Abstract
Background
Sleep problems in children have been increasingly recognized as a major public health issue. Previous research has extensively studied and presented many risk factors and potential mechanisms for children’s sleep problems. In this paper, we aimed to identify and summarize the consequences and implications of child sleep problems.
Data sources
A comprehensive search for relevant English language full-text, peer-reviewed publications was performed focusing on pediatric sleep studies from prenatal to childhood and adolescence in a variety of indexes in PubMed, SCOPUS, and Psych Info published in the past two decades. Both relevant data-based articles and systematic reviews are included.
Results
Many adverse consequences are associated with child sleep deficiency and other sleep problems, including physical outcomes (e.g., obesity), neurocognitive outcomes (e.g., memory and attention, intelligence, academic performance), and emotional and behavioral outcomes (e.g., internalizing/externalizing behaviors, behavioral disorders). Current prevention and intervention approaches to address childhood sleep problems include nutrition, exercise, cognitive–behavioral therapy for insomnia, aromatherapy, acupressure, and mindfulness. These interventions may be particularly important in the context of coronavirus disease 2019. Specific research and policy strategies can target the risk factors of child sleep as well as the efficacy and accessibility of treatments.
Conclusions
Given the increasing prevalence of child sleep problems, which have been shown to affect children’s physical and neurobehavioral wellbeing, understanding the multi-aspect consequences and intervention programs for childhood sleep is important to inform future research direction as well as a public health practice for sleep screening and intervention, thus improving sleep-related child development and health.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-022-00647-w.
Keywords: Behavior, Child sleep, Consequences, Implications, Neurocognitive, Physical, Prevention/intervention
Introduction
Unhealthy sleep in children and adolescents has been continually recognized as a major health issue and has received great attention in recent years by researchers, healthcare providers, families, caregivers, and school nurses/counselors [1–3]. It is estimated that approximately 20%–40% of infants and school-age children have poor sleep health, such as waking overnight, difficulty falling asleep, and difficulty sleeping alone [2, 4]. Also, up to 75% of high school students sleep less than recommended eight hours per night and report impaired sleep quality [5, 6]. Healthy sleep and circadian rhythm are critical to the physical, cognitive, and psychosocial development in children and adolescents [7–10]. For example, insufficient nighttime sleep and daytime sleepiness are associated with poor academic performance [11]. Persistent sleep problems in children predict clinical psychosocial symptoms during adolescence, such as aggression, attention deficits, social anxiety, and depression [12]. Additionally, insufficient sleep and poor sleep quality have been linked to cardiometabolic risk factors (i.e., obesity) [7], which increase the risk for cardiometabolic morbidity and mortality later in life [13–15]. Therefore, elucidating the health consequences of unhealthy sleep and identifying evidence-based sleep interventions will contribute to promoting health and well-being in children and adolescents.
In the current review, we aim to identify the physical, cognitive, and behavioral consequences of poor sleep; outline prevention and intervention strategies; and discuss recommendations for future research and practical applications. We reviewed data-based articles and systematic reviews on pediatric sleep health (2002–2022) for this study (detailed search strategies and inclusion/exclusion criteria were provided in the Supplementary Methods).
Consequences of sleep problems
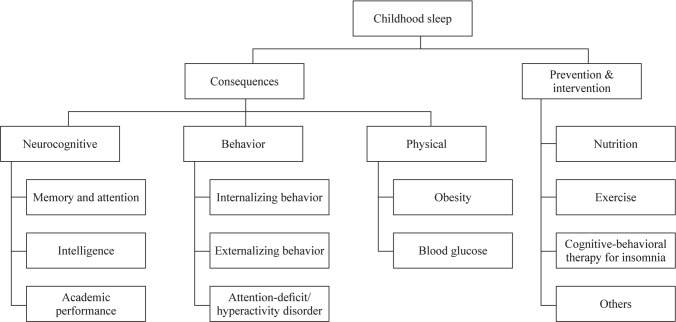
It is important to recognize the consequences of unhealthy sleep throughout childhood and adolescence, particularly during sensitive periods of development (e.g., at 5–6 years when starting school; when going through puberty) [16–18]. The following section details specific common consequences faced by children and adolescents experiencing poor sleep health, which are also outlined in Fig. 1.
Fig. 1.
Childhood sleep and consequences and implications
Physical health outcomes
Unhealthy sleep in children has been linked to many negative health outcomes. Here, we are particularly highlighting its potential role in cardiometabolic factors. Cardiometabolic risk factors (e.g., obesity, high blood pressure, impaired fasting glucose, high triglyceride and low high-density lipoprotein cholesterol levels) develop in childhood and adolescence [13–15]. About 10%–30% of children and adolescents worldwide are overweight or obese [19] and 6%–39% possess multiple cardiometabolic risk factors (e.g., metabolic syndrome) [13–15]. Cardiometabolic risk factors in early life predict cardiovascular disease, type 2 diabetes mellitus, and subsequent mortality in adulthood [13–15]. Unhealthy sleep indicators, such as short or very long sleep, fragmented sleep, insomnia symptoms and daytime sleepiness, are robust predictors of obesity, impaired glucose metabolism and high blood pressure among children and adolescents [7]. Unhealthy sleep may alter levels of appetite-regulating hormones (i.e., leptin, ghrelin), glucose metabolism and inflammatory biomarkers, thereby affecting cardiometabolic factors [20]. However, findings on the association between sleep duration and blood lipids are inconsistent [21]. The following section provides a literature review of the most common cardiometabolic risk factors (obesity and impaired glucose metabolism) among children and adolescents.
Obesity
Childhood and adolescent obesity has increased significantly in the past few decades and is now considered a major public health concern [22]. Many factors contribute to the rise of obesity, including screen media overuse [23], lack of exercise [23], and obesogenic diet [24]. However, more attention recently has been given to sleep as a risk factor [25, 26]. Prior research has consistently shown that shorter sleep duration is correlated with childhood obesity [25, 27–32], particularly among preschool-aged children [30, 32] and adolescents [28, 33]. A longitudinal study found that shorter sleep increased the risk of central obesity for girls [26]. Furthermore, both insufficient and excessive sleep were linked to short-term and long-term hyperglycemia in obese adolescents [34]. More current research reports that daytime sleepiness [35], midday napping [33], poor sleep quality [36], weekday–weekend sleep variability [37], greater sleep disturbances (e.g., night awakenings or overall disturbance score) [38], and a delayed sleep phase pattern [29] are also associated with obesity. However, the directionality of the relationship between sleep and obesity is unclear, as literature also reports that obese children are more likely to sleep for shorter durations and have more length variability between the weekdays and weekends compared with children classified as overweight, normal, or underweight [37]. Body processes that occur during sleep greatly impact the growth and wellbeing of children and adolescents through the regulation of hormone secretions [39, 40]. The potential mechanism for short sleep duration resulting in obesity could be that changes in hormone levels of leptin, ghrelin, insulin, cortisol, and growth hormone influence weight and nutrition [25, 39, 41]. Future research should focus on the connection between obesity and sleep duration, as well as the directionality of the relationship. Longitudinal studies may offer repeated measures of sleep assessments, in which potential confounding variables should be controlled such as physical activity, eating behaviors, and screen time.
Impaired glucose metabolism
Adolescents exhibit pubertal-related decreases in insulin sensitivity [42, 43] and changes in intrinsic sleep regulation, putting them at high risk for sleep-related impairment in glucose metabolism [43–45]. Despite mixed findings [46], a growing number of studies have shown that insufficient sleep and lower sleep efficiency are associated with lower insulin sensitivity [47], greater homeostatic model assessment for insulin resistance [48] and higher fasting glucose levels among adolescents with and without obesity [49]. In a sleep restriction study (6.5 hours/day), a split sleep schedule (5-hour nighttime sleep with a 1.5-hour afternoon nap) had a greater impact on blood glucose than continuous nighttime sleep. Indeed, the role of naps in glucose metabolism is controversial. Evidence suggests habitual midday naps (≥ 3 days/week) are associated with an increased risk of impaired glucose metabolism indicated by high fasting blood glucose, especially among adolescents who self-reported at least 9 hours of nighttime sleep. These findings highlight the importance of a moderate amount of sleep within a 24-hour period.
Neurocognitive outcomes
Cognitive development is one of the most important areas of development throughout childhood. Historically, research has largely focused on nutrition [50], environmental stimulation [51], and parenting [52] in relation to cognitive development. Over the past few decades, emerging research from both experimental and longitudinal observational studies also demonstrate its relationship with sleep [53, 54].
Insufficient sleep and impaired sleep quality have been shown to affect neurocognitive functions among children and adolescents, including attention, intelligence, and academic performance [9–11, 55]. For example, frequent nighttime awakenings are associated with poor cognitive functioning in toddlers [8]. Infants with nighttime awakenings for 2 times/night were found to have significantly higher mental development index score, as compared to those with more frequent nighttime awakenings [8]. We provide a literature review on primary neurocognitive outcomes below.
Memory and attention
Due to the importance of sleep in memory consolidation [33, 56], the occurrence of sleep problems has been shown to impair both memory retention and attentive behavior. Among school-aged children, those who frequently nap have faster reaction times on spatial memory functioning tasks than those who do not [33]. Additionally, those experiencing high-quality sleep of sufficient duration show better memory consolidation and recall of verbal and novel words [56]. Short sleep duration can adversely affect memory, including measurable impairments of short-term and working memory [9, 10].
Attention is also associated with sleep quality and duration in children and adolescents. In infants, lower sleep quality predicts compromised attention regulation [57]. Similarly, attention problems, such as hyperactive and inattentive behavior, in children [58] and adolescents [59] are associated with short sleep duration as a result of snoring and difficulty initiating and maintaining sleep. In regard to sleep duration, children who sleep for fewer hours during the night show measurable impairments in attention and greater levels of attention deficit hyperactivity disorder (ADHD)-like symptoms [60], such as greater levels of inattention [10] and distraction [61]. These associations are similarly seen in adolescents, particularly in classroom environments. Adolescents with shorter nighttime sleep durations demonstrate more inattentive and oppositional behaviors the following day [62] as well as diminished learning and unmindful manners in the classroom [53].
Intelligence
Longer sleep duration is associated with higher neurocognitive functioning, better overall IQ scores, and improved performance in perceptual reasoning and intellectual competence [63, 64]. Conversely, impaired performance on cognitive tasks is seen in both healthy and ADHD-diagnosed children with shortened sleep duration [65]. Sleep-disordered breathing (SDB), daytime sleepiness, and general sleep problems are also associated with decreased intelligence [66, 67]. Children with greater sleep problems and fatigue score lower on measures of verbal intelligence quotient, performance intelligence quotient, and full-scale intelligence quotient [55].
Academic performance
Consequences of disturbances to sleep can be reflected in the scholastic achievements of children and adolescents. In particular, daytime sleepiness has been demonstrated to significantly decrease academic achievement among studies in Finland [68], the Netherlands [69], China [11], and South Korea [70]. Less nighttime sleep can impair academic success, whereas longer sleep duration is positively associated with overall better academic performance [11, 63]. Furthermore, children with sleep problems during the night due to SDB and snoring show poor academic performance [71] and perform worse on measures of executive and academic functioning [66], mathematics, and spelling [58]. Specifically, among adolescents, those suffering from poor sleep quality are more likely to have both lower self-reported end-of-term grades and parent-reported academic performance [69, 72]. In China, during periods of high academic demand and stress, the vast majority (94.4%) of high school adolescents had a sleep duration of less than eight hours, and prolonged sleep latency was associated with poorer self-reported academic performance [73]. In actuality, sacrificing sleep to study longer does not lead to a better academic score [74].
Emotional and behavioral outcomes
Children and adolescents’ mental health well-being has been increasingly recognized as public health concerns, which contribute to child development and milestone achievement. The prevalence of behavioral and conduct disorders in children and adolescents has risen in recent years. These disorders include ADHD, anxiety, depression, oppositional defiant disorder (ODD) and conduct disorder. From the 2016 National Survey of Children's Health with a representative sample, it is estimated that about 7.4% of children have behavioral and/or conduct disorders, 7.1% have anxiety, and 3.2% experience depression in the United States [75]. ADHD may be prevalent in up to 9.8% of 3- to 17-year-old children in the United States [76]. While many factors have been contributed to these mental health problems, both laboratory and epidemiological studies have demonstrated the role of sleep in the development of the brain [8, 77, 78], which further impacts emotion and behavior [78]. Studies have shown that longer nighttime sleep duration and fewer sleep disturbances are associated with a more mature empathy pattern in young preschoolers [79]. The associations are more prominent in children at the higher end of the empathy spectrum and vary by sex [79]. Conversely, sleep problems can be both a predictor and outcome of negative behavior problems including internalizing behaviors (directed towards oneself) and externalizing behaviors (directed towards others) in children [80, 81]. For example, disruptive sleep in children and adolescents have been linked to depression, anxiety, aggression, and ADHD [80, 81]. We provide a detailed review in the following sections.
Internalizing behaviors
The most commonly observed internalizing behaviors among children and adolescents are depression and anxiety [80]. Studies show that shortened sleep durations are highly correlated to internalizing behaviors, as measured by parental and teacher reports as well as behavioral tasks [82, 83]. Specifically, depression is known to be associated with sleep deficiency [84, 85] and poor sleep quality [72]. Anxiety, a comorbid condition with depression, also increases with decreased sleep duration [86, 87]. School-aged children who experience persistent sleep problems have an increased risk of anxious and depressed mood [12]. Additionally, excessive daytime sleepiness is strongly associated with parental reports of anxiety and depression [35]. Furthermore, sleep problems in adolescents predict an increased prevalence of anxiety and depression symptoms over time [59] as well as predict diagnoses of generalized anxiety disorder [88].
Externalizing behaviors
Disruptions to normal sleep patterns may also increase externalizing behaviors. Shorter sleep is associated with more observed and parental-reported behavioral problems and more rule-breaking [60, 89], and predicts the occurrence of high externalizing behavior [82]. Among adolescents, those with variable sleep patterns (e.g., duration, timing) between the weekdays and weekends demonstrate higher levels of self- and parent-reported aggression [59, 90]. Furthermore, general difficulties with sleep and the occurrence of sleep problems may affect daytime behavior in children and adolescents [81]. In preschool-aged children, parental report of sleep difficulties is associated with teacher-reported externalized behaviors [83]. Additionally, children experiencing persistent sleep difficulties have an increased risk of aggression [12]. A longitudinal study of African American adolescents demonstrated that sleep disturbances were associated with adolescent aggression, such as carrying, handling, and utilizing a knife and/or gun and quick temperedness [91]. To note, sleep problems in preschoolers are associated with externalizing behaviors in adolescents [92].
ADHD and other behavioral disorders
Unhealthy sleep in children and adolescents may exacerbate the symptoms of behavioral disorders such as ADHD [93–95]. Both short [93] and long sleep duration [94], actigraphy-assessed sleep fragmentation (i.e., frequent and long wake after sleep onset) [95], more frequent daytime napping, insomnia, sleep terrors, sleep-talking, snoring, and bruxism [94] are associated with ADHD. Using self-reports and parental reports, a cross-sectional study found that ADHD-related behavioral problems were associated with difficulty falling and staying asleep in adolescents [96]. Children with shorter sleep duration are at increased risk of inattention, impulsivity, and hyperactivity [61, 97, 98] and higher scores on a parent-reported ADHD measure [97]. Shorter sleep duration and more sleep disturbances appear earlier in children who have ADHD, and these changes in sleep patterns are observed before the typical age of an ADHD clinical diagnosis [99]. Similarly, among adolescents with ADHD, changes in normal sleep patterns, such as regular daytime napping and later wake-up times, are associated with increased rates of inattention, hyperactivity, and impulsivity [94]. Consequences on other behavioral disorders, such as ODD and conduct disorder, are less clear. A longitudinal study followed up on 1420 children for 4–7 years and found that sleep problems predicted increases in the prevalence of later ODD, and in turn, ODD predicted increases in sleep problems over time [88]. Despite the limited evidence, screening children and adolescents for unhealthy sleep could offer promising opportunities for reducing the burden of mental and behavioral illness.
An important related question is whether sleep and behavior problems have a reciprocal relationship. An increasing number of studies are examining this in children and adolescents, but results across studies are inconsistent. There are studies showing bidirectional association [81, 100–102] and others finding no such relationship [103, 104]. For example, a recent longitudinal cohort study supports a bidirectional relationship: sleep problems in Chinese children at age six were associated with the development of new behavior problems at age 11.5, and vice versa [92]. This is in line with results from some [100, 102] but not all [81] studies. Quach and collegues reported that a bidirectional relationship existed between sleep and externalizing but not internalizing behavior. Assessing both sleep and behavior in pediatric settings is critically important for pediatric practitioners to identify other problems. Further research is required to fully understand this entwined relationship.
Prevention and interventions
Given the negative consequences of unhealthy sleep, there is a substantial need to identify effective measures to prevent and mitigate childhood sleep problems. At the system/community level, interventions including delaying school start time by just 30 minutes result in significantly increased sleep duration [105]. A recent study similarly found that delayed school start times could decrease the need to catch up on sleep during weekends [106]. In addition to sleeping more, students also reported greater sleep satisfaction and motivation [105], decreased daytime sleepiness and fatigue [107], and less depressed mood [108]. In this section, we further discuss evidence-based sleep interventions for children and adolescents on individual levels (Supplementary Table 1).
Nutritional intervention
Dietary intake and sleep are interwoven health behaviors associated with child development and may be amenable to intervention. In line with observational studies, experimental research shows the sleep benefits of improved nutritional intake. Among infants, those who receive iron–folic acid supplementation have longer nocturnal and total sleep duration. Zinc supplementation reduces the length of naps and increased total sleep duration [109]. However, there are no apparent sleep benefits of combined iron-folic acid and zinc supplements, suggesting a possible antagonism between iron-folic acid and zinc [109]. Additionally, exposure to omega-3 long-chain polyunsaturated fatty acids (LC-PUFA) through supplementation or dietary intervention may improve sleep organization and maturity in infants and improve sleep disturbance in children with clinical-level sleep problems [110, 111]. However, recommendations for taking omega-3 LC-PUFA to improve sleep outcomes should be taken with caution as this area of study is still inconclusive. Further investigation is also warranted to confirm the effect of omega-3 LC-PUFA intervention on sleep health in different populations. Furthermore, a recent randomized control trial was conducted on a sample of healthy lean and short children (5.6 years old) and utilized a range of nutritional supplements encompassing 25% of the recommended dietary reference intake for calories, was high in protein, and contained vitamins A, C and D, iron and zinc. Children who took at least 50% of the recommended dose had shorter sleep latency than those with poor adherence, and sleep duration was correlated with increased caloric intake, protein and carbohydrate intake per kilogram [112].
The type and timing of dietary intake may improve sleep–wake function, supporting the concept of chrononutrition. Accordingly, researchers have developed nighttime cereals enriched with sleep facilitators including tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate for infants and toddlers with sleep disorders. When ingested during night hours, infants with sleep disorders display longer nighttime sleep time, reduced sleep latency and improved sleep efficiency [113]. Similarly, earlier introduction of solid foods may improve infant sleep. Compared with infants exclusively breastfed until six months old, those who are introduced to solids at the age of three months exhibit longer sleep duration, less frequent waking at night, and fewer serious sleep problems [114].
Exercise
Exercise has been associated with improvement in sleep health [115, 116]. Slow-wave sleep is associated with cerebral restoration and recuperation [117] and is important for memory and learning [118]. Engaging in 30 minutes of high-intensity exercise significantly elevates the proportion of slow-wave sleep, increases sleep efficiency, and shortens sleep onset latency in school-aged children [119]. In a study of adolescents, those who ran 30 minutes every weekday morning for three consecutive weeks demonstrated improvements in both objectives (e.g., increased slow-wave sleep and decreased sleep latency) and subjective (e.g., enhanced sleep quality, mood, and concentration and reduced daytime sleepiness) sleep measures [120]. These findings suggest that promoting the involvement of children and adolescents in organized sports leagues or simply playing outside are potentially feasible and low-cost methods to mitigate sleep problems and subsequent adverse health outcomes.
Cognitive–behavioral therapy for insomnia (CBT-I)
CBT-I, the first-line treatment for adulthood insomnia, has been unitized to improve sleep health in adolescents with and without diagnosed insomnia [121–123]. Multimodal CBT-I consists of a combination of cognitive therapy (restructuring negative sleep beliefs or reducing excessive worry about sleep), behavioral interventions (e.g., sleep restriction and stimulus control), educational interventions (e.g., sleep hygiene) and relaxation (e.g., progressive muscle relaxation, guided imagery, and/or breathing techniques). CBT-I modifies the patterns of thinking and behavior underlying sleep disturbances, such as poor sleep hygiene, irregular sleep–wake schedules, delayed bedtimes, pre-sleep hyperarousal, and maladaptive sleep-related cognitions [122]. For early childhood, the cognitive component focuses on parental education programs as well as on altering sleep-related cognitions and beliefs of parents regarding the sleep of their child [124]. There are fewer studies on children and adolescents compared with the CBT-I studies on adults. However, emerging evidence has shown improvement in sleep latency, efficiency and quality, and wake-after-sleep onset after 4–12 weeks of CBT-I in children and adolescents, despite mixed findings of several sleep variables (e.g., total sleep time) [121–124]. In terms of delivery modality, self-administered digital CBT-I may overcome staff and cost constraints with a comparable sleep effect [121]. However, further high-quality RCTs are needed to test the cost-effectiveness of different CBT-I features, such as delivery format (i.e., individual vs. group, digital vs. face-to-face), length of treatment (i.e., short vs. long) and selection of CBT-I components.
Others: aromatherapy, acupressure, mindfulness
Researchers have begun investigating the potential use of aromatherapy among infant populations, though there is a lack of evidence on children and adolescents. Specifically, the use of lavender-scented lotion by mothers on their infants is associated with increased sleep duration and fewer nighttime sleep disturbances [125]. Similarly, infants bathed in lavender bath oil cry less prior to sleep and spend more time in deep sleep [126].
Acupressure therapy utilizes pressure and massage to stimulate the body [127]. Among a sample of adolescents with insomnia, those who receive acupressure stimulation during sleep via a wrist device demonstrate a significant increase in sleep duration as well as reductions in sleep onset latency, wake after sleep onset, and stage 2 sleep [128].
Finally, mindfulness has been identified as a potential nonpharmacologic intervention for better sleep health in children and adolescents. A middle school curriculum that combined Tai Chi with mindfulness practice demonstrated improved self-reports of sleep quality and sleep latency in students [129]. Among adolescents, similar improvements in sleep quality are linked to mindfulness-based treatments such as mediation practices [130] and structured programs including mindfulness-based stress reduction, yoga, and group discussion [131]. A potential mechanism of action is rumination: mindfulness reduces rumination, which is linked to poorer sleep quality, thus improving sleep quality [132].
Gaps in literature, future directions, and implications
This review discusses the current knowledge available regarding the adverse health consequences associated with poor sleep health, and potential interventions to prevent and mitigate these negative physical, mental, and behavioral health problems requiring further research. The majority of published articles on the topic of childhood and adolescent sleep utilize cross-sectional study designs. Additional research employing cohort or longitudinal study designs may elucidate the causality of these relationships. Furthermore, a recent concept analysis [133] calls for the employment of a comprehensive methodology in investigating the impact of sleep quality on children, which includes assessing how certain events (e.g., school breaks) may influence childhood sleep.
Prior research mainly focused on individual sleep characteristics in relation to health outcomes. However, sleep problems have multidimensional manifestations that often coexist in the pediatric population [2, 17]. Thus, using big data techniques to define high-risk sleep phenotypes (combination of sleep duration, quality, and circadian rhythm) that are associated with increased pediatric morbidity and mortality will inform more meaningful interventions for sleep-related health conditions. Also, sleep is often comorbid with other health conditions (e.g., depressive symptoms [88]) in influencing childhood functioning and diseases. Thus, symptom clusters should be considered in assessments and interventions for sleep-related health outcomes.
In terms of prevention and intervention techniques, current research is not sufficient to demonstrate and support their efficacy. Furthermore, increased focus on treatments that are not only effective but also simple, affordable, and easily accessible, such as exercise, are needed to ensure widespread use. Due to the importance of sleep on physical, mental, and behavioral health during developmentally sensitive periods [2, 17], the availability of such prevention and intervention strategies are critical in improving child and adolescent health. Community-based trials are warranted to test the efficacy of multi-level interventions in real-life settings, instead of highly-controlled clinical trial conditions.
In the context of the coronavirus disease 2019 (COVID-19) pandemic, recent studies have shown that children and adolescents are subject to sleep changes [134]. Promoting sleep health should be integrated into strategies to mitigate the effects of home confinement on children’s mental health during the COVID-19 outbreak [135].
Finally, given the significance of child sleep, it is important to highlight both practical and policy implications. Practical implications may focus on risk factors attributed to sleep as well as consistently assessing sleep quality in pediatric populations [133]. Policy implications are especially important to address the public health issue of child and adolescent sleep. As early as 2014, the American Academy of Pediatrics proposed the delay of school start times to extend child and adolescent sleep duration [136]. More recently, the American Academy of Nursing proposed addressing screen media practices to reduce sleep disturbances and adverse sleep outcomes [137]. Globally, the Ministry of Education of China launched a policy regarding delaying school start times for adolescents and enhancing sleep health, and this policy showed great benefits not only for child sleep health but also for physical and mental health as well as academic performance [138]. Given that there are many risk factors related to child sleep and given that there have been benefits from interventions targeted to these risk factors, we hope that future policies will continue to address these issues.
Conclusions
In conclusion, a variety of aspects of children’s growth and development is affected by childhood sleep. Considering that sleep problems are widespread among children of all age groups as well as their negative consequences on children’s physical and mental wellbeing, understanding and developing potential prevention and intervention efforts is important to guide future research and healthcare practices to mitigate sleep problems both in childhood and in later life.
Supplementary Information
Below is the link to the electronic supplementary material.
Author’s contribution
LJ conceptualized and designed the study, drafted the article, and revised it critically. JX drafted the article, and revised it critically. PS and RE drafted the article. LT, WG and JF revised the manuscript critically. All authors approve of the final manuscript to be published.
Funding
This study is funded by the National Institute of Child Health and Development (NIH/NICHD R01-HD087485). During the study, WG and JF were supported by the National Natural Science Foundation of China (Nos. 82073568, 82071493), Innovative Research Team of High-level Local Universities in Shanghai (Nos. SHSMU-ZDCX20211100, 20211900), Science and Technology Commission of Shanghai Municipality (No. 2018SHZDZX05), and Shanghai Municipal Health Commission (Nos. 2022XD056, 2020CXJQ01).
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethical approval
Not applicable.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. Author Fan Jiang is an Associate Editor for World Journal of Pediatrics. The paper was handled by the other Editor and has undergone rigorous peer review process. Author Fan Jiang was not involved in the journal's review of, or decisions related to this manuscript. The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ranum BM, Wichstrøm L, Pallesen S, Steinsbekk S. Prevalence and stability of insufficient sleep measured by actigraphy: a prospective community study. Pediatr Res. 2020;88:110–116. doi: 10.1038/s41390-020-0768-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Honaker SM, Meltzer LJ. Sleep in pediatric primary care: a review of the literature. Sleep Med Rev. 2016;25:31–39. doi: 10.1016/j.smrv.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Becker SP, Gregory AM. Editorial perspective: perils and promise for child and adolescent sleep and associated psychopathology during the COVID-19 pandemic. J Child Psychol Psychiatry. 2020;61:757–759. doi: 10.1111/jcpp.13278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson AA, Mindell JA, Hiscock H, Quach J. Child sleep behaviors and sleep problems from infancy to school-age. Sleep Med. 2019;63:5–8. doi: 10.1016/j.sleep.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleep Foundation. How electronics affect sleep. 2011. https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2011-technology-and-sleep. Accessed 8 Apr 2018.
- 6.Gradisar M, Gardner G, Dohnt H. Recent worldwide sleep patterns and problems during adolescence: a review and meta-analysis of age, region, and sleep. Sleep Med. 2011;12:110–118. doi: 10.1016/j.sleep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Matthews KA, Pantesco EJ. Sleep characteristics and cardiovascular risk in children and adolescents: an enumerative review. Sleep Med. 2016;18:36–49. doi: 10.1016/j.sleep.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W, Li SX, Jiang Y, Xu X, Spruyt K, Zhu Q, et al. A community-based study of sleep and cognitive development in infants and toddlers. J Clin Sleep Med. 2018;14:977–984. doi: 10.5664/jcsm.7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gradisar M, Terrill G, Johnston A, Douglas P. Adolescent sleep and working memory performance. Sleep Biol Rhythms. 2008;6:146–154. doi: 10.1111/j.1479-8425.2008.00353.x. [DOI] [Google Scholar]
- 10.Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J Pediatr Psychol. 2013;38:1058–1069. doi: 10.1093/jpepsy/jst033. [DOI] [PubMed] [Google Scholar]
- 11.Li S, Arguelles L, Jiang F, Chen W, Jin X, Yan C, et al. Sleep, school performance, and a school-based intervention among school-aged children: a sleep series study in China. PLoS One. 2013;8:e67928. doi: 10.1371/journal.pone.0067928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simola P, Liukkonen K, Pitkäranta A, Pirinen T, Aronen ET. Psychosocial and somatic outcomes of sleep problems in children: a 4-year follow-up study. Child Care Health Dev. 2014;40:60–67. doi: 10.1111/j.1365-2214.2012.01412.x. [DOI] [PubMed] [Google Scholar]
- 13.DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of metabolic syndrome as a predictor of cardiovascular disease between childhood and adulthood: the Princeton Lipid Research Cohort Study. J Am Coll Cardiol. 2015;66:755–757. doi: 10.1016/j.jacc.2015.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagman E, Danielsson P, Brandt L, Ekbom A, Marcus C. Association between impaired fasting glycaemia in pediatric obesity and type 2 diabetes in young adulthood. Nutr Diabetes. 2016;6:e227. doi: 10.1038/nutd.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y, Hou D, Zhao X, Wang L, Hu Y, Liu J, et al. Childhood obesity affects adult metabolic syndrome and diabetes. Endocrine. 2015;50:87–92. doi: 10.1007/s12020-015-0560-7. [DOI] [PubMed] [Google Scholar]
- 16.Galván A. The need for sleep in the adolescent brain. Trends Cogn Sci. 2020;24:79–89. doi: 10.1016/j.tics.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer LJ, Williamson AA, Mindell JA. Pediatric sleep health: it matters, and so does how we define it. Sleep Med Rev. 2021;57:101425. doi: 10.1016/j.smrv.2021.101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutil C, Walsh JJ, Featherstone RB, Gunnell KE, Tremblay MS, Gruber R, et al. Influence of sleep on developing brain functions and structures in children and adolescents: a systematic review. Sleep Med Rev. 2018;42:184–201. doi: 10.1016/j.smrv.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandner MA. Addressing sleep disturbances: an opportunity to prevent cardiometabolic disease? Int Rev Psychiatry. 2014;26:155–176. doi: 10.3109/09540261.2014.911148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun J, Wang M, Yang L, Zhao M, Bovet P, Xi B. Sleep duration and cardiovascular risk factors in children and adolescents: a systematic review. Sleep Med Rev. 2020;53:101338. doi: 10.1016/j.smrv.2020.101338. [DOI] [PubMed] [Google Scholar]
- 22.Sanyaolu A, Okorie C, Qi X, Locke J, Rehman S. Childhood and adolescent obesity in the United States: a public health concern. Glob Pediatr Health. 2019;6:2333794X19891305. doi: 10.1177/2333794X19891305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Riesch S, Tien J, Lipman T, Pinto-Martin J, O'Sullivan A. Screen media overuse and associated physical, cognitive, and emotional/behavioral outcomes in children and adolescents: an integrative review. J Pediatr Health Care. 2022;36:99–109. doi: 10.1016/j.pedhc.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liberali R, Kupek E, de Assis MAA. Dietary patterns and childhood obesity risk: a systematic review. Child Obes. 2020;16:70–85. doi: 10.1089/chi.2019.0059. [DOI] [PubMed] [Google Scholar]
- 25.Fu J, Wang Y, Li G, Han L, Li Y, Li L, et al. Childhood sleep duration modifies the polygenic risk for obesity in youth through leptin pathway: the Beijing Child and Adolescent Metabolic Syndrome cohort study. Int J Obes (Lond) 2019;43:1556–1567. doi: 10.1038/s41366-019-0405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma L, Ding Y, Chiu DT, Wu Y, Wang Z, Wang X, et al. A longitudinal study of sleep, weight status, and weight-related behaviors: Childhood Obesity Study in China Mega-cities. Pediatr Res. 2021;90:971–979. doi: 10.1038/s41390-021-01365-1. [DOI] [PubMed] [Google Scholar]
- 27.Chaput JP, Lambert M, Gray-Donald K, McGrath JJ, Tremblay MS, O’Loughlin J, et al. Short sleep duration is independently associated with overweight and obesity in Quebec children. Can J Public Health. 2011;102:369–374. doi: 10.1007/BF03404179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drescher AA, Goodwin JL, Silva GE, Quan SF. Caffeine and screen time in adolescence: associations with short sleep and obesity. J Clin Sleep Med. 2011;7:337–342. doi: 10.5664/JCSM.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarrin DC, McGrath JJ, Drake CL. Beyond sleep duration: distinct sleep dimensions are associated with obesity in children and adolescents. Int J Obes (Lond) 2013;37:552–558. doi: 10.1038/ijo.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang F, Zhu S, Yan C, Jin X, Bandla H, Shen X. Sleep and obesity in preschool children. J Pediatr. 2009;154:814–818. doi: 10.1016/j.jpeds.2008.12.043. [DOI] [PubMed] [Google Scholar]
- 31.Kong AP, Wing YK, Choi KC, Li AM, Ko GTC, Ma RC, et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011;12:659–665. doi: 10.1016/j.sleep.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Shi Z, Taylor AW, Gill TK, Tuckerman J, Adams R, Martin J. Short sleep duration and obesity among Australian children. BMC Public Health. 2010;10:609. doi: 10.1186/1471-2458-10-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji X, Li J, Liu J. The relationship between midday napping and neurocognitive function in early adolescents. Behav Sleep Med. 2019;17:537–551. doi: 10.1080/15402002.2018.1425868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koren D, Katz LEL, Brar PC, Gallagher PR, Berkowitz RI, Brooks LJ. Sleep architecture and glucose and insulin homeostasis in obese adolescents. Diabetes Care. 2011;34:2442–2447. doi: 10.2337/dc11-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calhoun SL, Vgontzas AN, Fernandez-Mendoza J, Mayes SD, Tsaoussoglou M, Basta M, et al. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep. Sleep. 2011;34:503–507. doi: 10.1093/sleep/34.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ischander MM, Lloyd RD., Jr Severe paediatric obesity and sleep: a mutual interactive relationship! J Sleep Res. 2021;30:e13162. doi: 10.1111/jsr.13162. [DOI] [PubMed] [Google Scholar]
- 37.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morrissey B, Taveras E, Allender S, Strugnell C. Sleep and obesity among children: a systematic review of multiple sleep dimensions. Pediatr Obes. 2020;15:e12619. doi: 10.1111/ijpo.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J, Zhang A, Li L. Sleep duration and overweight/obesity in children: review and implications for pediatric nursing. J Spec Pediatr Nurs. 2012;17:193–204. doi: 10.1111/j.1744-6155.2012.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 41.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ball GDC, Huang TTK, Gower BA, Cruz ML, Shaibi GQ, Weigensberg MJ, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and β-cell function during puberty. J Pediatr. 2006;148:16–22. doi: 10.1016/j.jpeds.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 43.Reinehr T. Metabolic syndrome in children and adolescents: a critical approach considering the interaction between pubertal stage and insulin resistance. Curr Diab Rep. 2016;16:8. doi: 10.1007/s11892-015-0695-1. [DOI] [PubMed] [Google Scholar]
- 44.Zimmet P, Alberti KGM, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 45.Arslanian S, Kim JY, Nasr A, Bacha F, Tfayli H, Lee S, et al. Insulin sensitivity across the lifespan from obese adolescents to obese adults with impaired glucose tolerance: who is worse off? Pediatr Diabetes. 2018;19:205–211. doi: 10.1111/pedi.12562. [DOI] [PubMed] [Google Scholar]
- 46.Fobian AD, Elliott L, Louie T. A systematic review of sleep, hypertension, and cardiovascular risk in children and adolescents. Curr Hypertens Rep. 2018;20:42. doi: 10.1007/s11906-018-0841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Bernardi Rodrigues AM, da Silva CC, Vasques AC, Camilo DF, Barreiro F, Cassani RS, et al. Association of sleep deprivation with reduction in insulin sensitivity as assessed by the hyperglycemic clamp technique in adolescents. JAMA Pediatr. 2016;170:487–494. doi: 10.1001/jamapediatrics.2015.4365. [DOI] [PubMed] [Google Scholar]
- 48.Peplies J, Börnhorst C, Günther K, Fraterman A, Russo P, Veidebaum T, et al. Longitudinal associations of lifestyle factors and weight status with insulin resistance (HOMA-IR) in preadolescent children: the large prospective cohort study IDEFICS. Int J Behav Nutr Phys Act. 2016;13:97. doi: 10.1186/s12966-016-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Li AM, Au CT, Kong APS, Zhang J, Wong CK, et al. Association between sleep architecture and glucose tolerance in children and adolescent. J Diabetes. 2015;7:10–15. doi: 10.1111/1753-0407.12138. [DOI] [PubMed] [Google Scholar]
- 50.Morley R, Lucas A. Nutrition and cognitive development. Br Med Bull. 1997;53:123–134. doi: 10.1093/oxfordjournals.bmb.a011595. [DOI] [PubMed] [Google Scholar]
- 51.Vandenbroucke L, Spilt J, Verschueren K, Piccinin C, Baeyens D. The classroom as a developmental context for cognitive development: a meta-analysis on the importance of teacher–student interactions for children’s executive functions. Rev Educ Res. 2018;88:125–164. doi: 10.3102/0034654317743200. [DOI] [Google Scholar]
- 52.Jeong J, Franchett EE, Ramos de Oliveira CV, Rehmani K, Yousafzai AK. Parenting interventions to promote early child development in the first 3 years of life: a global systematic review and meta-analysis. PLoS Med. 2021;18:e1003602. doi: 10.1371/journal.pmed.1003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beebe DW, Rose D, Amin R. Attention, learning, and arousal of experimentally sleep-restricted adolescents in a simulated classroom. J Adolesc Health. 2010;47:523–525. doi: 10.1016/j.jadohealth.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stormark KM, Fosse HE, Pallesen S, Hysing M. The association between sleep problems and academic performance in primary school-aged children: findings from a Norwegian longitudinal population-based study. PLoS One. 2019;14:e0224139. doi: 10.1371/journal.pone.0224139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Zhou G, Wang Y, Ai Y, Pinto-Martin J, Liu X. Sleep problems, fatigue, and cognitive performance in Chinese kindergarten children. J Pediatr. 2012;161:520–5.e2. doi: 10.1016/j.jpeds.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Henderson LM, Weighall AR, Brown H, Gareth GM. Consolidation of vocabulary is associated with sleep in children. Dev Sci. 2012;15:674–687. doi: 10.1111/j.1467-7687.2012.01172.x. [DOI] [PubMed] [Google Scholar]
- 57.Sadeh A, De Marcas G, Guri Y, Berger A, Tikotzky L, Bar-Haim Y. Infant sleep predicts attention regulation and behavior problems at 3–4 years of age. Dev Neuropsychol. 2015;40:122–137. doi: 10.1080/87565641.2014.973498. [DOI] [PubMed] [Google Scholar]
- 58.Brockmann PE, Urschitz MS, Schlaud M, Poets CF. Primary snoring in school children: prevalence and neurocognitive impairments. Sleep Breath. 2012;16:23–29. doi: 10.1007/s11325-011-0480-6. [DOI] [PubMed] [Google Scholar]
- 59.Coulombe JA, Reid GJ, Boyle MH, Racine Y. Sleep problems, tiredness, and psychological symptoms among healthy adolescents. J Pediatr Psychol. 2010;36:25–35. doi: 10.1093/jpepsy/jsq028. [DOI] [PubMed] [Google Scholar]
- 60.Pesonen AK, Räikkönen K, Paavonen EJ, Heinonen K, Komsi N, Lahti J, et al. Sleep duration and regularity are associated with behavioral problems in 8-year-old children. Int J Behav Med. 2010;17:298–305. doi: 10.1007/s12529-009-9065-1. [DOI] [PubMed] [Google Scholar]
- 61.Gruber R, Michaelsen S, Bergmame L, Frenette S, Bruni O, Fontil L, et al. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat Sci Sleep. 2012;4:33–40. doi: 10.2147/NSS.S24607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Becker SP, Epstein JN, Tamm L, Tilford AA, Tischner CM, Isaacson PA, et al. Shortened sleep duration causes sleepiness, inattention, and oppositionality in adolescents with attention-deficit/hyperactivity disorder: findings from a crossover sleep restriction/extension study. J Am Acad Child Adolesc Psychiatry. 2019;58:433–442. doi: 10.1016/j.jaac.2018.09.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gruber R, Laviolette R, Deluca P, Monson E, Cornish K, Carrier J. Short sleep duration is associated with poor performance on IQ measures in healthy school-age children. Sleep Med. 2010;11:289–294. doi: 10.1016/j.sleep.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Paavonen EJ, Räikkönen K, Pesonen AK, Lahti J, Komsi N, Heinonen K, et al. Sleep quality and cognitive performance in 8-year-old children. Sleep Med. 2010;11:386–392. doi: 10.1016/j.sleep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 65.Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34:315–323. doi: 10.1093/sleep/34.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bourke R, Anderson V, Yang JSC, Jackman AR, Killedar A, Nixon GM, et al. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011;12:489–496. doi: 10.1016/j.sleep.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 67.Bub KL, Buckhalt JA, El-Sheikh M. Children's sleep and cognitive performance: a cross-domain analysis of change over time. Dev Psychol. 2011;47:1504–1514. doi: 10.1037/a0025535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kronholm E, Puusniekka R, Jokela J, Villberg J, Urrila AS, Paunio T, et al. Trends in self-reported sleep problems, tiredness and related school performance among Finnish adolescents from 1984 to 2011. J Sleep Res. 2015;24:3–10. doi: 10.1111/jsr.12258. [DOI] [PubMed] [Google Scholar]
- 69.Boschloo A, Krabbendam L, Dekker S, Lee NC, De Groot R, Jolles J. Subjective sleepiness and sleep quality in adolescents are related to objective and subjective measures of school performance. Front Psychol. 2013;4:38. doi: 10.3389/fpsyg.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhie S, Lee S, Chae KY. Sleep patterns and school performance of Korean adolescents assessed using a Korean version of the pediatric daytime sleepiness scale. Korean J Pediatr. 2011;54:29–35. doi: 10.3345/kjp.2011.54.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Liu X, Ji X, Wang Y, Zhou G, Chen X. Sleep disordered breathing symptoms and daytime sleepiness are associated with emotional problems and poor school performance in children. Psychiatry Res. 2016;242:218–225. doi: 10.1016/j.psychres.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Short MA, Gradisar M, Lack LC, Wright HR. The impact of sleep on adolescent depressed mood, alertness and academic performance. J Adolesc. 2013;36:1025–1033. doi: 10.1016/j.adolescence.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Wang G, Ren F, Liu Z, Xu G, Jiang F, Skora E, et al. Sleep patterns and academic performance during preparation for college entrance exam in Chinese adolescents. J Sch Health. 2016;86:298–306. doi: 10.1111/josh.12379. [DOI] [PubMed] [Google Scholar]
- 74.Wang G, Jiang Y, Zhang Y, Liu S, Jiang F. Sacrificing sleep for scores: a cross-cultural perspective on the hidden costs of sleep loss in adolescents. Behav Sleep Med. 2016;14:581–583. doi: 10.1080/15402002.2016.1201966. [DOI] [PubMed] [Google Scholar]
- 75.Ghandour RM, Sherman LJ, Vladutiu CJ, Ali MM, Lynch SE, Bitsko RH, et al. Prevalence and treatment of depression, anxiety, and conduct problems in US children. J Pediatr. 2019;206:256–67.e3. doi: 10.1016/j.jpeds.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bitsko RH, Claussen AH, Lichstein J, Black LI, Jones SE, Danielson ML, et al. Mental health surveillance among children—United States, 2013–2019. MMWR Suppl. 2022;71:1–42. doi: 10.15585/mmwr.su7102a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/S0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- 78.Jones CE, Opel RA, Kaiser ME, Chau AQ, Quintana JR, Nipper MA, et al. Early-life sleep disruption increases parvalbumin in primary somatosensory cortex and impairs social bonding in prairie voles. Sci Adv. 2019;5:eaav5188. doi: 10.1126/sciadv.aav5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rong T, Sun X, Zhang Z, Li W, Deng Y, Wang Z, et al. The association between sleep and empathy in young preschoolers: a population study. J Sleep Res. 2022;31:e13530. doi: 10.1111/jsr.13530. [DOI] [PubMed] [Google Scholar]
- 80.Cremone A, de Jong DM, Kurdziel LBF, Desrochers P, Sayer A, LeBourgeois MK, et al. Sleep tight, act right: negative affect, sleep and behavior problems during early childhood. Child Dev. 2018;89:e42–59. doi: 10.1111/cdev.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quach JL, Nguyen CD, Williams KE, Sciberras E. Bidirectional associations between child sleep problems and internalizing and externalizing difficulties from preschool to early adolescence. JAMA Pediatr. 2018;172:e174363. doi: 10.1001/jamapediatrics.2017.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho S, Philbrook LE, Davis EL, Buss KA. Sleep duration and RSA suppression as predictors of internalizing and externalizing behaviors. Dev Psychobiol. 2017;59:60–69. doi: 10.1002/dev.21467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paavonen EJ, Porkka-Heiskanen T, Lahikainen AR. Sleep quality, duration and behavioral symptoms among 5–6-year-old children. Eur Child Adolesc Psychiatry. 2009;18:747–754. doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- 84.Bernaras E, Jaureguizar J, Garaigordobil M. Child and adolescent depression: a review of theories, evaluation instruments, prevention programs, and treatments. Front Psychol. 2019;10:543. doi: 10.3389/fpsyg.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lunsford-Avery JR, Judd CM, Axelson DA, Miklowitz DJ. Sleep impairment, mood symptoms, and psychosocial functioning in adolescent bipolar disorder. Psychiatry Res. 2012;200:265–271. doi: 10.1016/j.psychres.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sarchiapone M, Mandelli L, Carli V, Iosue M, Wasserman C, Hadlaczky G, et al. Hours of sleep in adolescents and its association with anxiety, emotional concerns, and suicidal ideation. Sleep Med. 2014;15:248–254. doi: 10.1016/j.sleep.2013.11.780. [DOI] [PubMed] [Google Scholar]
- 87.Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. J Child Psychol Psychiatry. 2014;55:180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shanahan L, Copeland WE, Angold A, Bondy CL, Costello EJ. Sleep problems predict and are predicted by generalized anxiety/depression and oppositional defiant disorder. J Am Acad Child Adolesc Psychiatry. 2014;53:550–558. doi: 10.1016/j.jaac.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Conway A, Miller AL, Modrek A. Testing reciprocal links between trouble getting to sleep and internalizing behavior problems, and bedtime resistance and externalizing behavior problems in toddlers. Child Psychiatry Hum Dev. 2017;48:678–689. doi: 10.1007/s10578-016-0692-x. [DOI] [PubMed] [Google Scholar]
- 90.Lemola S, Schwarz B, Siffert A. Interparental conflict and early adolescents’ aggression: is irregular sleep a vulnerability factor? J Adolesc. 2012;35:97–105. doi: 10.1016/j.adolescence.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 91.Umlauf MG, Bolland JM, Lian BE. Sleep disturbance and risk behaviors among inner-city African–American adolescents. J Urban Health. 2011;88:1130–1142. doi: 10.1007/s11524-011-9591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Glenn AL, Cui N, Raine A. Longitudinal bidirectional association between sleep and behavior problems at age 6 and 11 years. Sleep Med. 2021;83:290–298. doi: 10.1016/j.sleep.2021.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee SH, Kim HB, Lee KW. Association between sleep duration and attention-deficit hyperactivity disorder: a systematic review and meta-analysis of observational studies. J Affect Disord. 2019;256:62–69. doi: 10.1016/j.jad.2019.05.071. [DOI] [PubMed] [Google Scholar]
- 94.Chiang HL, Gau SSF, Ni HC, Chiu YN, Shang CY, Wu YY, et al. Association between symptoms and subtypes of attention-deficit hyperactivity disorder and sleep problems/disorders. J Sleep Res. 2010;19:535–545. doi: 10.1111/j.1365-2869.2010.00832.x. [DOI] [PubMed] [Google Scholar]
- 95.Mullin BC, Harvey AG, Hinshaw SP. A preliminary study of sleep in adolescents with bipolar disorder, ADHD, and non-patient controls. Bipolar Disord. 2011;13:425–432. doi: 10.1111/j.1399-5618.2011.00933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin CA, Hiscock H, Rinehart N, Heussler HS, Hyde C, Fuller-Tyszkiewicz M, et al. Associations between sleep hygiene and sleep problems in adolescents with ADHD: a cross-sectional study. J Atten Disord. 2020;24:545–554. doi: 10.1177/1087054718762513. [DOI] [PubMed] [Google Scholar]
- 97.Paavonen EJ, Raikkonen K, Lahti J, Komsi N, Heinonen K, Pesonen AK, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123:857–864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 98.Pesonen AK, Räikkönen K, Matthews K, Heinonen K, Paavonen JE, Lahti J, et al. Prenatal origins of poor sleep in children. Sleep. 2009;32:1086–1092. doi: 10.1093/sleep/32.8.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Scott N, Blair PS, Emond AM, Fleming PJ, Humphreys JS, Henderson J, et al. Sleep patterns in children with ADHD: a population-based cohort study from birth to 11 years. J Sleep Res. 2013;22:121–128. doi: 10.1111/j.1365-2869.2012.01054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang B, Isensee C, Becker A, Wong J, Eastwood PR, Huang RC, et al. Developmental trajectories of sleep problems from childhood to adolescence both predict and are predicted by emotional and behavioral problems. Front Psychol. 2016;7:1874. doi: 10.3389/fpsyg.2016.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kortesoja L, Vainikainen MP, Hotulainen R, Rimpelä A, Dobewall H, Lindfors P, et al. Bidirectional relationship of sleep with emotional and behavioral difficulties: a 5-year follow-up of Finnish adolescents. J Youth Adolesc. 2020;49:1277–1291. doi: 10.1007/s10964-020-01203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kelly RJ, El-Sheikh M. Reciprocal relations between children’s sleep and their adjustment over time. Dev Psychol. 2014;50:1137–1147. doi: 10.1037/a0034501. [DOI] [PubMed] [Google Scholar]
- 103.Mulraney M, Giallo R, Lycett K, Mensah F, Sciberras E. The bidirectional relationship between sleep problems and internalizing and externalizing problems in children with ADHD: a prospective cohort study. Sleep Med. 2016;17:45–51. doi: 10.1016/j.sleep.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 104.Pieters S, Burk WJ, Van der Vorst H, Dahl RE, Wiers RW, Engels RC. Prospective relationships between sleep problems and substance use, internalizing and externalizing problems. J Youth Adolesc. 2015;44:379–388. doi: 10.1007/s10964-014-0213-9. [DOI] [PubMed] [Google Scholar]
- 105.Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med. 2010;164:608–614. doi: 10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- 106.Widome R, Berger AT, Iber C, Wahlstrom K, Laska MN, Kilian G, et al. Association of delaying school start time with sleep duration, timing, and quality among adolescents. JAMA Pediatr. 2020;174:697–704. doi: 10.1001/jamapediatrics.2020.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minges KE, Redeker NS. Delayed school start times and adolescent sleep: a systematic review of the experimental evidence. Sleep Med Rev. 2016;28:86–95. doi: 10.1016/j.smrv.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boergers J, Gable CJ, Owens JA. Later school start time is associated with improved sleep and daytime functioning in adolescents. J Dev Behav Pediatr. 2014;35:11–17. doi: 10.1097/DBP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 109.Kordas K, Siegel EH, Olney DK, Katz J, Tielsch JM, Kariger PK, et al. The effects of iron and/or zinc supplementation on maternal reports of sleep in infants from Nepal and Zanzibar. J Dev Behav Pediatr. 2009;30:131–139. doi: 10.1097/DBP.0b013e31819e6a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dai Y, Liu J. Omega-3 long-chain polyunsaturated fatty acid and sleep: a systematic review and meta-analysis of randomized controlled trials and longitudinal studies. Nutr Rev. 2021;79:847–868. doi: 10.1093/nutrit/nuaa103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang J, Yan Y, Zheng JS, Mi J, Li D. Association between erythrocyte membrane phospholipid fatty acids and sleep disturbance in Chinese children and adolescents. Nutrients. 2018;10:344. doi: 10.3390/nu10030344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yackobovitch-Gavan M, Machtei A, Lazar L, Shamir R, Phillip M, Lebenthal Y. Randomised study found that improved nutritional intake was associated with better sleep patterns in prepubertal children who were both short and lean. Acta Paediatr. 2018;107:666–671. doi: 10.1111/apa.14205. [DOI] [PubMed] [Google Scholar]
- 113.Cubero J, Chanclón B, Sánchez S, Rivero M, Rodríguez AB, Barriga C. Improving the quality of infant sleep through the inclusion at supper of cereals enriched with tryptophan, adenosine-5′-phosphate, and uridine-5′-phosphate. Nutr Neurosci. 2009;12:272–280. doi: 10.1179/147683009X423490. [DOI] [PubMed] [Google Scholar]
- 114.Perkin MR, Bahnson HT, Logan K, Marrs T, Radulovic S, Craven J, et al. Association of early introduction of solids with infant sleep: a secondary analysis of a randomized clinical trial. JAMA Pediatr. 2018;172:e180739. doi: 10.1001/jamapediatrics.2018.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Buman MP, King AC. Exercise as a treatment to enhance sleep. Am J Lifestyle Med. 2010;4:500–514. doi: 10.1177/1559827610375532. [DOI] [Google Scholar]
- 116.Kline CE. The bidirectional relationship between exercise and sleep: implications for exercise adherence and sleep improvement. Am J Lifestyle Med. 2014;8:375–379. doi: 10.1177/1559827614544437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roth T. Slow wave sleep: does it matter? J Clin Sleep Med. 2009;5(2 Suppl):S4–5. doi: 10.5664/jcsm.5.2S.S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 119.Dworak M, Wiater A, Alfer D, Stephan E, Hollmann W, Strüder HK. Increased slow wave sleep and reduced stage 2 sleep in children depending on exercise intensity. Sleep Med. 2008;9:266–272. doi: 10.1016/j.sleep.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 120.Kalak N, Gerber M, Kirov R, Mikoteit T, Yordanova J, Pühse U, et al. Daily morning running for 3 weeks improved sleep and psychological functioning in healthy adolescents compared with controls. J Adolesc Health. 2012;51:615–622. doi: 10.1016/j.jadohealth.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 121.Werner-Seidler A, Johnston L, Christensen H. Digitally-delivered cognitive–behavioural therapy for youth insomnia: a systematic review. Internet Interv. 2018;11:71–78. doi: 10.1016/j.invent.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive–behavioral sleep interventions. Clin Child Fam Psychol Rev. 2017;20:227–249. doi: 10.1007/s10567-017-0234-5. [DOI] [PubMed] [Google Scholar]
- 123.Ma ZR, Shi LJ, Deng MH. Efficacy of cognitive behavioral therapy in children and adolescents with insomnia: a systematic review and meta-analysis. Braz J Med Biol Res. 2018;51:e7070. doi: 10.1590/1414-431x20187070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schlarb AA, Bihlmaier I, Velten-Schurian K, Poets CF, Hautzinger M. Short-and long-term effects of CBT-I in groups for school-age children suffering from chronic insomnia: the KiSS-program. Behav Sleep Med. 2018;16:380–397. doi: 10.1080/15402002.2016.1228642. [DOI] [PubMed] [Google Scholar]
- 125.Field T, Gonzalez G, Diego M, Mindell J. Mothers massaging their newborns with lotion versus no lotion enhances mothers’ and newborns’ sleep. Infant Behav Dev. 2016;45:31–37. doi: 10.1016/j.infbeh.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 126.Field T, Field T, Cullen C, Largie S, Diego M, Schanberg S, et al. Lavender bath oil reduces stress and crying and enhances sleep in very young infants. Early Hum Dev. 2008;84:399–401. doi: 10.1016/j.earlhumdev.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 127.Chen ML, Lin LC, Wu SC, Lin JG. The effectiveness of acupressure in improving the quality of sleep of institutionalized residents. J Gerontol A Biol Sci Med Sci. 1999;54:M389–M394. doi: 10.1093/gerona/54.8.M389. [DOI] [PubMed] [Google Scholar]
- 128.Carotenuto M, Gallai B, Parisi L, Roccella M, Esposito M. Acupressure therapy for insomnia in adolescents: a polysomnographic study. Neuropsychiatr Dis Treat. 2013;9:157–162. doi: 10.2147/NDT.S41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wall RB. Tai Chi and mindfulness-based stress reduction in a Boston public middle school. J Pediatr Health Care. 2005;19:230–237. doi: 10.1016/j.pedhc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 130.Bootzin RR, Stevens SJ. Adolescents, substance abuse, and the treatment of insomnia and daytime sleepiness. Clin Psychol Rev. 2005;25:629–644. doi: 10.1016/j.cpr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Biegel GM, Brown KW, Shapiro SL, Schubert CM. Mindfulness-based stress reduction for the treatment of adolescent psychiatric outpatients: a randomized clinical trial. J Consult Clin Psychol. 2009;77:855–866. doi: 10.1037/a0016241. [DOI] [PubMed] [Google Scholar]
- 132.Liu QQ, Zhou ZK, Yang XJ, Kong FC, Sun XJ, Fan CY. Mindfulness and sleep quality in adolescents: analysis of rumination as a mediator and self-control as a moderator. Pers Individ Dif. 2018;122:171–176. doi: 10.1016/j.paid.2017.10.031. [DOI] [Google Scholar]
- 133.Phillips SR, Johnson AH, Shirey MR, Rice M. Sleep quality in school-aged children: a concept analysis. J Pediatr Nurs. 2020;52:54–63. doi: 10.1016/j.pedn.2020.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Z, Tang H, Jin Q, Wang G, Yang Z, Chen H, et al. Sleep of preschoolers during the coronavirus disease 2019 (COVID-19) outbreak. J Sleep Res. 2021;30:e13142. doi: 10.1111/jsr.13142. [DOI] [PubMed] [Google Scholar]
- 135.Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. 2020;395:945–947. doi: 10.1016/S0140-6736(20)30547-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adolescent Sleep Working Group, Committee on Adolescence, Council on School Health School start times for adolescents. Pediatrics. 2014;134:642–649. doi: 10.1542/peds.2014-1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Riesch SK, Liu J, Kaufmann PG, Doswell WM, Cohen S, Vessey J. Preventing adverse health outcomes among children and adolescents by addressing screen media practices concomitant to sleep disturbance. Nurs Outlook. 2019;67:492–496. doi: 10.1016/j.outlook.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang G, Zhang J, Lam SP, Li SX, Jiang Y, Sun W, et al. Ten-year secular trends in sleep/wake patterns in Shanghai and Hong Kong school-aged children: a tale of two cities. J Clin Sleep Med. 2019;15:1495–1502. doi: 10.5664/jcsm.7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.