Abstract
Background:
Systemic sclerosis (SSc) is an autoimmune disease characterized by vasospasm and microvascular involvement. Iloprost (ILO), a prostaglandin analogous, is used for the treatment of SSc-related Raynaud’s phenomenon and digital ulcers. The suggested dose is 0.5–2 ng/kg/min for 6–8 h, and the maximum dose is decided upon the patient’s tolerance.
Objectives:
This study aims to analyze ILO infusion tolerance and possible predictive factors in patients with SSc.
Design:
This is a retrospective observational study.
Method:
We evaluated 113 patients with SSc beginning ILO intravenous (IV) infusion treatment between 2004 and 2021. We assessed the maximum tolerated ILO IV infusion rate, the incidence of adverse events (AEs), and the need for symptomatic therapy during the dose-finding sessions. We collected relevant demographic and medical and employed generalized linear models to assess possible predictors of maximum tolerated ILO infusion rate and AEs and logistic regression to assess predictors of AEs.
Results:
The median ILO infusion rate at the end of the dose-finding process was 0.88 ng/kg/min [interquartile range (IQR) = 0.37]. We found a significant inverse correlation between ILO infusion rate and body mass index (BMI) at the beginning of treatment. BMI was negatively associated with ILO infusion rate (β = −0.21, p = 0.02) after correction for relevant confounding factors. Overweight patients (BMI >26) presented a 13-fold increased risk of developing AEs during ILO titration [adjusted odds ratio = 13.979, 95% confidence interval (CI) = 2.359–82.845]. AEs during ILO titration occurred in 47.8% of patients, of whom 22.2% presented hypotension. Other AEs were headache, nausea, vomiting, diarrhea, and edema. Symptomatic therapy was needed in half of the patients at least once.
Conclusion:
This study showed that higher BMI was statistically associated with lower ILO infusion rate tolerance and higher AEs rate, underlying a possible BMI-dependent endothelial dysfunction. Individual ILO regimens still need to be tailored to the patient.
Plain Language Summary
Introduction: Systemic sclerosis is a rare a rheumatic disease characterized by skin thickening, vasospasm, and digital ulcers (DUs), as well as other organs involvement. Iloprost, which is administered as intravenous infusion, is one of the main treatments for this disease, and it is effective in reducing vasospasm and the frequency of DUs. Even if there is a suggested dose range, the exact dose must be tailored on each patient, because the tolerance to the drug is variable. Tolerance is limited by dose-dependent unwanted effects, as headache, low blood pressure, dizziness, and sickness. This study aimed to identify possible predictors of such tolerance.
Materials and Methods: We collected data from our patients with systemic sclerosis beginning the treatment with iloprost between January 2004 and November 2021 at our hospital facility in Verona, Italy, and analyzed different factors that could be associated with a better tolerance, as age, sex, disease duration, smoking habit, body mass index (a measure of body fatness), blood pressure, concomitant medications, and different patterns of the disease.
Results: We found that a higher body mass index was associated with lower iloprost tolerance and higher adverse events rate in patients with systemic sclerosis, while we did not find a correlation with other factors. We believe overweight and obese patients (who have a higher body mass index) have a defect in the vasodilatation mechanism and can therefore be more susceptible to the effect of this medication.
Conclusions: While preliminary, our results could provide a good starting point to develop a predictive tool to limit adverse events during this therapy.
Keywords: systemic sclerosis, iloprost, body mass index, prostanoids
Introduction
Systemic sclerosis (SSc) is an autoimmune disease characterized by autoantibodies expression and multiple organs involvement (skin, lung, gastrointestinal, myocardial, renal) arising from chronic reversible vasospasm and vascular remodeling, clinically expressed as Raynaud’s phenomenon (RP) and skin fibrosis.1,2 Many drugs have shown efficacy in SSc-related RP, as calcium channel blockers (CCBs), iloprost (ILO), endothelin inhibitors (bosentan, macitentan), and phosphodiesterase 5 inhibitors (PDE5i) (sildenafil, tadalafil).3
ILO is a stable analogous to prostacyclin PGI2, and, as such, it is active on platelets, fibroblasts, and endothelial cells leading to reduced aggregations, vasodilatation, and fibrotic remodeling.4 It is currently recommended by the EULAR guidelines for SSc DU and SSc-related RP.5 The delay in the beginning ILO therapy was found to be a significant risk factor for ischemic DUs.6 The manufacturer datasheet suggests a dose of 0.5–2 ng/kilogram of body weight (kg)/min for intravenous (IV) infusion. Clinical efficacy appears to not differ significantly using lower doses or higher doses, as shown by Kawald et al. in a small randomized trial. In the high-dose group, however, only 12 of 25 patients reached the maximal dose (2.0 ng/kg/min); the others (52%) showed transient side effects requiring dose reduction to tolerated doses.7 Negrini et al.8 recently summarized current clinical practice in various tertiary centers in Italy, reporting a monthly 6–8 h/day administration at a mean rate of 1.5 ng/kg/min.
In current clinical practice, ILO infusion rate is tailored to individual patient tolerance: titration is usually achieved by slowly increasing infusion rate up to the maximum tolerated dose,9 with the most common ILO side effects being nausea, hypotension, tachycardia, vomit, diarrhea, painful digital swelling, flushing, and headache.10,11 This method, however, is imprecise and might lead to an excess of adverse events (AEs), albeit they are usually mild and quickly reversible. A better understanding of the factors that predict tolerance to ILO might help the tailoring of the ILO infusion rate, avoiding unnecessary AEs. This study aims to identify possible predictors of ILO tolerance in patients with SSc.
Methods
We retrospectively evaluated clinical records of patients with an established diagnosis of SSc according to ACR/EULAR classification criteria,12 who started IV ILO therapy at our hospital facility between January 2004 and November 2021 for SSc DU or SSc-related RP.
We evaluated the patients at the baseline of their ILO IV therapy: patients had to be naïve to such therapy, and patients with basal systolic blood pressure inferior to 90 mmHg, heart ischemic disease, and coagulation diseases were excluded from ILO IV therapy. We included only patients with complete data and without variations in the ILO infusion rate in the 12 months following the dose-finding process.
A homogeneous dose-finding process was followed at our clinic during this timeframe. The initial ILO IV rate was 0.5 ng/kg/min for the first hour, and then it was increased by 0.25 ng/kg/min every other hour. The ILO IV rate was increased until the development of AEs, up to 2 ng/kg/min. In case of AEs, the ILO IV rate was reduced halfway to the previous dose, and the patient was reassessed after 1 h. Symptomatic therapy was administered as needed and noted in the patient’s clinical record. We defined the maximum tolerated ILO IV rate as the highest ILO IV rate tolerated in three consecutive sessions, expressed as ng/kg/min. Each session lasted 6 h. Vasoactive drugs (such as CCB or endothelin inhibitors) were withdrawn on the ILO infusion days. Premedication was not routinely administered during the first three sessions.
We collected the relevant demographic, clinical, and therapeutic data from the patients’ electronic records. Skin involvement was assessed using the modified Rodnan’s skin score (mRSS).13 The body mass index (BMI, kg/m2) of patients was calculated without shoes and in light indoor clothes using a balance beam scale and a fixed stadiometer. The gastroesophageal involvement was assessed by esophageal manometry or esophageal transit study, or a diagnosis of small intestinal bacterial overgrowth (SIBO).14 The interstitial lung disease (ILD) involvement was assessed through computerized tomography (CT) chest scans15 and pulmonary arterial hypertension (PAH) by right heart catheterization.16 Hypotension was defined as systolic blood pressure below 90 mmHg or a reduction >20 mmHg of the initial systolic blood pressure.
The reporting of this study followed the ‘Strengthening the Reporting of Observational Studies in Epidemiology’ (STROBE) guidelines for reporting observational studies.17 The STROBE checklist is available as Supplementary Material.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 26 (IBM Corp., Armonk, NY, USA). Significance was given for p < 0.05, and all tests are two-tailed. We used the Kolmogorov–Smirnov test to assess the normal distribution of the data. Continuous parametric variables are reported as mean values ± standard deviation (SD) and nonparametric variables as medians and interquartile range (IQR). Categorical variables are reported as frequency and proportions. We performed the Mann–Whitney U test to compare continuous nonparametric variables and Student’s t test to compare continuous parametric variables. We performed Pearson’s correlation coefficient to test associations between continuous variables and employed generalized linear models with robust estimators to identify determinants of maximum tolerated ILO dose (expressed as ng/kg/min). Model 1 included gender, age, disease duration, mean arterial pressure, and smoking habit. Model 2 was further adjusted for disease pattern (diffuse versus limited cutaneous involvement), calcinosis, and ulcers before the beginning of the ILO treatment, while model 3 (fully adjusted) took into account also concomitant therapy with CCB and PDE5i. We analyzed BMI both as a continuous variable in the generalized linear models and as a categorical variable, dividing the population into tertiles and comparing the median ILO IV rate between them using a Mann–Whitney U test. Binary logistic regression was employed to assess predictors of AEs. BMI was included in this analysis as a dichotomous variable, using the World Health Organization (WHO) definition for overweight (BMI > 25) as the cut-off value.
Results
We screened 149 consecutive patients with SSc undergoing ILO IV therapy. A total of 113 patients had complete clinical basal data and met the inclusion criteria. All the relevant demographic and clinical characteristics of the cohort are summarized in Table 1.
Table 1.
Clinical characteristics of patients with systemic sclerosis beginning iloprost infusion at our facility between 2004 and 2021.
| Characteristics | n = 113 |
|---|---|
| Female, n (%) | 94 (83.2) |
| BMI, mean ± SD | 24.19 ± 3.94 |
| Age (years), mean ± SD | 49.28 ± 13.57 |
| Current smoker, n (%) | 34 (30.6) |
| Disease duration (years), mean ± SD | 2.28 ± 2.8 |
| Iloprost rate (ng/kg/min), median (IQR) | 0.88 (0.37) |
| mRSS, mean ± SD | 7.4 ± 4.2 |
| Diffuse pattern, n (%) | 42 (37.2) |
| Prior digital ulcers, n (%) | 45 (40) |
| Autoantibodies | |
| Anti-Topo I antibodies, n (%) | 28 (24.8) |
| Anti-centromeric antibodies, n (%) | 50 (44.2) |
| Anti-RNA polymerase III antibodies, n (%) | 6 (5.3) |
| Anti-RNP antibodies, n (%) | 5 (4.4) |
| Anti-SSA antibodies, n (%) | 8 (7.1) |
| Other antibodies, n (%) | 16 (14.1) |
| Major organ involvement | |
| Gastrointestinal manifestation, n (%) | 56 (50) |
| Interstitial lung disease, n (%) | 37 (32.7) |
| Pulmonary artery hypertension, n (%) | 5 (4.4) |
| Myocardial or pericardial involvement, n (%) | 16 (14.1) |
| Ongoing therapies | |
| CCB combination therapy, n (%) | 43 (38.1) |
| Endothelin inhibitors combination therapy, n (%) | 16 (14.2) |
| Immunosuppressants, n (%) | 47 (41.6) |
BMI, body mass index; CCB, calcium channel blocker; ILO, iloprost; IQR, interquartile range; mRSS, modified Rodnan’s skin score; RNP, ribonucleoprotein; SD, standard deviation; SSA, Sjogren’s syndrome–related A; Topo I, topoisomerase I.
Immunosuppressive therapy includes methotrexate, leflunomide, mycophenolate mofetil, rituximab, and tocilizumab.
The median maximum ILO IV infusion rate of our patients was 0.88 ng/kg/min (IQR = 0.37). We found a significant inverse correlation between ILO infusion rate and BMI (r = −0.299; p = 0.001), while we did not find a significant correlation between ILO infusion rate and age (p = 0.360) or disease duration (p = 0.147) at the beginning of ILO therapy. Mean ILO IV rate did not differ significantly between men and women (p = 0.870), smokers and not smokers (p = 0.562), limited or diffuse disease (p = 0.782), anti-Scl70 antibodies and anti-centromere antibodies (p = 0.684), CCB treated and not treated (p = 0.165), endothelin inhibitors users and nonusers (p = 0.556), and history of DUs and no previous DUs (p = 0.899). In addition, patients with different organ involvement show no difference in ILO infusion rate.
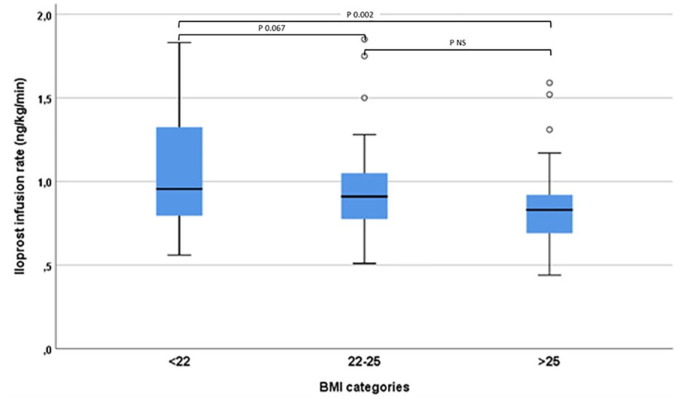
In the unadjusted analyses, we found an association between BMI and ILO infusion rate (ρ = −0.29, p < 0.001). In the fully adjusted model, BMI was still negatively associated with ILO infusion rate (β = −0.29, p = 0.001). The fitting of the fully adjusted model was good [deviance/degree of freedom (df) = 0.765]. The results of the generalized linear models are presented in Table 2. When BMI was analyzed as a categorical variable, the difference in the mean ILO infusion rate was statistically significant between the higher (BMI = 25.1) and the lower (BMI = 22.4) tertiles (p = 0.004), as shown in Figure 1.
Table 2.
Association between BMI and iloprost tolerance in patients with systemic sclerosis.
| Model | BMI, −β (95% CI) | p value |
|---|---|---|
| Unadjusted | −0.024 (−0.040 to −0.007) | <0.001 |
| Model 1 | −0.026 (−0.043 to −0.009) | 0.003 |
| Model 2 | −0.025 (−0.042 to −0.007) | 0.006 |
| Model 3 (fully adjusted) | −0.021 (−0.039 to −0.003) | 0.020 |
Model 1 adjusted for gender, age, disease duration, and smoking habit. Model 2 adjusted for gender, age, disease duration, smoking habit, disease pattern (diffuse versus limited cutaneous involvement), calcinosis, and ulcers before the beginning of the iloprost treatment. Model 3 (main model) adjusted for gender, age, disease duration, smoking habit, disease pattern (diffuse versus limited cutaneous involvement), calcinosis, ulcers before the beginning of the iloprost treatment, and concomitant therapy with CCB and PDE5i.
BMI, body mass index; CCB, calcium channel blocker; PDE5i, phosphodiesterase 5 inhibitors.
Figure 1.
Maximum tolerated iloprost infusion rate in patients with systemic sclerosis stratified by body mass index tertiles.
BMI, body mass index.
A total of 71 AEs occurred in 47 (47.8%) patients during ILO titration. AEs are summarized in Table 3. Symptomatic therapy was needed at least once during the examined period in half of the patients, the most common being metoclopramide (27%) and acetaminophen (24%). Binary logistic regression analysis showed that overweight patients (BMI > 25, according to the WHO definition) had a 13-fold increased risk of developing AEs during ILO titration [adjusted odds ratio = 13.979, 95% confidence interval (CI) = 2.359–82.845, p < 0.001], while gender, age, disease duration, disease pattern, main organ involvement (heart, lung, gastrointestinal), mean blood pressure, and smoking did not result predictive of AEs (as shown in Table 4). Higher BMI was correlated with higher mean arterial blood pressure (p = 0.001; r = 0.345); however, mean arterial blood pressure was not significantly different in patients who developed AEs (other than hypotension) compared with patients without AEs (124.5 ± 15.1 mmHg versus 120 ± 12.3 mmHg; p = 0.192).
Table 3.
Adverse reactions during iloprost infusion dose-finding process.
| Adverse reactions, n = 71 in 47 patients | |
|---|---|
| Headache, n (%) | 22 (46.8) |
| Nausea, n (%) | 19 (40.4) |
| Hypotension, n (%) | 12 (25.6) |
| Vomit, n (%) | 3 (6.3) |
| Diarrhea, n (%) | 3 (6.3) |
| Leg swelling/oedema, n (%) | 3 (6.3) |
| Palpitations, n (%) | 2 (4.4) |
| Syncope, n (%) | 2 (4.3) |
| General malaise, n (%) | 2 (4.3) |
| Epistaxis, n (%) | 1 (2.1) |
| Muscle pain, n (%) | 1 (2.1) |
| Hearing loss, n (%) | 1 (2.1) |
Table 4.
Predictors of adverse reactions during iloprost dose-finding process.
| Variable | p value | OR (95% CI) |
|---|---|---|
| BMI (>25) | <0.001 | 13.979 95% (2.359–82.845) |
| Male sex | 0.587 | |
| Age | 0.769 | |
| Disease duration | 0.217 | |
| Disease pattern (diffuse versus limited) | 0.170 | |
| Main organ involvement (versus none) | 0.668 | |
| Mean blood pressure | 0.129 | |
| Smoking | 0.427 |
BMI, body mass index; CI, confidence interval; OR, odd ratio.
Discussion
We conducted a retrospective observational study on the factors associated with the maximum tolerated ILO IV rate in a cohort of patients with SSc. Overall, we found that high BMI was an independent predictor of ILO tolerance, regarded both as ILO IV rate and AEs development. ILO infusion was well-tolerated in more than half of the patients, and the reported AEs were generally mild and promptly reversible, consistently with previous findings by Negrini et al.8 and Barsotti et al.18 both as reported frequency and characteristics. The median ILO infusion rate in this study population was slightly lower than the mean ILO infusion rate found by Negrini et al.,8 but the same suggested range (0.5–2 ng/kg/min). The authors, however, reported that 34% of the patients showed an increased ILO tolerance over years. In addition, a significant proportion (5/8) of the involved centers adopted a fixed premedication before ILO infusion and the concomitant CCB treatment suspension strategy varied between centers; therefore, these findings are not directly comparable.
A higher BMI correlated with lower ILO infusion rates and overweight patients showed a higher incidence of AEs compared with patients with normal body weight, including hypotension. In addition, mean basal arterial pressure was not associated with AEs, and higher blood pressure at the beginning of the ILO infusion was not protective against AEs. The pathogenesis of AEs following therapy with ILO is not entirely comprehended, but the main driver is probably the abnormal response to exogenous vasodilatation, even if other mechanisms, such as liquid leakage at digital and the brain blood circulation level, could also play a role.4,10 Our findings are supported by a plausible biologic rationale. In patients with SSc, loss of intracellular junction and hypoxia–reperfusion injury lead to endothelial dysfunction, which is characterized by vascular leaking and altered response to vasodilatation molecules and linked to increased DU risk.19–21 In addition, also obese patients without rheumatic diseases displayed endothelial dysfunction.22 Endothelial dysfunction in obese patients is driven by lower basal nitric oxide availability and overexpression of prostaglandin receptor E2, which is one of the molecular targets of the ILO.23,24 Some studies explored a possible pathogenetic role of different adipokines, which were correlated with endothelial inflammation and profibrotic unbalance in patients with SSc.25 For these reasons, overweight and obese SSc patients might have an ever more deficient counter-regulatory endothelial mechanism linked to both vascular constriction and dilatation alterations. Supporting our hypothesis there is evidence that, in patients with primary Raynaud’s phenomenon, a higher BMI correlates with a greater decline in skin temperature.26 In summary, the increased expression of prostaglandin receptor E2 together with endothelial dysfunctions might well explain our findings.
Lower BMI, malnutrition, and changes in body composition are common features in SSc and are associated with disease duration, gastrointestinal involvement, and ILD.27–29 Coherently with the short disease duration (<3 years) of the study cohort, BMI was mostly in the normality range; therefore, we can consider our patients a good proxy for ILO-naïve patients in the clinical practice. Our findings suggest that body composition and adipose tissue could play a role also in the pathogenesis and in the response to the treatment of SSc and that different bodyweight at the beginning of the disease could reflect different clinical phenotypes in patients with SSc. We believe this study could provide a good starting point in developing a predictive tool to limit AEs incidence during the dose-finding process for ILO therapy in patients with SSc. Clinicians should also be aware that higher basal blood pressure is not protective against ILO-related adverse effects.
This study has strengths and limitations. The key strength of this study is the large population with access to complete medical history and medications. The main limitation is its retrospective nature. In addition, other nonmeasured confounders, such as lean mass and comorbidities, might contribute to ILO tolerance, possibly affecting our results.
Conclusion
This study showed that a gradual titration of ILO IV therapy was well-tolerated in >50% of the patients with SSc. We found that a higher BMI was statistically associated with poor tolerance of ILO infusion rate and higher incidence of AEs, possibly due to endothelial dysfunction related to body composition, as already described in obese and overweight patients without rheumatic diseases. Further studies involving patients undergoing ILO treatment for different diseases could help understand whether the correlation between ILO tolerance and BMI is disease-specific or an expression of impaired vascular adaptation to exogenous dilatation. Overall, our findings could provide a novel insight into the mechanism of action of this drug and promote further research into the body composition role in SSc pathogenesis and stratification of treatment. Individual ILO regimens still need to be tailored to each patient, however.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X221137125 for Higher body mass index is associated with a lower iloprost infusion rate tolerance and higher iloprost-related adverse events in patients with systemic sclerosis by Riccardo Bixio, Giovanni Adami, Eugenia Bertoldo, Alessandro Giollo, Andrea Morciano, Davide Bertelle, Giovanni Orsolini, Luca Idolazzi, Maurizio Rossini and Ombretta Viapiana in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Riccardo Bixio  https://orcid.org/0000-0001-9335-3371
https://orcid.org/0000-0001-9335-3371
Giovanni Adami  https://orcid.org/0000-0002-8915-0755
https://orcid.org/0000-0002-8915-0755
Alessandro Giollo  https://orcid.org/0000-0001-9355-7673
https://orcid.org/0000-0001-9355-7673
Davide Bertelle  https://orcid.org/0000-0002-5901-7742
https://orcid.org/0000-0002-5901-7742
Luca Idolazzi  https://orcid.org/0000-0002-7254-4686
https://orcid.org/0000-0002-7254-4686
Maurizio Rossini  https://orcid.org/0000-0001-9692-2293
https://orcid.org/0000-0001-9692-2293
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Riccardo Bixio, Rheumatology Section, Department of Medicine, University of Verona, Policlinico G.B. Rossi 10, 37134 Verona, Italy.
Giovanni Adami, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Eugenia Bertoldo, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Alessandro Giollo, Division of Rheumatology, University of Padova, Padova, Italy.
Andrea Morciano, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Davide Bertelle, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Giovanni Orsolini, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Luca Idolazzi, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Maurizio Rossini, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Ombretta Viapiana, Rheumatology Section, Department of Medicine, University of Verona, Verona, Italy.
Declarations
Ethics approval and consent to participate: The studies involving human participants were reviewed and approved by our local Ethics Committee (Verona, Italy), and the study was conducted within the protocol 1483CESC, in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written informed consent was obtained from all individual participants included. All patients/participants provided their written informed consent to participate in this study.
Consent for publication: All patients/participants provided their written informed consent to publish the findings of this study.
Author contributions: Riccardo Bixio: Conceptualization; Data curation; Investigation; Methodology; Validation; Writing – original draft; Writing – review & editing.
Giovanni Adami: Data curation; Methodology; Writing – review & editing.
Eugenia Bertoldo: Writing – review & editing.
Alessandro Giollo: Conceptualization; Supervision; Writing – review & editing.
Andrea Morciano: Data curation; Investigation.
Davide Bertelle: Data curation; Investigation.
Giovanni Orsolini: Supervision; Visualization.
Luca Idolazzi: Validation; Visualization.
Maurizio Rossini: Supervision; Validation; Visualization; Writing – review & editing.
Ombretta Viapiana: Validation; Visualization.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The Associate Editor of Therapeutic Advances in Musculoskeletal Disease (Giovanni A is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor was not involved in the decision-making process.
Availability of data and materials: No additional data available.
References
- 1. Di Benedetto P, Ruscitti P, Berardicurti O, et al. Endothelial-to-mesenchymal transition in systemic sclerosis. Clin Exp Immunol. Epub ahead of print 26 March 2021. DOI: 10.1111/cei.13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nagaraja V, Matucci-Cerinic M, Furst DE, et al. Current and future outlook on disease modification and defining low disease activity in systemic sclerosis. Arthritis Rheumatol 2020; 72: 1049–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. García de la Peña Lefebvre P, Nishishinya MB, Nishishinya MB, Pereda CA, et al. Efficacy of Raynaud’s phenomenon and digital ulcer pharmacological treatment in systemic sclerosis patients: a systematic literature review. Rheumatol Int 2015; 35: 1447–1459. [DOI] [PubMed] [Google Scholar]
- 4. Tsou PS, Palisoc PJ, Flavahan NA, et al. Dissecting the cellular mechanism of prostacyclin analog iloprost in reversing vascular dysfunction in scleroderma. Arthritis Rheumatol 2021; 73: 520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 2017; 76: 1327–1339. [DOI] [PubMed] [Google Scholar]
- 6. Caramaschi P, Martinelli N, Volpe A, et al. A score of risk factors associated with ischemic digital ulcers in patients affected by systemic sclerosis treated with iloprost. Clin Rheumatol 2009; 28: 807–813. [DOI] [PubMed] [Google Scholar]
- 7. Kawald A, Burmester GR, Huscher D, et al. Low versus high-dose iloprost therapy over 21 days in patients with secondary Raynaud’s phenomenon and systemic sclerosis: a randomized, open, single-center study. J Rheumatol 2008; 35: 1830–1837. [PubMed] [Google Scholar]
- 8. Negrini S, Magnani O, Matucci-Cerinic M, et al. Iloprost use and medical management of systemic sclerosis-related vasculopathy in Italian tertiary referral centers: results from the PROSIT study. Clin Exp Med 2019; 19: 357–366. [DOI] [PubMed] [Google Scholar]
- 9. Ingegnoli F, Schioppo T, Allanore Y, et al. Practical suggestions on intravenous iloprost in Raynaud’s phenomenon and digital ulcer secondary to systemic sclerosis: systematic literature review and expert consensus. Semin Arthritis Rheum 2019; 48: 686–693. [DOI] [PubMed] [Google Scholar]
- 10. Bellando-Randone S, Bruni C, Lepri G, et al. The safety of iloprost in systemic sclerosis in a real-life experience. Clin Rheumatol 2018; 37: 1249–1255. [DOI] [PubMed] [Google Scholar]
- 11. Grant SM, Goa KL. Iloprost a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs 1992; 43: 889–924. [DOI] [PubMed] [Google Scholar]
- 12. Hoogen F, van den Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann Rheum Dis 2013; 72: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 13. Valentini G, D’Angelo S, Della Rossa A, et al. European Scleroderma Study Group to define disease activity criteria for systemic sclerosis – IV: assessment of skin thickening by modified Rodnan skin score. Ann Rheum Dis 2003; 62: 904–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McMahan ZH. Gastrointestinal involvement in systemic sclerosis: an update. Curr Opin Rheumatol 2019; 31: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffmann-Vold A-M, Maher TM, Philpot EE, et al. The identification and management of interstitial lung disease in systemic sclerosis: evidence-based European consensus statements. Lancet Rheumatol 2020; 2: e71–83. [DOI] [PubMed] [Google Scholar]
- 16. Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) – endorsed by Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 17. von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barsotti S, Lorenzoni V, Di Battista M, et al. Prostanoids in scleroderma microangiopathy: clinical and pharmacoeconomic comparison between two intravenous regimens. Scand J Rheumatol 2021; 50: 307–313. [DOI] [PubMed] [Google Scholar]
- 19. Bruni C, Frech T, Manetti M, et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed. Front Immunol 2018; 9: 2045. https://www.frontiersin.org/article/10.3389/fimmu.2018.02045 (accessed 16 March 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schlez A, Kittel M, Braun S, et al. Endothelium-dependent regulation of cutaneous microcirculation in patients with systemic scleroderma. J Invest Dermatol 2003; 120: 332–334. [DOI] [PubMed] [Google Scholar]
- 21. Silva I, Teixeira A, Oliveira J, et al. Endothelial dysfunction, microvascular damage and ischemic peripheral vasculopathy in systemic sclerosis. Clin Hemorheol Microcirc 2017; 66: 117–130. [DOI] [PubMed] [Google Scholar]
- 22. Li X, Liu H, Zhang Y, et al. A prediction equation to estimate vascular endothelial function in different body mass index populations. Front Cardiovasc Med 2022; 9: 766565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Williams IL, Wheatcroft SB, Shah AM, et al. Obesity, atherosclerosis and the vascular endothelium: mechanisms of reduced nitric oxide bioavailability in obese humans. Int J Obes Relat Metab Disord 2002; 26: 754–764. [DOI] [PubMed] [Google Scholar]
- 24. Elisia I, Lam V, Cho B, et al. Exploratory examination of inflammation state, immune response and blood cell composition in a human obese cohort to identify potential markers predicting cancer risk. PLoS ONE 2020; 15: e0228633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Frommer KW, Neumann E, Müller-Ladner U. Role of adipokines in systemic sclerosis pathogenesis. Eur J Rheumatol 2020; 7: S165–S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giurgea G-A, Mlekusch W, Charwat-Resl S, et al. Brief report: relationship of age and body mass index to skin temperature and skin perfusion in primary Raynaud’s phenomenon. Arthritis Rheumatol 2015; 67: 238–242. [DOI] [PubMed] [Google Scholar]
- 27. Caimmi C, Caramaschi P, Venturini A, et al. Malnutrition and sarcopenia in a large cohort of patients with systemic sclerosis. Clin Rheumatol 2018; 37: 987–997. [DOI] [PubMed] [Google Scholar]
- 28. Rivas-Vargas D. Analysis of body mass index and risk factors of interstitial lung disease in systemic sclerosis patients. Rev Colomb Reumatol Engl Ed 2020; 27: 9–19. [Google Scholar]
- 29. Nagy A, Palmer E, Polivka L, et al. Treatment and systemic sclerosis interstitial lung disease outcome: the overweight paradox. Biomedicines 2022; 10: 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X221137125 for Higher body mass index is associated with a lower iloprost infusion rate tolerance and higher iloprost-related adverse events in patients with systemic sclerosis by Riccardo Bixio, Giovanni Adami, Eugenia Bertoldo, Alessandro Giollo, Andrea Morciano, Davide Bertelle, Giovanni Orsolini, Luca Idolazzi, Maurizio Rossini and Ombretta Viapiana in Therapeutic Advances in Musculoskeletal Disease