Abstract
Background
Patients with multiple myeloma are at higher risk for infections due to disease pathogenesis and administered therapies. The purpose of this study was to estimate the risk for any grade and severe infections associated with the use of anti-CD38 monoclonal antibodies in patients with multiple myeloma.
Methods
We searched PubMed and EMBASE for randomized controlled trials (RCTs) that included patients with multiple myeloma who received CD38-targeting monoclonal antibody regimens and reported outcomes of infection and performed a random-effects meta-analysis to estimate the relative risk for infections.
Results
After screening 673 citations, we retrieved 17 studies providing data on 11 RCTs. Overall, the included reports evaluated 5316 patients (2797 in the intervention arm and 2519 in the control arm). The relative risk (RR) for both any grade or severe infections was 1.27 (95% CI, 1.17–1.37 and 1.14–1.41, respectively). The cumulative incidence of any grade infections for patients who received anti-CD38 agents was 77% (95% CI, 68%–86%), while for severe infections it was 28% (95% CI, 23%–34%). Patients treated with anti-CD38 agents had a 39% higher risk for any grade pneumonia (RR, 1.39; 95% CI, 1.12–1.72) and a 38% higher risk for severe pneumonia (RR, 1.38; 95% CI, 1.09–1.75). For upper respiratory tract infections, the relative risk was 1.51 and 1.71 for any grade and severe infections, respectively. Regarding varicella-zoster virus (VZV) reactivation, we found no evidence of increased risk (RR, 3.86; 95% CI, 0.66–22.50).
Conclusions
Patients with multiple myeloma treated with regimens that included an anti-CD38 monoclonal antibody were at higher risk for any grade or severe infections without an associated higher mortality rate during the follow-up period of the retrieved studies. No evidence of increased risk for VZV reactivation was noted, but there was a significant association between CD38-targeting treatment and pneumonia risk. Increased surveillance for infections, development of effective prophylactic strategies, and studies with long follow-up are needed for patients with multiple myeloma treated with anti-CD38-based regimens.
Keywords: multiple myeloma, monoclonal antibodies, infections, daratumumab, isatuximab
Among patients with multiple myeloma who received anti-CD38 monoclonal antibody-based treatment the relative risk for infection was 1.27, with a 28% incidence of severe infection. Consequently, we describe the importance of surveillance and prophylactic strategies across our studied patient population.
Patients with multiple myeloma have up to 7–10 times higher risk for infections compared with the general population [1, 2]. Moreover, infections represent one of the leading causes of death in patients with multiple myeloma [3, 4], and almost 10% of patients with newly diagnosed multiple myeloma die because of an infection even before treatment initiation [5].
Available treatments for multiple myeloma, including proteasome inhibitors, immunomodulatory agents (such as pomalidomide and lenalidomide, glucocorticoids), and monoclonal antibodies targeting specific myeloma cell antigens, further predispose to infection [3, 6]. Corticosteroid treatment lowers monocyte and lymphocyte cell counts, inhibits monocyte and lymphocyte function, and diminishes neutrophil and monocyte trafficking to inflammatory sites [7]. Accordingly, in comparison with steroid-naïve patients, individuals receiving glucocorticoid treatment exhibit increased risk of cellulitis, herpes zoster infections, bloodstream infections, candidiasis, and lower respiratory tract infections [8, 9]. A recent meta-analysis on immunomodulatory drugs revealed an elevated rate of severe infections among patients who received immunomodulatory agents that ranged from 13% to 22%, depending on the treatment setting (transplant-eligible, nontransplant, relapsed/refractory, maintenance) [10]. Also, a different meta-analysis found that patients who receive lenalidomide have an increased risk of high-grade infection by more than double [11]. The rate of severe infections among patients who received proteasome inhibitor–based regimens ranged from 9.7% to 23.3% [10]. Taken in their totality, these findings suggest that the benefit imparted by existing therapeutic options is associated with an increase in infection rates.
CD38 is a transmembrane glycoprotein highly expressed in multiple myeloma cells that, at relatively low levels, is also expressed in normal immune cells [12–14]. CD38 is involved in B-cell differentiation, neutrophil and monocyte chemotaxis, and T-cell activation and proliferation [15]. Depending on pH levels, CD38 functions as an extracellular enzyme, acting as a metabolic sensor that catalyzes the extracellular conversion of NAD+ to calcium signaling regulators such as adenosine [16]. In addition, CD31 is a nonsubstrate ligand that is naturally expressed by endothelial cells as a cell adhesion protein that interacts with CD38 [17, 18].
Multiple myeloma cells have a high expression of CD38, a surface glycoprotein that allows adhesion to the local microenvironment [19]. Several studies have shown that only plasma cells highly express CD38 antigens in the bone marrow and that malignant plasma cells are not detected in either the CD38-negative cell subpopulation or the proportion of cells that weakly express CD38 antigens [19–21]. However, B, T, and NK cells, once activated, also increase their CD38 surface expression to levels comparable to plasma cells [22]. Daratumumab, a CD38-targeting monoclonal antibody, is effective in the clearance of cell subsets that express CD38, which include immunosuppressive regulatory T and B cells and myeloid-derived suppressor cells [22]. Furthermore, an interesting outcome of daratumumab treatment is the upregulation of cytotoxic T-cell number, activity, and clonality, along with interferon-gamma production in extensively pretreated relapsed and refractory patients with multiple myeloma [22]. These findings demonstrate a more effective adaptive immune response that develops with treatment of multiple myeloma using an anti-CD38 monoclonal antibody.
Monoclonal antibodies targeting CD38 (daratumumab and isatuximab) have shown great efficacy and transformed the multiple myeloma treatment landscape [23–25]. Both daratumumab and isatuximab bind to distinct epitopes on CD38 [26, 27]. An additional difference between these 2 monoclonal antibodies is that daratumumab is a fully humanized agent compared with isatuximab, which is a chimeric agent [12]. Furthermore, unlike isatuximab, daratumumab is currently recommended for the treatment of newly diagnosed patients with multiple myeloma [27], and isatuximab is now under clinical study for use in treating patients with newly diagnosed multiple myeloma. Both of our drugs of interest have nonlinear pharmacokinetics, which is in accordance with the target-mediated drug disposition that both mediate [28, 29].
To the best of our knowledge, our study is the first meta-analysis to evaluate the incidence of infection, risk of infection, and risk of death attributed to infection among patients who receive anti-CD38 monoclonal antibody–based regimens. In this context, we performed a systematic review and meta-analysis of randomized clinical trials (RCTs) to estimate the risk of infections in patients with multiple myeloma who received anti-CD38 monoclonal antibody–based regimens compared with those treated with backbone myeloma regimens that did not include anti-CD38 agents.
METHODS
Approach
This systematic review and meta-analysis was performed based on the approach detailed in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) statement checklist [30].
Data Sources
We searched the PubMed/MEDLINE and EMBASE databases for literature in English using the following search term: “(daratumumab OR isatuximab OR anti-CD38) AND randomized” [31].
Study Selection
A randomized controlled trial was considered eligible for our analysis if it fulfilled the following criteria: (a) randomized patients with multiple myeloma, (b) had an intervention arm regimen with an anti-CD38 monoclonal antibody (daratumumab or isatuximab), (c) compared the effect of the intervention arm with backbone multiple myeloma regimens that only differed in the absence of an anti-CD38 agent, and (d) included extractable data of at least 1 outcome of interest.
For studies with multiple extended follow-up reports, we also included the latest published reports. We excluded review articles, case reports, meeting abstracts, case–control studies, cross-sectional studies, observational studies, and reports from nonrandomized, single-arm trials.
Primary and Secondary Outcomes
By collecting the number of patients who developed infections, pneumonia, upper respiratory tract infection, varicella-zoster virus (VZV) reactivation, and death attributed to infection, we evaluated the relative risk of infection (any grade and severe) among patients who received an anti-CD38 monoclonal antibody (compared with those who received the exact same regimen without the anti-CD38 agent). For secondary outcomes, we evaluated the relative risk of pneumonia, upper respiratory tract infections, varicella zoster virus reactivation, and death attributed to infection. We performed relative risk assessment both for the primary report and the latest report of the retrieved RCTs. The grade of infectious complications was based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE). Using these criteria, we were able to assess the risk ratio of infections that occurred during the follow-up period of retrieved studies [32]. As defined by the CTCAE, grade 3 infections are severe or medically significant infections that require hospitalization but are not life-threatening, grade 4 infections endanger patient lives and require urgent intervention, and grade 5 infections are the cause of death of a patient [32].
Data Extraction and Quality Assessment
Two reviewers (S.V. and A.V.) independently determined study eligibility by screening titles and abstracts and performed full-text review of selected studies. Potential disagreements were discussed and resolved by consensus. We extracted data that included randomized safety populations, regimens in the intervention and control arms, treatment setting (newly diagnosed or relapsed/refractory), primary and secondary outcomes, duration of follow-up period, and information related to study quality. Consequently, we retrieved pertinent data on patients who presented infectious manifestations. For quality assessment, we evaluated the risk of bias using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2), which evaluates the validity and bias in randomized controlled trials across 5 domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result [33].
Data Synthesis and Analysis
We used Stata, version 17 (Stata Corporation, College Station, TX, USA), for data synthesis and analysis. For RCTs that published multiple study reports of different follow-up periods of the same study population, we retrieved the first and the latest published articles and grouped the studies into 2 different pools of first and latest extended follow-up reports, respectively. We performed a separate analysis on both the first published articles and the latest follow-up reports for the same predetermined variables in question. We used the DerSimonian and Laird approach [34] to estimate the relative risk of any grade and severe infections for patients who received daratumumab or isatuximab vs those who received multiple myeloma regimens that did not include an anti-CD38 agent. Notably, we use the term “cumulative incidence” to refer to the infection rate measured during the follow-up period of retrieved RCTs [35].
For the initial analysis, we used the primary reports of the retrieved RCTs. We also utilized primary reports to estimate our secondary outcomes. We used a random-effects approach, as we assumed the effects are heterogeneous because of variances in study design, drug combinations, follow-up periods, and drug dosages. To stabilize the variances, we used Freeman Tukey double arcsine transformation [36].
For this systematic review and meta-analysis, we utilized 95% CIs and estimated heterogeneity using the I2 statistic [37]. A guide to interpretation of heterogeneity with the I2 statistic is as follows: 0%–40% might not be important, 30%–60% may represent moderate heterogeneity, 50%–90% may represent substantial heterogeneity, 75%–100% for considerable heterogeneity [38]. Finally, we used Egger’s test to evaluate for publication bias and to assess for small study effects [39].
RESULTS
Overall Data
We retrieved 673 citations from PubMed and EMBASE searches. After title and abstract screening, we excluded 652 studies and performed detailed full-text evaluation of 21 publications. We identified 17 publications, from 11 different RCTs, that fulfilled the inclusion criteria of this meta-analysis (Figure 1) [40–56]. Six articles [51–56] reported the latest extended follow-up report of an RCT, and 11 articles [40–50] provided primary data. Baseline characteristics and the overall risk of bias for studies with extractable outcomes are presented in Supplementary Table 1.
Figure 1.
Flow diagram for selection of studies included in the systematic review and meta-analysis. Abbreviations: REML, restricted maximum likelihood; RR, relative risk or risk ratio.
Eligible studies contributed information on 5316 patients, with 2797 patients receiving a regimen containing an anti-CD38 monoclonal antibody and 2519 patients receiving control regimens without anti-CD38. Two RCTs with 3 reports [46, 47, 54] studied the anti-CD38 monoclonal antibody isatuximab, and the rest assessed daratumumab [40–45, 48–53, 55, 56]. Of note, all studies presented their results by reporting the number of patients who developed infections and not the number of infectious events.
Four RCTs with 6 published reports [40, 43, 45, 49, 51, 55] studied patients with newly diagnosed multiple myeloma, and 7 RCTs with 11 reports [41, 42, 44, 46–48, 50, 52–54, 56] studied patients with relapsed/refractory multiple myeloma. Glucocorticoids (dexamethasone or prednisone) were part of the treatment in all studies. In 7 [41, 42, 47, 48, 53, 55, 56] out of the 11 RCTs, the cumulative dose of dexamethasone administered (orally or intravenously) was 160 mg per cycle. Only 1 RCT used a different cumulative dose of dexamethasone per cycle, at 120 mg [45]. One RCT [51] used oral prednisone with a cumulative dose of 240 mg per cycle. The Cassiopeia RCT [43] used oral or intravenous dexamethasone with a completely different protocol compared with the rest of the studies (40 mg on days 1, 2, 8, 9, 15, 16, 22, and 23 of induction cycles 1 and 2 and days 1 and 2 of induction cycles 3 and 4 and 20 mg on days 8, 9, 15, and 16 of induction cycles 3 and 4 and days 1, 2, 8, 9, 15, and 16 of both consolidation cycles). One RCT with 2 reports [40, 51] used melphalan, and 6 studies with 9 reports [41, 43, 45, 46, 49, 50, 54–56] used an immunomodulatory agent such as thalidomide, pomalidomide, or lenalidomide as part of the treatment regimens. Four RCTs with 7 reports did not use a proteasome inhibitor in their regimens [41, 46, 49, 50, 54–56].
Regarding infection prophylaxis, 5 RCTs provided data [41, 42, 47, 51, 55]. Notably, study protocols recommended that Pneumocystis jirovecii pneumonia prophylaxis be considered as per institutional guidelines [41, 42, 47, 51, 55]. Also, antiviral prophylaxis with valacyclovir, acyclovir, or famciclovir was recommended for prophylaxis against herpes zoster reactivation [41, 42, 47, 51, 55]. Of note, 1 study [41] also allowed for prophylactic antibiotic treatment.
The median age of randomized patients ranged between 61 and 74 years, and the average proportion of males was 55%. Regarding primary reports, the median duration of follow-up ranged from 7.4 to 28 months, and for RCTs that published extended follow-up reports, the median duration of monitoring ranged from 27.8 to 56.2 months.
In Table 1, we present the relative risk for infections, severe infections, pneumonia, severe pneumonia, upper respiratory tract infections (URIs), severe URIs, varicella zoster virus (VZV) reactivation, and death of patients who received an anti-CD38 monoclonal antibody–based regimen compared with those who received backbone therapies that did not include an anti-CD38 monoclonal antibody. Egger’s test for publication bias showed no evidence of small study effects (bias = 0.03; P = .98), while the heterogeneity among the results ranged from might not be important to considerable (I2 = 0%–95.09%).
Table 1.
Relative Risk for all Outcomes of Interest
| Infectious Outcome | Relative Risk | 95% CI |
|---|---|---|
| Any grade infections | 1.27 | 1.17–1.37 |
| Severe infections | 1.27 | 1.14–1.41 |
| Any grade pneumonia | 1.39 | 1.12–1.72 |
| Severe pneumonia | 1.38 | 1.09–1.75 |
| URIs | 1.51 | 1.35–1.70 |
| Severe URIs | 1.71 | 1.00–2.90 |
| VZV reactivation | 3.86 | 0.66–22.50 |
| Death attributed to infection | 1.35 | 0.76–2.40 |
Abbreviations: URIs, upper respiratory tract infections; VZV, varicella-zoster virus.
Relative Risk for Infections
Any Grade Infections
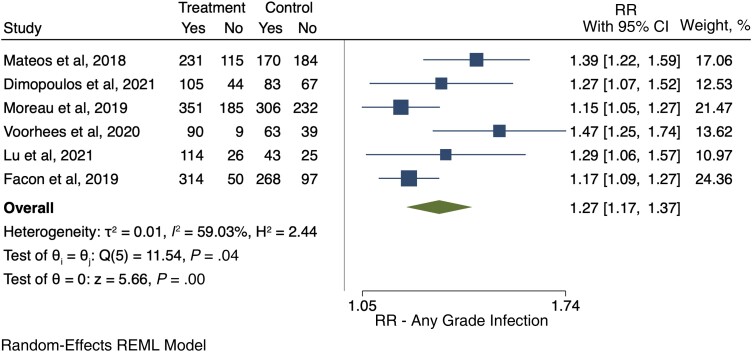
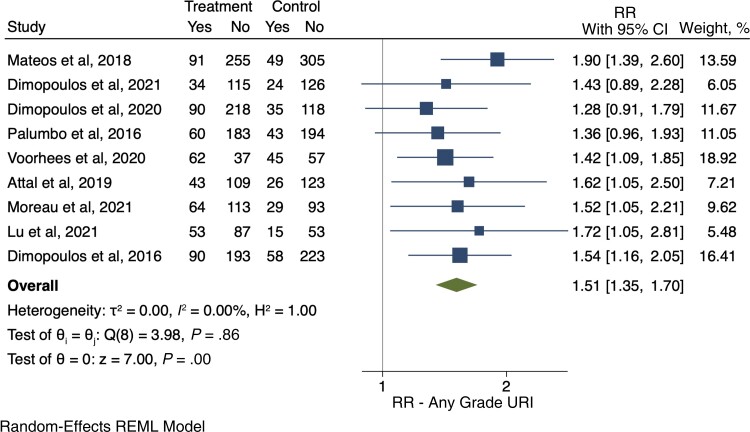
We retrieved 6 RCTs [40, 41, 43, 45, 48, 49] that provided data on the development of infections of any grade. Overall, in these studies 1634 patients received anti-CD38-based therapy, and 1205 of them developed an infection of any grade (73.7%). In the control arms, 933 of the 1577 patients developed an infection (59.2%). Among anti-CD38-treated patients, the relative risk for any grade of infection compared with control was 1.27 (95% CI, 1.17–1.37) (Figure 2). The pooled cumulative incidence of any grade infections for patients who received anti-CD38 monoclonal antibodies was 77% (95% CI, 68%–86%) (Supplementary Figure S1).
Figure 2.
Relative risk of any grade infections. Individual and combined estimates of the relative risk of any grade infections with 95% CIs. Abbreviations: REML, restricted maximum likelihood; RR, relative risk or risk ratio.
Severe Infections (Grade 3 or 4)
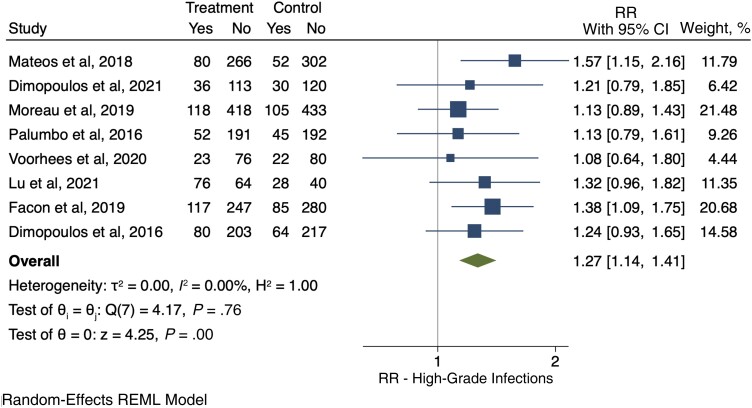
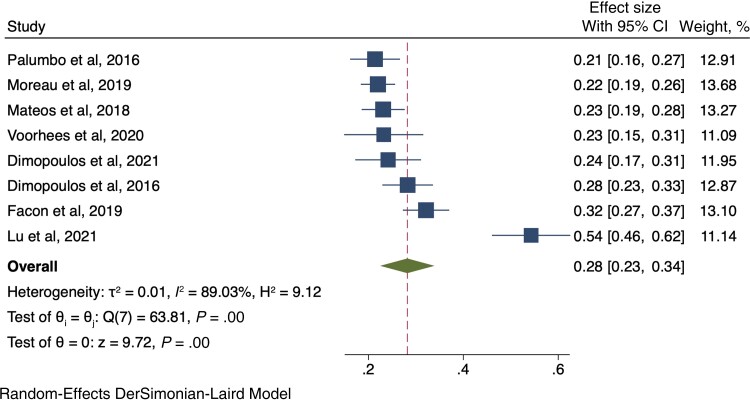
Eight RCTs [40, 41, 43–45, 48–50] evaluated the development of severe infections. Among the 2160 patients who received anti-CD38 compounds, 582 (26.9%) developed a severe infection, while among 2095 patients in the control arms, 431 (20.6%) developed a severe infection (RR, 1.27; 95% CI, 1.14–1.41) (Figure 3). The pooled cumulative incidence of severe infections for patients who received anti-CD38 monoclonal antibodies was 28% (95% CI, 23%–34%) (Figure 4).
Figure 3.
Relative risk of severe infections. Individual and combined estimates of the relative risk of severe infections with 95% CIs. Abbreviations: REML, restricted maximum likelihood; RR, relative risk or risk ratio.
Figure 4.
Cumulative incidence rate of severe infections among those receiving anti-CD38 monoclonal antibodies. Individual and combined estimates of the incidence of severe infections for patients treated with anti-CD38 monoclonal antibodies with 95% CIs. Abbreviation: ES, effect size (cumulative incidence).
Infection-Associated Mortality
Eight studies [40–47] provided data on infection-related mortality. In these studies, 2009 patients received anti-CD38 treatment, and 1656 patients were evaluated in the control arms. Similar rates of infection-induced death were found between the anti-CD38 (32/2009, 1.59%) and control (19/1656, 1.14%) groups (RR, 1.35; 95% CI, 0.76–2.40) (Supplementary Figure 2).
Upper Respiratory Tract Infection
Nine RCTs [40–42, 44–48, 50] reported the development of URIs among 1897 patients in the intervention arm and 1616 patients in the control arm. Among them, 587 and 324 patients, respectively, developed a URI. There was a 51% greater chance of URI among patients who were treated with anti-CD38 agents compared with patients in the control arms (RR, 1.51; 95% CI, 1.35–1.70) (Figure 5). Among these, 53 patients in the intervention arms and 23 in the control arms developed severe URIs (RR of severe URIs, 1.71; 95% CI, 1.00–2.90).
Figure 5.
Relative risk for upper respiratory tract infections (any grade). Individual and combined estimates of the relative risk of any grade upper respiratory tract infections with 95% CIs. Abbreviations: REML, restricted maximum likelihood; RR, relative risk or risk ratio; URI, upper respiratory tract infection.
Risk for Pneumonia
Any Grade
Ten RCTs [40–42, 44–50] evaluated the development of pneumonia. In the anti-CD38 group, 401 out of 2261 patients (17.7%) developed any grade pneumonia, compared with 242 out of 1981 patients (12.2%) in the control arms (RR, 1.39; 95% CI, 1.12–1.72) (Supplementary Figure 3).
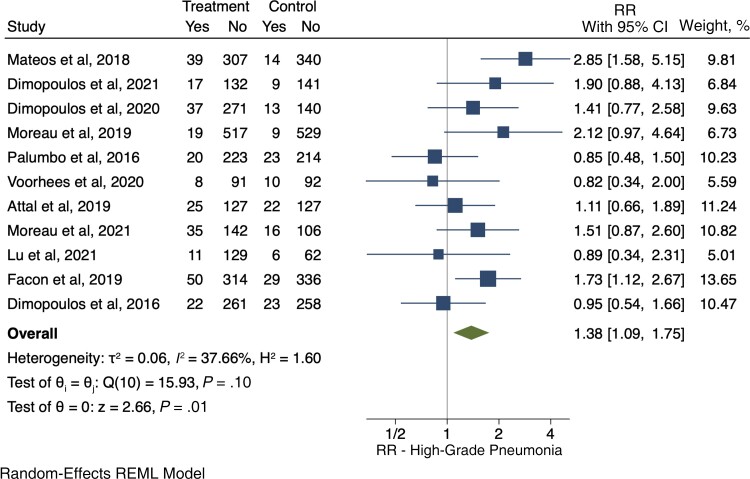
Severe Pneumonia
Eleven RCTs [40–50] evaluated the development of severe pneumonia, and there was an increased RR for severe pneumonia (1.38; 95% CI, 1.09–1.75) for the anti-CD38 group (283/2797, 10.1%), compared with the control group (196/2519, 7.78%) (Figure 6).
Figure 6.
Relative risk for severe pneumonia. Individual and combined estimates of the relative risk of severe pneumonia with 95% CIs. Abbreviations: REML, restricted maximum likelihood; RR, relative risk or risk ratio.
Risk for Varicella Zoster Virus Reactivation
Three RCTs [41, 42, 45] evaluated the development of VZV among 556 patients in the intervention arms and 407 patients in the control arms, and there were few cases of VZV reactivation in both groups (anti-CD38 group: 6/556, 1.08%; control group: 0/407, 0%), with an RR of 3.86 (95% CI, 0.66–22.50) (Supplementary Figure 4).
Outcomes from Extended Reports
In Supplementary Table 2, we present relevant results that were derived from analyzing data retrieved by the extended reports of available RCTs. Severe infection development had a relative risk of 1.49 (95% CI, 1.29–1.72). Additionally, the relative risk for pneumonia and severe pneumonia was 1.61 (95% CI, 1.21–2.16) and 1.60 (95% CI, 1.20–2.14), respectively. Regarding upper respiratory tract infections, the relative risk was 1.73 (95% CI, 1.51–1.97).
DISCUSSION
Treatment of multiple myeloma is transitioning to include the use of monoclonal antibodies, such as CD38-targeting agents [57–59]. As a result, the inclusion of CD-38-targeting monoclonal antibodies to therapy regimens has improved the survival of multiple myeloma patients [23, 60, 61]. In our systematic review and meta-analysis, we evaluated the relative risk of infections (any grade and severe) for patients with multiple myeloma who received anti-CD38-based regimens. Infections were 1.27 times more likely among patients receiving anti-CD38-based treatment, compared with those who received backbone multiple myeloma regimens that did not include an anti-CD38 monoclonal antibody. Interestingly, almost 4 out of 5 patients who received anti-CD38 agents developed an infection, and >1 out of 4 developed severe infections. Notably, as provided by the follow-up data of the retrieved studies, we found no evidence of increased mortality due to infection for anti-CD38 groups compared with controls.
The higher risk of infections in patients treated with anti-CD38 treatment could be, at least partially, attributed to the rapid depletion of gamma globulins and natural killer cells (CD56+) that is induced by anti-CD38 agents [62]. A recent study evaluating 171 patients assessed the rate of infections, hospitalizations attributed to infections, absolute numbers of lymphocyte populations, and 90-day survival of patients who received daratumumab [63]. The study found that 36.5% of patients eventually developed an infection. In comparison to patients without infection, those who developed one had a statistically significantly decreased median nadir absolute neutrophil count, absolute lymphocyte count, and CD56+ cell count. Based on these findings, the researchers hypothesized that one plausible explanation for the development of infection among patients treated with anti-CD38 treatment might be the decrease in absolute neutrophil count, lymphocyte count, and natural killer cell count. Notably, a second study that assessed the effect of NK cell counts on the safety of daratumumab treatment among patients taking part in 2 phase II trials [64, 65] reported that a decline in natural killer cell count is not associated with an increase in infections [66]. The study showed a similar incidence of infections among participants regardless of their baseline NK cell counts, maximum NK-cell reduction levels, or recovery rate of NK cells. Taken together, these studies indicate that the factors that lead to the considerably higher risk of infection in patients treated with anti-CD38 monoclonal antibodies are not yet determined, and further studies are needed to pinpoint specific variables and patients at higher risk for infection.
The presence of a bimodal surge in the incidence of bacterial (with peaks at 4–6 months and 70–72 months) and viral infections (with peaks at 7–9 months and 52–54 months) post–multiple myeloma diagnosis highlights the importance of long-term follow-up [67], particularly for patients receiving targeted treatment such as anti-CD38 monoclonal antibodies. The presence of elevated infection risk and the requirement for ongoing treatment intervention highlight the significance of long-term infection surveillance for the patient population of our study.
Looking into specific types of infection, we found that there was a higher relative risk for pneumonia and severe pneumonia in the anti-CD38 group (1.39 and 1.38, respectively). A previous meta-analysis included 7 RCTs evaluating the use of daratumumab and found a significant relative risk of 1.65 for pneumonia [68]. The European Myeloma Network recommends vaccination against influenza and encapsulated bacteria (pneumococci, Haemophilus influenzae, and meningococci) before treatment initiation [69]. Effective vaccination, infection prevention, and prophylaxis protocols should be implemented and evaluated in patients who receive anti-CD38 agents. Importantly, a recent reassuring study showed development of protective immunoglobulin (Ig)G antibodies following vaccination, to a comparable level as that seen in controls [70].
A number of studies have suggested a connection between daratumumab treatment and hypogammaglobulinemia, which could result in increased risk for infection among daratumumab-treated individuals [70, 71]. In a recent study evaluating the effect of daratumumab on normal plasma cells, polyclonal immunoglobulin levels—levels of polyclonal IgA, IgM, and IgE—significantly decreased, while levels of IgG remained stable [70]. Treatment with anti-CD38 monoclonal antibodies is also associated with the emergence of neutropenia, an additional predilecting factor to infection development [72], and in the real-world setting, almost 1 out of 2 patients on daratumumab treatment eventually develops neutropenia, and ∼1 out of 3 develops grade 3 or higher neutropenia [72].
The European Myeloma Network also recommends vaccination against VZV. Our meta-analysis did not show any evidence of increased risk for VZV reactivation among patients treated with anti-CD38 compounds. However, other agents used in the treatment of multiple myeloma, such as proteasome inhibitors, have been associated with an increased incidence of VZV reactivation [73]. As a result, guidelines support the use of VZV vaccination [69], and most of the studies included in our analysis included antiviral prophylaxis for all patients. Notably, recent pharmacovigilance data from the Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) showed increased risk for VZV reactivation (OR, 3.48; 95% CI, 2.63–4.61) for patients treated with daratumumab compared with the composite of all adverse events reported in the database [74]. Pharmacovigilance data also revealed increased risk (OR, 1.59; 95% CI, 1.19–2.11) for VZV reactivation for patients who received daratumumab compared with multiple myeloma patients who received different treatments.
Interestingly, we found no evidence of increased risk for death attributed to infection between the anti-CD38 and control groups as assessed using the follow-up data of retrieved studies. Although this is a reassuring finding, it could potentially be related to the earlier diagnosis and aggressive treatment of severe infections, especially in the setting of a randomized clinical trial. Interestingly, a recent cohort study of patients who received daratumumab showed an increased risk for severe infections in patients with persistence of severe lymphopenia [75]. The study revealed an increased rate of severe infection development in patients with high-grade lymphopenia compared with non–severely lymphopenic patients (44% vs 22%). In addition, infection-attributed deaths only occurred in the group of patients who were severely lymphopenic. For patients who received daratumumab, presence of high-grade lymphopenia was associated with the development of infection-attributed death in comparison to the nonlymphopenic group. Therefore, high-grade lymphopenia could be a factor in the development of worse infectious outcomes. Accordingly, it is necessary for studies and registries with long follow-up to define additional factors that contribute to unfavorable infectious outcomes (including infection-attributed deaths) in patients treated with anti-CD38 agents. Considering and analyzing strategic preventative strategies that could considerably reduce infection risk are crucial.
This systematic review and meta-analysis has some limitations. The RCT reports that we retrieved did not detail the timing of infection development, and many of the studies only described some of the outcomes of interest. Corticosteroids were administered to every patient across the RCTs we retrieved, so we could not control for the presence of steroid treatment and discover how it affected the observed findings. Due to lack of relevant data, we were not able to clarify the specific causes of the significant finding of increased pneumonia risk for patients who receive anti-CD38 treatment. Data regarding VZV reactivation were limited, as only 4 reports provided extractable information in their manuscripts, highlighting the need for a universal way of reporting safety results for RCTs. Regarding COVID-19 development, data were limited and could not be assessed in our meta-analysis. Additionally, evaluating the relative impact of each drug on infections is challenging as the therapeutic landscape of multiple myeloma most frequently requires the use of combination regimens.
CONCLUSIONS
There was an almost 30% higher risk for infection development in patients with multiple myeloma receiving anti-CD38 monoclonal antibodies compared with anti-CD38-naive individuals. Indeed, patients treated with anti-CD38-based regimens had a notable 28% incidence rate of severe infections. This increased risk, however, did not translate to a higher risk for infection-related death. Based on the reassuring result of lack of evidence of higher risk for infection-related death and the established efficacy of anti-CD38 regimens, future studies could focus on identifying patients at particularly increased risk. Studies and registries with long follow-up can help us identify patients at higher risk for infection and recognize effective vaccination and prophylaxis strategies and antimicrobial stewardship protocols.
Supplementary Material
Acknowledgments
Financial support. None.
Author contributions. S.V., A.V., M.K., F.S., and E.M. conceptualized and designed the study and participated in data interpretation. S.V. and A.V. participated in data collection and extraction. S.V. and F.S. prepared tables and figures and performed the statistical analysis. S.V. drafted the initial manuscript. A.V., M.K., F.S., and E.M. reviewed and revised the manuscript. All authors read and approved the final manuscript as submitted and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Patient consent. Our study does not include factors necessitating patient consent.
Contributor Information
Stephanos Vassilopoulos, Infectious Diseases Division, Rhode Island Hospital, Providence, Rhode Island, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Athanasios Vassilopoulos, Infectious Diseases Division, Rhode Island Hospital, Providence, Rhode Island, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Markos Kalligeros, Infectious Diseases Division, Rhode Island Hospital, Providence, Rhode Island, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Fadi Shehadeh, Infectious Diseases Division, Rhode Island Hospital, Providence, Rhode Island, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA; School of Electrical and Computer Engineering, National Technical University of Athens, Athens, Greece.
Eleftherios Mylonakis, Infectious Diseases Division, Rhode Island Hospital, Providence, Rhode Island, USA; Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Brioli A, Klaus M, Sayer H, et al. The risk of infections in multiple myeloma before and after the advent of novel agents: a 12-year survey. Ann Hematol 2019; 98:713–22. [DOI] [PubMed] [Google Scholar]
- 2. Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica 2015; 100:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nucci M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis 2009; 49:1211–25. [DOI] [PubMed] [Google Scholar]
- 4. Mai EK, Haas E-M, Lücke S, et al. A systematic classification of death causes in multiple myeloma. Blood Cancer J 2018; 8:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Augustson BM, Begum G, Dunn JA, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002–Medical Research Council Adult Leukaemia Working Party. J Clin Oncol 2005; 23:9219–26. [DOI] [PubMed] [Google Scholar]
- 6. Cowan AJ, Green DJ, Kwok M, et al. Diagnosis and management of multiple myeloma: a review. JAMA 2022; 327:464–77. [DOI] [PubMed] [Google Scholar]
- 7. Caro J, Braunstein M, Williams L, et al. Inflammation and infection in plasma cell disorders: how pathogens shape the fate of patients. Leukemia 2022; 36:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parrillo a JE, Fauci AS. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol 1979; 19:179–201. [DOI] [PubMed] [Google Scholar]
- 9. Fardet L, Petersen I, Nazareth I. Common infections in patients prescribed systemic glucocorticoids in primary care: a population-based cohort study. PLoS Med 2016; 13:e1002024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teh BW, Harrison SJ, Worth LJ, Thursky KA, Slavin MA. Infection risk with immunomodulatory and proteasome inhibitor–based therapies across treatment phases for multiple myeloma: a systematic review and meta-analysis. Eur J Cancer 2016; 67:21–37. [DOI] [PubMed] [Google Scholar]
- 11. Ying L, YinHui T, Yunliang Z, Sun H. Lenalidomide and the risk of serious infection in patients with multiple myeloma: a systematic review and meta-analysis. Oncotarget 2017; 8:46593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van de Donk N, Richardson PG, Malavasi F. CD38 antibodies in multiple myeloma: back to the future. Blood 2018; 131:13–29. [DOI] [PubMed] [Google Scholar]
- 13. Santonocito AM, Consoli U, Bagnato S, et al. Flow cytometric detection of aneuploid CD38(++) plasmacells and CD19(+) B-lymphocytes in bone marrow, peripheral blood and PBSC harvest in multiple myeloma patients. Leuk Res 2004; 28:469–77. [DOI] [PubMed] [Google Scholar]
- 14. Glaría E, Valledor AF. Roles of CD38 in the immune response to infection. Cells 2020; 9:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malavasi F, Deaglio S, Funaro A, et al. Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology. Physiol Rev 2008; 88:841–86. [DOI] [PubMed] [Google Scholar]
- 16. Aarhus R, Graeff RM, Dickey DM, Walseth TF, Hon CL. ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP+ (∗). J Biol Chem 1995; 270:30327–33. [DOI] [PubMed] [Google Scholar]
- 17. Deaglio S, Mallone R, Baj G, et al. CD38/CD31, a receptor/ligand system ruling adhesion and signaling in human leukocytes. Chem Immunol 2000; 75:99–120. [PubMed] [Google Scholar]
- 18. Watt SM, Gschmeissner SE, Bates PA. PECAM-1: its expression and function as a cell adhesion molecule on hemopoietic and endothelial cells. Leuk Lymphoma 1995; 17:229–44. [DOI] [PubMed] [Google Scholar]
- 19. Costa F, Dalla Palma B, Giuliani N. CD38 expression by myeloma cells and its role in the context of bone marrow microenvironment: modulation by therapeutic agents. Cells 2019; 8:1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harada H, Kawano MM, Huang N, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood 1993; 81:2658–63. [PubMed] [Google Scholar]
- 21. Bataille R, Jégo G, Robillard N, et al. The phenotype of normal, reactive and malignant plasma cells. Identification of” many and multiple myelomas” and of new targets for myeloma therapy. Haematologica 2006; 91:1234–40. [PubMed] [Google Scholar]
- 22. Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38 + immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016; 128:384–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giri S, Grimshaw A, Bal S, et al. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: a systematic review and meta-analysis. JAMA Oncol 2020; 6:1759–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Plesner T, Krejcik J. Daratumumab for the treatment of multiple myeloma. Front Immunol 2018; 9:1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajkumar SV, Kumar S. Multiple myeloma current treatment algorithms. Blood Cancer J 2020; 10:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van de Donk NWCJ, Janmaat ML, Mutis T, et al. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev 2016; 270:95–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shen F, Shen W. Isatuximab in the treatment of multiple myeloma: a review and comparison with daratumumab. Technol Cancer Res Treat 2022; 21:15330338221106563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu XS, Dimopoulos MA, Sonneveld P, et al. Pharmacokinetics and exposure–response analyses of daratumumab in combination therapy regimens for patients with multiple myeloma. Adv Ther 2018; 35:1859–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frampton JE. Isatuximab: a review of its use in multiple myeloma. Target Oncol 2021; 16:675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.PubMed search. Available at: https://pubmed.ncbi.nlm.nih.gov/?term=%28daratumumab+OR+isatuximab+OR+anti-CD38%29+AND+randomized. Accessed 1 September 2022.
- 32. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), version 5.0. Available at: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 1 September 2022.
- 33. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 34. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–88. [DOI] [PubMed] [Google Scholar]
- 35. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. JBI Evid Implement 2015; 13:147–53. [DOI] [PubMed] [Google Scholar]
- 36. Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health 2014; 72:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–58. [DOI] [PubMed] [Google Scholar]
- 38. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, version 6.3. 2022. Available at: www.training.cochrane.org/handbook2022. Accessed 1 September 2022. [Google Scholar]
- 39. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006; 295:676–80. [DOI] [PubMed] [Google Scholar]
- 40. Mateos MV, Dimopoulos MA, Cavo M, et al. Daratumumab plus bortezomib, melphalan, and prednisone for untreated myeloma. N Engl J Med 2018; 378:518–28. [DOI] [PubMed] [Google Scholar]
- 41. Dimopoulos MA, Terpos E, Boccadoro M, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol 2021; 22:801–12. [DOI] [PubMed] [Google Scholar]
- 42. Dimopoulos M, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020; 396:186–97. [DOI] [PubMed] [Google Scholar]
- 43. Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019; 394:29–38. [DOI] [PubMed] [Google Scholar]
- 44. Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375:754–66. [DOI] [PubMed] [Google Scholar]
- 45. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020; 136:936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet 2019; 394:2096–107. [DOI] [PubMed] [Google Scholar]
- 47. Moreau P, Dimopoulos MA, Mikhael J, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet 2021; 397:2361–71. [DOI] [PubMed] [Google Scholar]
- 48. Lu J, Fu W, Li W, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in chinese patients with relapsed or refractory multiple myeloma: phase 3 LEPUS (MMY3009) study. Clin Lymphoma Myeloma Leuk 2021; 21:e699–709. [DOI] [PubMed] [Google Scholar]
- 49. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019; 380:2104–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 2016; 375:1319–31. [DOI] [PubMed] [Google Scholar]
- 51. Mateos MV, Cavo M, Blade J, et al. Overall survival with daratumumab, bortezomib, melphalan, and prednisone in newly diagnosed multiple myeloma (ALCYONE): a randomised, open-label, phase 3 trial. Lancet 2020; 395:132–41. [DOI] [PubMed] [Google Scholar]
- 52. Usmani SZ, Quach H, Mateos MV, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): updated outcomes from a randomised, multicentre, open-label, phase 3 study. Lancet Oncol 2022; 23:65–76. [DOI] [PubMed] [Google Scholar]
- 53. Mateos MV, Sonneveld P, Hungria V, et al. Daratumumab, bortezomib, and dexamethasone versus bortezomib and dexamethasone in patients with previously treated multiple myeloma: three-year follow-up of CASTOR. Clin Lymphoma Myeloma Leuk 2020; 20:509–18. [DOI] [PubMed] [Google Scholar]
- 54. Richardson PG, Perrot A, San-Miguel J, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Lancet Oncol 2022; 23:416–27. [DOI] [PubMed] [Google Scholar]
- 55. Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol 2021; 22:1582–96. [DOI] [PubMed] [Google Scholar]
- 56. Bahlis NJ, Dimopoulos MA, White DJ, et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020; 34:1875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jain A, Ramasamy K. Evolving role of daratumumab: from backbencher to frontline agent. Clin Lymphoma Myeloma Leuk 2020; 20:572–87. [DOI] [PubMed] [Google Scholar]
- 58. Moreau P, Kumar SK, San Miguel J, et al. Treatment of relapsed and refractory multiple myeloma: recommendations from the International Myeloma Working Group. Lancet Oncol 2021; 22:e105–18. [DOI] [PubMed] [Google Scholar]
- 59. Offidani M, Corvatta L, Morè S, et al. Daratumumab for the management of newly diagnosed and relapsed/refractory multiple myeloma: current and emerging treatments. Front Oncol 2020; 10:624661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lakshman A, Abeykoon JP, Kumar SK, et al. Efficacy of daratumumab-based therapies in patients with relapsed, refractory multiple myeloma treated outside of clinical trials. Am J Hematol 2017; 92:1146–55. [DOI] [PubMed] [Google Scholar]
- 61. Dima D, Dower J, Comenzo RL, Varga C. Evaluating daratumumab in the treatment of multiple myeloma: safety, efficacy and place in therapy. Cancer Manag Res 2020; 12:7891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vitkon R, Netanely D, Levi S, et al. Daratumumab in combination with proteasome inhibitors, rapidly decreases polyclonal immunoglobulins and increases infection risk among relapsed multiple myeloma patients: a single center retrospective study. Ther Adv Hematol 2021; 12:20406207211035272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnsrud AJ, Johnsrud JJ, Susanibar SA, et al. Infectious and immunological sequelae of daratumumab in multiple myeloma. Br J Haematol 2019; 185:187–9. [DOI] [PubMed] [Google Scholar]
- 64. Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016; 387:1551–60. [DOI] [PubMed] [Google Scholar]
- 65. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015; 373:1207–19. [DOI] [PubMed] [Google Scholar]
- 66. Casneuf T, Xu XS, Adams HC III, et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv 2017; 1:2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Teh BW, Harrison SJ, Worth LJ, Spelman T, Thursky KA, Slavin MA. Risks, severity and timing of infections in patients with multiple myeloma: a longitudinal cohort study in the era of immunomodulatory drug therapy. Br J Haematol 2015; 171:100–8. [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Li Y, Chai Y. Efficacy and safety of daratumumab in the treatment of multiple myeloma: a systematic review and meta-analysis. J Int Med Res 2021; 49:3000605211038135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia 2021; 35:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Frerichs KA, Bosman PWC, van Velzen JF, et al. Effect of daratumumab on normal plasma cells, polyclonal immunoglobulin levels, and vaccination responses in extensively pre-treated multiple myeloma patients. Haematologica 2020; 105:e302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ejaz K, Roback JD, Stowell SR, Sullivan HC. Daratumumab: beyond multiple myeloma. Transfus Med Rev 2021; 35:36–43. [DOI] [PubMed] [Google Scholar]
- 72. Kobayashi H, Tsushima T, Terao T, et al. Evaluation of the safety and efficacy of daratumumab outside of clinical trials. Int J Hematol 2019; 109:665–72. [DOI] [PubMed] [Google Scholar]
- 73. Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Myeloma 2008; 8:237–40. [DOI] [PubMed] [Google Scholar]
- 74. Burns EA, Ensor JE, Anand K, et al. Opportunistic infections in patients receiving daratumumab regimens for multiple myeloma (MM). Blood 2021; 138(Suppl 1):4740. [Google Scholar]
- 75. Cottini F, Huang Y, Williams N, et al. Real world experience of daratumumab: evaluating lymphopenia and adverse events in multiple myeloma patients. Front Oncol 2020; 10:575168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.