Abstract
Neuromodulation is recommended by major international guidelines as a fourth-line treatment in bladder pain syndrome/interstitial cystitis (BPS/IC) patients after failure of behavioural, oral and intravesical pharmacological treatments, including hydrodistension. A non-systematic review of studies identified by electronic search of MEDLINE was performed with no time limitation. A narrative synthesis of the existing evidence regarding the results of sacral, tibial and pudendal nerve stimulation in the management of BPS/IC was developed. Neuromodulation in pelvic chronic pain disorders, including BPS/IC, is a useful tool for refractory patients to conventional treatments. Sacral neuromodulation may be effective in patients with BPS without Hunner’s lesions, and the effect seems to be maintained in the mid- and long-term. Posterior tibial nerve stimulation can be offered to patients with BPS/IC in the context of a multidisciplinary approach. When pudendal neuralgia is suspected, selective pudendal nerve stimulation has a high response rate. The aetiology of the pain can influence the outcomes in the mid- and long-term of the different neuromodulation approaches, thus careful diagnosis is recommended.
Keywords: bladder pain syndrome, chronic pelvic pain, interstitial cystitis, neuromodulation, treatment
Introduction
As defined by the International Continence Society (ICS), chronic pelvic pain is characterized by persistent pain lasting longer than 6 months or recurrent episodes of abdominal/pelvic pain, hypersensitivity or discomfort often associated with elimination changes, and sexual dysfunction often in the absence of organic aetiology.1 It potentially includes urologic, gynaecologic, gastrointestinal, musculoskeletal, neurologic and rheumatologic aetiology, with psychosocial aspects.1,2 Phenotyping or describing the condition by its symptoms, signs and, where possible, by investigations, has been demonstrated to have clinical and research validity, but it should only be used if there is adequate evidence to support its use.2
Following the latest edition of the European Association of Urology (EAU) Guidelines on Chronic Pelvic Pain, primary bladder pain syndrome (PBPS) is the occurrence of persistent or recurrent pain perceived in the urinary bladder region, accompanied by at least one other symptom, such as pain worsening with bladder filling and day-time or night-time urinary frequency.2,3 Similarly, the ICS defines bladder pain syndrome (BPS) as a persistent or recurrent chronic pelvic pain, pressure or discomfort perceived to be related to the urinary bladder accompanied by at least one other urinary symptom, such as an urgent need to void or urinary frequency.1
Recent clinical guidelines for BPS/IC of the Japanese Urological Association distinguish between Hunner-type interstitial cystitis (IC) and BPS without macroscopic urothelial damage and absence of inflammatory changes.4 Histopathological differences are the background of this division, with immunological inflammatory reaction and epithelial denudation in the first, and functional defects of urothelial barrier, neurogenic inflammation, neural hyperactivity or extra-bladder disorders in the latter.5 However, current treatment trends are focused on both disorders in the correction of the dysfunctional epithelial barrier, mast cell activation and neurogenic inflammation. Conservative treatment including dietary control, medical treatment with oral medication and intravesical instillations/injections and surgical treatment with hydrodistension, fulguration, urinary diversion ± cystectomy or neurostimulation are the mainstay of their management.2–4 Other new therapeutic approaches are being studied like the intravesical injection of platelet-rich plasma for the reparation of urothelial defects and regeneration of the epithelium in patients with BPS/IC.6
The effect of neuromodulation on pain disorders is usually explained by the gate theory proposed by Melzack and Wall.7 They suggested that pain perceived to have a visceral origin, which stimulates primary afferent fibres and travels to the brain via transmission cells, could be blocked by converging impulses arising from a somatic origin (by non-nociceptive fibres at the same dermatome) that activate inhibitory interneurons located in the dorsal horn of the spinal cord. Impulses from the dorsal horn are controlled by a descending system containing fibres from the brainstem, thalamus, and limbic lobes, and thereby, neuromodulation controls the pain sensations at the spinal segmental gate and modulates pain sensation at higher brain centres.8
Although the role of different neuromodulation techniques is more robust in overactive bladder and faecal incontinence, it is still limited for pain.2 Therefore, we aimed to summarize the evidence available in the literature supporting the utilization of the most used neuromodulation procedures and devices in the urological and urogynaecological field for the management of BPS and other chronic pelvic pain disorders with urological involvement.
Methods
In July 2021, a non-systematic review was performed according to the items of SANRA,9 a scale specifically designed for the quality assessment of narrative review articles.
The studies and reviews were identified by electronic search of MEDLINE with no time limitation. The search strategy included [(neuromodulation) OR (modulation) OR (stimulation)] AND [(sacral) OR (pudendal) OR (tibial)] AND [(pain) OR (cystitis)]. We considered randomized controlled trials (RCTs), comparative non-RCTs and single-arm cohort studies, and narrative and systematic reviews. No limitation in the number of patients in case series was set. We additionally searched the reference list of all included studies and any relevant review articles. Non-English texts were excluded.
A narrative synthesis of the existing evidence regarding the results of sacral, tibial and pudendal nerve stimulation in the management of BPS/IC was developed.
Sacral nerve stimulation
Sacral nerve stimulation (SNS) or sacral neuromodulation was developed during the 70s by Thanago and Smith, who observed in animal models how electrical stimulation of S3 was able to modulate detrusor–sphincter activity, thus regulating micturition cycle (Figure 1). They performed the first implant in humans in 1981, and after some technical improvements, the US Food and Drug Administration approved in 1997 SNS for the treatment of urge urinary incontinence.
Figure 1.
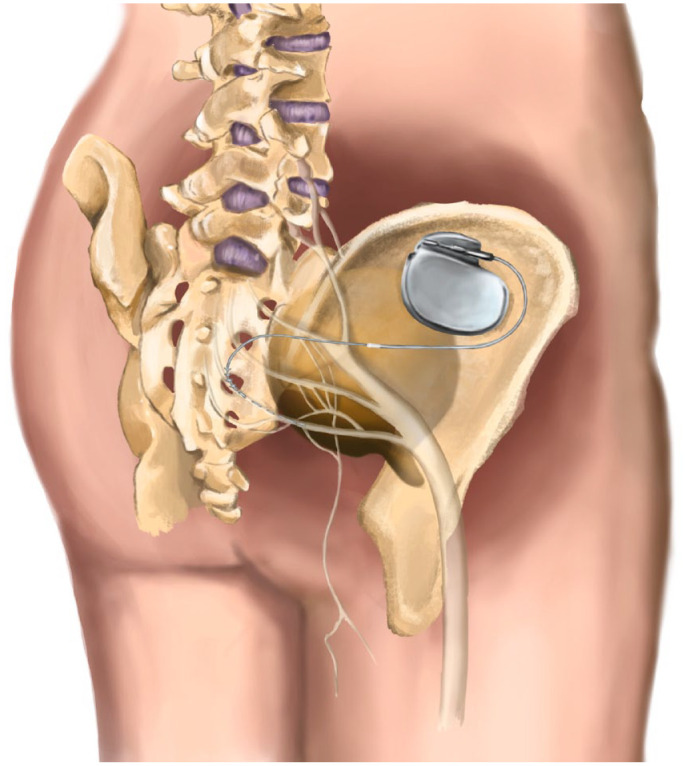
Electrode and implantable pulse generator positioning in SNS.
SNS hypothetically balances excitatory and inhibitory impulses from and to the pelvic organs at sacral and suprasacral centres through the stimulation of afferent nerves in the pelvis. The electric pulses are supposed to modulate not only the spinal cord reflexes but also brain networks.10 Despite no high-level evidence, SNS is recommended by major international guidelines as a fourth-line treatment in BPS/IC patients after failure of behavioural, oral and intravesical pharmacological treatments, including hydrodistension. Last update of American Urological Association (AUA) Guideline on BPS/IC management proposed an SNS trial before considering oral cyclosporine or major surgeries (cystoplasty or urinary diversion with or without cystectomy).11 In the same line, the latest version of the EAU Guidelines on Chronic Pelvic Pain (2022 Edition) also recommends SNS before considering more invasive surgeries.3
First evidences of SNS effectiveness in BPS/IC were reported in 1999 by Shaker and Hassouna.12 They observed in patients implanted by urgency/frequency syndrome but also affected by pelvic pain, how neuromodulation was effective in reducing both kind of symptoms. However, 1 year after this publication, Chai et al.13 reported their results with percutaneous S3 stimulation in six patients with clinical symptoms (increased voiding frequency, urgency and pain) and cystoscopic findings (glomerulations) suggestive of IC, who have failed previous oral and intravesical therapies. Subjective symptoms, such as pain and urgency, improved significantly but also objective parameters analysed (voiding frequency, urinary heparin-binding epidermal growth factor and urinary antiproliferative factor activity) tended to normalize.
Peters et al.14 compared the results of SNS following the traditional procedure [percutaneous nerve evaluation (PNE) with a monopolar lead followed by open surgical implant of the permanent quadripolar lead] with the staged procedure (implanting the permanent quadripolar lead in the first stage). Both techniques of SNS improved urinary symptoms, pain and quality of life, but a positive response to test phase followed by implant was much more frequently observed in the staged technique (94% test to implant rate versus 52% for the traditional PNE). Other variables of efficacy in symptoms reduction of refractory BPS/IC patients, such as narcotics requirement reduction, have also been demonstrated with SNS.15
A non-systematic review of articles between 1990 and 2010 was conducted by Marcelissen et al.,16 identifying 11 articles reporting results of SNS in refractory BPS/IC, and two additional publications reporting its effects in patients with intractable non-specific pelvic or urogenital pain. All studies yielded positive results of SNS, but the authors raised some concerns. Most studies were retrospective, evaluated a limited number of patients or had a limited follow-up. Therefore, clear diagnostic criteria were also lacking in some cases, making comparisons between studies difficult. They concluded than although preliminary results seem to be promising, larger prospective trials and longer follow-up studies were still required to clearly establish the efficacy of this minimally invasive therapy.
A systematic review by Wang J was conducted in 2017, with more than 500 patients included in one RCT, eight prospective cohort series and eight retrospective case series. Follow-up ranged from 0 (test) to 86 months. Their analysis showed marked improvements in pain [with a mean reduction in visual analogue scale (VAS) of 3.99 points], objective variables (such as day-time frequency, nocturia, 24-h micturitions and average voided volume) and also subjective variables [urgency and specific BPS/IC symptoms evaluated through Interstitial Cystitis Symptom Index (ICSI)–Interstitial Cystitis Problem Index (ICPI) questionnaires].17
Recently, we published our long-term data on SNS for patients with BPS/IC refractory to third-line therapies, with a mean follow-up of 96 (range: 12–204) months. We observed than 6 out of 10 patients benefit from SNS in the mid- and long-term, with no serious complications except for a lead rupture during the test phase requiring open surgery for extraction. Reintervention rate was high (75%), but most surgeries were indicated for battery replacement.10
Limitations of the studies
Some authors and societies advocate for BPS/IC categorization based on initial endoscopic and histologic classification, differentiating between patients with and without Hunner lesions.4 In patients with Hunner lesions, bladder-directed therapies such as steroid injection, Hunner lesions resection or coagulation, intravesical instillation or oral cyclosporine must be favoured. However, patients with non-Hunner BPS might be better approached with neuromodulation and a multidisciplinary team management (including pain specialists, psychosocial therapists, etc.) to obtain the best results.18 However, most studies have a mixed profile of patients and lack of homogeneous definitions of BPS/IC, making it difficult to contrast the results between them.10
Another handicap in the comparison between studies is the different tests selected by the authors for the definition of outcomes and the heterogeneity in the threshold of symptomatic improvement to indicate pulse generator implantation.10 Furthermore, subacute stimulation can be both performed with PNE and quadripolar tined lead, although it is usually specified in most papers.10
Implications for research
It has to be determined if phenotypical classification of IC/BPS is useful for predicting the success of SNS in these patients. A possible response can be the decrease in neuroinflammatory urine markers, such as nerve growth factor, P-substance or adenosine triphosphate (ATP).
Posterior tibial nerve stimulation
Posterior tibial nerve stimulation (PTNS) is a technique derived from traditional Chinese medicine, which consists of electrically stimulating a point along the path of the common peroneal or posterior tibial nerve to modulate bladder activity. The posterior tibial nerve is a mixed nerve (contains sensory and motor fibres) from the L5-S3 roots (the same spinal segments from which the parasympathetic innervation of the bladder originates). In this way, it allows an entry pathway to the sacral plexus (posterior tibial approach) in a simple and safe way to electrically modulate voiding reflexes (Figures 2 and 3).
Figure 2.

Simulation of the entry pathway to the sacral plexus of the tibial nerve stimulation.
Figure 3.
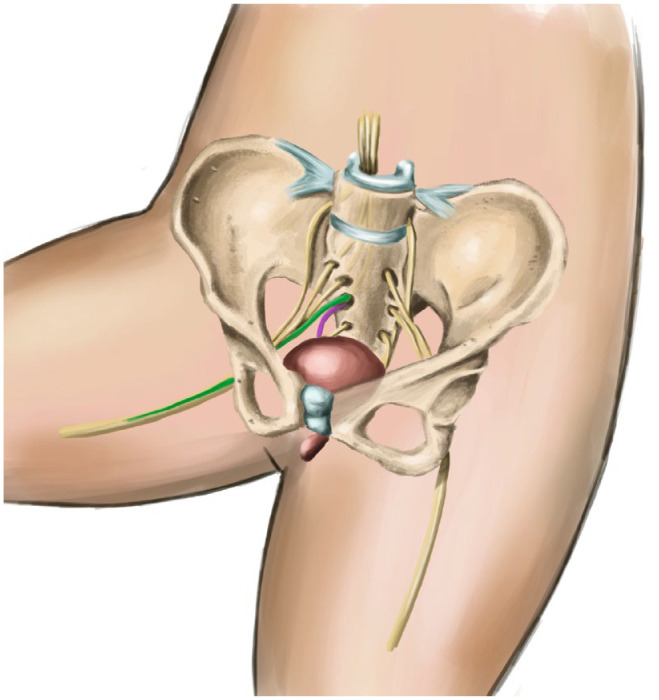
Afferent pulses of the posterior tibial nerve (green) entering the S3 root, and efferent pulses (rose) modulating bladder function.
Although PTNS has mainly been used for overactive bladder treatment, some studies have reported its efficacy in different types of pelvic pain. Table 1 summarizes the case series of PTNS in pelvic pain disorders.19–24
Table 1.
Case series reporting outcomes of PTNS in pelvic pain disorders.
| Study | Type | N | Women/men | Mean age (years) | Diagnosis | Mean variables | Success rate (%) | Follow-up (weeks) |
|---|---|---|---|---|---|---|---|---|
| McGuire et al.19 | R | 5 | – | – | IC | Subjective improvement | 80 | – |
| Van Balken et al.20 | P | 33 | 11/22 | 51 | Pelvic pain | VAS, McGill pain questionnaire, SF-36 | 42 | 12 |
| Kim et al.21 | P | 15 | 10/5 | 60 | Pelvic pain | VAS, GRA, IPSS | 30–60 | 12 |
| Kabay et al.22 | P | 45 | 0/45 | 37 | Pelvic pain | NIH-CPSI, VAS | 40–66 | 12 |
| Ragab et al.23 | P | 20 | 20/0 | 41 | IC (NIDDK criteria) | VAS, ICSI/ICPI, GRA | 10 | 12 |
| Sudol et al.24 | P | 16 | 16/0 | 49 | IC/BPS (SUFU criteria) | GRA, VAS, ICSI/ICPI, PUF | 30–70 | 12 |
GRA, Global Response Assessment scale; IC, interstitial cystitis; IC/BPS, interstitial cystitis/bladder pain syndrome; ICSI/ICPI, O’Leary-Sant Interstitial Cystitis Symptom and Problem Index Questionnaire; IPSS, International Prostate Symptoms Score; NIH-CPSI, National Institutes of Health Chronic Prostatitis Symptom Index; P, prospective; PUF, Pelvic Pain and Urgency/Frequency Patient Symptom Scale; R, retrospective; SF-36, 36-Item Short Form Health Survey questionnaire; VAS, Visual Analogue Scale for pain.
McGuire and his group19 published the first report of PTNS including patients with neurogenic and non-neurogenic overactive bladder, IC (five patients) and radiation cystitis. Four out of five patients in the BPS/IC group showed improvements according to authors, but no specific variables are described in this article except for the reduction on urinary frequency and improved bladder discomfort.
Van Balken et al. reported in 2003 the results of a prospective multicentre trial including 22 men and 11 women with chronic pelvic pain of different origins. They evaluated the VAS for pain, McGill questionnaire for pain and SF-36 questionnaire for the assessment of quality of life. After 12 weeks of treatment, a subjective response (patient’s request to continue treatment) was observed in 42%, with 21% of patients reporting VAS reductions of > 50% and 18% reporting reductions of > 25%. In all patients, an improvement in both quality of life and the total pain rate intensity (McGill questionnaire) was noted.20 Although their results are moderately good, authors state in their conclusions that a significant placebo effect cannot be ruled out.
Kabay et al. compared PTNS versus sham treatment in a RCT with 89 male patients affected by chronic pelvic pain (referred to the bladder, groyne, perineum, genitals and lower abdomen). Using the VAS of pain and the National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI), they reported statistically significant improvements after 12 weeks of treatment in pain (40%) and symptoms score (66.6%).22
Specifically in BPS/IC population, PTNS has shown conflicting results in the few studies available. Ragab et al. published a prospective case series in 20 women diagnosed with BPS/IC according to the National Institute of Diabetes and Kidney Diseases (NIDDK) criteria. Patients underwent 12 weekly sessions of percutaneous tibial nerve stimulation for 30 min, being evaluated through VAS for pain, O’Leary-Sant ICSI, O’Leary-Sant ICPI and global response assessment (GRA) score. At the end of the study, only two patients (10%) reported slight–moderate symptoms improvement. No significant changes were observed in VAS, ICSI/ICPI scores and GRA (p > 0.5).23
Conversely, a pilot study conducted more recently by Sudol et al. including 16 patients of whom 10 completed the protocol, showed positive results. All patients met SUFU criteria for BPS/IC and received PTNS sessions for 30 min once a week for 12 weeks. Primary outcome was change in a GRA scale and secondary outcomes included VAS for pain and ICSI/ICPI scores for symptom bother. Response rate according to GRA at the end of the study was as follows: two patients slightly worse, two patients without change, four patients slightly improved, two patients moderately improved and one patient markedly improved. Six subjects reported improvement in VAS and there were also non-statistically significant reductions in ICSI/ICPI scores. The authors concluded than although no statistically robust improvements were observed, PTNS had favourable clinical results. This, together with the very few adverse events reported, supports the use of PTNS as an off-label option for BPS/IC patients.24
Some authors have evaluated PTNS in combination with other BPS/IC therapies, with good results. Baykal et al.25 evaluated PTNS in combination with intravesical heparin in 12 patients with a longer follow-up (mean 13 months), with improvements in pain scores, cystometric capacity and voiding frequency sustained during the study period. Their positive results suggest that the combination of PTNS with other BPS/IC therapies is an option that must be explored in further studies. According to their modest results, and specially taking into account that it is a procedure with nearly no side effects, PTNS should be considered as an off-label treatment for BPS/IC patients, probably with better results in the setting of a multimodal approach.
Limitations of the studies and implications for research
Small patient populations, retrospective studies mostly without control arms, lack of homogeneity and patient stratification, and no consensus in which the tools are to evaluate the effectiveness of PTNS are the main limitations of the evidence in this area. RCTs with clear and restricted inclusion criteria are missing to dilucidate if PTNS is clinically effective for the different phenotypes of chronic pelvic pain patients. This is a knowledge gap that must be overcome to be able to propose PTNS with a high degree of recommendation. However, and although evidence is very limited, we think that PTNS can be offered to chronic pelvic pain patients ideally in the setting of a multimodal approach, as it has virtually no side effects and at least follows the Hippocratic principle of ‘first, do not harm’.
Pudendal nerve stimulation
The pudendal nerve originates from the second through the fourth sacral nerves in the sacral plexus. It exits the pelvis through the greater sciatic foramen, crosses over the ischial spine and then re-enters the pelvis through the lesser sciatic foramen.26 The pudendal nerve provides motor innervation to the anal sphincters and the urethral sphincter, and the sensory distribution course is generally via the three terminal branches:26,27
The inferior rectal branch supplies the skin around the anus and communicates with the perineal branch of the posterior femoral cutaneous nerve and its terminal branch, the posterior scrotal (labia majora) nerve;
The perineal branch has two superficial sensory branches, the medial and lateral posterior scrotal (labial) nerves;
The dorsal nerve of the penis/clitoris runs along the dorsal face of the penis/clitoris, supplying the overlying skin.
This division is important when evaluating patients with possible pudendal neuralgia, who could present with pain in the distribution of some or all branches. The pudendal nerve may be damaged at the level of the piriformis muscle, the sacrospinal/ sacrotuberous ligaments, within Alcock’s canal or even at multiple levels.2
Pudendal nerve stimulation has been long used for the treatment of idiopathic overactive bladder28 (and also for neurogenic detrusor overactivity)29 and pudendal neuralgia. Although most case series report the results with the percutaneous implantation of tined leads, there are also descriptions of open and laparoscopic lead placement,30,31 and with implantable devices.32 Little evidence supports the management of BPS/IC with pudendal-only neuromodulation; thus, a review of chronic pelvic-perineal pain syndromes was undergone.
In 1989, Schimdt explained the posterior approach for PNE and block.33 In his original report, the patient is placed in the prone position with slight flexion of the hips, and the position of the ischial spine is identified topographically using the ischial tuberosities, greater trochanter and lower edge of the ischial-tuberous ligament. Reitz et al.34 described later another posterior approach to the pudendal nerve from a dorsal and tangential direction in cadaveric models. They located the site of insertion of the needle by drawing a horizontal line starting from the great trochanter and a vertical one starting from the extremity of the ischiatic tuberosity. The intersection of the two lines matches below the ischiatic spine, under which runs the ischiatic nerve.
A perineal approach for the insertion of the tined lead in neuro-urological patients has also been described by Spinelli et al.,29 which begins with the insertion of the needle perpendicularly above the ischial tuberosity, and then tilted medially and dorsally towards the recto-ischial fossa until it reaches the Alcock’s canal below and behind the ischial spine. They also recommend neurophysiology guidance with the measurement of pudendal nerve terminal motor latency. To avoid bowel or vagina puncture during needle insertion, the surgeon’s finger is placed into the rectum or the vagina, and this step was emphasized by Bock in his description of the technique for patients with faecal incontinence.35
Heinze et al.36 defined a new percutaneous approach with fluoroscopic and neurophysiological guidance. They defined the STAR triangle by marking the middle of the acetabulum (A), the centre of the ischial tuberosity (T), the ischial spine (S) and the anal rim (R). The centre of this STAR triangle serves as the starting point for puncture, and the trunk of the pudendal nerve meets the apical corner of the triangle.
A group of urologists of the Renji Hospital and the Shanghai Jiao Tong University School of Medicine has also reported the practicability of printing a 3D template for needle insertion with previous anatomical study with computed tomography scan for the sacral bone and magnetic resonance imaging for nerves and blood vessels.37
Possover described in 2004 the feasibility of laparoscopic pelvic autonomous and somatic nerve exposure and evaluation.38 However, 3 years later, he leaded the report of the Laparoscopic Implantation Of Neuroprosthesis (LION) procedure30 for patients with pelvic intractable neuralgia using the On-Point™ peripheral nerve-stimulating electrode and the Interstim™ permanent neurostimulation system (Medtronic Inc., Minneapolis, MN, USA).
In 2019, the endoscopic transgluteal minimal invasive technique (ENTRAMI) for implantation of a pudendal electrode was described in cadaveric models,39 and clinical effectiveness in patients with chronic perineal pain in the pudendal or cluneal nerve area was demonstrated in 15 patients after nerve release.40 In another pilot study, 16 patients underwent both nerve release and implantation of a PNE lead through a posterior approach with full visual control.41
Recently, the group by Hoang Roberts et al.32 reported the effectiveness of Stimwave® Freedom Stimulators™ (Stimwave, Pompano Beach, FL, USA) in the management of pudendal neuralgia.
Table 2 summarizes the reports of different case series focused on chronic pudendal nerve stimulation in pelvic pain disorders.30–32,36,37,42–45
Table 2.
Case series reporting outcomes of chronic pudendal nerve stimulation in pelvic pain disorders.
| Author | No. of Patients | F/M ratio | Mean age [years (SD)] | Diagnosis (no. of patients) | Approach | Procedure, positive response criteria | Outcome | Mean follow-up since implant (months) |
Complications (no. of patients) | Reintervention | Explantation rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peters et al.42 | 22 | 19/3 | 48 | IC (22) | Percutaneous, posterior | Tined lead (sacral and pudendal), > 50% symptomatic improvement | 17/22 with positive response in first stage 13/17 chose definitive pudendal implant, improvement in ICSI/PI score after 6 months (12.8 points) 4/17 chose definitive sacral implant, improvement in ICSI/PI score after 6 months (8.8 points) |
6 | Sterile seromas around the IPG (2) | None | Not reported |
| Possover et al.30 | 3 | 2/1 | 58 (14.0) | Right genitofemoral and pudendal neuralgia (1), phantom limb, stump neuroma pain, and permanent pain in lower limb and entire gluteal region (1), sciatica and OAB (1) | Laparoscopic | On-PointTM peripheral nerve stimulating electrode, not reported | Median improvement in VAS score in 7.6 points (SD 0.57) | 4 | Not reported | Not reported | Not reported |
| Carmel et al.43 | 3 | 3/0 | 50.3 (11.8) | Refractory chronic pelvi-perineal pain compatible with pudendal neuralgia (3) | Percutaneous, posterior | Tined lead, ⩾ 60% of subjective clinical improvement | ⩾ 80% of subjective clinical improvement | 20 | Not reported | One change of implantable pulse generator | Not reported |
| Peters et al.44 | 84 | 66/18 | 51.8 (16.9) | BPS/IC (42), pelvic/bladder pain without BPS/IC (2), dry/wet OAB (26), NOUR (13) | Percutaneous, ischial-rectal (posterior) | Tined lead, > 50% symptomatic improvement |
60/84 with positive response in first stage 55/84 with definitive pudendal implant BPS/IC group: improvement in ICSI/PI score after 3 (7.6 points) and 6 months (9.1 points) |
21.4 | Lead migration (3) Pain and uncomfortable stimulation (2) Symptoms worsening after a fall (1) Localized wound infection (1) |
Revision of migrated leads: three new pudendal leads two sacral leads Second pudendal lead for bilat. stimulation (2) Battery replacements (2) |
5/55 |
| Heinze et al.36 | 10 | Not reported | Not reported | Chronic pelvic pain syndromes (10) | STAR (posterior) | Tined lead, not reported |
90% IPG implantation rate Decrease in millimetric VAS from 85–40 mm |
1 | - | - | - |
| Peters et al.45 | 19 | 12/7 | 54.8 (16.4) | Pudendal neuralgia (19) | Percutaneous, posterior | Tined lead, > 50% symptomatic improvement |
Complete pain relief (3) Almost complete pain relief (3) Significant/remarkable pain relief (10) Small/slight pain relief (3) |
Not reported | Not reported | Three revised pudendal leads | 5/19 |
| Li et al.31 | 1 | 1/0 | 48 | Intense acyclic pelvic pain, voiding dysfunction | Laparoscopic | Bilat. S3 and pudendal tined leads, not reported | Complete resolution of pain | >36 | Partial thrombosis of the external iliac artery (1) | None | No |
| Gu et al.37 | 16 | 9/7 | 50.6 | Perineal pain (7), frequency and urgency (3), poor urination (6) | Percutaneous, posterior and perineal with 3D template | Two sacral leads and nine pudendal tined leads, not reported | Improvement in pain (6.33 ± 1.63 points), urinary
frequency (3.71 ± 1.95) and dysuria (4.25 ± 1.71) |
Not reported | None | Not reported | Not reported |
| Hoang Roberts et al.32 | 13 | 12/1 | 50 | Pudendal neuralgia | Percutaneous, posterior | StimWave, > 50% symptomatic improvement | 10/13 with positive response, 9 underwent implantation | 9 | Lead migration (2), broken wire (1), nonfunctioning antenna (2) |
Explantation (2) | 2/9 |
Bilat., bilateral; BPS/IC, bladder pain syndrome/ interstitial cystitis; F/M, female/male; ICSI/PI, Interstitial Cystitis Symptom and Problem Index; IPG, implantable pulse generator; NOUR, non-obstructive urinary retention; NPRS, numeric pain rating scale; OAB, overactive bladder; VAS, visual analogue scale.
In the study by Peters et al.,44 42 patients with BPS/IC were included. Compared with non-BPS/IC patients, those reported more severe symptoms at baseline, 3- and 6-months checkpoints, and even at 12 months, mean scores were returning to baseline values. Heinze et al.36 reported on the application of a new percutaneous implantation technique in patients with chronic pelvic pain disorders (as mentioned before), including patients with BPS/IC and pudendal neuralgia; however, they only described the results after 1 month of stimulation and no reference to differences between groups according to the initial diagnosis was made. Thus, there is not enough evidence to assure that patients with BPS/IC have a worse response to chronic pudendal nerve stimulation, but a careful selection of the patients is advisable.
Limitations of the studies
Again, the evidence favouring the use of chronic pudendal nerve stimulation in pelvi-perineal pain syndromes is limited by the paucity of RCTs or well-designed case–control studies.
Implications for research
Clearly defined outcomes are essential to make comparisons between studies and approaches.
Other neuromodulation routes
With the development of new techniques of neuromodulation, case series reporting the outcomes with diverse methods have been published. Spinal cord stimulation of the conus medullaris can be attempted in patients with refractory pudendal neuralgia.46 Motor cortex stimulation in refractory pelvic and perineal pain has been described by a multidisciplinary group in Nantes,47 indicated when other treatments, including pudendal nerve release and spinal cord stimulation, have failed. Moreover, dorsal root ganglion stimulation has been offered to patients with refractory chronic pelvic pain.48
Other outpatient treatments such as pudendal electroacupuncture have been explored to treat perineal and urethral pain with good results in selected patients.49,50
Conclusion
Clinical studies exploring the outcomes of neuromodulation in chronic pelvic pain disorders including BPS/IC show that it is a useful tool for refractory patients to conventional treatments. The evidence is limited by study’s design and the lack of target-population definition in most investigations. The aetiology of the pain can influence the outcomes in the mid- and long-term of the different neuromodulation approaches, thus careful diagnosis is recommended.
Acknowledgments
The authors acklowledge Eduardo Ruiz-Torres who served as illustrator, and Thomas Paterson for proofreading activity.
Footnotes
ORCID iDs: Bárbara Padilla-Fernández  https://orcid.org/0000-0002-8566-6033
https://orcid.org/0000-0002-8566-6033
David M. Castro-Díaz  https://orcid.org/0000-0002-4484-9159
https://orcid.org/0000-0002-4484-9159
Contributor Information
Bárbara Padilla-Fernández, Department of Urology, Complejo Hospitalario Universitario de Canarias, Carretera La Cuesta, s/n, San Cristóbal de La Laguna 38320, Tenerife, Spain; Departamento de Cirugía, Facultad de Medicina, Universidad de La Laguna, San Cristóbal de La Laguna, Spain.
David Hernández-Hernández, Department of Urology, Complejo Hospitalario Universitario de Canarias, San Cristóbal de La Laguna, Spain.
David M. Castro-Díaz, Department of Urology, Complejo Hospitalario Universitario de Canarias, San Cristóbal de La Laguna, Spain Departamento de Cirugía, Facultad de Medicina, Universidad de La Laguna, San Cristóbal de La Laguna, Spain.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Bárbara Padilla-Fernández: Conceptualization; Methodology; Writing – original draft; Writing – review & editing.
David Hernández-Hernández: Conceptualization; Methodology; Writing – original draft.
David M. Castro-Díaz: Methodology; Supervision; Visualization.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: B.P.-F. and D.M.C-D. have served as speakers for Medtronic. D.H.-H. has no conflict to declare regarding this article.
Availability of data and materials: Not applicable.
References
- 1. Doggweiler R, Whitmore KE, Meijlink JM, et al. A standard for terminology in chronic pelvic pain syndromes: a report from the chronic pelvic pain working group of the international continence society. Neurourol Urodyn 2017; 36: 984–1008. [DOI] [PubMed] [Google Scholar]
- 2. Engeler D, Baranowski AP, Berghmans B, et al. EAU guidelines on chronic pelvic pain, 2021, https://uroweb.org/guidelines/archive/chronic-pelvic-pain/
- 3. Engeler D, Baranowski AP, Berghmans B, et al. EAU guidelines on chronic pelvic pain, 2022, https://uroweb.org/guidelines/chronic-pelvic-pain
- 4. Homma Y, Akiyama Y, Tomoe H, et al. Clinical guidelines for interstitial cystitis/bladder pain syndrome. Int J Urol 2020; 27: 57820200414–57820200589. [DOI] [PubMed] [Google Scholar]
- 5. Yoshimura N, Oguchi T, Yokoyama H, et al. Bladder afferent hyperexcitability in bladder pain syndrome/interstitial cystitis. Int J Urol 2014; 21(Suppl. 1): 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Trama F, Illiano E, Marchesi A, et al. Use of intravesical injections of platelet-rich plasma for the treatment of bladder pain syndrome: a comprehensive literature review. Antibiotics Basel 2021; 10: 20211001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965; 150: 971–979. [DOI] [PubMed] [Google Scholar]
- 8. Tahseen S. Role of sacral neuromodulation in modern urogynaecology practice: a review of recent literature. Int Urogynecol J 2018; 29: 1081–1091. [DOI] [PubMed] [Google Scholar]
- 9. Baethge C, Goldbeck-Wood S, Mertens S. SANRA – a scale for the quality assessment of narrative review articles. Res Integr Peer Rev 2019; 4: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hernández-Hernández D, Padilla-Fernández B, Navarro-Galmés MÁ, et al. Sacral neuromodulation in the management of bladder pain syndrome/interstitial cystitis. Current Bladder Dysfunction Reports 2020; 15: 83–92. [Google Scholar]
- 11. Hanno PM, Burks DA, Clemens JQ, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome. American Urological Association Education and Research, American urological Association, 2014. [Google Scholar]
- 12. Shaker H, Hassouna MM. Sacral root neuromodulation in the treatment of various voiding and storage problems. Int Urogynecol J Pelvic Floor Dysfunct 1999; 10: 336–343. [DOI] [PubMed] [Google Scholar]
- 13. Chai TC, Zhang C, Warren JW, et al. Percutaneous sacral third nerve root neurostimulation improves symptoms and normalizes urinary HB-EGF levels and antiproliferative activity in patients with interstitial cystitis. Urology 2000; 55: 643–646. [DOI] [PubMed] [Google Scholar]
- 14. Peters KM, Carey JM, Konstandt DB. Sacral neuromodulation for the treatment of refractory interstitial cystitis: outcomes based on technique. Int Urogynecol J Pelvic Floor Dysfunct 2003; 14: 223–228; discussion 228. [DOI] [PubMed] [Google Scholar]
- 15. Peters KM, Konstandt D. Sacral neuromodulation decreases narcotic requirements in refractory interstitial cystitis. BJU Int 2004; 93: 777–779. [DOI] [PubMed] [Google Scholar]
- 16. Marcelissen T, Jacobs R, van Kerrebroeck P, et al. Sacral neuromodulation as a treatment for chronic pelvic pain. J Urol 2011; 186: 387–393. [DOI] [PubMed] [Google Scholar]
- 17. Wang J, Chen Y, Chen J, et al. Sacral neuromodulation for refractory bladder pain syndrome/interstitial cystitis: a global systematic review and meta-analysis. Sci Rep 2017; 7: 11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akiyama Y, Luo Y, Hanno PM, et al. Interstitial cystitis/bladder pain syndrome: the evolving landscape, animal models and future perspectives. Int J Urol 2020; 27: 491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGuire EJ, Zhang SC, Horwinski ER, et al. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol 1983; 129: 78–79. [DOI] [PubMed] [Google Scholar]
- 20. van Balken MR, Vandoninck V, Messelink BJ, et al. Percutaneous tibial nerve stimulation as neuromodulative treatment of chronic pelvic pain. Eur Urol 2003; 43: 158–163; discussion 163. [DOI] [PubMed] [Google Scholar]
- 21. Kim SW, Paick JS, Ku JH. Percutaneous posterior tibial nerve stimulation in patients with chronic pelvic pain: a preliminary study. Urol Int 2007; 78: 58–62. [DOI] [PubMed] [Google Scholar]
- 22. Kabay S, Kabay SC, Yucel M, et al. Efficiency of posterior tibial nerve stimulation in category IIIB chronic prostatitis/chronic pelvic pain: a sham-controlled comparative study. Urol Int 2009; 83: 33–38. [DOI] [PubMed] [Google Scholar]
- 23. Ragab MM, Tawfik AM, Abo El-enen M, et al. Evaluation of percutaneous tibial nerve stimulation for treatment of refractory painful bladder syndrome. Urology 2015; 86: 707–711. [DOI] [PubMed] [Google Scholar]
- 24. Sudol NT, Guaderrama N, Adams-Piper E, et al. Percutaneous tibial nerve stimulation for the treatment of interstitial cystitis/bladder pain syndrome: a pilot study. Int Urogynecol J 2021; 32: 2757–2764. [DOI] [PubMed] [Google Scholar]
- 25. Baykal K, Senkul T, Sen B, et al. Intravesical heparin and peripheral neuromodulation on interstitial cystitis. Urol Int 2005; 74: 361–364. [DOI] [PubMed] [Google Scholar]
- 26. Farber AJ, Wilckens JH, Jarvis MAJCG. Chapter 25 – pelvic pain in the athlete. In: Seidenberg PH, Beutler AI. (eds) The sports medicine resource manual. Philadelphia, PA: W.B. Saunders, 2008, pp. 306–327. [Google Scholar]
- 27. Benson JT, Griffis K. Pudendal neuralgia, a severe pain syndrome. Am J Obstet Gynecol 2005; 192: 1663–1668. [DOI] [PubMed] [Google Scholar]
- 28. Benson JT, Griffis K, Fuller E, et al. Pudendal nerve stimulation therapy for urinary urge incontinence and urgency-frequency syndrome: a pilot study. J Pelv Surg 2003; 9: 310. [Google Scholar]
- 29. Spinelli M, Malaguti S, Giardiello G, et al. A new minimally invasive procedure for pudendal nerve stimulation to treat neurogenic bladder: description of the method and preliminary data. Neurourol Urodyn 2005; 24: 305–309. [DOI] [PubMed] [Google Scholar]
- 30. Possover M, Baekelandt J, Chiantera V. The Laparoscopic Implantation of Neuroprothesis (LION) procedure to control intractable abdomino-pelvic neuralgia. Neuromodulation 2007; 10: 18–23. [DOI] [PubMed] [Google Scholar]
- 31. Li ALK, Marques R, Oliveira A, et al. Laparoscopic implantation of electrodes for bilateral neuromodulation of the pudendal nerves and S3 nerve roots for treating pelvic pain and voiding dysfunction. Int Urogynecol J 2018; 29: 1061–1064. [DOI] [PubMed] [Google Scholar]
- 32. Hoang Roberts L, Vollstedt A, Volin J, et al. Initial experience using a novel nerve stimulator for the management of pudendal neuralgia. Neurourol Urodyn 2021; 40: 1670–1677. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt RA. Technique of pudendal nerve localization for block or stimulation. J Urol 1989; 142: 1528–1531. [DOI] [PubMed] [Google Scholar]
- 34. Reitz A, Gobeaux N, Mozer P, et al. Topographic anatomy of a new posterior approach to the pudendal nerve for stimulation. Eur Urol 2007; 51: 1350–1355; discussion 1355. [DOI] [PubMed] [Google Scholar]
- 35. Bock S, Folie P, Wolff K, et al. First experiences with pudendal nerve stimulation in fecal incontinence: a technical report. Tech Coloproctol 2010; 14: 41–44. [DOI] [PubMed] [Google Scholar]
- 36. Heinze K, Hoermann R, Fritsch H, et al. Comparative pilot study of implantation techniques for pudendal neuromodulation: technical and clinical outcome in first 20 patients with chronic pelvic pain. World J Urol 2015; 33: 289–294. [DOI] [PubMed] [Google Scholar]
- 37. Gu Y, Lv T, Jiang C, et al. Neuromodulation of the pudendal nerve assisted by 3D printed: a new method of neuromodulation for lower urinary tract dysfunction. Front Neurosci 2021; 15: 619672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Possover M. Laparoscopic exposure and electrostimulation of the somatic and autonomous pelvic nerves: a new method for implantation of neuroprothesis in paralyzed patients? Gynecol Surg 2004; 1: 87–90. [Google Scholar]
- 39. Jottard K, Bonnet P, Bruyninx L, et al. The ENTRAMI technique: endoscopic transgluteal minimal invasive technique for implantation of a pudendal electrode under full visual control: a cadaver study. Neurourol Urodyn 2019; 38: 130–134. [DOI] [PubMed] [Google Scholar]
- 40. Jottard K, Bruyninx L, Bonnet P, et al. Endoscopic trans gluteal minimal-invasive approach for nerve liberation (ENTRAMI technique) in case of pudendal and/or cluneal neuralgia by entrapment: one-year follow-up. Neurourol Urodyn 2020; 39: 2003–2007. [DOI] [PubMed] [Google Scholar]
- 41. Jottard K, Bruyninx L, Bonnet P, et al. Pilot study: pudendal neuromodulation combined with pudendal nerve release in case of chronic perineal pain syndrome. the ENTRAMI technique: early results. Int Urogynecol J 2021; 32: 2765–2770. [DOI] [PubMed] [Google Scholar]
- 42. Peters KM, Feber KM, Bennett RC. A prospective, single-blind, randomized crossover trial of sacral vs pudendal nerve stimulation for interstitial cystitis. BJU Int 2007; 100: 835–839. [DOI] [PubMed] [Google Scholar]
- 43. Carmel M, Lebel M, Tu le M. Pudendal nerve neuromodulation with neurophysiology guidance: a potential treatment option for refractory chronic pelvi-perineal pain. Int Urogynecol J 2010; 21: 613–616. [DOI] [PubMed] [Google Scholar]
- 44. Peters KM, Killinger KA, Boguslawski BM, et al. Chronic pudendal neuromodulation: expanding available treatment options for refractory urologic symptoms. Neurourol Urodyn 2010; 29: 1267–1271. [DOI] [PubMed] [Google Scholar]
- 45. Peters KM, Killinger KA, Jaeger C, et al. Pilot study exploring chronic pudendal neuromodulation as a treatment option for pain associated with pudendal neuralgia. Low Urin Tract Symptoms 2015; 7: 138–142. [DOI] [PubMed] [Google Scholar]
- 46. Buffenoir K, Rioult B, Hamel O, et al. Spinal cord stimulation of the conus medullaris for refractory pudendal neuralgia: a prospective study of 27 consecutive cases. Neurourol Urodyn 2015; 34: 177–182. [DOI] [PubMed] [Google Scholar]
- 47. Louppe JM, Nguyen JP, Robert R, et al. Motor cortex stimulation in refractory pelvic and perineal pain: report of two successful cases. Neurourol Urodyn 2013; 32: 53–57. [DOI] [PubMed] [Google Scholar]
- 48. Hunter CW, Yang A. Dorsal root ganglion stimulation for chronic pelvic pain: a case series and technical report on a novel lead configuration. Neuromodulation 2019; 22: 87–95. [DOI] [PubMed] [Google Scholar]
- 49. Chen S, Wang S, Gao Y, et al. Bilateral electrical pudendal nerve stimulation as additional therapy for lower urinary tract dysfunction when stage II sacral neuromodulator fails: a case report. BMC Urol 2021; 21: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li T, Feng XY, Feng XM, et al. The short-term efficacy of electrical pudendal nerve stimulation versus intravesical instillation for the urethral pain syndrome: a randomized clinical trial. World J Urol 2021; 39: 3993–3998. [DOI] [PubMed] [Google Scholar]


