Abstract
The ability of Rhodococcus equi to induce pneumonia in foals depends on the presence of an 85- to 90-kb plasmid. In this study, we evaluated whether plasmid-encoded products mediate virulence by modulating the cytokine response of foals. Foals infected intrabronchially with a virulence plasmid-containing strain of R. equi had similar gamma interferon (IFN-γ) and interleukin-12 (IL-12) p35 but significantly higher IL-1β, IL-10, IL-12 p40, and tumor necrosis factor alpha (TNF-α) mRNA expression in lung tissue compared to foals infected with the plasmid-cured derivative. IFN-γ mRNA expression levels in CD4+ T lymphocytes isolated from bronchial lymph nodes (BLN) were similar for the two groups of R. equi-infected foals on day 3 postinfection. However, on day 14, in association with pneumonia and marked multiplication of virulent R. equi but with complete clearance of the plasmid-cured derivative, IFN-γ mRNA expression in BLN CD4+ T lymphocytes was significantly (P < 0.001) higher in foals infected with the plasmid-cured derivative. These results suggests an immunomodulating role for R. equi virulence plasmid-encoded products in downregulating IFN-γ mRNA expression by CD4+ T lymphocytes.
Rhodococcus equi, a gram-positive facultative intracellular pathogen of macrophages, is one of the most important causes of disease in foals between 1 and 5 months of age. R. equi has also emerged as a significant opportunistic pathogen in immunosuppressed people, especially those infected with the human immunodeficiency virus (1, 6, 10). Infection in either species is most commonly characterized by a life-threatening pyogranulomatous pneumonia. R. equi is widespread in the environment of horse breeding farms. Unlike most environmental R. equi, isolates from pneumonic foals typically contain an 85- to 90-kb plasmid encoding a highly immunogenic, lipid-modified virulence-associated protein (VapA) (30, 32–34). Plasmid-cured derivatives of virulent R. equi strains lose the ability to replicate and survive in macrophages and fail to induce pneumonia in foals, confirming the absolute necessity of the large plasmid for the virulence of R. equi (7, 36).
Study of the pathogenesis of R. equi infections has been complicated by the fact that typical granulomatous lung lesions have not been reproduced by R. equi infection in any immunocompetent species other than young horses. The normal murine lung can progressively clear an inoculum of R. equi sufficient to induce severe pneumonia in foals, suggesting that the results of studies on the pathogenesis of this infection in mice may not necessarily be extrapolated to foals. In mice, pulmonary clearance of virulent (i.e., containing the VapA-encoding large plasmid) R. equi requires functional T lymphocytes (3, 37). Although both CD4+ and CD8+ T cells contribute to host defense against R. equi in mice, CD4+ T lymphocytes play the major role and are absolutely required for complete pulmonary clearance (13, 21, 24). Immunocompetent BALB/c mice experimentally infected with virulent R. equi develop a Th1 cytokine response and progressively clear the infection (14). In contrast, mice in which a Th2 cytokine response was induced by administration of monoclonal antibodies (MAbs) against gamma interferon (IFN-γ) fail to clear the infection and develop pulmonary granulomas (14). More recently, adoptive transfer of R. equi-specific Th1 or Th2 cell lines to R. equi-susceptible nude mice has clearly shown that a Th1 response is sufficient to effect pulmonary clearance whereas a Th2 response is detrimental (15).
The Th1/Th2 paradigm defined for murine Th cell clones has provided a useful framework for understanding immune response in infectious diseases, but it remains to be established whether this paradigm can be applied to the horse. The reasons for the peculiar susceptibility of foals to R. equi infections are unknown. Study of the equine immune response to infectious agents including R. equi has been limited by the lack of species-specific reagents available for measuring cytokines. However, the recent development of sensitive and reproducible reverse transcription (RT)-PCR assays has made quantitation of equine cytokine mRNA expression possible (9, 29).
Analogy to human immunodeficiency virus-related R. equi pneumonia in humans suggests either that foals are immunocompromised in some way or that infection with virulent R. equi alters immune response in foals, or both. As the basis for the present study, we hypothesized that plasmid-encoded products mediate the virulence of R. equi by modulating the cytokine response of infected foals. To address this hypothesis, we compared interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p35, IL-12 p40, IFN-γ, and tumor necrosis factor alpha (TNF-α) mRNA expression in the lungs and bronchial lymph node (BLN) CD4+ T lymphocytes of foals infected with a virulent plasmid-containing strain of R. equi to that of foals infected with a plasmid cured derivative of the same strain.
MATERIALS AND METHODS
Bacteria.
The virulent R. equi strain 103+, originally isolated from a pneumonic foal, which contains an 85-kb plasmid and produces VapA, and its avirulent plasmid-cured VapA-negative derivative (strain 103−) were used (5). Aliquots of the two strains were stored at −70°C. Prior to use for infection of foals, the aliquots were grown on Trypticase soy agar plates for 48 h at 37°C. Bacteria were harvested with 4 ml of sterile phosphate-buffered saline (PBS) per plate, the optical density of the resulting suspension was read at 540 nm, and the bacterial concentration was estimated from a standard curve. The bacterial suspension was diluted with sterile PBS to a final concentration of 5 × 107 bacteria/ml. The concentration of the inoculum actually derived was determined retrospectively by counting CFU.
Infection of foals.
Twenty-two healthy mixed-breed pony foals were used in this study, in conjunction with another study on the role of the 85-kb plasmid and VapA in virulence of R. equi (7). Adequate passive transfer of immunoglobulin was confirmed in foals 12 to 24 h after birth, using an enzyme-linked immunosorbent assay (ELISA) kit for semiquantitative measurement of total immunoglobulin G (IgG) (Cite test; Idexx Laboratories, Westbrook, Maine). Foals were reared with their mothers on pasture and were monitored weekly for seroconversion to R. equi by using an ELISA as previously described (23). At 18 to 23 days of age, foals were moved with their dams to individual box stalls in an isolation facility. Criteria for inclusion in the study were normality in physical examination, lung sounds on auscultation, temperature, radiographs of the lungs, and lack of seroconversion to R. equi by ELISA. Foals meeting these criteria were randomly assigned to three experimental groups and infected 1 or 2 days after arrival in the isolation facility. There were no differences in IgG antibody titers against R. equi between groups at the time of infection.
Foals were sedated with xylazine hydrochloride (Rompun; 0.5 mg/kg; Bayer Inc., Etobicoke, Ontario, Canada) and butorphanol tartrate (Torbugesic; 0.07 mg/kg; Ayerst Laboratories, Montreal, Québec, Canada) intravenously. A flexible fiberoptic endoscope was used to deliver 1.25 × 109 bacteria suspended in 25 ml of sterile PBS into both main bronchi (total dose, 2.5 × 109 bacteria in 50 ml of PBS). Eight foals were infected with 103+ and eight foals were infected with 103−; six foals that received only PBS were used as controls. The day of infection was designated day 0. Half of the foals in each group (103+, 103−, and controls) were euthanized at 3 days postinfection, and half were euthanized at 14 days postinfection by intravenous administration of a lethal dose of pentobarbital sodium. Immediately after euthanasia, BLN were collected aseptically and placed in sterile PBS for subsequent isolation of CD4+ T lymphocytes. Lung samples (approximately 0.5 g) were collected from a preselected site in the left cranioventral lung lobe, rapidly frozen in liquid nitrogen, and stored at −70°C until used for total RNA extraction.
Isolation of CD4+ T lymphocytes.
CD4+ T lymphocytes were isolated from the freshly collected BLN by immunomagnetic separation using a previously characterized mouse IgG anti-horse CD4 MAb (CVS4) (17). This antibody was generously donated by Paul Lunn, University of Wisconsin, and was used as hybridoma supernatant. Briefly, BLN were cut into in 0.5-cm3 pieces, and the cells were separated in glass tissue grinders. Mononuclear cells were separated by density gradient centrifugation using endotoxin-free Ficoll-Paque (specific gravity, 1.077; Pharmacia Biotech, Baie d’Urfée, Québec, Canada) and washed twice with cold PBS. The cells were counted and 4 × 107 cells were incubated at 4°C for 25 min with MAb CVS4. After being washed twice in cold PBS, the cells were incubated at 4°C for 25 min with a rat anti-mouse IgG fluorescein isothiocyanate (FITC)-labeled MAb. The cells were then washed in PBS, resuspended in magnetic cell sorter buffer (0.5% bovine serum albumin and 2 mM EDTA in PBS), and incubated at 4°C for 15 min with anti-FITC microbeads (Miltenyi Biotec, Auburn, Calif.). CD4+ T lymphocytes were obtained by positive selection magnetic cell sorting (VarioMACS; Miltenyi Biotec) as specified by the manufacturer. CD4+ T-lymphocyte purity of all BLN preparations was determined before and after immunomagnetic separation by flow cytometric analysis. CD4+ T lymphocytes were lysed in denaturing solution (4 M guanidinium thiocyanate, 25 mM sodium citrate, 0.5% sodium N-lauroylsarcosine, 0.1 M 2-mercaptoethanol) and kept frozen at −70°C until used for total RNA extraction.
RNA isolation, DNase treatment of RNA samples, and cDNA synthesis.
Total RNA was isolated by a modification of the single-step guanidinium thiocyanate procedure as previously described (4). RNA concentration was measured by optical density at 260 nm (GeneQuant II; Pharmacia Biotech). All RNA samples were treated with amplification-grade DNase I (Gibco BRL, Burlington, Ontario, Canada) to remove any traces of genomic DNA contamination. Briefly, 1 U of DNase I and 1 μl of 10× DNase I reaction buffer were mixed with 1.5 μg of total RNA to yield a 10-μl reaction mixture. The mixture was incubated for 10 min at room temperature and then inactivated by addition 1 μl of 25 mM EDTA and heating at 65°C for 10 min.
cDNA was synthesized with a first-strand cDNA synthesis kit (Clontech, Palo Alto, Calif.) as specified by the manufacturer. Briefly, 1.5 μg of total RNA was mixed with 1 μl of oligo(dT)18 primer (20 μM) and heated at 70°C for 2 min. After cooling to room temperature, the following reagents were added: 4 μl of 5× reaction buffer (250 mM Tris-HCl [pH 8.3], 375 mM KCl, 15 mM MgCl2), 1 μl of deoxynucleoside triphosphates (10 mM each), 0.5 μl of RNase inhibitor (40 U/μl), and 1 μl of Moloney murine leukemia virus reverse transcriptase (200 U/μl). The mixture was incubated at 42°C for 1 h, heated at 94°C for 5 min, diluted to a final volume of 100 μl, and stored at −70°C until used for PCR analysis.
PCR analysis.
PCR primer pairs specific for equine β-actin, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p35, IL-12 p40, IFN-γ, and TNF-α have been previously described (9). cDNA prepared as described above (2 μl) was amplified in a 50-μl PCR in the presence of 50 pmol of each primer, deoxynucleoside triphosphates (0.2 mM each), 5 μl of 10× reaction buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl), 1.5 mM MgCl2, and 2 U of Taq DNA polymerase (AmpliTaq; Perkin-Elmer, Branchburg, N.J.). PCR was performed with an initial denaturation step at 94°C for 2 min and 40 cycles of amplification followed by a 7-min extension at 72°C. Each cycle included denaturation at 94°C for 45 s, annealing at 60°C for 45 s, and extension at 72°C for 2 min. Amplified PCR products were visualized by electrophoresis of 10 μl of the reaction mixture on a 1.6% agarose gel followed by ethidium bromide staining. Samples without cDNA were always included in the amplification reactions to check for contamination. cDNA obtained from concanavalin A-stimulated equine blood mononuclear cells was used as a positive control. RNA samples were also subjected to PCR using the β-actin primers to confirm the absence of genomic DNA contamination. The specificities of the amplified bands were confirmed by visualizing a single band of predicted size based on a molecular weight standard (100-bp DNA ladder; Pharmacia Biotech).
Quantitation of mRNA by competitive PCR.
mRNA expression of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p35, IL-12 p40, IFN-γ, TNF-α, and β-actin was determined quantitatively by competitive PCR as previously described (9). Briefly, equal amounts of cDNA were amplified in the presence of 2 μl of six fourfold serial dilutions of heterologous DNA fragments (mimics). PCR was performed as described above for 35 to 40 cycles. Following gel electrophoresis and ethidium bromide staining, densitometric analysis of the bands corresponding to the target and the mimic was performed with a gel video system (Molecular Analyst; Bio-Rad, Hercules, Calif.). The densitometric analysis results were used to plot a standard curve from which the amount of target cDNA was determined. To account for variation in the amount and quality of starting material, all results were corrected to the mean β-actin value.
Statistical analysis.
The effects of bacterial strains and time postinfection on the cytokine response of foals were analyzed by a two-factorial analysis of variance (ANOVA) (27), and adjusted means (least square [LS] means) were calculated by the general linear model procedure of SAS (SAS System for Windows, version 6.10; SAS Institute, Cary, N.C.). In preliminary analysis, the effect of sex, as assumed, was not significant and was therefore removed from the final model. Data that were not normally distributed, as indicated by the univariate procedure of SAS, were transformed to the natural logarithm. For graphic presentation, LS means were converted back to original units from loge transformed data. Consequently, standard errors of means are not reported. Comparisons between bacterial groups at each time point were made by performing a t test on LS means (least significant difference test) (27). In rare instances when normal distribution of the data was not achieved even after loge transformation, the results of the ANOVA were verified by the Kruskal-Wallis test. Results were considered statistically significant if the value of P was ≤0.05, and trends were reported if the P value was ≤0.10.
RESULTS
Experimentally induced R. equi bronchopneumonia.
The complete clinicopathologic description of the foals used in this study has been reported elsewhere (7). Briefly, all foals infected with R. equi 103+ developed macroscopic lesions ranging from mild to moderate consolidation of the cranioventral lung lobes on day 3 to severe consolidation involving 60 to 70% of the lung area on day 14. The BLN of 103+-infected foals were slightly enlarged on day 3 and markedly enlarged on day 14. The lungs and BLN of 103−-infected foals and PBS controls were macroscopically normal. Histologically, 103+-infected foals developed lesions of suppurative to granulomatous bronchopneumonia, whereas the lesions of 103−-infected foals were limited to mild atelectasis and slight hypercellularity of the interalveolar septae. On day 3 postinfection, the mean number of R. equi (log10 per gram of lung ± standard deviation) in the lungs of foals infected with strain 103+ (3.67 ± 1.35) was significantly higher than in those infected with strain 103− (1.43 ± 0.73). On day 14, the 103+ numbers had increased significantly (9.45 ± 1.0), whereas strain 103− could no longer be cultured. R. equi was not cultured from the control foals (7).
Cytokine mRNA expression in the lungs of foals infected with virulent and avirulent R. equi.
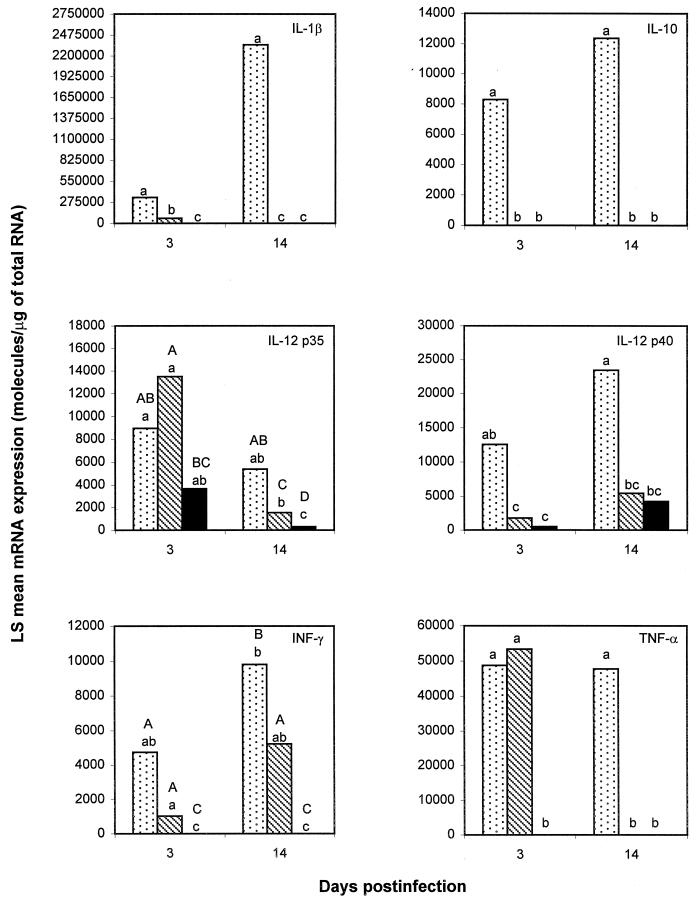
IL-1β, IL-2, IL-10, IL-12 p35, IL-12 p40, IFN-γ, and TNF-α mRNA expression was detected in the lungs of the foals used in this study. On day 3 postinfection, IL-1β mRNA concentrations were significantly greater in 103+-infected foals than in those infected with 103− or the controls (Fig. 1). Foals infected with 103− had significantly greater IL-1β mRNA concentrations than the controls on day 3, but these levels returned to baseline values on day 14 postinfection (Fig. 1). IL-10 mRNA expression was detected only in the lungs of 103+-infected foals (Fig. 1). IL-12 p35 mRNA expression was not statistically different among the three groups on day 3, but there was a trend toward greater IL-12 p35 mRNA expression in 103+-infected foals on day 14 (Fig. 1). IL-12 p40 mRNA expression was significantly higher in 103+-infected foals than in those infected with 103− or the controls on both day 3 and day 14 (Fig. 1). IFN-γ mRNA expression was significantly greater in R. equi-infected foals than in the controls. Although mean lung IFN-γ mRNA concentrations were consistently greater in 103+-infected foals than in those infected with 103−, this difference was not statistically significant on day 3. There was, however, a trend toward higher IFN-γ mRNA expression for 103+-infected foals on day 14 (Fig. 1). Levels of TNF-α mRNA expression were similar in the two groups of R. equi infected foals on day 3, while low expression of this cytokine was detected in only one control; on day 14, TNF-α mRNA expression had not changed in 103+-infected foals, while expression of this cytokine had returned to baseline values in the foals infected with 103− (Fig. 1). Very low IL-2 mRNA expression was detected qualitatively in the lungs of all R. equi-infected foals and four controls (data not shown), but mRNA expression was too low for accurate quantitation by competitive PCR. IL-4, IL-5, and IL-6 mRNA expression was not detected. Statistically significant differences in cytokine mRNA expression in lung tissues between the two groups of R. equi-infected foals are summarized in Table 1.
FIG. 1.
LS mean IL-1β, IL-10, IL-12 p35, IL-12 p40, IFN-γ, and TNF-α mRNA concentrations in the lungs of control foals (solid bars; n = 6) and foals infected intrabronchially with either virulent plasmid-containing R. equi 103+ (dotted bars; n = 8) or its plasmid-cured derivative 103− (striped bars; n = 8). Half of the foals in each group (103+, 103−, and controls) were euthanized at 3 days postinfection, and the remainder were euthanized at 14 days postinfection. mRNA expression was quantitated by RT-competitive PCR. Lowercase letters differing between groups indicate a statistically significant difference in mRNA expression (P ≤ 0.05); capital letters differing between groups indicate a trend toward a statistically significant difference in mRNA expression (P ≤ 0.10). When at least one letter is common between two groups, the difference is not statistically significant.
TABLE 1.
Summary of significant differences (P ≤ 0.05) in cytokine mRNA expression between foals infected with R. equi 103+ and foals infected with R. equi 103−
| Cytokine | Differencea
|
|||
|---|---|---|---|---|
| Lung tissue
|
BLN CD4+ T cells
|
|||
| 3 days p.i. | 14 days p.i. | 3 days p.i. | 14 days p.i. | |
| IL-1β | ↑ | ↑ | ND | ND |
| IL-2 | — | — | — | — |
| IL-4 | ND | ND | ND | — |
| IL-5 | ND | ND | ND | ND |
| IL-6 | ND | ND | ↑ | ↑ |
| IL-10 | ↑ | ↑ | — | — |
| IL-12 p35 | — | — | ↑ | ↑ |
| IL-12 p40 | ↑ | ↑ | ND | ND |
| IFN-γ | — | — | — | ↓ |
| TNF-α | — | ↑ | ND | ND |
mRNA expression was measured in lung tissue and BLN CD4+ T lymphocytes by RT-competitive PCR on days 3 and 14 postinfection (p.i.). Results are expressed as significantly higher (↑) or lower (↓) mRNA expression in 103+-infected foals compared to that in 103−-infected foals, no cytokine mRNA expression detected (ND), or mRNA expression in 103+-infected foals not statistically different from that in 103−-infected foals (—).
Isolation of CD4+ T lymphocytes from BLN of foals infected with virulent and avirulent R. equi.
Because differences in cytokine mRNA expression in lung tissue may have resulted from different proportions of some cell populations between groups, and because we were interested in investigating possible Th1/Th2 cytokine responses of foals infected with virulent and avirulent R. equi, CD4+ T lymphocytes were isolated from BLN by magnetic cell sorting. Percentage of CD4+ T cells as assessed by flow cytometry ranged from 88.5 to 99.1%, with a mean ± standard deviation of 95.1% ± 4.2%. There were no statistically significant differences in the percentage of CD4+ T lymphocytes between groups of foals.
Cytokine mRNA expression in CD4+ T lymphocytes isolated from the BLN of foals infected with virulent and avirulent R. equi.
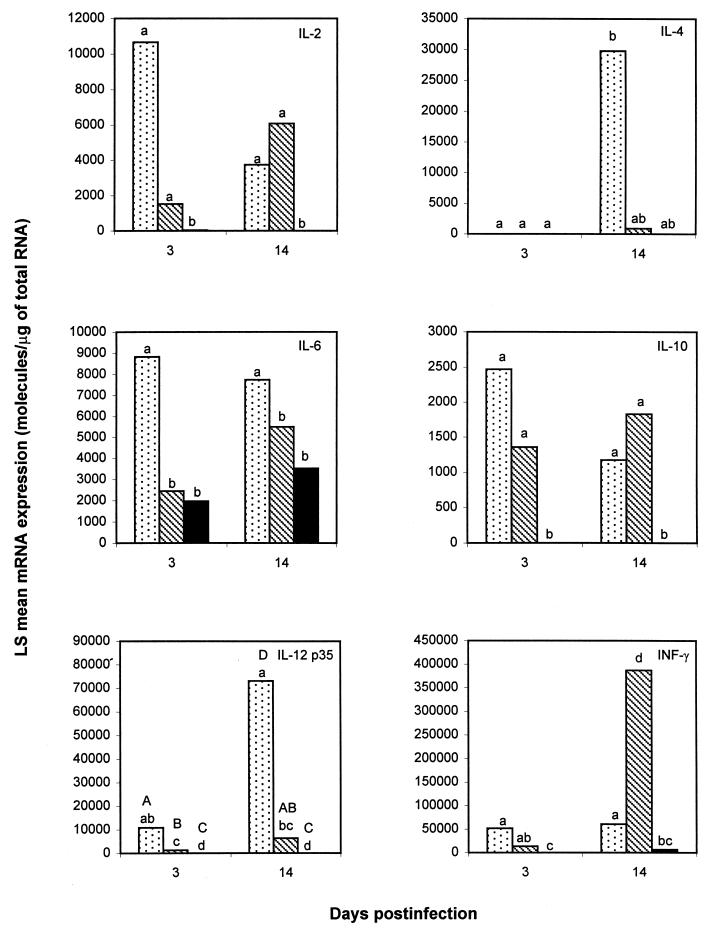
IL-12 p35 mRNA concentrations were significantly higher in 103+-infected foals than in those infected with 103−, but IL-12 p35 was not detected in the controls (Fig. 2). On day 3, IFN-γ mRNA expression was not significantly different between the two groups of R. equi-infected foals. However, on day 14 (when 103+-infected foals had severe pyogranulomatous bronchopneumonia and very large numbers of R. equi in their lungs, and 103−-infected foals were free of macroscopic lung lesions and had completely cleared R. equi from their lungs), mean IFN-γ mRNA concentrations were significantly (P < 0.001) greater in 103−-infected foals. Despite a 600,000-fold increase in bacterial numbers between day 3 and day 14 in 103+-infected foals, the amount of IFN-γ mRNA expression did not change in this group (Fig. 2). IL-2 and IL-10 mRNA expression was significantly higher in R. equi-infected foals than in the controls, but there were no statistically significant differences in mRNA expression of these cytokines between 103+- and 103−-infected foals (Fig. 2). IL-4 mRNA expression was not detected on day 3. On day 14, IL-4 mRNA expression was detected from all four foals infected with 103+, from two of four foals infected with 103−, and from one of three controls, but mean IL-4 mRNA expression was not statistically different between groups. IL-6 mRNA concentrations were significantly higher in 103+-infected foals than in the other two groups, but IL-6 mRNA expression in foals infected with 103− was not significantly different from that of the control group (Fig. 2). Statistically significant differences in cytokine mRNA expression in BLN CD4+ T lymphocytes between the two groups of R. equi infected foals are summarized in Table 1.
FIG. 2.
LS mean IL-2, IL-4, IL-6, IL-10, IL-12 p35, and IFN-γ mRNA concentrations in BLN CD4+ T lymphocytes of control foals (solid bars; n = 6) and foals infected intrabronchially with either virulent plasmid-containing R. equi 103+ (dotted bars; n = 8) or its plasmid-cured derivative 103− (striped bars; n = 8). For details, see the legend to Fig. 1.
DISCUSSION
Both a virulent plasmid-containing strain of R. equi and its avirulent plasmid-cured derivative induced in vivo expression of mRNA for several inflammatory and regulatory cytokines in the natural host, the foal. This study demonstrated drastic differences in cytokine induction between virulent and avirulent R. equi, supporting the hypothesis that plasmid-encoded products play an immunomodulatory role by modifying cytokine response.
The virulent, plasmid-containing strain of R. equi induced significantly more IL-1β, IL-10, and IL-12 p40 mRNA expression in lung tissues than did the plasmid-cured derivative (Fig. 1). Levels of TNF-α mRNA expression were similar in the two groups of R. equi-infected foals on day 3 postinfection. However, on day 14, when the foals infected with avirulent R. equi had completely cleared the infection and were free of visible lung lesions, their TNF-α mRNA expression had returned to baseline values (Fig. 1). These results demonstrate that prolonged induction of inflammatory cytokine in R. equi-infected foals requires establishment of infection which, as previously shown, depends on the presence of the 85-kb plasmid (7, 36). As opposed to foals where the 85-kb plasmid is absolutely required for virulence, opportunistic infections in immunosuppressed people do not always result from R. equi strains containing the large plasmid and expressing VapA (31). The present results in foals differ from those of a recent study of mice in which intravenous inoculation with another virulent plasmid-containing strain of R. equi resulted in IFN-γ and TNF-α production in the spleen, liver, and lungs, whereas only minimal concentrations of these cytokines were detected in mice infected with the plasmid-cured derivative (16). In the same study, IL-4 and IL-10 were not detected in mice infected with either strain of R. equi (16). These differences likely reflect species differences but may also reflect the greater sensitivity of RT-PCR as used here than of ELISA performed on the murine tissue homogenates.
Differences in cytokine mRNA expression in lungs of R. equi 103+- and 103−-infected foals may have resulted in part from different proportions of some cell populations. Because we were interested in investigating the possible relevance and existence of Th1/Th2 cytokine patterns in foals infected with virulent and avirulent strains of R. equi, CD4+ T cells were isolated from BLN by magnetic cell sorting. Preliminary attempts to isolate CD4+ T cells via negative selection by depletion of CD8+ T lymphocytes were unsuccessful, likely because of the high proportion of CD4− CD8− T lymphocytes in the lungs of young horses (2). Positive immunomagnetic separation by using a MAb to equine CD4 resulted in a mean percentage of CD4+ T lymphocytes of 95%. Although positive immunomagnetic selection using a MAb to CD4 may, by itself, induce cytokine production (26), this effect, if present, was likely minimal in the present study, as assessed by the very low induction of most cytokines in cells from control foals (Fig. 2). Others have also shown that positive immunomagnetic separation of CD4+ T lymphocytes results in preservation of various cell functions, including cytokine generation (12, 18).
IL-6 and IL-12 p35 mRNA expression was significantly greater in CD4+ T lymphocytes of foals infected with virulent plasmid-containing R. equi (Fig. 2). Although statistically significant, differences in IL-12 p35 mRNA expression between the two groups of R. equi-infected foals are unlikely to have biological significance because IL-12 p40 was not detected and IL-12 is biologically active only when secreted as a heterodimer. In mice and humans, a wide variety of cells (including T cells) have the ability to produce IL-12 p35 (35). However, only macrophages, neutrophils, and B lymphocytes can also produce the p40 subunit and therefore secrete biologically active IL-12 (35). The significantly higher IFN-γ mRNA expression in 103−-infected foals than in those infected with 103+ on day 14 postinfection suggests a role for plasmid-encoded products in downregulating IFN-γ mRNA expression by CD4+ T lymphocytes in association with progression of infection. The downregulation of IFN-γ mRNA expression observed on day 14 in BLN CD4+ T lymphocytes was not, however, reflected in lung tissues, where there was a trend toward higher IFN-γ mRNA in 103+-infected foals (Fig. 1). Virulence plasmid-associated downregulation of IFN-γ induction has also been found in mice infected with Yersinia pestis, another facultative intracellular pathogen with a large virulence plasmid (20). In mice, IFN-γ-producing CD4+ T lymphocytes are essential for the clearance of virulent R. equi (15). The present results therefore support the hypothesis that plasmid-associated downregulation of IFN-γ mRNA expression by CD4+ T lymphocytes in vivo in foals plays an important role in the pathogenesis of R. equi-induced disease.
It is unclear whether the reduced IFN-γ induction by CD4+ T cells associated with progression of infection with virulent R. equi was associated with an enhanced Th2-like response. Although mean BLN CD4+ T-cell IL-4 mRNA concentrations were more than 30 times higher in 103+-infected foals than in those infected with 103− on day 14, this difference was not statistically significant, given the variability in IL-4 mRNA expression and the small number of foals sampled. If the differences between groups and variance observed in this study are adequate estimates of the population, detection of significant differences among 103+- and 103−-infected foals in IL-4 mRNA expression with an 80% probability at a P ≤ 0.05 level would have required a sample size of 16 foals per group.
Suppression of IFN-γ induction has also been reported for mycobacterial infections. Compared with healthy human tuberculin reactors, Mycobacterium tuberculosis-stimulated blood mononuclear cells from clinical tuberculosis patients had diminished mRNA expression and production of the Th1 cytokines IL-2 and IFN-γ (38). Similarly, in cattle, progression of Mycobacterium paratuberculosis infections to clinical stages is associated with reduced IFN-γ mRNA expression (28). As is the case in the present study, these two reports failed to demonstrate that the reduced IFN-γ response was associated with increased IL-4 response. Th2-independent mechanisms that could suppress a Th1 response may include dysregulation of costimulatory molecules, anergized T lymphocytes, or enhanced production of immunosuppressive monokines such as transforming growth factor β (38). In mice, IL-10 can downregulate the progression of Th cells toward the Th1 cytokine profile. Although IL-10 inhibited production of several Th1 and inflammatory cytokines, its effect was particularly marked on IFN-γ secretion (22). It may therefore be relevant that IL-10 gene expression was detected in the lungs of all the foals infected with virulent R. equi but not from any of the lungs of foals infected with the plasmid-cured derivative. Whether or not IL-10 production in lung tissue prevented the increase in IFN-γ mRNA by CD4+ T cells in 103+-infected foals remains to be determined, but in those cells, there was no difference in IL-10 production between the two groups of R. equi-infected foals.
Optimal adherence of R. equi to macrophages requires complement and is mediated almost exclusively by Mac-1, a complement receptor type 3 (11). Signaling through complement receptor type 3 has recently been shown to reduce macrophage production of IL-12, thereby suppressing IFN-γ production and Th1-driven cell-mediated immunity (19). In a recent study, however, infection of mouse macrophages with R. equi 103+ or 103− failed to induce significant differences in IL-1β, IL-6, IL-10, IL-12, and TNF-α mRNA expression or cytokine production between the two groups over a 48-h period (8). In the present study, levels of IL-12 p35 mRNA expression in lung tissues were similar in both groups of R. equi-infected foals, and IL-12 p40 mRNA expression was significantly higher in 103+-infected foals. Therefore, downregulation of IFN-γ mRNA expression in CD4+ T lymphocytes by virulent R. equi does not appear to occur through suppression of IL-12 induction. Further studies are therefore needed to identify the mechanisms underlying the depressed IFN-γ response by CD4+ T lymphocytes associated with the progression of the disease caused by virulent plasmid-containing R. equi.
In conclusion, this study has shown that virulent R. equi can modulate the cytokine response of the foal, its natural host. Downregulation of IFN-γ mRNA expression in CD4+ T lymphocytes by virulent R. equi may play an important role in the pathogenesis R. equi-induced disease in foals. However, definitive understanding of the role of these cytokines in the pathogenesis of R. equi infections in foals awaits production of the equine-specific anticytokine MAbs required to modulate the cytokine response of horses.
ACKNOWLEDGMENTS
This work was supported by the Natural Sciences and Engineering Research Council of Canada, by the E. P. Taylor Equine Research Fund, and by the Ontario Ministry of Agriculture, Food and Rural Affairs. S. Giguère is the recipient of a fellowship from the Medical Research Council of Canada.
We thank William Matthes-Sears for assistance with statistical analysis.
REFERENCES
- 1.Arlotti M, Boboli G, Moscatelli G L, Magnati G, Maserati R, Borghi V, Andreoni M, Libanore M, Bonazzi L, Piscina A, Ciammarughi R. Rhodococcus equi infection in HIV-positive subjects: a retrospective analysis of 24 cases. Scand J Infect Dis. 1996;28:463–467. doi: 10.3109/00365549609037941. [DOI] [PubMed] [Google Scholar]
- 2.Balson G A, Smith G D, Yager J A. Immunophenotypic analysis of foal bronchoalveolar lymphocytes. Vet Microbiol. 1997;56:237–246. doi: 10.1016/s0378-1135(97)00092-8. [DOI] [PubMed] [Google Scholar]
- 3.Balson G A, Yager J A, Croy B A. SCID/beige mice in the study of immunity to Rhodococcus equi. In: Rossdale P D, Wade J F, editors. Equine infectious diseases. 6th ed. New Market, United Kingdom: R&W Publications; 1992. pp. 49–53. [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.de la Pena-Moctezuma A, Prescott J F. Association with HeLa cells by Rhodococcus equi with and without the virulence plasmid. Vet Microbiol. 1995;46:383–392. doi: 10.1016/0378-1135(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 6.Donisi A, Suardi M G, Casari S, Longo M, Cadeo G P, Carosi G. Rhodococcus equi infection in HIV-infected patients. AIDS. 1996;10:359–362. doi: 10.1097/00002030-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Giguère S, Hondalus M K, Yager J A, Darrah P, Mosser D, Prescott J F. Role of the 85-kb plasmid and plasmid-encoded virulence-associated protein A in intracellular survival and virulence of Rhodococcus equi. Infect Immun. 1999;67:3548–3557. doi: 10.1128/iai.67.7.3548-3557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giguère S, Prescott J F. Cytokine induction in murine macrophages infected with virulent and avirulent Rhodococcus equi. Infect Immun. 1998;66:1848–1854. doi: 10.1128/iai.66.5.1848-1854.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giguère S, Prescott J F. Quantitation of equine cytokine mRNA expression by reverse transcription-competitive polymerase chain reaction. Vet Immunol Immunopathol. 1999;67:1–15. doi: 10.1016/s0165-2427(98)00212-8. [DOI] [PubMed] [Google Scholar]
- 10.Harvey R L, Sunstrum J C. Rhodococcus equi infections in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991;13:139–145. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- 11.Hondalus M K, Diamond M S, Rosenthal L A, Springer T A, Mosser D M. The intracellular bacterium Rhodococcus equi requires Mac-1 to bind to mammalian cells. Infect Immun. 1993;61:2919–2929. doi: 10.1128/iai.61.7.2919-2929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenne L, Kilwinski J, Scheffold W, Kern P. IL-5 expressed by CD4+ lymphocytes from Echinococcus multilocularis-infected patients. Clin Exp Immunol. 1997;109:90–97. doi: 10.1046/j.1365-2249.1997.4031299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanaly S T, Hines S A, Palmer G H. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993;61:4929–4932. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaly S T, Hines S A, Palmer G H. Cytokine modulation alters pulmonary clearance of Rhodococcus equi and development of granulomatous pneumonia. Infect Immun. 1995;63:3037–3041. doi: 10.1128/iai.63.8.3037-3041.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanaly S T, Hines S A, Palmer G H. Transfer of a CD4+ Th1 cell line to nude mice effects clearance of Rhodococcus equi from the lungs. Infect Immun. 1996;64:1126–1132. doi: 10.1128/iai.64.4.1126-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasuga-Aoki H, Takai S, Sasaki Y, Tsubaki S, Madarame H, Nakane A. Tumor necrosis factor and interferon-γ are required in host resistance against virulent Rhodococcus equi infection in mice: cytokine production depends on the virulence levels of R. equi. Immunology. 1999;96:122–127. doi: 10.1046/j.1365-2567.1999.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lunn D P, Holmes M A, Duffus P H. Three monoclonal antibodies identifying antigens on all equine T-lymphocytes, and two mutually exclusive T-lymphocyte subsets. Immunology. 1991;74:251–257. [PMC free article] [PubMed] [Google Scholar]
- 18.Manyonda I T, Soltys A J, Hay F C. A critical evaluation of the magnetic cell sorter and its use in the positive and negative selection of CD45RO+ cells. J Immunol Methods. 1992;149:1–10. doi: 10.1016/s0022-1759(12)80042-1. [DOI] [PubMed] [Google Scholar]
- 19.Marth T, Kelsall B L. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima R, Brubaker R R. Association between virulence of Yersinia pestis and suppression of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1993;61:23–31. doi: 10.1128/iai.61.1.23-31.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nordman P, Ronco E, Nauciel C. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect Immun. 1992;60:2748–2752. doi: 10.1128/iai.60.7.2748-2752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Powrie F, Bean D, Moore K W. Interleukin 10. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. 2nd ed. New York, N.Y: Marcel Dekker Inc.; 1997. pp. 143–152. [Google Scholar]
- 23.Prescott J F, Fernandez A S, Nicholson V M, Patterson M A, Yager J A, Viel L, Perkins G. Use of a virulence-associated protein based enzyme-linked immunosorbent assay for Rhodococcus equi serology in horses. Equine Vet J. 1996;28:344–349. doi: 10.1111/j.2042-3306.1996.tb03103.x. [DOI] [PubMed] [Google Scholar]
- 24.Ross T L, Balson G A, Miners J S, Smith G D, Shewen P E, Prescott J F, Yager J A. Role of CD4+, CD8+ and double negative T-cells in the protection of SCID/beige mice against respiratory challenge with Rhodococcus equi. Can J Vet Res. 1997;60:186–192. [PMC free article] [PubMed] [Google Scholar]
- 25.Sekizaki T, Takai S, Egawa Y, Ikeda T, Ito H, Tsubaki S. Sequence of the Rhodococcus equi gene encoding the virulence-associated 15-17-kDa antigens. Gene. 1995;155:135–136. doi: 10.1016/0378-1119(95)00009-u. [DOI] [PubMed] [Google Scholar]
- 26.Stanciu L A, Shute J, Holgate S T, Djukanovic R. Production of IL-8 and IL-4 by positively and negatively selected CD4+ and CD8+ human T cells following a four-step cell separation method including magnetic cell sorting (MACS) J Immunol Methods. 1996;189:107–115. doi: 10.1016/0022-1759(95)00240-5. [DOI] [PubMed] [Google Scholar]
- 27.Steel R G D, Torrie J H. Principle and procedures of statistics. A biometrical approach. 2nd ed. New York, N.Y: McGraw-Hill; 1980. [Google Scholar]
- 28.Sweeney R W, Jones D E, Habecker P, Scott P. Interferon-γ and interleukin 4 gene expression in cows infected with Mycobacterium paratuberculosis. Am J Vet Res. 1998;59:842–847. [PubMed] [Google Scholar]
- 29.Swiderski C E, Klei T R, Horohov D W. Quantitative measurement of equine cytokine mRNA expression by polymerase chain reaction using target-specific standard curves. J Immunol. 1999;222:155–169. doi: 10.1016/s0022-1759(98)00193-8. [DOI] [PubMed] [Google Scholar]
- 30.Takai S, Anzai T, Sasaki Y, Tsubaki S, Kamada M. Virulence of Rhodococcus equi isolated from lesions of infected foals. Bull Equine Res Inst. 1993;30:9–14. [Google Scholar]
- 31.Takai S, Imai Y, Fukunaga N, Uchida Y, Kamisawa K, Sasaki Y, Tsubaki S, Sekizaki T. Identification of virulence-associated antigens and plasmids in Rhodococcus equi from patients with AIDS. J Infect Dis. 1995;172:1306–1311. doi: 10.1093/infdis/172.5.1306. [DOI] [PubMed] [Google Scholar]
- 32.Takai S, Sekizaki T, Ozawa T, Sugawara T, Watanabe Y, Tsubaki S. Association between large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991;59:4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takai S, Watanabe Y, Ikeda T, Ozawa T, Matsukura S, Tamada Y, Tsubaki S, Sekizaki T. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993;31:1726–1729. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan C, Prescott J F, Patterson M C, Nicholson V M. Molecular characterization of a lipid-modified virulence-associated protein of Rhodococcus equi and its potential in protective immunity. Can J Vet Res. 1995;59:51–59. [PMC free article] [PubMed] [Google Scholar]
- 35.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 36.Wada R, Kamada M, Anzai T, Nakanishi A, Kanemaru T, Takai S, Tsubaki S. Pathogenicity and virulence of Rhodococcus equi in foals following intratracheal challenge. Vet Microbiol. 1997;56:301–312. doi: 10.1016/s0378-1135(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 37.Yager J A, Prescott C A, Kramar D P, Honnah H, Balson G A, Croy B A. The effect of experimental infection with Rhodococcus equi on immunodeficient mice. Vet Microbiol. 1991;28:363–376. doi: 10.1016/0378-1135(91)90071-m. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Lin Y, Iyer D V, Gong J, Abrams J S, Barnes P F. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:3231–3234. doi: 10.1128/iai.63.8.3231-3234.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]