory humoral immunity in the normal human vaginal tract. Scand J Immunol. 1995;42:269–274. doi: 10.1111/j.1365-3083.1995.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 14.Iwahi T, Abe Y, Nakao M, Imada A, Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by Escherichia coli in mice. Infect Immun. 1983;39:1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson J R, Stamm W E. Urinary tract infections in women. Ann Intern Med. 1989;111:906–917. doi: 10.7326/0003-4819-111-11-906. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson A, Carlsson S R, Dahlgren C. Identification of the lysosomal membrane glycoprotein Lamp-1 as a receptor for type-1-fimbriated (mannose-specific) Escherichia coli. Biochem Biophys Res Commun. 1996;219:168–172. doi: 10.1006/bbrc.1996.0200. [DOI] [PubMed] [Google Scholar]
- 17.Kisielius P V, Schwan W R, Amundsen S K, Duncan J L, Schaeffer A J. In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect Immun. 1989;57:1656–1662. doi: 10.1128/iai.57.6.1656-1662.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langermann S, Palaszynski S, Barnhart M, Auguste G, Pinkner J S, Burlein J, Barren P, Koenig S, Leath S, Jones H C, Hultgren S J. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science. 1997;276:607–611. doi: 10.1126/science.276.5312.607. [DOI] [PubMed] [Google Scholar]
- 19.Mantle M, Husar S D. Binding of Yersinia enterocolitica to purified, native small intestinal mucins from rabbits and humans involves interactions with the mucin carbohydrate moiety. Infect Immun. 1994;62:1219–1227. doi: 10.1128/iai.62.4.1219-1227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neu H C. Urinary tract infections. Am J Med. 1992;92:63S–70S. doi: 10.1016/0002-9343(92)90312-y. [DOI] [PubMed] [Google Scholar]
- 21.Ofek J, Beachy E H. Bacterial adherence. London, United Kingdom: Chapman and Hall; 1980. General concepts and principles of bacterial adherence in animals and humans; pp. 3–29. [Google Scholar]
- 22.Rajan N, Tsarbopoulos A, Kumarasamy R, O’Donnell R O, Taremi S S, Baldwin S W, Seelig G F, Fan X, Pramanik B, Le H V. Characterization of recombinant human interleukin receptor from CHO cells: role of N-linked oligosaccharides. Biochem Biophys Res Commun. 1995;206:694–702. doi: 10.1006/bbrc.1995.1098. [DOI] [PubMed] [Google Scholar]
- 23.Ramphal R, Carnoy C, Fierve S, Michalski J C, Houdret N, Lamblin G, Strecker G, Roussel P. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (GalB1-3-GlcNA) or type 2 (GalB1-4GlcNAc) disaccharide units. Infect Immun. 1991;59:700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramphal R, Pyle M. Evidence for mucins and sialic acid as receptors for Pseudomonas aeruginosa to mucin glycopeptides from sputa of patients with cystic fibrosis and chronic bronchitis. Infect Immun. 1983;41:339–344. doi: 10.1128/iai.41.1.339-344.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Ortega M, Ofek I, Sharon N. Membrane glycoproteins of human polymorphonuclear leukocytes that act as receptors for mannose-specific Escherichia coli. Infect Immun. 1987;55:968–973. doi: 10.1128/iai.55.4.968-973.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sajjan U S, Corey M, Karmali M A, Forstner J F. Binding of Pseudomonas cepacia to normal human intestinal mucin and respiratory mucin from patients with cystic fibrosis. J Clin Invest. 1992;89:648–656. doi: 10.1172/JCI115631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaeffer A J, Amundsen S K, Schmidt L N. Adherence of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1979;24:753–759. doi: 10.1128/iai.24.3.753-759.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaeffer A J, Jones J M, Dunn J K. Association of in vitro Escherichia coli adherence to vaginal and buccal epithelial cells with susceptibility to women to recurrent urinary tract infections. N Engl J Med. 1981;304:1062–1066. doi: 10.1056/NEJM198104303041802. [DOI] [PubMed] [Google Scholar]
- 29.Simon P M, Goode P L, Mobasseri A, Zopf D. Inhibition of Helicobacter pylori binding to gastrointestinal epithelial cells by sialic acid-containing oligosaccharides. Infect Immun. 1997;65:750–757. doi: 10.1128/iai.65.2.750-757.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith H W. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977;41:475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamey T A, Howell J J. Studies of introital colonization in women with recurrent urinary infections. IV. The role of local vaginal antibodies. J Urol. 1976;115:413–415. doi: 10.1016/s0022-5347(17)59222-8. [DOI] [PubMed] [Google Scholar]
- 32.Stromberg N, Nyholm P G, Pascher I, Normark S. Saccharide orientation at the cell surface affects glycolipid receptor function. Proc Natl Acad Sci USA. 1991;88:9340–9344. doi: 10.1073/pnas.88.20.9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svanborg-Eden C, Svennerholm A M. Secretory immunoglobulin A and G antibodies prevent adhesion of Escherichia coli to human urinary tract epithelial cells. Infect Immun. 1978;22:790–797. doi: 10.1128/iai.22.3.790-797.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thankavel K, Madison B, Ikeda T, Malaviya R, Shaw A H, Arumugam P M, Abraham S N. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J Clin Invest. 1997;100:1123–1136. doi: 10.1172/JCI119623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokronegoro A, Sirisinha S. Quantitative analysis of immunoglobulins and albumin in secretion of female reproductive tract. Fertil Steril. 1975;26:413–417. doi: 10.1016/s0015-0282(16)41112-x. [DOI] [PubMed] [Google Scholar]
- 36.Usala S, Usala F, Haciski R, Holt J, Schumacher G. IgG and IgA content of vaginal fluid during the menstrual cycle. J Reprod Med. 1989;34:292–294. [PubMed] [Google Scholar]
- 37.Venegas M F, Navas E L, Gaffney R A, Duncan J L, Anderson B E, Schaeffer A J. Binding of type 1 piliated Escherichia coli to vaginal mucus. Infect Immun. 1995;63:416–422. doi: 10.1128/iai.63.2.416-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheater P R, Burkitt H G, Daniels V G. Functional histology. New York, N.Y: Churchill Livingstone; 1979. Female reproductive system; pp. 255–271. [Google Scholar]
- 39.Wold A E, Mestecky J, Tomana M, Kobata A, Ohbayashi H, Endo T, Svanborg Eden C. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect Immun. 1990;58:3073–3077. doi: 10.1128/iai.58.9.3073-3077.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wold A E, Motas C, Svanborg C, Mestecky J. Lectin receptors on IgA isotypes. Scand J Immunol. 1994;39:195–201. doi: 10.1111/j.1365-3083.1994.tb03360.x. [DOI] [PubMed] [Google Scholar]
- 41.Wu X R, Sun T T, Medina J. In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci USA. 1996;93:9630–9635. doi: 10.1073/pnas.93.18.9630. [DOI] [PMC free article] [PubMed] [Google Scholar]