Abstract
Background
Human immunodeficiency virus drug resistance (HIVDR) can negatively impact the effectiveness of antiretroviral therapy (ART). We aimed to estimate the prevalence of pretreatment HIVDR (PDR) among ART initiators and the prevalence of viral load (VL) suppression and acquired HIVDR among individuals receiving ART for 12 ± 3 months (ADR12) and ≥48 months (ADR48) in El Salvador.
Methods
Nationally representative cross-sectional PDR, ADR12 and ADR48 surveys were conducted among adults with HIV from October 2018 to August 2019, following World Health Organization-recommended methods. Demographic and clinic data and blood specimens were collected.
Results
Two hundred sixty participants were enrolled in the PDR survey, 230 in ADR12 and 425 in ADR48. Twenty-seven percent (95% confidence interval [CI], 17.1%–39.9%) of ART initiators had PDR to efavirenz or nevirapine. The prevalence of VL suppression was 88.8% (95% CI, 83.1%–92.8%) in ADR12 and 80.5% (95% CI, 76.6%–84.0%) in ADR48 surveys. Among people with HIV receiving a first-line nonnucleoside reverse transcriptase inhibitor (NNRTI)-based ART regimens and with unsuppressed VL, the prevalence of ADR to efavirenz or nevirapine was 72.0% (95% CI, 32.3%–93.3%) and 95.0% (68.5%–99.4%) in the ADR12 and ADR28 surveys, respectively. ADR12 to boosted protease inhibitors (PI/r) or integrase strand transfer inhibitors (INSTIs) was not observed. ADR48 was 1.3% (95% CI, 0.2%–9.6%) and 2.1% (0.3%–13.7%), respectively.
Conclusions
Programmatic improvements in ART delivery are urgently needed in El Salvador to address the high levels of resistance to efavirenz or nevirapine among ART initiators and the low VL suppression prevalence among individuals on treatment.
Keywords: Central America, drug resistance surveillance, El Salvador, HIV, WHO
Human immunodeficiency virus drug resistance (HIVDR) can increase the number of AIDS-associated deaths, new HIV infections, and the costs of the antiretroviral therapy (ART) program [1, 2]. Therefore, World Health Organization (WHO) recommends the surveillance of pretreatment drug resistance (PDR) among ART initiators and acquired HIV drug resistance (ADR) among people with HIV (PHIV) receiving ART for 12 ± 3 months (ADR12) and ≥48 months (ADR48) [3–5].
Several Latin American and Caribbean countries have reported nationally representative estimates of PDR to nonnucleoside reverse transcriptase inhibitors (NNRTIs) >10% [6–11], the WHO-recommended threshold to urge transition to a non-NNRTI-containing first-line ART regimen [2]. Significantly higher levels of HIV resistance to NNRTI (odds ratio [OR], 3.4 [95% confidence interval {CI}, 1.8%–6.9%]; P < .0001) have been reported among ART initiators with previous exposure to antiretroviral (ARV) drugs (ie, ART discontinuation or HIV prophylaxis) compared with those without previous exposure to ARV drugs [12].
In 2008, the prevalence of PDR to any ARV drug in El Salvador was 10.3% (95% CI, 2.8%–24.2%), as reported in a study including 47 female sex workers and 9.0% (95% CI, 3.6%–17.6%) among 98 men who have sex with men [13]. In 2011, a sentinel study reported a prevalence of PDR to any ARV drug of 5.7% (95% CI, 2.4%–12.6%) among 88 ART initiators without previous exposure to ARV drugs, 2.3% (95% CI, .6%–7.9%) resistance to both nucleoside reverse transcriptase inhibitors (NRTIs) and NNRTIs, and 1.4% (95% CI, .2%–7.6%) to boosted protease inhibitors (PI/r) [14].
To better understand the current HIVDR context in El Salvador, a nationally representative study was conducted among adults initiating and receiving ART from October 2018 to August 2019.
METHODS
The national HIV program has provided universal ART in El Salvador since 2018. At the time of the study, there were 24 000 PHIV in El Salvador, and 48% had started ART in 19 clinics [15]. According to the national ART guidelines, the regimen of tenofovir (TDF), emtricitabine or lamivudine (FTC/3TC), and efavirenz (EFV) was the preferred first-line ART, and a PI/r-based regimen was the preferred second-line ART [16].
Study Design
A nationally representative cross-sectional study was conducted following WHO-recommended methods [17, 18]. Clinics with a small number of adults receiving ART (representing <10% of the national cohort of adults receiving ART) were excluded from the sampling frame following the WHO-recommended methods [17, 18]. Twelve clinics were selected to participate in the study.
The sample size was calculated using the WHO standardized Microsoft Excel–based calculator [19]. The sample size per survey was calculated based on (i) ADR48: the total number of PHIV receiving ART 4 years before survey initiation (5842); (ii) ADR12: the number of ART initiators 2 years before survey initiation (1400); and (iii) PDR: the number of ART initiators 1 year before survey initiation (744). The default model assumptions recommended by WHO were used for sample size calculation: (i) 10% PDR; (ii) 85% viral load (VL) suppression; (iii) 25% previous ARV drug exposure prevalence among ART initiators; (iv) 100% individuals initiating NNRTI-based regimens; (v) 95% PHIV receiving a first-line ART regimen (ie, ARV drugs prescribed to initiate ART); (vi) 100% PHIV on first-line ART receiving NNRTI-based regimens; and (vii) a target CI half-width of ±5%. The sample size was 249 for the PDR survey, 284 for the ADR12 survey, and 400 for the ADR48 survey. The sample distribution into the 12 clinics included in the sampling frame was proportional to the cohort of PHIV receiving ART in each clinic.
Patient Consent Statement
The institutional review boards of Universidad del Valle de Guatemala and the National Institute of Respiratory Diseases in Mexico (protocol number E02-17) approved the study. The staff involved in recruiting participants were trained on appropriate study procedures. All participants provided written informed consent before enrollment.
Participant Enrollment
ART initiators (≥18 years of age), with or without previous exposure to ARV drugs, were eligible for the PDR survey. ART initiators were defined as individuals starting or restarting first-line ART. Previous exposure to ARV drugs was defined as ART discontinuation or HIV prophylaxis. PHIV (≥18 years of age) receiving ART for 12 ± 3 months were eligible for the ADR12 survey, and those receiving ART for ≥48 months were eligible for the ADR48 survey. Eligible individuals who consented were consecutively enrolled from October 2018 to August 2019. Deidentified demographic and clinical data and blood specimens were collected.
Laboratory Procedures
Blood specimens were collected from both adults initiating ART or receiving ART who were enrolled in the study. Plasma for VL testing was separated from whole blood within 6 hours of collection. VL (Abbott m2000 system, Abbott Molecular, Des Plaines, Illinois) and CD4 (Cytomics FC 500 system, Beckman Coulter, Brea, California) were measured in 2 reference laboratories in El Salvador.
Dried blood spots were prepared for HIVDR testing using either venous blood or finger pricks (depending on the participating centers’ operational capabilities and staff availability). Specimens for HIVDR testing were handled and stored following the WHO/HIV ResNet Laboratory Operational Framework [20]. HIVDR testing was performed on specimens with VL ≥1000 copies/mL at the National Institute of Respiratory Diseases in Mexico, a WHO-designated regional laboratory for HIVDR surveillance. HIV RNA was extracted from 1 dried blood spot using silica columns (QIAamp Viral RNA Kit; Qiagen, Valencia, California). HIV protease and reverse transcriptase were amplified and sequenced using in-house validated protocols (HXB2: 2268-3302) [21]. Integrase was amplified and sequenced separately, using an in house–validated protocol (HXB2: 4013-5265) [7, 22]. Sanger sequencing was performed on a 3730xl Genetic Analyzer (ThermoFisher, Waltham, Massachusetts). The sequences were assembled using ReCall (British Columbia Centre for Excellence in HIV/AIDS [BCCfE], Vancouver, Canada) [23]. The WHO/BCCfE HIVDR quality control tool was used for posttesting quality assurance [20]. Sequence clusters with genetic distance <0.5% within the same sequencing batch were repeated and confirmed.
Data Analysis
As per WHO-recommended methods, VL suppression was defined as <1000 viral copies/mL and HIVDR was defined as a penalty score ≥15 using the Stanford HIVdb tool (version 8.9) [17, 18, 24, 25]. HIV subtype was assigned using the Stanford HIVdb subtyping tool (version 8.9) and confirmed with the REGA HIV-1 subtyping tool (version 3) and the Recombinant Identification Program (version 3.0) [24, 26–28].
Weighted statistical analysis was performed using Stata version 15.1 (StataCorp, College Station, Texas), following the WHO recommendations [17, 18]. The weights were defined per participating clinic, considering the estimated eligible population sizes and the number of enrolled individuals. PDR analysis was weighted considering the number of specimens successfully sequenced. VL suppression estimates were weighted based on the VL results available. ADR analysis was weighted by the number of sequences available among the individuals who were not virally suppressed. VL suppression among PHIV receiving ART for 12 (±3) months was adjusted by retention using the country estimate of adults receiving ART 12 months after ART initiation (706/800 [88.3%]) [29]. Retention-adjusted VL suppression was calculated as the product of the retention and VL suppression rates, and a Wald 95% CI was computed using the associated variance. The weighted analysis accounted for underenrollment, actual patient accrual rates, retention in care, and viral amplification failure.
Phylogenetic Analysis
An HIV transmission network analysis was performed to identify and characterize transmission clusters. After removing codons associated with HIVDR according to the Stanford HIVdb tool version 8.9 [24, 25], sequences from participants of the ADR and PDR surveys were aligned by codons using Mega v6 [30]. Phylogenetic trees were constructed using the maximum likelihood method based on the Tamura-Nei model with 1000 bootstrap repetitions. Reference sequences for HIV subtypes obtained from the Los Alamos HIV Sequences Database (http://www.hiv.lanl.gov) were included in the trees. The trees were visualized and colored in Mega v6 [30]. Using HIV-TRACE [31], putative transmission links were defined and resolved between individuals whose HIV sequences had a genetic distance <1.5%. A multivariable logistic regression model including gender, age, VL, state of residence, HIVDR, and previous ARV exposure was applied to find variables associated with clustering. Analyses were performed using Stata version 15.1 software (StataCorp, College Station, Texas).
RESULTS
Clinical and Demographic Characteristics
A total of 260 ART initiators were enrolled in the PDR survey (104% of the target sample size), 72.5% (95% CI, 64.0%–79.7%) were male, and 77.7% (95% CI, 69.7%–84.1%) were >25 years old (Table 1). Among ART initiators, 32.6% (95% CI, 25.6%–40.4%) had CD4 count <200 cells/µL, 44.6% (95% CI, 35.6%–54.0%) had CD4 count 200–500 cells/µL, and 22.8% (95% CI, 15.7%–31.9%) had CD4 count >500 cells/µL. The majority (91.7% [95% CI, 87.6%–94.5%]) initiated ART with TDF, FTC/3TC, and EFV (Table 1). HIV-1 subtype B was observed in 99.5% (95% CI, 96.5%–99.9%) of ART initiators and the CRF12_BF in 0.5% (95% CI, .1%–3.5%).
Table 1.
Demographic and Clinical Characteristics of Individuals Initiating and Receiving Antiretroviral Therapy in El Salvador, 2018–2019a
| Characteristic | ART Initiators (n = 260) | PHIV Receiving ART for 12 ± 3 mo (n = 230) | PHIV Receiving ART for ≥48 mo (n = 425) | |||
|---|---|---|---|---|---|---|
| No. | % (95% CI) | No. | % (95% CI) | No. | % (95% CI) | |
| Gender | ||||||
| Female | 54 | 23.9 (17.3–32.2) | 62 | 22.6 (17.8–28.2) | 211 | 47.1 (42.2–52.0) |
| Male | 200 | 72.5 (64.0–79.7) | 164 | 75.6 (69.7–80.6) | 213 | 52.6 (47.6–57.4) |
| Transgender | 6 | 3.5 (1.4–8.7) | 4 | 1.8 (.6–5.5) | 1 | 0.4 (.1–2.3) |
| Age, y | ||||||
| ≤25 | 57 | 22.3 (15.9–30.3) | 42 | 19.0 (11.3–30.2) | 13 | 4.2 (2.4–7.3) |
| >25 | 203 | 77.7 (69.7–84.1) | 188 | 81.0 (69.8–88.7) | 412 | 95.8 (92.7–97.6) |
| Current ART line | ||||||
| First-line | 260 | 100.0 (98.5–100.0) | 229 | 99.7 (98.6–99.9) | 337 | 83.4 (80.4–86.0) |
| Second-line | NA | … | 1 | 0.3 (.1–1.4) | 87 | 16.5 (13.8–19.5) |
| Third-line | NA | … | 0 | 0.0 (0–1.6) | 1 | 0.2 (.0–.6) |
| Current ART regimen | ||||||
| TDF + 3TC/FTC + EFV | 235 | 91.7 (87.6–94.5) | 216 | 93.5 (89.1–96.2) | 113 | 23.8 (20.1–27.9) |
| TDF + 3TC/FTC + NVP | 2 | 1.6 (.3–8.8) | 1 | 0.2 (.2–.2) | 10 | 2.3 (1.3–3.9) |
| ZDV + 3TC/FTC + EFV | 9 | 2.9 (1.7–4.8) | 7 | 4.5 (2.1–9.1) | 167 | 44.9 (40.2–49.7) |
| ZDV + 3TC/FTC + NVP | 2 | 0.6 (.2–1.5) | 0 | 0.0 (.0–1.6) | 30 | 8.5 (6.0–11.8) |
| PI-based | 3 | 0.7 (.4–1.2) | 1 | 0.3 (.1–1.4) | 93 | 17.7 (15.0–20.7) |
| Other | 9 | 2.5 (1.6–3.9) | 5 | 1.5 (.8–3.0) | 12 | 2.9 (1.6–5.2) |
Abbreviations: 3TC/FTC, lamivudine/emtricitabine; ART, antiretroviral therapy; CI, confidence interval; EFV, efavirenz; NA, not applicable; NVP, nevirapine; PI, protease inhibitor; PHIV, people with human immunodeficiency virus; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Study design–weighted proportions and 95% CIs.
The proportion of ART initiators without previous ARV drug exposure was 79.2% (95% CI, 73.1%–84.2%). The proportion of ART initiators who reported unknown previous ARV drug exposure was low (0.6% [95% CI, .6%–.64%]). Previous exposure to ARV drugs was reported by 20.3% (95% CI, 15.3%–26.4%) of ART initiators, 16.7% (95% CI, 11.6%–23.5%) among males and 30.7% (95% CI, 18.2%–46.8%) among females. All participants with previous ARV drug exposure reported ART discontinuation.
A total of 230 PHIV receiving ART were enrolled in the ADR12 survey (81% of the target sample size) and 425 in the ADR48 survey (106% of the target sample size). The percentage of females increased with follow-up time receiving ART (ADR12 = 22.6% [95% CI, 17.8%–28.2%] vs ADR48 = 47.1% [95% CI, 42.2%–52.0%]). The proportion of PHIV on a first-line NNRTI-based regimen was 99.7% for ADR12 and 83.4% for ADR48. The most frequently prescribed regimen among PHIV receiving ART for 12 ± 3 was TDF, FTC/3TC, and EFV. Zidovudine (ZDV), FTC/3TC, and EFV was the most frequently prescribed regimen among PHIV receiving ART for ≥48 months (Table 1). The proportion of PHIV receiving ART for ≥48 months using second-line ART was 17.7% (95% CI, 13.8%–22.4%) among females and 14.8% (95% CI, 11.1%–19.4%) among males.
Pretreatment HIV Drug Resistance
The prevalence of PDR to EFV or nevirapine (NVP) was 27.0% (95% CI, 17.1%–39.9%), to any NRTI was 10.3% (95% CI, 4.1%–23.6%), to integrase strand transfer inhibitors (INSTIs) was 3.5% (95% CI, 2.9%–19.5%), and to PI/r was 1.2% (95% CI, .6%–2.4%) (Table 2). The prevalence of PDR to EFV or NVP was higher than 10% in both ART initiators with previous exposure to ARV drugs (37.4% [95% CI, 22.3%–55.4%]) and those without previous ARV exposure (24.4% [95% CI, 13.2%–40.7%]). The prevalence of PDR to EFV or NVP was 29.1% (95% CI, 17.7%–44.0%) among males and 14.9% (95% CI, 7.3%–28.0%) among females. K103NS was the mutation most frequently observed among ART initiators (20.1% [95% CI, 11.3%–33.2%]) (Supplementary Table 1).
Table 2.
Prevalence of Pretreatment Human Immunodeficiency Virus Drug Resistance in El Salvador, 2018–2019a
| Drug Class | All | ART Initiators Without Previous ARV Drug Exposure | ART Initiators With Previous ARV Drug Exposure | |||
|---|---|---|---|---|---|---|
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | |
| NRTI | ||||||
| Any | 14/209 | 10.3 (4.1–23.6) | 7/159 | 9.6 (2.8–28.4) | 7/47 | 13.3 (6.9–24.1) |
| ABC | 9/209 | 8.0 (3.0–23.0) | 2/159 | 7.0 (1.0–30.0) | 7/47 | 13.0 (7.0–24.0) |
| 3TC or FTC | 9/209 | 8.0 (3.0–23.0) | 2/159 | 7.0 (1.0–30.0) | 7/47 | 13.0 (7.0–24.0) |
| TDF | 3/209 | 3.0 (1.0–11.0) | 1/159 | 2.0 (.0–17.0) | 2/47 | 3.0 (1.0–9.0) |
| ZDV | 6/209 | 2.0 (1.0–4.0) | 5/159 | 2.0 (1.0–5.0) | 1/47 | 2.0 (.0–9.0) |
| NNRTI | ||||||
| EFV or NVP | 39/209 | 27.0 (17.1–39.9) | 21/159 | 24.4 (13.2–40.7) | 18/47 | 37.4 (22.3–55.4) |
| DOR | 23/209 | 12.0 (7.0–19.0) | 10/159 | 9.0 (4.0–18.0) | 13/47 | 23.0 (14.0–35.0) |
| EFV | 38/209 | 26.8 (16.9–39.7) | 21/159 | 24.4 (13.2–40.7) | 17/47 | 36.3 (21.3–54.5) |
| ETR | 14/209 | 10.0 (4.0–23.0) | 6/159 | 9.0 (2.0–29.0) | 8/47 | 14.0 (8.0–23.0) |
| NVP | 39/209 | 27.0 (17.1–39.9) | 21/159 | 24.4 (13.2–40.7) | 18/47 | 37.4 (22.3–55.4) |
| RPV | 21/209 | 12.0 (6.0–25.0) | 13/159 | 12.0 (4.0–29.0) | 8/47 | 14.0 (8.0–23.0) |
| PI/r | ||||||
| ATV/r, DRV/r, or LPV/r | 4/209 | 1.2 (.6–2.4) | 4/159 | 1.5 (.7–3.1) | 0/47 | 0.0 (.0–7.6) |
| ATV/r | 3/209 | 1.0 (.0–2.0) | 3/159 | 1.0 (.0–3.0) | 0/47 | 0.0 (.0–7.6) |
| DRV/r | 0/209 | 0.0 (.2.0) | 0/159 | 0.0 (.0–2.0) | 0/47 | 0.0 (.0–7.6) |
| LPV/r | 2/209 | 1.0 (.0–2.0) | 2/159 | 1.0 (.0–3.0) | 0/47 | 0.0 (.0–7.6) |
| INSTI | ||||||
| Any | 2/197 | 3.5 (2.9–19.5) | 1/149 | 3.9 (3.8–27.1) | 1/45 | 2.1 (1.4–8.7) |
| BIC | 0/197 | 0.0 (.0–1.9) | 0/149 | 0.0 (.0–2.5) | 0/45 | 0.0 (.0–7.9) |
| CAB | 0/197 | 0.0 (.0–1.9) | 0/149 | 0.0 (.0–2.5) | 0/45 | 0.0 (.0–7.9) |
| DTG | 0/197 | 0.0 (.0–1.9) | 0/149 | 0.0 (.0–2.5) | 0/45 | 0.0 (.0–7.9) |
| EVG | 1/197 | 3.0 (.3–21.5) | 1/149 | 3.9 (.4–27.1) | 0/45 | 0.0 (.0–7.9) |
| RAL | 1/197 | 0.5 (.1–2.6) | 0/149 | 0.0 (.0–2.5) | 1/45 | 2.1 (1.4–8.7) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ARV, antiretroviral; ATV/r, atazanavir/ritonavir; BIC, bictegravir; CAB, cabotegravir; CI, confidence interval; DOR, doravirine; DRV/r, darunavir/ritonavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV/r, lopinavir/ritonavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI/r, boosted protease inhibitor; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Study design–weighted proportions and 95% CIs. The “All” group includes 3 ART initiators with unknown previous ARV drug exposure. HIV drug resistance was defined as the presence of a penalty score ≥15 using the Stanford HIVdb algorithm.
The prevalence of PDR to TDF was 3.0% (95% CI, 1.0%–11.0%) and to FTC/3TC was 8.0% (95% CI, 3.0%–23.0%) (Table 2). Resistance to elvitegravir was 3.0% (95% CI, .3%–21.5%) and 0.5% (95% CI, .1%–2.6%) to raltegravir. The mutations associated with reduced susceptibility to elvitegravir and raltegravir were S147G (3.0% [95% CI, .3%–21.5%]), E138AKT (1.2% [95% CI, .5%–3.1%]), and Y143CHR (0.5% [95% CI, .1%–2.6%]). Mutations associated with resistance to dolutegravir (DTG), cabotegravir, and bictegravir were not detected (Supplementary Table 1).
VL Suppression
The prevalence of VL suppression was 88.8% (95% CI, 83.1%–92.8%) among PHIV receiving ART for 12 (±3) months, decreasing to 78.4% (95% CI, 73.9%–83.0%) when adjusted by retention on ART after 12 (±3) months of ART initiation. The prevalence of VL suppression among PHIV receiving ART for ≥48 months was 80.5% (95% CI, 76.6%–84.0%), 82.6% (95% CI, 78.3%–86.3%) among those on first-line regimen and 69.8% (95% CI, 59.2%–78.7%) among those on second-line ART.
Acquired HIV Drug Resistance
Among PHIV on ART for 12 (±3) months receiving a first-line NNRTI-based regimen and with unsuppressed VL, the prevalence of ADR to EFV or NVP was 72.0% (95% CI, 39.1%–91.2%), and 61.2% (95% CI, 32.9%–83.5%) to any NRTI (Table 3). Resistance to PI/r or INSTI was not observed.
Table 3.
Prevalence of Acquired Human Immunodeficiency Virus Drug Resistance Among Individuals Receiving Antiretroviral Therapy With Unsuppressed Viral Load in El Salvador, 2018–2019a
| Drug Class | PHIV on ART for 12 ± 3 mo | PHIV on ART for 12 ± 3 mo Receiving First-Line NNRTI-Based Regimen | PHIV on ART for ≥48 mo | PHIV on ART for ≥48 mo Receiving First-Line NNRTI-Based Regimen | PHIV on ART for ≥48 mo Receiving Second-Line PI/r-Based Regimen | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | no./No. | % (95% CI) | |
| NRTI | ||||||||||
| Any | 9/15 | 61.2 (32.9–83.5) | 9/15 | 61.2 (32.9–83.5) | 50/61 | 83.5 (72.0–90.8) | 39/42 | 91.2 (77.5–96.9) | 11/19 | 65.7 (42.4–83.3) |
| ABC | 9/15 | 61.2 (32.9–83.5) | 9/15 | 61.2 (32.9–83.5) | 50/61 | 83.5 (72.0–90.8) | 39/42 | 91.2 (77.5–96.9) | 11/19 | 65.7 (42.4–83.3) |
| 3TC or FTC | 9/15 | 61.2 (32.9–83.5) | 9/15 | 61.2 (32.9–83.5) | 47/61 | 77.2 (65.1–86.1) | 39/42 | 91.2 (77.5–96.9) | 8/19 | 45.3 (24.0–68.5) |
| TDF | 8/15 | 49.1 (24.1–74.5) | 8/15 | 49.1 (24.1–74.5) | 28/61 | 42.4 (30.8–55.0) | 21/42 | 47.4 (33.4–62.0) | 7/19 | 31.0 (14.2–54.9) |
| ZDV | 0/15 | 0.0 (.0–20.4) | 0/15 | 0.0 (.0–20.4) | 26/61 | 45.9 (33.5–58.7) | 19/42 | 48.8 (34.3–63.6) | 7/19 | 39.1 (19.0–63.7) |
| NNRTI | ||||||||||
| EFV or NVP | 12/15 | 72.0 (39.1–91.2) | 12/15 | 72.0 (39.1–91.2) | 53/61 | 84.8 (71.5–92.5) | 41/42 | 95.0 (72.0–99.3) | 12/19 | 61.5 (36.9–81.3) |
| DOR | 9/15 | 62.8 (34.2–84.5) | 9/15 | 62.8 (34.2–84.5) | 39/61 | 63.4 (50.7–74.5) | 35/42 | 80.8 (64.2–90.8) | 4/19 | 23.7 (8.4–51.3) |
| EFV | 12/15 | 72.0 (39.1–91.2) | 12/15 | 72.0 (39.1–91.2) | 52/61 | 83.8 (70.7–91.8) | 41/42 | 95.0 (72.0–99.3) | 11/19 | 58.4 (34.6–78.9) |
| ETR | 6/15 | 48.1 (23.3–73.9) | 6/15 | 48.1 (23.3–73.9) | 18/61 | 29.3 (19.6–41.4) | 16/42 | 37.7 (24.7–52.8) | 2/19 | 10.2 (3.0–30.0) |
| NVP | 12/15 | 72.0 (39.1–91.2) | 12/15 | 72.0 (39.1–91.2) | 53/61 | 84.8 (71.5–92.5) | 41/42 | 95.0 (72.0–99.3) | 12/19 | 61.5 (36.9–81.3) |
| RPV | 7/15 | 53.3 (27.3–77.6) | 7/15 | 53.3 (27.3–77.6) | 28/61 | 43.5 (32.2–55.6) | 22/42 | 52.9 (38.1–67.2) | 6/19 | 22.2 (11.1–39.4) |
| PI/r | ||||||||||
| ATV/r, DRV/r, or LPV/r | 0/15 | 0.0 (.0–20.4) | 0/15 | 0.0 (.0–20.4) | 5/61 | 9.8 (3.7–23.3) | 1/42 | 1.3 (.4–4.9) | 4/19 | 29.0 (11.0–57.4) |
| ATV/r | 0/15 | 0.0 (.0–20.4) | 0/15 | 0.0 (.0–20.4) | 5/61 | 9.8 (3.7–23.3) | 1/42 | 1.3 (.4–4.9) | 4/19 | 29.0 (11.0–57.4) |
| DRV/r | 0/15 | 0.0 (.0–20.4) | 0/15 | 0.0 (.0–20.4) | 3/61 | 2.8 (1.3–5.9) | 1/42 | 1.3 (.4–4.9) | 2/19 | 6.1 (2.2–15.5) |
| LPV/r | 0/15 | 0.0 (.0–20.4) | 0/15 | 0.0 (.0–20.4) | 5/61 | 9.8 (3.7–23.3) | 1/42 | 1.3 (.4–4.9) | 4/19 | 29.0 (11.0–57.4) |
| INSTI | ||||||||||
| Any | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 1/61 | 5.1 (1.3–18.7) | 1/44 | 2.1 (.8–5.6) | 1/16 | 14.9 (2.2–57.1) |
| BIC | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 1/61 | 3.6 (.5–21.4) | 0/44 | 0.0 (.0–8.0) | 1/16 | 14.9 (2.2–57.1) |
| CAB | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 1/61 | 3.6 (.5–21.4) | 0/44 | 0.0 (.0–8.0) | 1/16 | 14.9 (2.2–57.1) |
| DTG | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 2/61 | 3.6 (.5–21.4) | 0/44 | 0.0 (.0–8.0) | 1/16 | 14.9 (2.2–57.1) |
| EVG | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 2/61 | 5.1 (1.3–18.7) | 1/44 | 2.1 (.8–5.6) | 1/16 | 14.9 (2.2–57.1) |
| RAL | 0/17 | 0.0 (.0–18.4) | 0/17 | 0.0 (.0–18.4) | 2/61 | 5.1 (1.3–18.7) | 1/44 | 2.1 (.8–5.6) | 1/16 | 14.9 (2.2–57.1) |
Abbreviations: 3TC, lamivudine; ABC, abacavir; ART, antiretroviral therapy; ATV/r, atazanavir/ritonavir; BIC, bictegravir; CAB, cabotegravir; CI, confidence interval; DOR, doravirine; DRV/r, darunavir/ritonavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; EVG, elvitegravir; FTC, emtricitabine; INSTI, integrase strand transfer inhibitor; LPV/r, lopinavir/ritonavir; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI/r, boosted protease inhibitor; PHIV, people with human immunodeficiency virus; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir disoproxil fumarate; ZDV, zidovudine.
Study design–weighted proportions and 95% CIs. HIV drug resistance was defined as the presence of a penalty score ≥15 using the Stanford HIVdb algorithm.
The PHIV on ART for ≥48 months receiving a first-line NNRTI-based regimen and with VL ≥1000 copies/mL had a prevalence of ADR to EFV or NVP of 95.0% (95% CI, 72.0%–99.3%), 1.3% (95% CI, .4%–4.9%) to PI/r, and 2.1% (95% CI, .8%–5.6%) to INSTI (Table 3). The prevalence of resistance to ZDV and FTC/3TC among PHIV on first-line NNRTI and TDF-based regimens with VL ≥1000 copies/mL was 14.4% (95% CI, 2.7%–50.8%). On the other hand, resistance to both TDF and FTC/3TC was 47.0% (95% CI, 26.4%–68.7%) among the PHIV on first-line NNRTI and ZDV-based regimens with VL ≥1000 copies/mL.
The most frequent mutations among PHIV receiving ART for ≥48 months with unsuppressed VL were M184IV (77.2% [95% CI, 60.9%–88.1%]), K103NS (59.5% [95% CI, 42.7%–74.3%]), and T215FY (35.6% [95% CI, 22.9%–50.6%]) (Supplementary Table 2). The only INSTI-associated resistance mutation observed was R263K.
Among PHIV receiving ART for ≥48 months with VL ≥1000 copies/mL and receiving a PI/r-based second-line regimen, 29.0% (95% CI, 11.0%–57.4%) were resistant to ritonavir-boosted lopinavir or atazanavir, 6.1% (95% CI, 2.2%–15.5%) were resistant to ritonavir-boosted darunavir, and 14.9% (95% CI, 2.2%–57.1%) were resistant to any INSTI (Table 3).
Phylogenetic Analysis
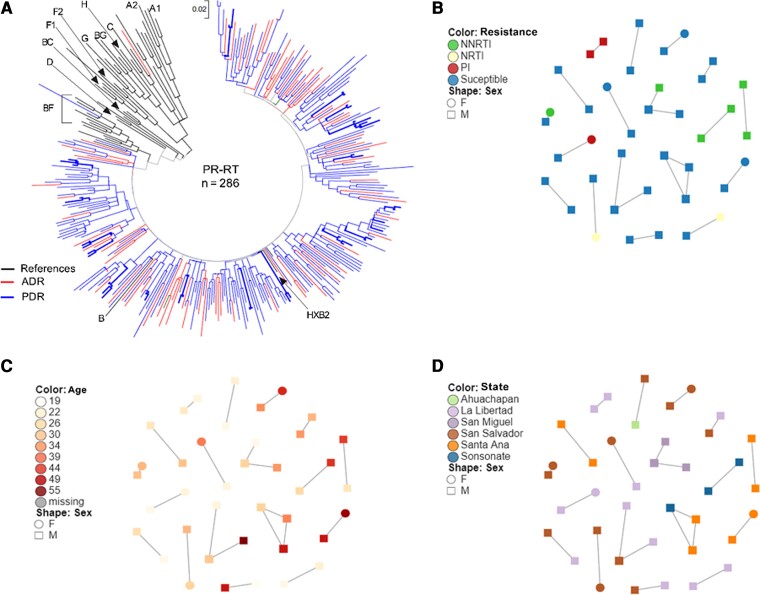
Although all individuals with available HIV sequences participating in the ADR12, ADR48, and PDR surveys were included in the phylogenetic analysis, only clusters including individuals participating in the PDR survey were observed. Eighteen clusters of 2–3 individuals, including 39 individuals, were observed (Figure 1). Frequent links between persons with large age differences were observed, suggesting HIV transmission between younger and older persons. As expected, persons forming clusters frequently resided in the same geographic area, which implies HIV is spreading locally. PDR prevalence in clustering individuals was 28% (11/39), with 3 clusters suggesting transmission of drug-resistant variants. Considering only participants of the PDR survey, after multivariable adjustment, persons aged 33–40 years were less likely to form clusters compared to persons aged <25 years (adjusted OR, 0.23 [95% CI, .07–.77]; P = .017) and persons with previous ARV exposure were less likely to be included in clusters (aOR, 0.14 [95% CI, .03–.65]; P = .013). Associations of clustering with gender, geographic state of residence, VL, or HIVDR were not observed after multivariable adjustment (Table 4).
Figure 1.
Phylogenetic tree and clustering. A, Sequences from 286 individuals included in the pretreatment drug resistance and acquired drug resistance surveys were included in the analysis. Reference human immunodeficiency virus (HIV) type 1 sequences of different subtypes obtained from the Los Alamos HIV Database were also included. Sequences were aligned by codon using ClustalW on Mega v6 after removing codons associated with HIV drug resistance according to the Stanford HIVdb tool (v8.9). A maximum likelihood tree was constructed based on the Tamura-Nei model with 1000 bootstrap repetitions. Using HIV-TRACE, putative transmission links were defined between individuals whose HIV sequence had a genetic distance <1.5%. Sequences forming clusters are shown in bold in the tree. Putative transmission links are detailed by drug resistance (B), age (C), and state of residence (D). Abbreviations: ADR, acquired drug resistance; F, female; M, male; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PDR, pretreatment drug resistance; PI, protease inhibitor; PR-RT, protease–reverse transcriptase.
Table 4.
Variables Associated With Clustering in Individuals Initiating Antiretroviral Therapy Within El Salvador's Human Immunodeficiency Virus Genetic Network
| Variable | Clustering No. (%) |
Crude OR (95% CI) |
P Value | Adjusted OR (95% CI)a |
P Value |
|---|---|---|---|---|---|
| Gender | |||||
| Male (n = 160) | 33 (20.6) | 1.00 | 1.00 | ||
| Female (n = 43) | 6 (14.0) | 0.62 (.24–1.61) | .325 | 0.93 (.32–2.74) | .901 |
| Transgender (n = 6) | 0 (0.0) | … | … | ||
| Age category, y | |||||
| ≤25 (n = 51) | 16 (31.4) | 1.00 | 1.00 | ||
| 26–32 (n = 47) | 10 (21.3) | 0.59 (.23–1.49) | .261 | 0.59 (.21–1.66) | .320 |
| 33–40 (n = 55) | 5 (9.1) | 0.22 (.07–.69) | .004 | 0.23 (.07–.77) | .017 |
| >40 (n = 55) | 8 (14.6) | 0.37 (.14–.99) | .040 | 0.55 (.19–1.64) | .285 |
| Viral load category | |||||
| Quartile 1 (n = 51) | 10 (19.6) | 1.00 | 1.00 | ||
| Quartile 2 (n = 52) | 11 (21.2) | 1.10 (.42–2.89) | .846 | 1.25 (.43–3.61) | .675 |
| Quartile 3 (n = 51) | 4 (7.8) | 0.35 (.10–1.23) | .086 | 0.38 (.10–1.44) | .156 |
| Quartile 4 (n = 52) | 13 (25.0) | 1.27 (.53–3.50) | .513 | 2.03 (.69–5.97) | .197 |
| State of residence | |||||
| San Salvador (n = 95) | 12 (12.6) | 1.00 | 1.00 | ||
| Other (n = 114) | 27 (23.7) | 2.15 (1.01–4.56) | .042 | 1.74 (.75–4.05) | .196 |
| Any HIVDR | |||||
| No (n = 162) | 28 (17.3) | 1.00 | 1.00 | ||
| Yes (n = 47) | 11 (23.4) | 1.46 (.66–3.23) | .344 | 2.47 (.97–6.33) | .059 |
| Previous ARV exposure | |||||
| No (n = 162) | 37 (22.8) | 1.00 | 1.00 | ||
| Yes (n = 47) | 2 (4.3) | .15 (.03–.67) | .004 | 0.14 (.03–.65) | .013 |
Statistically significant associations (P < .05) are marked in bold.
Abbreviations: ARV, antiretroviral; CI, confidence interval; HIVDR, human immunodeficiency virus drug resistance; OR, odds ratio.
Logistic regression model including gender, age category, viral load, state of residence, presence of HIVDR, and previous ARV exposure. Number of observations in the model: 199. Missing data for age: 1, viral load: 3.
DISCUSSION
As previously described in other low- and middle-income countries [6–11], this study suggests that the ART program in El Salvador faces important HIVDR challenges. The majority of PHIV initiated ART with an EFV- or NVP-based regimen. The level of PDR to EFV and NVP was found to be high (24.4% in ART-naive persons, 37.4% in persons with previous ARV drug exposure, and 27.0% overall), around 3-fold higher than the WHO-recommended threshold (10%) to urgently shift to a first-line non-NNRTI-containing regimen [2]. A systematic review informing the WHO guidelines on the public health response to PDR concluded that PHIV with PDR to NNRTIs who initiated with an NNRTI-based regimen were less likely to suppress VL, more likely to interrupt ART, and more likely to experience virologic failure or death when compared with PHIV with PDR to NNRTIs who initiated with a non-NNRTI-based regimen [2, 32]. Increases in AIDS-associated deaths, new HIV infections, and ART program costs have been predicted in settings with PDR levels >10% [1]. The results of this study were presented to El Salvador's Ministry of Health and HIV Programme authorities in late 2019. Given the high rates of PDR to EFV and NVP, the Ministry of Health updated the national guidelines to prescribe the globally recommended DTG-based regimen as the preferred first-line option for ART initiators [2, 33, 34].
The HIV transmission network analysis showed clusters of drug-resistant variants that suggest PDR spread among ART-naive persons and locally. Of note, persons with ADR were not observed in the network, suggesting either a limited role of this group in HIVDR transmission in El Salvador or a limited sampling density in the network. A similar observation was made for persons 33–40 years of age, who were less likely to form clusters than persons under 25, which has also been observed in other Latin American countries [35]. These observations, although limited by sample size, can help support and design HIV prevention policies and activities such as preexposure prophylaxis for adolescents and young adults.
Concerning the PHIV receiving ART, the retention-adjusted VL suppression estimate among PHIV receiving ART for 12 (±3) and the VL suppression rate among PHIV receiving ART for ≥48 months were below the global 2020 target of 90% of all PHIV receiving ART with VL suppression [36]. In addition, PHIV receiving ART with VL ≥1000 copies/mL in El Salvador had high rates of HIVDR to NNRTI-based regimens, the most frequently used in the country. Considering these observations, a comprehensive action framework is needed to strengthen the performance of the national ART program and improve the quality of HIV services in El Salvador to improve VL suppression rates. For example, as per WHO recommendations, PHIV receiving NNRTI-based first-line ART regimens should be transitioned to an optimal DTG-based regimen [33, 37]. In addition, the routine monitoring of early warning indicators of HIVDR should be used to identify clinics with suboptimal performance and to design focused interventions [38–40]. For example, the assessment of attrition from ART should trigger focused interventions to identify and overcome the barriers to retention in care and treatment [41, 42]. Also, the VL testing coverage in El Salvador was 66%, considerably below the WHO-recommended target of >95% [43, 44]. Therefore, enhancing the VL coverage and monitoring the VL testing cascade at the national and clinic levels could help identify programmatic gaps and underserved populations related to VL coverage, ART adherence counseling, and timely ART change for PHIV who are not suppressed [45–49].
In 2020, El Salvador updated the national ART guidelines adopting the WHO-recommended DTG-based second-line ART regimen in combination with an optimized NRTI backbone for those failing a first-line NNRTI-based regimen, prescribing ZDV (with FTC/3TC) if TDF was used in the failing first-line regimen and vice versa [33, 34]. DTG, administered with at least 1 fully active NRTI, has been reported to be superior to ritonavir-boosted lopinavir–based regimens in individuals failing an NNRTI-based first-line regimen [50]. In this study, we observed dual resistance to NRTIs used as the backbone in second-line ART among PHIV receiving first-line NNRTI-based regimens with unsuppressed VL. HIVDR testing could be a valuable tool in El Salvador to avoid a functional DTG monotherapy among PHIV with dual resistance to the NRTI backbone, which could lead to DTG resistance [51–53]. To preserve the NRTI backbone among PHIV receiving ART in El Salvador, prolonged exposure to suboptimal ART should be avoided. Prompt clinical response to high VL and timely ART switch prevents drug resistance–associated mutation accumulation [54–57].
As previously reported by other countries in Central America [8, 10], a limitation of this study was the underenrollment for the ADR12 survey (81.0% [230/284] sample goal attainment) despite an extension of the enrollment period due to logistic constraints experienced by many clinics and slow enrollment response. This limitation was accounted for by weighting the available data per clinic, considering the estimated eligible population sizes, the number of individuals enrolled, and the number of successful genotypes. Similarly, the study was designed to provide nationally representative PDR and ADR prevalence estimates, but not subnational or subpopulation-specific estimates that could help guide focused interventions. Finally, risk behavior data were not collected, limiting more in-depth analysis of the HIV transmission network in El Salvador.
In conclusion, important challenges for the ART program were observed in the first nationally representative HIVDR study in El Salvador, including high levels of resistance to EFV and NVP among ART initiators and low VL suppression prevalence among individuals on treatment. The prompt response of the Ministry of Health introducing DTG-based regimens as preferred first-line and alternative second-line ART [34] is expected to contribute to addressing HIVDR in the country. Other prioritizing actions are the transition of PHIV receiving ART to an optimal DTG-based regimen and monitoring the VL cascade to ensure that individuals with confirmed virologic failure are rapidly switched to an optimal regimen to avoid the accumulation of ARV-associated resistance mutations. Additional studies to identify programmatic issues in specific geographical areas or populations are warranted to further adapt and strengthen responses to HIVDR in El Salvador.
Supplementary Material
Contributor Information
Amalia Girón-Callejas, Centro de Estudios en Salud, Universidad del Valle de Guatemala, Guatemala City, Guatemala.
Claudia García-Morales, Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases, Mexico City, Mexico.
Ricardo Mendizabal-Burastero, Centro de Estudios en Salud, Universidad del Valle de Guatemala, Guatemala City, Guatemala.
Alma Quezada, Ministerio de Salud de El Salvador, San Salvador, El Salvador.
Lisette Ruiz, Ministerio de Salud de El Salvador, San Salvador, El Salvador.
Nelly Arguera, Ministerio de Salud de El Salvador, San Salvador, El Salvador.
Salvador Sorto, Ministerio de Salud de El Salvador, San Salvador, El Salvador.
Ana I Nieto, Ministerio de Salud de El Salvador, San Salvador, El Salvador.
Daniela Tapia-Trejo, Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases, Mexico City, Mexico.
Dulce M López-Sánchez, Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases, Mexico City, Mexico.
Marissa Pérez-García, Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases, Mexico City, Mexico.
Luis Cruz, Centro de Estudios en Salud, Universidad del Valle de Guatemala, San Salvador, El Salvador.
Raúl Andino, Centro de Estudios en Salud, Universidad del Valle de Guatemala, San Salvador, El Salvador.
Edgar Sajquim, Centro de Estudios en Salud, Universidad del Valle de Guatemala, Guatemala City, Guatemala.
Sandra I Juárez, US Centers for Disease Control and Prevention, Central American Region, Guatemala City, Guatemala.
Nasim Farach, US Centers for Disease Control and Prevention, Central American Region, Guatemala City, Guatemala.
Giovanni Ravasi, Pan-American Health Organization, Washington, District of Columbia, USA.
Sanny Northbrook, US Centers for Disease Control and Prevention, Central American Region, Guatemala City, Guatemala.
Gustavo Reyes-Terán, Coordinating Commission of the Mexican National Institutes of Health, Mexico City, Mexico.
Santiago Ávila-Ríos, Centre for Research in Infectious Diseases, National Institute of Respiratory Diseases, Mexico City, Mexico.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank all participants for their generosity. We are grateful to the healthcare staff from the clinics participating in this study for their valuable support in implementing the survey.
Disclaimer. The funders had no role in study design, data collection and analysis, publication decision, or manuscript preparation. The findings and conclusions of this manuscript are those of the authors and do not necessarily represent the official position of the funding agencies.
Financial support. This work was supported by the US President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention, under the terms of the cooperative agreement with Universidad del Valle de Guatemala (GH001285) and the Mexican Government (Comisión de Equidad y Género de las Legislaturas LX-LXI, Comisión de Igualdad de Género de la Legislatura LXII de la H. Cámara de Diputados de la República Mexicana) and Consejo Nacional de Ciencia y Tecnología (CONACyT SALUD-2013-01-202475).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Phillips AN, Stover J, Cambiano V, et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality. New infections, and antiretroviral therapy program costs in sub-Saharan Africa. J Infect Dis 2017; 215:1362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization (WHO) . Guidelines on the public health response to pretreatment HIV drug resistance: July. Geneva, Switzerland: WHO; 2017:84. [Google Scholar]
- 3. Bertagnolio S, Beanland RL, Jordan MR, Doherty M, Hirnschall G. The World Health Organization's response to emerging human immunodeficiency virus drug resistance and a call for global action. J Infect Dis 2017; 216(Suppl 9):S801–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO) . HIV drug resistance surveillance guidance. Update. Geneva, Switzerland: WHO; 2015:20. [Google Scholar]
- 5. World Health Organization (WHO) . Global action plan on HIV drug resistance. 2021. Geneva, Switzerland: WHO; 2017:40. [Google Scholar]
- 6. Bissio E, Barbás MG, Bouzas MB, et al. Pretreatment HIV-1 drug resistance in Argentina: results from a surveillance study performed according to WHO-proposed new methodology in 2014–15. J Antimicrob Chemother 2017; 72:504–10. [DOI] [PubMed] [Google Scholar]
- 7. Avila-Rios S, Garcia-Morales C, Valenzuela-Lara M, et al. HIV-1 drug resistance before initiation or re-initiation of first-line ART in eight regions of Mexico: a sub-nationally representative survey. J Antimicrob Chemother 2019; 74:1044–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giron-Callejas A, Garcia-Morales C, Mendizabal-Burastero R, et al. High levels of pretreatment and acquired HIV drug resistance in Nicaragua: results from the first nationally representative survey 2016. J Int AIDS Soc 2019; 22:e25429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Machado LY, Blanco M, López LS, et al. National survey of pre-treatment HIV drug resistance in Cuban patients. PLoS One 2019; 14:e0221879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giron-Callejas A, Garcia-Morales C, Mendizabal-Burastero R, et al. High level of pre-treatment and acquired HIV drug resistance in Honduras: a nationally representative survey 2016–17. J Antimicrob Chemother 2020; 75:1932–42. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization (WHO) . HIV drug resistance report. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- 12. Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Murillo W, de Rivera I L, Albert J, Guardado ME, Nieto AI, Paz-Bailey G. Prevalence of transmitted HIV-1 drug resistance among female sex workers and men who have sex with men in El Salvador, Central America. J Med Virol 2012; 84:1514–21. [DOI] [PubMed] [Google Scholar]
- 14. Holguin A, Yebra G, Martin L, et al. Transmitted drug-resistance in human immunodeficiency virus–infected adult population in El Salvador, Central America. Clin Microbiol Infect 2013; 19:E523–32. [DOI] [PubMed] [Google Scholar]
- 15. AIDSinfo . Fact sheets: El Salvador 2018. 2022. https://aidsinfo.unaids.org/. Accessed August 2022.
- 16. Ministerio de Salud de El Salvador . Guía clínica para la atención integral en salud de las personas con VIH. San Salvador: Ministerio de Salud de El Salvador, 2014:107. [Google Scholar]
- 17. World Health Organization (WHO) . Surveillance of HIV drug resistance in populations initiating antiretroviral therapy (pre-treatment HIV drug resistance). Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 18. World Health Organization (WHO) . Surveillance of HIV drug resistance in adults receiving ART (acquired HIV drug resistance). Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 19. World Health Organization (WHO) . Sample size calculators to design PDR and ADR surveys. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 20. World Health Organization (WHO) . WHO/HIVRESNET HIV drug resistance laboratory operational framework. Geneva, Switzerland: WHO; 2017:73. [Google Scholar]
- 21. Garcia-Morales C, Tapia-Trejo D, Quiroz-Morales VS, et al. HIV pretreatment drug resistance trends in three geographic areas of Mexico. J Antimicrob Chemother 2017; 72:3149–58. [DOI] [PubMed] [Google Scholar]
- 22. Van Laethem K, Schrooten Y, Covens K, et al. A genotypic assay for the amplification and sequencing of integrase from diverse HIV-1 group M subtypes. J Virol Methods 2008; 153:176–81. [DOI] [PubMed] [Google Scholar]
- 23. Woods CK, Brumme CJ, Liu TF, et al. Automating HIV drug resistance genotyping with RECall, a freely accessible sequence analysis tool. J Clin Microbiol 2012; 50:1936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rhee SY, Gonzales MJ, Kantor R, Betts BJ, Ravela J, Shafer RW. Human immunodeficiency virus reverse transcriptase and protease sequence database. Nucl Acids Res 2003; 31:298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis 2006; 42:1608–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stanford University HIV Drug Resistance Database . HIVDB subtyping program. 2019. https://hivdb.stanford.edu/page/hiv-subtyper/. Accessed August 2022.
- 27. Siepel AC, Halpern AL, Macken C, Korber BT. A computer program designed to screen rapidly for HIV type 1 intersubtype recombinant sequences. AIDS Res Hum Retrovir 1995; 11:1413–6. [DOI] [PubMed] [Google Scholar]
- 28. Pineda-Pena AC, Faria NR, Imbrechts S, et al. Automated subtyping of HIV-1 genetic sequences for clinical and surveillance purposes: performance evaluation of the new REGA version 3 and seven other tools. Infect Genet Evol 2013; 19:337–48. [DOI] [PubMed] [Google Scholar]
- 29. Ministerio de Salud de El Salvador . Informe nacional: construcción de la cascada el continuo de la atención en VIH, a diciembre 2018, a nivel nacional y clínicas de atención integral y datos nacionales del instituto salvadoreño del seguro social. San Salvador: Ministerio de Salud de El Salvador; 2019. [Google Scholar]
- 30. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30:2725–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pond SL K, Weaver S, Leigh Brown AJ, Wertheim JO. HIV-TRACE (transmission cluster engine): a tool for large scale molecular epidemiology of HIV-1 and other rapidly evolving pathogens. Mol Biol Evol 2018; 35:1812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization (WHO). Systematic reviews and meta-analyses informing the guidelines on the public health response to pretreatment HIV drug resistance. Web annex 2. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 33. World Health Organization (WHO). Updated guidance on first-line and second-line antiretroviral regimens. Geneva, Switzerland: WHO; 2019:14. [Google Scholar]
- 34. Ministerio de Salud de El Salvador. Guía clínica para la atención integral en salud de las personas con VIH. San Salvador: Ministerio de Salud de El Salvador; 2020:104. [Google Scholar]
- 35. Matías-Florentino M, Chaillon A, Ávila-Ríos S, et al. Pretreatment HIV drug resistance spread within transmission clusters in Mexico City. J Antimicrob Chemother 2020; 75:656–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Joint United Nations Programme on HIV/AIDS. 90-90-90: an ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 2014:40. [Google Scholar]
- 37. World Health Organization (WHO). Transition to new antiretroviral drugs in HIV programmes: clinical and programmatic considerations. Technical update. Geneva, Switzerland: WHO; 2017:40. [Google Scholar]
- 38. St-Jean M, Harrigan PR, Sereda P, Montaner J, Lima VD. An assessment of the relationship between the World Health Organization HIV drug resistance early warning indicators and HIV drug resistance acquisition. HIV Med 2017; 18:342–53. [DOI] [PubMed] [Google Scholar]
- 39. World Health Organization (WHO) . Meeting report on assessment of World Health Organization HIV drug resistance early warning indicators: report of the early advisory indicator panel meeting. Geneva, Switzerland: WHO; 2012:9241503947. [Google Scholar]
- 40. World Health Organization (WHO). Maintaining and improving quality of care within HIV clinical services. Geneva, Switzerland: WHO; 2019:16. [Google Scholar]
- 41. Shubber Z, Mills EJ, Nachega JB, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hall BJ, Sou KL, Beanland R, et al. Barriers and facilitators to interventions improving retention in HIV care: a qualitative evidence meta-synthesis. AIDS Behav 2017; 21:1755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. World Health Organization (WHO) . HIV drug resistance strategy update. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- 44. Joint United Nations Programme on HIV/AIDS . 90-90-90. HIV testing and treatment indicators—El Salvador—2019.2019.https://aidsreportingtool.unaids.org. Accessed 1 October 2022.
- 45. World Health Organization (WHO) . Considerations for developing a monitoring and evaluation framework for viral load testing. Geneva, Switzerland: WHO; 2019. [Google Scholar]
- 46. Etoori D, Ciglenecki I, Ndlangamandla M, et al. Successes and challenges in optimizing the viral load cascade to improve antiretroviral therapy adherence and rationalize second-line switches in Swaziland. J Int AIDS Soc 2018; 21:e25194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Glass TR, Motaboli L, Nsakala B, et al. The viral load monitoring cascade in a resource-limited setting: a prospective multicentre cohort study after introduction of routine viral load monitoring in rural Lesotho. PLoS One 2019; 14:e0220337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ford N, Orrell C, Shubber Z, Apollo T, Vojnov L. HIV viral resuppression following an elevated viral load: a systematic review and meta-analysis. J Int AIDS Soc 2019; 22:e25415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonner K, Mezochow A, Roberts T, Ford N, Cohn J. Viral load monitoring as a tool to reinforce adherence: a systematic review. J Acquir Immune Defic Syndr 2013; 64:74–8. [DOI] [PubMed] [Google Scholar]
- 50. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]
- 51. Wijting I, Rokx C, Boucher C, et al. Dolutegravir as maintenance monotherapy for HIV (DOMONO): a phase 2, randomised non-inferiority trial. Lancet HIV 2017; 4:e547–54. [DOI] [PubMed] [Google Scholar]
- 52. Blanco JL, Rojas J, Paredes R, et al. Dolutegravir-based maintenance monotherapy versus dual therapy with lamivudine: a planned 24 week analysis of the DOLAM randomized clinical trial. J Antimicrob Chemother 2018; 73:1965–71. [DOI] [PubMed] [Google Scholar]
- 53. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the randomized noninferiority monotherapy of TiviCAY trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 54. Boender TS, Kityo CM, Boerma RS, et al. Accumulation of HIV-1 drug resistance after continued virological failure on first-line ART in adults and children in sub-Saharan Africa. J Antimicrob Chemother 2016; 71:2918–27. [DOI] [PubMed] [Google Scholar]
- 55. Cozzi-Lepri A, Phillips AN, Martinez-Picado J, et al. Rate of accumulation of thymidine analogue mutations in patients continuing to receive virologically failing regimens containing zidovudine or stavudine: implications for antiretroviral therapy programs in resource-limited settings. J Infect Dis 2009; 200:687–97. [DOI] [PubMed] [Google Scholar]
- 56. Sigaloff KC, Hamers RL, Wallis CL, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr 2011; 58:23–31. [DOI] [PubMed] [Google Scholar]
- 57. Gupta RK, Hill A, Sawyer AW, et al. Virological monitoring and resistance to first-line highly active antiretroviral therapy in adults infected with HIV-1 treated under WHO guidelines: a systematic review and meta-analysis. Lancet Infect Dis 2009; 9:409–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.