Abstract
The ongoing 2022 monkeypox virus outbreak has disproportionately impacted men who have sex with men and is associated with an increased frequency of atypical symptoms. The impetus for this outbreak is currently unknown. Experts suggest it may be related to the cessation of routine smallpox vaccination globally and biological changes in the monkeypox virus itself. Human monkeypox infection is classically associated with a fever prodrome followed by the eruption of small macules at the site of inoculation and when disseminating. The lesions then develop into a papule within 1–2 days and turn it a vesicle that pustulate with central umbilication within 5–7 days. They may ulcerate as they heal but will eventually begin to scab and new skin will form which often leaves a hyperpigmented or pitting scar. The overall process can take 2–3 weeks to heal entirely depending on the immune status of the host and other factors, such as antiviral treatment and previous vaccination. Primary inoculation of the monkeypox virus in the perianal region can lead to the development of single or multiple vesiculopustular lesions. They can appear similar to other sexually transmitted infections which could lead to a misdiagnosis. We present two separate cases of human monkeypox infection in men who have sex with men and concomitant human immunodeficiency virus (HIV) disease who both presented for anogenital lesions and proctitis who were successfully treated with tecovirimat. Treatment with tecovirimat has been shown to reduce symptoms and duration of illness. However, the unique features of the 2022 monkeypox virus outbreak necessitate further research to better understand the efficacy of this antiviral in the current monkeypox outbreak.
Keywords: human monkeypox infection, monkeypox virus, perianal lesions, tecovirimat
Introduction
Human monkeypox infection is a zoonotic viral disease caused by the monkeypox virus, which is a double-stranded deoxyribonucleic acid (DNA) virus within the Orthopoxvirus genus of the Poxviridae family.1,2 The Orthopoxvirus genus also contains the variola virus and cowpox virus that cause the infectious diseases smallpox and cowpox, respectively.1,2 Though the monkeypox virus is not a direct descendent of the variola virus, they do share a close genetic relationship.2 The clinical presentation of both monkeypox and smallpox infections can be similar based off previous reports during a time when smallpox was still circulating naturally in endemic regions.1–5 Given the similarities in both viruses, previous observations have shown that 85% of those with a smallpox vaccination were protected from developing human monkeypox infection.6
The monkeypox virus can infect a diverse range of mammals. This includes non-human primates who appear to be an incidental host.7 The sylvatic reservoir of the monkeypox virus is currently unknown but there is some evidence that it may be small rodents.5,8 Prior to 2022, monkeypox virus outbreak transmission of the virus to a human was most commonly linked to animal contact.5 Human-to-human transmission may occur due to contact with respiratory secretions, saliva, or infectious material from cutaneous lesions.5 Other forms of transmission may be related to exposure to the virus during preparation or consumption of bushmeat5 and possible transplacental infection in an infected mother.9 Human sexual transmission is being investigated but it is not clear at this time whether infection can occur from semen or vaginal fluids. Monkeypox virus DNA has been detected from the semen of men with known infection, but more research is needed to better understand if this is a risk factor.1,10
The clinical symptoms of human monkeypox infection closely resemble smallpox. Still, there is a lower risk of transmission and mortality.1,3–5 The classic course of illness is a prodromal systemic illness preceding the eruption of cutaneous lesions.5
Human monkeypox infection was first reported in the 1970s1,5,11 but considered rare.12 From 1970 to 1979, all reported cases occurred in certain regions located in Western and Central Africa with limited evidence of human-to-human transmission being described.12 However, human monkeypox infection has been increasing since the 1980s which has correlated with the cessation of routine smallpox vaccination.5,13 According to the World Health Organization, the latest global trends for ongoing 2022 monkeypox virus outbreak in nonendemic countries have resulted in more than 75,790 confirmed cases worldwide among 109 reporting countries as of October 24, 2022.14 The Centers for Disease Control and Prevention (CDC) have reported a total of 28,004 confirmed cases detected in all 50 states, but case counts are dropping since vaccination and tecovirimat treatment were made readily available globally to at-risk populations.14,15 This ongoing outbreak is unique and has disproportionately affected men who have sex with men (MSM) without an associated with recent travel to an endemic area.1,3,16,17
The disproportionate impact of the 2022 monkeypox virus outbreak on an already vulnerable population is further complicated by the potential misdiagnosis of cutaneous monkeypox lesions as a sexually transmitted infection (STI) or concomitant STI.1,3,16,17 This current outbreak demonstrates a growing need to understand the clinical spectrum of disease, associated complications, and management of monkeypox infection. We herein describe two cases of monkeypox infection in men living with human immunodeficiency virus (HIV) who were treated with tecovirimat and showed clinical response to therapy with the resolution of symptoms.
Case 1
A 40-year-old Caucasian male living in Florida with past medical history of well-controlled HIV disease [HIV RNA < 20 copies/ml; CD4 count (29%) 742 cells/µl] presented with a new painful, itchy, vesiculopustular rash surrounding the anus and perianal region [Figure 1(a)]. Patient is adherent to oral once-daily single-pill (bictegravir, emtricitabine, and tenofovir alafenamide) antiretroviral therapy (ART) and takes no other medications. He denied any surgical history or known drug allergies. Patient denied any recreational substance use and consumes occasional alcohol during social events. He reported on the day he received the modified vaccinia Ankara vaccine (JYNNEOS) that he had receptive anal intercourse with a new male partner. He had not had any recent domestic or international travel. Within 2 days of that sexual encounter, he noticed subjective fever, myalgia, malaise, and itchiness in the anus. He originally presented to an urgent care facility due to concerns for rectal chlamydial or gonococcal infection. Rash was not seen then, and he was given one intramuscular injection of ceftriaxone and oral doxycycline. The vesiculopustular rash appeared 5 days after the new sexual encounter and is he now being seen in infectious diseases clinic. Due to the current human monkeypox outbreak in Florida, vesiculopustular fluid was sent to Florida Department of Health laboratory for monkeypox virus polymerase chain reaction (PCR) testing. Given his clinical presentation, patient was empirically started on tecovirimat (TPOXX®) 600 mg every 12 h within 48 h after receiving the medication from the US CDC. Rectal swabs for Chlamydia trachomatis and Neisseria gonorrhoeae nucleic acid amplification and syphilis serological testing were negative. Monkeypox virus PCR testing was positively confirmed from vesiculopustular fluid several days later.
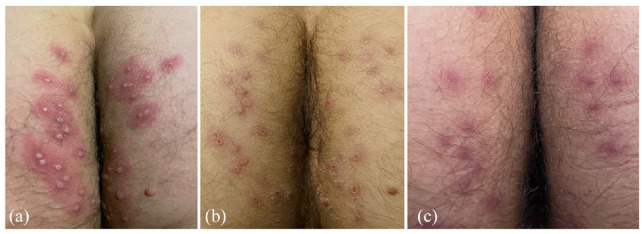
Figure 1.
(a) Monkeypox infection located in the perianal region with central umbilicated vesiculopustular rash and erythematous base. (b) Crusting and scabbing on day 7 of tecovirimat therapy. (c) Complete resolution of vesiculopustular lesions 21 days since initial symptoms began and after completing a 14-day course of tecovirimat therapy which are now exhibiting pigmented scarring.
After starting oral tecovirimat and over the next 2–3 days, new singular, painful, vesiculopustular lesions had arisen on the posterior left upper extremity and right anterior wrist, right anterior thigh, bilateral hairline, and one lesion on the exterior upper lip. No new perianal lesions appeared and mild fever, myalgias, and malaise began to improve. However, new significant anorectal pain developed with accompanying constipation due to fear of defecation. He was started on oral stool softeners to help with constipation and oral gabapentin, and topical lidocaine to reduce the neuropathic pain. On day 7 of tecovirimat therapy, systemic symptoms had abated, anorectal pain improved, and no new vesiculopustular lesions noted. Resolving features of the existing vesiculopustular rash were seen with crusting and scaling of the lesions [Figure 1(b)]. Anorectal pain had completely subsided at the end of the 14-day course of tecovirimat. Clinical exam conducted 21 days after initial symptoms and yielded a complete resolution of the vesiculopustular lesions. New skin formed in the areas of the previous rash, with most lesions exhibiting pigmented scarring as seen on the wrist (Figure 2) and perianal lesions [Figure 1(c)].
Figure 2.
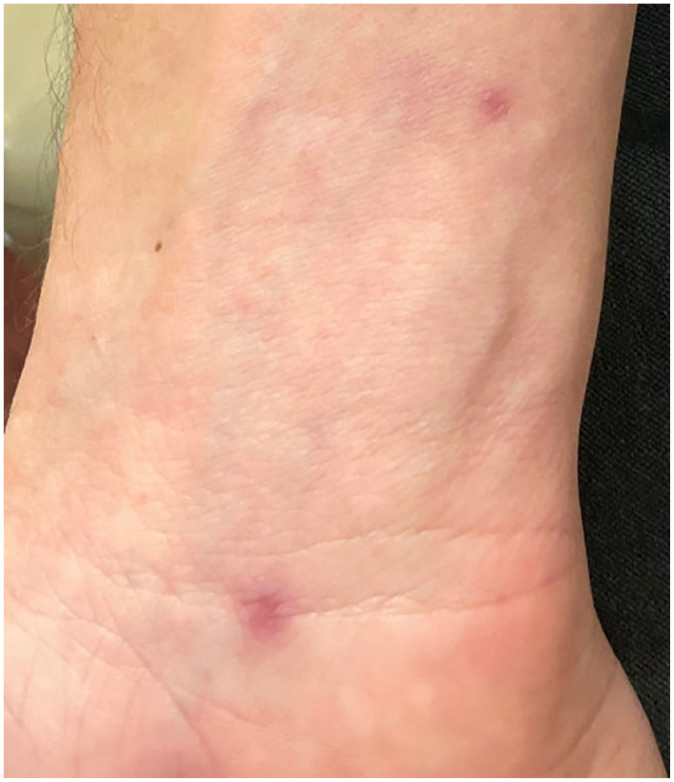
Pigmented scarring of healed monkeypox lesion located on the wrist.
Case 2
A 35-year-old Caucasian male living in Colorado with past medical history of well-controlled HIV disease [HIV RNA < 20 copies/ml; CD4 count (37%) 666 cells/µl] on oral once-daily single-pill (bictegravir, emtricitabine, and tenofovir alafenamide) ART, major depressive disorder, migraine disorder, gastroesophageal reflex disorder, and anal condylomas presented to the outpatient infectious diseases clinic with a 4-day history of body aches, fevers, and chills. A review of systems was positive for watery diarrhea, lower back pain, rectal pain, and painful swollen inguinal lymphadenopathy. Patient reported history of only sex with other men. His most recent sexual encounter (1 month prior to presentation) was receptive anal intercourse. He stated that 2 weeks before presentation, he went to a crowded dance club with friends for which there was close but non-intimate contact. He also noted regular attendance at his local gym and used the steam room often. He denied recent domestic or international travel. Denied any surgical history. He endorsed adherence to his ART and oral sumatriptan for migraine disorder when needed. His current allergies include lactose intolerance and trimethoprim/sulfamethoxazole.
At the first infectious diseases clinic visit, routine laboratory findings revealed normal complete blood count, normal comprehensive metabolic panel, negative gastrointestinal pathogen PCR panel, normal urinalysis, negative triple-site (urethra/anus/nasopharyngeal) gonorrhea/chlamydia probe, and a nonreactive rapid plasma reagin (RPR). However, 1 day later, he returned to the clinic with acute onset of vesiculopustular rash in several areas of his body, including his back, left axilla, and left groin. Lesions appeared papular with surrounding erythema and central umbilication. These lesions were swabbed and resulted in positive non-variola Orthopoxvirus identification by PCR, and the presumptive diagnosis of monkeypox infection was made.
Over the course of the next few days, new lesions appeared on the bottom of his feet and in his rectum, causing severe pain with bowel movements. In total, he counted approximately 40 lesions over his entire body. Due to this severe pain in his anorectal region and HIV disease, the patient was started on oral tecovirimat 600 mg every 12 h on illness day 8 with a tentative plan for a total of 14 days. Within 72 h of starting tecovirimat, the patient noted significant improvement in pain, his lesions began crusting over, and he had resolution of myalgias, lymphadenopathy, and fevers. He noticed approximately three new lesions appear after starting tecovirimat but on day 7 of treatment, all lesions had sustained crusting when examined in infectious diseases clinic. He finished the 14-day course without complications and resolution of his cutaneous lesions detailed over the phone. Therefore, 1 month after completing treatment, he had sustained resolution of previous cutaneous lesions and no clinical evidence of recurrence.
Discussion
We described two clinical cases of presumed monkeypox-associated proctitis with a quick resolution of symptoms using the antiviral tecovirimat. Monkeypox infection typically presents as a febrile systemic illness that precedes the eruption of a vesiculopustular rash.1,3–5 The course of illness after infection has three phases: an incubation period, prodromal period, and rash period.5 The incubation period is variable but is approximately 7–17 days and followed by a prodromal period.5 The prodromal period lasts approximately 1–4 days and is characterized by fever, myalgia, fatigue, headache, and lymphadenopathy.5 After this prodromal period, there is a rash eruption.5 The lesions may be painful or painless and are typically centrifugal in distribution with majority of cutaneous lesions presenting in one stage of development, well-circumscribed, and demonstrating distinct central umbilication.5 The presence of lymphadenopathy may help differentiate monkeypox infection from smallpox, and the single stage of development is useful in differentiating the rash from varicella.5
However, the ongoing 2022 monkeypox virus outbreak is unique in that it has disproportionately impacted MSM.1,3,16,17 Therefore, this outbreak has had unique features which are different than previous outbreaks associated with animal contact.1,3,4,13 Among 528 reported cases across 16 countries, 98% of infections were among MSM, 41% had concomitant HIV infection, and 95% of transmission occurred via sexual activity.1 In the 30 patients for whom data were available, 13 (43%) did not report prodromal symptoms,1 which corresponds with 42% of persons not experiencing prodromal symptoms as reported by the CDC.4 Though the classical manifestations of cutaneous lesions and fever were present in 95% and 62% of patients, atypical manifestations were reported: 21% reported pharyngitis, 14% reported proctitis or anorectal pain, and 10% reported low mood.1 The cutaneous lesions were most commonly described as vesiculopustular (58%), multiple ulcers (19%), or single ulcer (11%); however, it should be noted that a rash was absent in 5% of patients.1 Of those with cutaneous lesions, the most frequent was the anogenital area at 73%, and patients most frequently presented to an HIV clinic or sexual health clinic, 29% and 23%, respectively.1 Thornhill et al.1 describe 377 patients who underwent STI testing, of whom 109 (29%) were found to have a concomitant STI, with syphilis being the most frequent at 9%. These data presented have significant implications. As the most common sites of presentation were HIV clinics and sexual health clinics, clinical providers must be diligent to avoid anchoring bias to prevent the misdiagnosis of monkeypox as another STI. This is further complicated by the prevalence of concomitant STI in those with active human monkeypox infection. The anogenital lesions of monkeypox may resemble primary syphilis and the presence of cutaneous lesions on the palms or soles may resemble secondary syphilis or disseminated gonorrhea infection. In the aforementioned study, 5% of patient received antiviral treatment, with cidofovir and tecovirimat being the most common.1
Both patients reported here were men living with controlled HIV disease who were adherent with ART and CD4 counts > 200 cells/µl but presented with debilitating perianal disease with presumed proctitis due to human monkeypox infection. The CDC currently recommends treatment for those at risk of severe disease or those with lesions in anatomical locations which can cause significant scarring or the future development of strictures or in regions which are at risk for serious secondary bacterial infections that may require debridement.17 In the two cases presented, treatment was elected due to the presence of lesions in anorectal region and with proctitis with concerns for preventing bowel movements due to severe pain.
At this time, it is unclear whether the monkeypox virus can be transmitted through sexual intercourse but further research is being done. One study analyzing 32 men with active monkeypox infection revealed that 91% had detection of monkeypox virus DNA among collected semen specimens.1 This finding likely merits further study as it is unknown if the viral DNA in the samples is capable of replication and transmissibility.1,10
Tecovirimat is an antiviral medication which was approved in the United States for the treatment of smallpox under the Food and Drug Administration (FDA) Animal Rule (code of Federal Regulations title 21, part 314, subpart I).18,19 This route of approval was established by the FDA for drugs using suitable animal models when it is not ethical to perform efficacy studies in humans for serious or life-threatening conditions.18,19 The mechanism of action of tecovirimat is known and targets the Orthopoxvirus protein F13 (also known as VP37 or p37) which is highly conserved among this genus. The F13 protein is needed for the intracellular formation of Orthopoxvirus extracellular enveloped virions. Inhibiting the F13 protein prevents spread to other uninfected cells. The available in vitro data have demonstrated that tecovirimat has broad antiviral activity against a diverse range of orthopoxviruses, including Variola virus, Vaccinia virus, Rabbitpox virus, and monkeypox virus.19 Safety in humans is limited; however, among 359 healthy volunteers, only mild adverse events were reported and were similar in those in the placebo arm.20,21 Recent data provided by the CDC among those being treated for human monkeypox infection in the United States have shown that among this convenience cohort, only 3.5% (n = 12/360) reported adverse side effects while on tecovirimat treatment.20 Given the lack of human clinical trial data, it is not known to what extent tecovirimat can interact with other drugs but it can inhibit (weak) CYP2C19 and CYP2C8, and induces (weak) CYP3A4 hepatic metabolism, thus have a potential effect on a broad range of medication classes, including anti-epileptics, hormonal contraceptives, sirolimus, and tacrolimus.23 Dosing of these medications if given concurrently may need to be evaluated while on tecovirimat and discussed with a pharmacy expert.
In the United States, human monkeypox infection was rapidly emerging, with 2891 cases from 43 states reported by the CDC as of July 22, 2022.4 Cases continued to rise in July 2020 and actions were taken to improve access to tecovirimat (TPOXX) and modified vaccinia Ankara vaccine (JYNNEOS) to combat the outbreak here in the United States on August 18, 2022.16 After these measures and other public health actions were taken, we now have seen a drastic drop in case count trends since the middle of August 2022.15 The use of tecovirimat for the current outbreak needs further study but appears to show promise in reducing symptoms in those infected, with up to 92% resolution of lesions and pain by day 21.24 The CDC on September 9, 2022 released data collected from the intake forms from the tecovirimat expanded access investigational new drug protocol among information provided from 549 patients.22 Among 119 patients who had provided outcome data to the CDC, 89.5% reported that all lesions had either crusted or healed with a new skin following a standard 14-day tecovirimat therapy.22 One observation from the study conducted by O’ Laughlin et al.22 revealed that some individuals did develop new lesions after starting tecovirimat, including 13.2% who reported new lesions within the first week and 2.2% at the post-treatment visit with their health care provider. In both our patients, they did develop limited new lesions within the first 2–3 days of starting treatment but at the 1-week mark had crusting of all lesions and no evidence of new cutaneous findings.
Given the global impact of the 2022 monkeypox virus outbreak, it will be important to assess the efficacy and safety of tecovirimat in a randomized clinical trial. There is currently several clinical trials enrolling patients in the United States and Democratic Republic of Congo to evaluate the safety and efficacy of tecovirimat for human monkeypox treatment.18,25 To better understand whether tecovirimat treatment is clinically efficacious, further data are needed from randomized clinical trials to assess efficacy, indications for treatment, and subsequent duration for those with human monkeypox infection. Among populations at risk for severe disease or the presence of lesions in sensitive regions of the body, clinicians and those practicing travel medicine should be aware of the vesiculopustular appearance of monkeypox infection and regional access to tecovirimat therapy.
Acknowledgments
None.
Footnotes
ORCID iDs: Norman L. Beatty  https://orcid.org/0000-0002-3032-5741
https://orcid.org/0000-0002-3032-5741
Andrés F. Henao Martinez  https://orcid.org/0000-0001-7363-8652
https://orcid.org/0000-0001-7363-8652
Contributor Information
Norman L. Beatty, Division of Infectious Diseases and Global Medicine, Department of Medicine, College of Medicine, University of Florida, 1600 SW Archer Rd. PO Box 100289, Gainesville, FL, 32610, USA.
Coulter Small, Division of Infectious Diseases and Global Medicine, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, USA.
Tyler Degener, Division of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA.
Andrés F. Henao Martinez, Division of Infectious Diseases, School of Medicine, University of Colorado, Aurora, CO, USA
Declarations
Ethics approval and consent to participate: Ethical approval was not required. The present report is in compliance with the Health Insurance Portability and Accountability Act (HIPAA).
Consent for publication: Both patients have provided written consent for the publication of the case report and photographs.
Author contributions: Norman L. Beatty: Conceptualization; Investigation; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Coulter Small: Writing – review & editing.
Tyler Degener: Investigation; Validation; Writing – original draft.
Andrés F. Henao Martinez: Conceptualization; Investigation; Methodology; Supervision; Validation; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that there is no conflict of interest. The Editor in Chief of Therapeutic Advances in Infectious Disease is a co-author of this paper. Therefore, the peer-review process was managed by alternative members of the Editorial Board and the submitting Editor had no involvement in the decision-making process.
Availability of data and materials: All data are available as part of this report.
References
- 1. Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries: April-June 2022. N Engl J Med 2022; 387: 679–691. [DOI] [PubMed] [Google Scholar]
- 2. Shchelkunov SN, Totmenin AV, Safronov PF, et al. Analysis of the monkeypox virus genome. Virology 2002; 297: 172–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ortiz-Martinez Y, Rodriguez-Morales AJ, Franco-Paredes C, et al. Monkeypox: a description of the clinical progression of skin lesions: a case report from Colorado, USA. Ther Adv Infect Dis 2022; 9: 117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases: United States, May 17-July 22, 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis 2014; 58: 260–267. [DOI] [PubMed] [Google Scholar]
- 6. Fine PE, Jezek Z, Grab B, et al. The transmission potential of monkeypox virus in human populations. Int J Epidemiol 1988; 17: 643–650. [DOI] [PubMed] [Google Scholar]
- 7. Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between1958 and 2012. Future Virol 2013; 8: 129–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynolds MG, Carroll DS, Karem KL. Factors affecting the likelihood of monkeypox’s emergence and spread in the post-smallpox era. Curr Opin Virol 2012; 2: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis 2017; 216: 824–828. [DOI] [PubMed] [Google Scholar]
- 10. Lapa D, Carletti F, Mazzotta V, et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis 2022; 22: 1267–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ 1972; 46: 593–597. [PMC free article] [PubMed] [Google Scholar]
- 12. Breman JG, Steniowski MV, Zanotto E, et al. Human monkeypox, 1970–79. Bull World Health Organ 1980; 58: 165–182. [PMC free article] [PubMed] [Google Scholar]
- 13. Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis 2019; 13: e0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The WHO 2022 monkeypox outbreak: global trends, https://worldhealthorg.shinyapps.io/mpx_global/ (accessed 24 October 2022).
- 15. CDC monkeypox U.S. map & case count, https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html (accessed 24 October 2022).
- 16. House TW. FACT SHEET: White House announces new actions to combat monkeypox outbreak, 2022, https://www.whitehouse.gov/briefing-room/statements-releases/2022/08/18/fact-sheet-white-house-announces-new-actions-to-combat-monkeypox-outbreak/
- 17. CDC Clinical Guidance. Treatment information for healthcare professionals, https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html (accessed 24 October 2022).
- 18. Sherwat A, Brooks JT, Birnkrant D, et al. Tecovirimat and the treatment of monkeypox: past, present, and future considerations. N Engl J Med 2022; 387: 579–581. [DOI] [PubMed] [Google Scholar]
- 19. Chan-Tack KM, Harrington PR, Choi SY, et al. Assessing a drug for an eradicated human disease: US Food and Drug Administration review of tecovirimat for the treatment of smallpox. Lancet Infect Dis 2019; 19: e221–e224. [DOI] [PubMed] [Google Scholar]
- 20. Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med 2018; 379: 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Food and Drug Administration 2018 meeting materials, Antiviral Drugs Advisory Committee (formerly known as the Anti-Infective Drugs Advisory Committee), https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/ucm587657.htm (accessed 24 October 2022).
- 22. O’Laughlin K, Tobolowsky FA, Elmor R, et al. Clinical use of tecovirimat (Tpoxx) for treatment of monkeypox under an investigational new drug protocol: United States, May–August 2022. MMWR Morb Mortal Wkly Rep 2022; 71: 1190–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tecovirimat. Lexi-drugs. Hudson, OH: Lexicomp, 2022, https://www.uptodate.com/contents/tecovirimat-united-states-availability-limited-to-health-department-cdc-expanded-access-protocol-drug-information (accessed 24 October 2022). [Google Scholar]
- 24. Desai AN, Thompson GR, 3rd, Neumeister SM, et al. Compassionate use of tecovirimat for the treatment of monkeypox infection. JAMA 2022; 328: 1348–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. A5418 Clinical Trial Study of tecovirimat for human monkeypox virus (STOMP), https://www.stomptpoxx.org/main (accessed 24 October 2022).