Abstract
Purpose
To report the characteristics of a case series of ocular inflammatory events following COVID-19 vaccination in Japan.
Study design
Retrospective multicenter study
Methods
In this retrospective multicenter survey, a questionnaire was sent to 16 Japanese hospitals that had uveitis specialty clinics. Information on patients who developed ocular inflammatory events within 14 days of COVID-19 vaccination between February 2021 and December 2021 was collected.
Results
Thirty-seven patients were diagnosed with ocular inflammatory events following COVID-19 vaccination. The mean age was 53.4 ± 16.4 years (range, 26-86 years), and the mean time to onset after vaccination was 6.3 ± 4.2 days (range, 1-14 days). Vogt-Koyanagi-Harada disease (VKH) was the most common event (n = 17 patients, 46%), followed by anterior uveitis (n = 6), infectious uveitis (n = 3), acute zonal occult outer retinopathy (AZOOR) (n = 2), sarcoidosis-associated uveitis (n = 1), acute posterior multifocal placoid pigment epitheliopathy (APMPPE) (n = 1), optic neuritis (n = 1), multiple evanescent white dot syndrome (MEWDS) (n = 1), Posner-Schlossman syndrome (n = 1), and unclassified uveitis (n = 4). Twenty-eight cases occurred after BNT162b2 vaccination (Pfizer-BioNTech) and 8 after mRNA-1273 vaccination (Moderna), whilst 1 patient had no information about vaccine type.
Conclusions
COVID-19 vaccination can be related to various types of ocular inflammatory events. When we encounter patients with ocular inflammatory disease, we should consider that it may be an adverse effect of COVID-19 vaccination.
Keywords: COVID-19 vaccination, Vaccine-associated ocular inflammation, Multicenter survey
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a worldwide pandemic, involving more than 500 million infections and 6 million deaths. Vaccines against SARS-CoV-2 were rapidly developed, and currently 4 types are available: messenger ribonucleic acid-type vaccines (Pfizer-BioNTech [1] and Moderna [2]), viral vector-type vaccines (Janssen Johnson & Johnson [3] and Oxford-AstraZeneca [4]), inactivated-type vaccines (Sinovac [5] and Sinopharm [6]), and a protein subunit-type vaccine (Novavax [7]). The common systemic and local adverse events reported after vaccination are fever, headache, fatigue, myalgia, arthralgia, nausea, vomiting, chills, pain, erythema, swelling, and lymphadenopathy [1]. Ocular adverse events after vaccination have also been reported, including facial nerve palsy, central venous sinus thrombosis, acute anterior uveitis, acute macular neuroretinopathy, corneal graft rejection, anterior scleritis, and panuveitis [8]. We herein report the results of a large number of cases obtained in a multicenter survey in Japan and describe 2 representative cases of ocular inflammatory disease after mRNA COVID-19 vaccination. The purpose of this study was to identify uveitis diseases occurring after COVID-19 vaccination and to inform patients and health care providers.
Methods
Sixteen Japanese institutions that provide uveitis consultations participated in this retrospective survey. The ethics review committee of Kyushu University and of each participating facility approved the study protocol, and the study was conducted in accordance with the tenets of the Declaration of Helsinki. Patients diagnosed with ocular inflammatory events within 14 days of COVID-19 vaccination between February 2021 and December 2021 were included, including both new-onset and relapse cases, and both first- and second-vaccination cases. The time period of within 14 days (2 weeks) after vaccination was established on the basis of data from a study of uveitis associated with vaccines other than the COVID-19 vaccine, in which the mean time from vaccination to disease onset was 16 days [9]. A questionnaire was sent to each institution, and clinicians filled out the following information from the patient’s medical record: age, sex, type of vaccine, number of vaccination (first or second), day of onset, type of ocular inflammatory event, visual acuity and intraocular pressure at onset day, ocular findings, results of clinical examination, and clinical course. The cases that had definite onset and vaccination dates and that occurred within 14 days of vaccination were analyzed.
Results
Sixteen institutions responded to the survey, providing data on a total of 46 cases. We selected the 37 cases that had exact dates of vaccination and disease onset that occurred within 14 days of vaccination. The study population, type of vaccine, and time after vaccination are described in Table 1 according to the number of vaccinations. The mean age was 53.4 ± 16.4 years (range 26-86 years); 15 patients (41%) were male. Fifteen cases occurred after the first vaccination, and 22 cases, after the second vaccination. Twenty-eight patients had been administered the BNT162b2 vaccine (Pfizer-BioNTech), and 8 patients, the mRNA-1273 vaccine (Moderna), whilst 1 patient had no information about the type of vaccine. The mean time to onset after vaccination was 6.3 ± 4.2 days (range 1-14 days), and 29 of the cases were new cases, and 8, recurrent cases. The details of the ocular inflammatory events are shown in Fig. 1. Vogt-Koyanagi-Harada disease (VKH), including 2 cases of VKH-like uveitis, was the most common, occurring in 17 cases (46%, 95% CI = 31%-62%), followed by anterior uveitis in 6 cases (16%, 95% CI = 7%-32%), infectious uveitis in 3 cases (8%, 95% CI = 2%-22%), sarcoidosis-associated uveitis in 1 case (3%, 95% CI = -0.75%-15%), other events in 6 cases (16%, 95% CI = 7%-32%), and unclassified uveitis in 4 cases (11%, 95% CI = 4%-25%). Infectious cases consisted of 2 cases of acute retinal necrosis and 1 case of tuberculous uveitis, and the other ocular inflammatory events included 2 cases of acute zonal occult outer retinopathy (AZOOR), 1 case of acute posterior multifocal placoid pigment epitheliopathy (APMPPE), 1 case of optic neuritis, 1 case of multiple evanescent white dot syndrome (MEWDS), and 1 case of Posner-Schlossman syndrome. In terms of vaccine type, VKH was the most common event among those vaccinated with either the BNT162b2 vaccine or the mRNA-1273 vaccine (12 cases with the BNT162b2 vaccine and 4 cases with the mRNA-1273 vaccine). Two representative cases are shown below.
Table 1.
Description of the study population according to the number of vaccinations
| Total | After first vaccination | After second vaccination | ||
|---|---|---|---|---|
| Background | No. of patients | 37 | 15 | 22 |
| Age, y | 53.4±16.4 (26–86) | |||
| Sex, male | 15 | 3 | 12 | |
| Sex, female | 22 | 12 | 10 | |
| Vaccine | BNT162b2 | 28 | 11 | 17 |
| mRNA-1273 | 8 | 3 | 5 | |
| Unknown | 1 | 1 | 0 | |
| Other | Time after vaccination (days) | 6.3±4.2 (1–14) | 6.5±3.9 (1–13) | 6.9±4.3 (1–14) |
| New-onset | 29 | 9 | 20 | |
| Relapse | 8 | 6 | 2 |
Fig. 1.
Distribution of ocular inflammatory diseases of all patients (left column), BNT162b2-vaccinated patients (middle column), and mRNA-1273-vaccinated patients (right column)
Case reports
Case 1
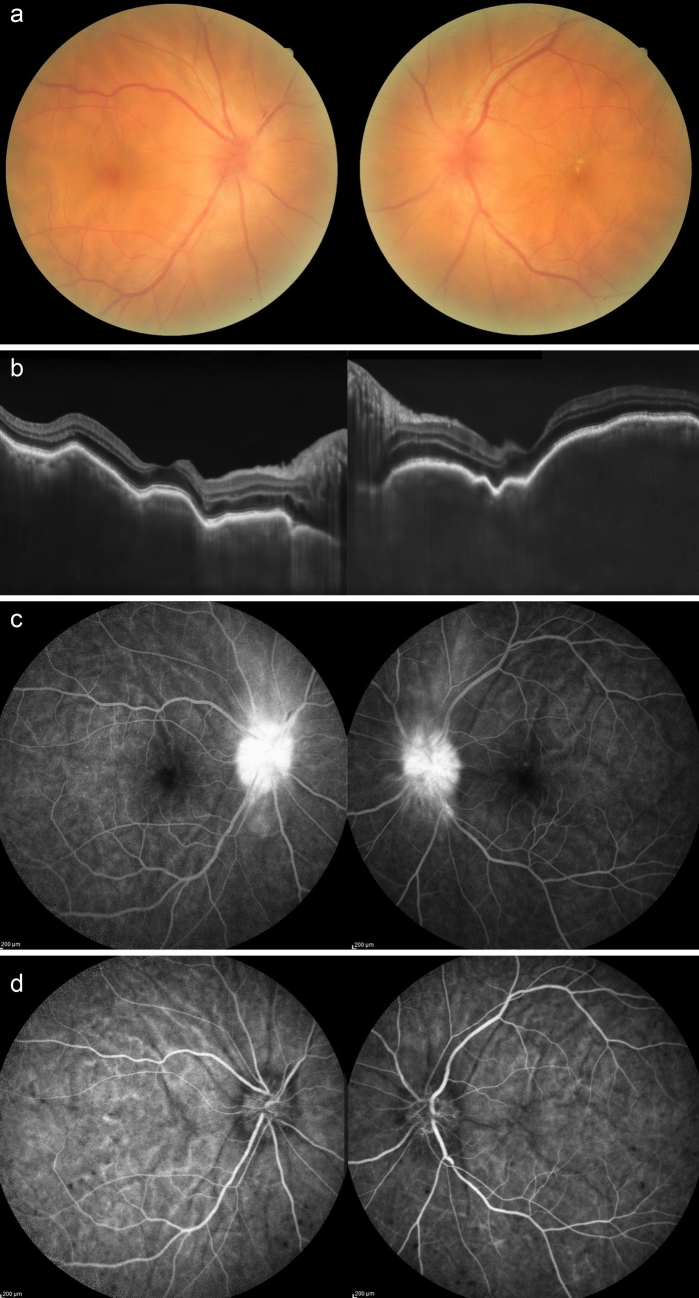
The patient was a 78-year-old woman who had no history of ocular diseases and had a history of diabetes mellitus and heart failure. She became aware of vision loss in both eyes 9 days after receiving her first BNT162b2 vaccine and came to Kyushu University Hospital 7 weeks after vaccination. She was also aware of headaches and tinnitus. Examination revealed fine keratic precipitates and an anterior chamber cell grade of 1+ in both eyes. Fundoscopy revealed optic disc swelling and retinal folds, both of which are findings of panuveitis (Fig. 2a). Optical coherence tomography revealed remarkable retinal-choroidal folding, serous retinal detachment, and choroidal thickening (Fig. 2b). In fundus fluorescein and indocyanine green angiography, we could see multifocal areas of pinpoint fluorescein dye leakage and vigorous optic disc hyperfluorescence of both eyes (Fig. 2c, d). In addition to these clinical findings, we diagnosed VKH because the patient had cerebrospinal fluid pleocytosis and findings positive for HLA-DR4. The patient was treated with intravenous methylprednisolone of 1 g/day for 3 days, intravenous dexamethasone of 8 mg/day for 3 days, and of 6 mg/day for 4 days, followed by oral prednisolone at an initial dosage of 40 mg/day. The oral prednisolone was gradually tapered after the initial therapy. The choroidal thickness gradually reversed to normal, and the choroidal fold and serous retinal detachment disappeared in 3 weeks.
Fig. 2.
a Remarkable optic disc swelling was seen in both eyes in the fundus photograph. b Optical coherence tomography revealed retinal-choroidal folding, serous retinal detachment, and choroidal thickening in both eyes. c Fundus fluorescein angiography showed high fluorescein in the optic disc in both eyes. d Indocyanine green angiography showed dark spots in the choroid
Case 2
The patient was a 71-year-old man who had no history of ocular diseases. He complained of vision loss in both eyes 13 days after receiving his second BNT162b2 vaccine and went for a check-up at a nearby hospital about 5 weeks after vaccination, where he was diagnosed with suspected optic neuropathy. He was referred to Kyushu University Hospital about 7 weeks after vaccination. Examination revealed fine keratic precipitates (partially resembling mutton-fat precipitates) and an anterior chamber cell grade of 1+ in both eyes. Optic disc swelling and retinal folds were seen on funduscopy (Fig. 3a). Optical coherence tomography showed remarkable retinal-choroidal folding and choroidal thickening, but serous retinal detachment was not seen (Fig. 3b). Fundus fluorescein and indocyanine green angiography showed multifocal areas of pinpoint fluorescein leakage, vigorous optic disc high fluorescence, and dark spots in the choroid in both eyes (Fig. 3c, d). Because he also had cerebrospinal fluid pleocytosis and was HLA-DR4 positive, we diagnosed VKH and prescribed the same steroid therapy as in case 1. The inflammatory findings were improved at 10 days after the treatment.
Fig. 3.
a The fundus photographs showed marked swelling and reddishness of the optic disc of both eyes. b Optical coherence tomography revealed retinal-choroidal folding and choroidal thickening in both eyes. c Fundus fluorescein angiography showed vigorous optic disc high fluorescence in both eyes. d Indocyanine green angiography showed dark spots in the choroid
Discussion
Many virus vaccines have been developed and put into practical use, preventing the devastating effects of numerous infectious diseases. On the other hand, adverse reactions to vaccines can sometimes be fatal and should always be taken into consideration. Several vaccines have also been developed for COVID-19, which has been pandemic since 2019. The efficacy of these vaccines has been proven in clinical trials, and vaccination is well underway; however, as the administration in various countries progresses, reports of the appearance of systemic adverse events have emerged, as with other virus vaccines. There have also been reports of ocular diseases, including uveitis, anterior uveitis, macular neuroretinopathy, retinal vascular occlusion, corneal graft rejection, and scleritis [10–12]. Most of these reports are case reports or single-center reports, with only a few reports including a large number of cases at multiple centers [13, 14]. Ours is the first report of a multicenter survey of COVID-19 vaccine-associated uveitis in Japan.
In this study, the most common ocular inflammatory disease after COVID-19 vaccination was VKH. VKH was diagnosed according to the Revised Diagnostic Criteria for VKH [15]. The 2 cases shown here were diagnosed on the basis of the typical ocular findings included in the diagnostic criteria, such as serous retinal detachments, choroidal thickening, and fluorescein angiography findings, as well as the presence of cerebrospinal fluid pleocytosis. Two cases reported in the survey form were also described as having VKH-like disease because of their ocular findings, but these cases did not fully meet the diagnostic criteria. One case had no choroidal thickening and the other had no neurologic findings. It is possible that the pathogenetic mechanism of inflammation in vaccine-induced uveitis differs from that of typical uveitis, resulting in different ocular findings. Other reports to date have shown a higher frequency of anterior uveitis. In their multinational case series, Testi and colleagues reported 70 patients with ocular inflammatory events after COVID-19 vaccination [13]. The patients they analyzed experienced the inflammatory events within 14 days of vaccination, as did the patients of our survey. Anterior uveitis was the most common event (n = 41, 58.6%) followed by posterior uveitis (n = 9, 12.9%) and scleritis (n = 7, 10.0%). Bolletta and colleagues reported on 42 eyes of 34 patients with uveitis and other ocular inflammatory complications after COVID-19 vaccination in Italy [14]. Their patients had various types of diseases, such as anterior uveitis (n = 5, 11.9%), retinal vein occlusion (n = 5, 11.9%), herpetic keratitis (n = 3, 7.1%), multiple evanescent white dot syndrome (MEWDS) (n = 3, 7.1%), toxoplasma retinochoroiditis (n = 3, 7.1%), VKH reactivation (n = 2, 4.8%), and anterior scleritis (n = 2, 4.8%). Chen and colleagues reported a case series in Asia in which 10 eyes of 7 patients exhibited ocular complications after vaccination [16]. Unlike in the above-mentioned reports by Testi and colleagues and Bolletta and colleagues, most of the cases in the study by Chen and colleagues were diagnosed with VKH (n = 3, 30.0%), as in our study. A recent epidemiologic survey of uveitis in Japan revealed that sarcoidosis was the most common (10.6%), followed by VKH (8.1%) [17]. The fact that the frequency of VKH (n = 17, 46%) was higher than that of sarcoidosis (n = 1, 3%) in the present survey suggests that the present group of cases was not collected by chance, but a collection of cases due to vaccination and that the pathogenesis of the disease may be similar to that of VKH.
In Japan, the BNT162b2 vaccine (Pfizer-BioNTech), mRNA-1273 vaccine (Moderna), and ChAdOx1-S vaccine (Oxford-AstraZeneca) are currently available. Most people are vaccinated with the BNT162b2 or mRNA-1273 vaccine, which means the majority of vaccinations are of the mRNA-type, and only these 2 vaccines were found to cause adverse reactions of uveitis in this survey. Although the number of cases of uveitis caused by the BNT162b2 vaccine in Japan is high, this may be due to the high rate of vaccination with the BNT162b2 vaccine in Japan, and the difference in the frequency of cases caused by different vaccines is unknown.
Several possible mechanisms of adverse vaccine reactions have been discussed, including reactions to the adjuvants that are added to vaccines to enhance their efficacy. Adjuvants are thought to induce autoimmune reactions in predisposed or genetically susceptible individuals [18]. Other possibilities include molecular similarity of the vaccine peptide to the uveitic peptide [19], but the precise mechanism of uveitis development following COVID-19 vaccination remains unknown.
The main limitation of this study is that it was a retrospective questionnaire survey, making it difficult to prove a definite cause-and-effect relationship between vaccination and the development of uveitis. This study does not provide evidence that vaccination was associated with the onset of ocular inflammatory events. However, because the vaccination rate in Japan is already over 80%, it would be difficult to conduct a prospective study with a nonvaccinated control group. In addition, in the present study, cases in which symptoms developed within 14 days of vaccination were included as vaccine-related uveitis, but there may also have been cases in which vaccination-related symptoms developed more than 14 days after vaccination, making it difficult to determine the exact incidence of the disease. Finally, because the main objective of this study was to determine the frequency of vaccine-related cases and to provide patients and health care providers with information on the symptoms that may occur in patients with postvaccination uveitis, we did not analyze whether the frequency of onset differs depending on the presence or absence of underlying disease or whether there is a difference in response to treatment or prognosis compared with nonvaccine-related uveitis. Further detailed investigations on these issues are needed.
In this survey, we reported on the actual onset and characteristics of ocular inflammatory events following vaccination in a multicenter study. Although vaccination is the most effective means of avoiding severe symptoms caused by COVID-19, it should be kept in mind that vaccination can sometimes cause ocular side effects, including ocular inflammatory events.
Acknowledgements
We thank Dr Koju Kamoi of Tokyo Medical and Dental University for cooperation in collecting case data. We thank the institutions that participated in this survey: Hokkaido University, Yamagata University, National Defense Medical College, Kyorin University, Tokyo Medical and Dental University, Tokyo Medical University, Jichi Medical University Saitama Medical Center, Kyoto Prefectural University of Medicine, Japan Community Health Care Organization Osaka Hospital, Osaka University, Kindai University, Kobe University, Hyogo College of Medicine, Saneikai Tsukazaki Hospital, Yamaguchi University, and Kyushu University.
Conflicts of interest
Y. Yasaka, None; E. Hasegawa, None; H. Keino, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Novartis, Nikon, Kowa, ZEISS, Mitsubishi Tanabe, Santen, Bayer, Senju, AbbVie); Y. Usui, None; K. Maruyama, None; Y. Yamamoto, None; T. Kaburaki, Grants or contracts (AbbVie, Santen, Alcon, Senju, HOYA, Novartis), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Novartis, Eisai, AbbVie, Mitsubishi Tanabe, Kowa, Wakamoto, Senju, Ono); D. Iwata, None; M. Takeuchi, None; S. Kusuhara, None; H. Takase, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Novartis, AbbVie, Senju, Eisai, Otsuka), Patents planned, issued or pending (Japanese Patent Application No. 2019-107483); K. Nagata, Grants or contracts (AMO), Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Novartis, Senju, Kowa, AbbVie, Santen); R. Yanai, None; Y. Kaneko, None; C. Iwahashi, None; A. Fukushima, Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events (Santen, Senju, Johnson & Johnson, Novartis, Rohto Nitten, Mitsubishi Tanabe); N. Ohguro, None; K. Sonoda, None.
Footnotes
Corresponding author: Eiichi Hasegawa
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoff J, Gray G, Vandebosch A, Cárdenas V, Shukarev G, Grinsztejn B, et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against Covid-19. N Engl J Med. 2021;384:2187–201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao Q, Bao L, Mao H, Wang L, Xu K, Yang M, et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science. 2020;369:77–81. doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath PT, Galiza EP, Baxter DN, Boffito M, Browne D, Burns F, et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng XL, Betzler BK, Ng S, Chee SP, Rajamani L, Singhal A, et al. The eye of the storm: COVID-19 vaccination and the eye. Ophthalmol Ther. 2022;11:81–100. doi: 10.1007/s40123-021-00415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benage M, Fraunfelder FW. Vaccine-associated uveitis. Mo Med. 2016;113:48–52. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MTM, Niederer RL, McGhee CNJ, Danesh-Meyer HV. COVID-19 vaccination and the eye. Am J Ophthalmol. 2022;240:79–98. doi: 10.1016/j.ajo.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng XL, Betzler BK, Testi I, Ho SL, Tien M, Ngo WK, et al. Ocular adverse events after COVID-19 vaccination. Ocul Immunol Inflamm. 2021;29:1216–1224. doi: 10.1080/09273948.2021.1976221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haseeb AA, Solyman O, Abushanab MM, Abo Obaia AS, Elhusseiny AM. Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines (Basel) 2022;10:342. doi: 10.3390/vaccines10020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Testi I, Brandão-de-Resende C, Agrawal R, Pavesio C. Ocular inflammatory events following COVID-19 vaccination: a multinational case series. J Ophthalmic Inflamm Infect. 2022;12:4. doi: 10.1186/s12348-021-00275-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolletta E, Iannetta D, Mastrofilippo V, De Simone L, Gozzi F, Croci S, et al. Uveitis and other ocular complications following COVID-19 vaccination. J Clin Med. 2021;10:5960. doi: 10.3390/jcm10245960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease: report of an international committee on nomenclature. Am J Ophthalmol. 2001;131:647–652. doi: 10.1016/S0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Li X, Li H, Li M, Gong S. Ocular adverse events after inactivated COVID-19 vaccination in Xiamen. Vaccines (Basel) 2022;10:482. doi: 10.3390/vaccines10030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonoda KH, Hasegawa E, Namba K, Okada AA, Ohguro N, Goto H, et al. Epidemiology of uveitis in Japan: a 2016 retrospective nationwide survey. Jpn J Ophthalmol. 2021;65:184–190. doi: 10.1007/s10384-020-00809-1. [DOI] [PubMed] [Google Scholar]
- 18.Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9:435. doi: 10.3390/vaccines9050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham ET, Moorthy RS. Vaccine-associated posterior uveitis. Retina. 2020;40:595–598. doi: 10.1097/IAE.0000000000002816. [DOI] [PubMed] [Google Scholar]