Abstract
HIV infection is a significant independent risk factor for severe coronavirus disease 2019 (COVID-19) disease and death. We summarize COVID-19 vaccine responses in people with HIV (PWH). A systematic literature review of studies from January 1, 2020, to March 31, 2022, of COVID-19 vaccine immunogenicity in PWH from multiple databases was performed. Twenty-eight studies from 12 countries were reviewed. While 22 (73%) studies reported high COVID-19 vaccine seroconversion rates in PWH, PWH with lower baseline CD4 counts, CD4/CD8 ratios, or higher baseline viral loads had lower seroconversion rates and immunologic titers. Data on vaccine-induced seroconversion in PWH are reassuring, but more research is needed to evaluate the durability of COVID-19 vaccine responses in PWH.
Keywords: COVID-19 vaccines, people with HIV, SARS-CoV-2 antibody, seroconversion
Globally, over 500 million people have been infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), with >6 million reported deaths [1]. HIV infection has been associated with severe presentation of COVID-19 and death [2]. By the end of 2020, ∼38 million people with HIV (PWH) globally were at risk of severe morbidity and mortality from SARS-CoV-2 [3]. PWH with low CD4 counts or nonsuppressed HIV viral loads (VLs) are at increased risk of severe COVID-19 disease when infected with SARS-CoV-2 [4]. Excess risk of severe outcomes is also associated with older age and the presence of comorbidities, including obesity, hypertension, and diabetes, all of which are becoming more common in PWH [5]. PWH on antiretroviral treatment (ART) with suppressed VLs and CD4 counts >200 cells/mm3 appear to have the same risk for severe COVID-19 as people who do not have HIV [6].
Since 2020, a number of COVID-19 vaccines have received emergency use listing (EUL) by the World Health Organization (WHO) [7], and although humoral immune response as measured by antibody levels to COVID-19 vaccines is associated with neutralization titers [8, 9], there remains a significant deficit in the understanding of the correlates of immune protection and vaccine efficacy or effectiveness. Data on antibodies elicited by COVID-19 vaccines in groups with immunocompromising conditions are mixed but have generally shown lower seropositivity among those who are more profoundly immunocompromised [9]. Given the risk for severe morbidity, hospitalization, and death in PWH with COVID-19, it is important to understand the immunologic response to COVID-19 vaccines in PWH. We conducted a systematic literature review of the available evidence of COVID-19 vaccine immunogenicity and seroconversion rates in PWH.
METHODS
Study Design
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) [10].
Ethics
No formal ethics approval was sought because this study retrieved and synthesized data from already published studies and did not involve the collection or transfer of data, samples, or specimens.
Search Strategy
Searches of databases MEDLINE via PubMed, Embase (OVID) 1988-, Global Health (OVID), Cochrane Library, CINAHL (EbscoHost), Scopus, and the WHO Global COVID Literature Database were conducted for published and preprint articles in the English language from January 1, 2020, to March 31, 2022. The search was conducted according to a study protocol using a different search strategy for each database (Supplementary Data 1). Preprint manuscripts and original research articles published in peer-reviewed, scientific journals were screened.
Study Selection
A 2-staged screening method was adopted, screening first by title and abstract, and then screening the full text of potentially eligible articles. For each article identified, the title, abstract, and full text were screened independently by 4 members of the review team (H.M.C., K.M., E.A., and I.Z.), with discrepancies resolved after discussion. We included randomized trials and observational studies published in English that reported data from human participants who received a COVID-19 vaccine of any emergency use listed by the WHO; included PWH of any age, race, or gender; and reported data of serologic measurements of either seroconversion or serologic titers after COVID-19 vaccination. Studies that were duplicates, unavailable as full text, or abstract only were excluded.
Data Management
Data were extracted by the same review team members according to a predetermined proforma in Microsoft Excel, version 2018. All available key extracted data were reviewed and checked for completeness at the end of the data extraction phase. Study characteristics comprised setting, study design, and sample size criteria. Participant data reviewed included HIV and antiretroviral therapy (ART) status, baseline CD4 count, and HIV VL before or at the time of first COVID-19 vaccine dose. Intervention-related data included COVID-19 vaccine type, test interval after the final reported administered dose, and the number of subjects receiving each type of vaccine. Outcome-related data included seroconversion rate, SARS-CoV-2 antibody assay (IgG) to receptor binding domain (RBD) titer, unit of IgG antibody measurement, assay type and positivity threshold, and whether SARS-CoV-2 variants were assessed.
For any studies that had an overlapping group of PWH due to follow-up study design [11, 12], the calculation of total number of PWH included in this review did not include the follow-up study. For any studies that potentially included the same individuals due to drawing from the same data source but for which the degree of overlap was not possible to determine [13, 14], a lower bound on the total number of patients included in this review was estimated assuming full overlap.
Risk of Bias
The risk of bias was assessed using the Downs and Black scale [15]. Two authors (H.M.C., K.M.) independently performed component quality analysis, reviewed all inconsistent assessments, and resolved disagreements by consensus.
Statistical Analysis and Data Visualization
Data on humoral immune response were summarized descriptively. Seroconversion rates were used to assess vaccine effectiveness. For studies that reported seroconversion rates, 95% confidence intervals were calculated using the Wilson score method. Where studies reported seroconversion rates for both HIV-positive and HIV-negative individuals, odds ratios and corresponding 95% confidence intervals were calculated; in the case of studies reporting 0 seronegative cases in 1 or both groups, the Haldane-Anscombe continuity-corrected odds ratio was calculated by adding 0.5 to each cell of the corresponding contingency table. All statistical procedures and data visualization were performed in Python 3.7.6.
RESULTS
The screening strategy identified 28 articles in total (Supplementary Figure 1) reporting outcomes for >3700 vaccinated PWH.
Study Characteristics
Studies were conducted across different settings including South Africa (2 studies) [16, 17], Europe (12 studies) [12, 13, 18, 19, 20–27], Canada (2 studies) [28, 29], Israel (2 studies) [13, 14], the United States (5 studies) [9, 30–34], and China (4 studies) [35–38] (Tables 1 and 2). There were 4 retrospective studies [20, 23, 24, 32] and 24 prospective studies (of which 2 were randomized controlled trials [17, 26], with sample sizes ranging from 12 to 697 PWH adults. All 9 studies initially included as preprints have subsequently been published in full, and data extracted from the preprints were verified against the final publication. One study was a follow-up [12] of the same patients from another study [11], with 2 studies having potential overlapping patients due to drawing from the same data source [13, 14].
Table 1.
Study Characteristics
| Author/Year/Location | Study Design | No. | CD4 Cell Count Baseline (PWH) | HIV VL Baseline (PWH) | COVID-19 Vaccine(s) | Test Interval (After Final Dose) | Total/% Sero-converted | Seroconversion Definition | IgG Value | IgG Range | IgG Unit | Assay Type; Positivity Threshold | SARS-CoV-2 Variant |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| González de Aledo, 2022, Spain | Prospective observational cohort | 100 PWH | 96% >200 cells/mm3 | 98% <50 copies/mL | mRNA-1273 (90); BNT162b2 (10) | 4 wk | 100% | Ab concentration ≥33.8 BAU/mL | mRNA-1273: 69% >2080; BNT162b2: 50% >2080 | NR | BAU/mL | LIAISON SARS-CoV-2 TrimericS IgG (DiaSorin, Saluggia, Italy); ≥33.8 BAU/mL | NR |
| Antinori, 2022, Italy | Prospective observational cohort | 166 PWH, 169 controls | 32 <200 cells/mm3, 56 200–500 cells/mm3, 78 >500 cells/mm3 | <50 copies/mL: 68.8% of patients with CD4 <200 cells/mm3, 92.9% 200–500 cells/mm3, 100% >500 cells/mm3 | BNT162b2 (all controls, 57% PWH), mRNA-1273 (43% PWH) | 1 mo | 86.7% among CD4 <200 cells/mm3, 100% among CD4 200–500 cells/mm3, 98.7% among CD4 >500 cells/mm3 | IgG ≥7.1 BAU/mL | 507 CD4 <200 cells/mm3, 1477 CD4 200–500 cells/mm3, 1782 CD4 >500 cells/mm3, 2353 controls | IQR: 212–1143 CD4 <200, 471–2056 CD4 200–500, 989–2769 CD4 >500, 1378–3758 controls | BAU/mL | ARCHITECT SARS-CoV-2 IgG II Quantitative (Abbott Laboratories, Wiesbaden, Germany); ≥7.1 BAU/mL | NR |
| Brumme, 2022, Canada | Prospective observational cohort | 100 PWH, 152 controls | Median 710 (IQR 525–935) cells/mm3 | 95% <50 copies/mL, 5% 71–162 copies/mL | mRNA-1273, BNT162b2, ChAdOx1 | 1 mo | 96% PWH, 99% controls | Anti-RBD Ab titer | 3.86 | IQR 3.63–4.07 | Log10 U/mL | Elecsys Anti-SARS-CoV-2 S; ≥0.4 U/mL | Delta |
| Feng, 2021, China | Open-label 2-arm nonrandomized study | 42 PWH, 28 controls | 100% ≥200 cells/mm3 | 22 undetectable VL, 8 detectable VL <20 copies/mL, 12 VL >20 copies/mL | BBIBP-CorV | 4 wk | 57.1% PWH; 92.9% controls | ≥3-fold increase in RBD from baseline | NR | NR | NR | In-house ELISA; NR | NR |
| Frater, 2021, UK | Single-arm open-label substudy of a phase 2/3 clinical trial | 54 PWH | 100% ≥350 cells/mm3 with median CD4 count 694.0 cells/mm3 (IQR 573.5–859.5) | 100% viral load <50 copies/mL | ChAdOx1 | 28 d | 100% | IgG ≥10 EU | 941 | IQR 531–1445 | EU | Meso Scale Discovery MIA against spike and RBD; IgG ≥10 EU | NR |
| Ogbe, 2021, UK | Open-label, nonrandomized substudy | 54 PWH, 50 controls | 694 cells/mm3 (IQR 573.5–859.5) | 100% <50 copies/mL | ChAdOx1 | 154 d | NR | >13 EU | NR | NR | EU (ELISA units) | In-house total IgG ELISA; >13 EU | WT, Alpha, Beta, Delta, Gamma |
| Haidar, 2022, USA | Prospective cohort study | 94 PWH, 172 controls | 87.2% >200 cells/mm3 | 97.9% virally suppressed | mRNA-1273 (29 PWH, 73 controls); BNT162b2 (63 PWH, 96 controls); Ad26.COV2.S (2 PWH, 3 controls) | Median (IQR): 85.5 d (62.0–105.0) PWH, 132.5 d (118.0–150.0) controls | 79.8% PWH (86.6% of those with CD4 >200 cells/mm3); 92.4% controls | ≥1.00 S/CO ratio | Median: 4.8 PWH, 5.2 controls | IQR: 1.6–12.5 PWH, 2.5–11.5 controls | S/CO ratio | Beckman Coulter SARS-CoV-2 anti-S protein RBD IgG; extinction coefficient S/CO ratio reactive (≥1.0) | NR |
| Hassold, 2022, France | Retrospective study | 105 PWH | 18 <200 cells/mm3, 36 200–500 cells/mm3, 51 >500 cells/mm3 | <50 copies/mL: 44% CD4 <200 cells/mm3, 83.3% CD4 200–500 cells/mm3, 96% CD4 >500 cells/mm3 | BNT162b2 (75%); mRNA-1273 (8.5%); ChAdOx1 (16.5%) | 73 d (IQR 29–97) | 78% among CD4 <200 cells/mm3, 94.5% among CD4 200–500 cells/mm3, 100% among CD4 >500 cells/mm3 | IgG ≥7.1 BAU/mL | Median: 247.9 CD4 <200 cells/mm3, 396.5, 200–500 cells/mm3, 623.8 CD4 >500 cells/mm3 | IQR: 5.88–434.9 CD4 <200 cells/mm3, 105.8–1174 CD4 200–500 cells/mm3, 262.2–2288 >500 cells/mm3 | BAU/mL | Abbott Architect SARS-COV-2 IgG Quant II; 7.1 BAU/mL | NR |
| Huang, 2022, China | Prospective cross-sectional study | 120 PWH, 53 controls | 630.5 cells/mm3 (IQR 499.5–848.8) | 58.1% ≤60 copies/mL, 25.6% 61–200 copies/mL, 16.3% >200 copies/mL | Sinovac CoronaVac (55% PWH, 30.2% controls); Sinopharm BBIBP-CorV (45% PWH, 69.8 controls) | 0–84+ d | 96.2% PWH, 100% controls | A/CO ratio ≥1.0 | Median: 6.8 PWH, 10.1 controls | IQR: 3.3–12.1 PWH, 6.5–19.4 controls | A/CO ratio | Wantai SARS-CoV-2 IgG total and anti-S-IgG; A/CO ratio ≥1.0 | NR |
| Jedicke, 2021, Germany | Prospective observational cohort | 52 PWH, 41 controls | Mean 716 cells/mm3 (range 151–1558) | 100% ≤200 copies/mL, 96.5% < 50 copies/mL | BNT162b2 | Mean (range): 35 d (1–128) PWH, 26 d (18–37) controls | NR | SARS-CoV-2 spike IgG level | 246.2 PWH, 502.5 controls | IQR: 218.7 PWH, 118.8 controls | RU/mL | Euroimmun Anti-SARS-CoV-2 QuantiVac ELISA IgG; NR | Wuhan |
| Khan, 2021, South Africa | Phase 3b open-label clinical trial | Vaccinated: 26 PWH, 73 controls Unvaccinated: 34 PWH, 28 controls |
Median (IQR): 581 cells/mm3 (328–794) for COVID-infected unvaccinated, 852 cells/mm3 (730–1184) for infected and vaccinated, 735 cells/mm3 (458–863) uninfected and vaccinated | 1/26 vaccinated PWH had HIV VL >40 copies/mL; 10/34 unvaccinated PWH had HIV VL >40 copies/mL, with median (IQR) 3060 (1224–30 160) copies/mL for those with detectable VL | Ad26.CoV2.S | Median (range): 56 d (19–98) | NR | 2 × standard deviation of prepandemic controls | NR | NR | NR | ImmuSAFE COVID-19 microarray-based anti-SARS CoV-2 IgG against N and S proteins assay; NR | Delta |
| Levy, 2021, Israel | Prospective observational | 143 PWH, 261 controls | Mean 700 cells/mm3 (95% CI 648–757) | 95% <40 copies/mL | BNT162b2 | Median (IQR): 18 d (14–21) PWH, 26 d (24–27) controls | 97.2% PWH, 98.9% controls | Titers ≥1.1 | GMT: 5.2 PWH, 6.1 controls | 95% CI: 4.8–5.5 PWH, 5.8–6.4 controls | GMT | In-house ELISA for anti-RBD IgG; ≥1.1 | NR |
| Rahav, 2021, Israel | Prospective cohort study | 156 PWH, 272 controls | Mean 700 cells/mm3 | 95% with an undetectable VL | BNT162b2 | Median (IQR): 19 d (14–21) PWH, 26 d (24–27) controls | 98.7% PWH, 98.9% controls | ≥1.1 S/CO ratio | GMT: 5.14 PWH, 5.98 controls | 95% CI: 4.84–5.46 PWH, 5.70–6.28 controls | S/CO | NR; ≥1.1 S/CO ratio | NR |
| Liu, 2021, China | Cross-sectional prospective observational study | 55 PWH, 21 controls | Median (IQR): 580 cells/mm3 (447–723) | 100% ≤50 copies/mL | Sinovac CoronaVac | Median (IQR): 5 wk (3.71–8.71) PWH, 4.86 wk (3–9.14) controls | NR | NR | Median: 22.4 PWH CD4 ≥350 cells/mm3, 11.2 PWH CD4 <350 cells/mm3, 16 controls | IQR: 17–24.4 PWH CD4 ≥350 cells/mm3, 4.6–21.2 PWH CD4 <350 cells/mm3, 11.3–23.2 controls | U/mL | Wantai SARS-CoV-2 anti-S-IgG; NR | NR |
| Lombardi, 2022, Italy | Prospective cohort study | 71 PWH, 10 controls | Median (IQR): 747 (593–942) cells/mm3 | All PWH had HIV VL <50 copies/mL Except for 5 patients with HIV VL 85-–2 930 000 copies/mL |
mRNA-1273 | 28 d | NR | NR | Median: 2173 CD4 <350 cells/mm3, 5763 CD4 350–500 cells/mm3, 2449 CD4 >500 cells/mm3, 1425 controls | IQR: 987–4109 CD4 <350 cells/mm3, 4801–>12 500 CD4 350–500 cells/mm3, 1524–5704 CD4 >500 cells/mm3, 599–6131 controls | U/mL | Elecsys anti-SARS-CoV-2 S; ≥0.80 U/mL | NR |
| Lv, 2021, China | Prospective cohort study | 24 PWH, 24 controls | 20 CD4 <500 cells/mm3 | NR | Sinovac CoronaVac; Sinopharm BBIBP-CorV | 40 d | PWH 79.2%, controls 87.5% | ≥20% inhibition | NR | NR | NR | ELISA anti-RBD; NR | NR |
| Madhi, 2021, South Africa | Multicenter double-blind RCT | 100 PWH, 46 controls | Median (IQR): 742 cells/mm3 (540–953) | 25% <50 copies/mL | ChAdOx1 | 14 d | PWH 87.5, controls 95.7 | ≥26 BAU/mL | GMC: 347.8 PWH, 364.2 controls | 95% CI: 195.0–620.4 PWH, 238.6–555.8 controls | BAU/mL | ELISA for recombinant SARS-CoV-2 RBD; NR | B.1.351 |
| Milano, 2022, Italy | Retrospective observational | 697 PWH | 14 CD4 <200 cells/mm3 | 90.7% <20 copies/mL | BNT162b2 | 3 mo | 98.6% | ≥50 AU/mL | 1305.9 | Range 9.8–109 881.6 | AU/mL | Abbott IgG anti-RBD chemiluminescent assay, ≥50 AU/mL | NR |
| Nault, 2022, Canada | Prospective cohort | 106 PWH, 20 controls | 6 CD4 <250 cells/mm3, 18 CD4 250–500 cells/mm3, 82 CD4 >500 cells/mm3 | 96% <40 copies/mL | mRNA-1273 (all PWH), BNT162b2 (all controls) | 3–4 wk after first dose | 94.3% PWH, 95% controls | Mean of prepandemic plasma + 3 standard deviations | Mean: 1.35 CD4 <250 cells/mm3, 3.52 CD4 >250 cells/mm3 & controls | NR | log RLU | ELISA; 0.94 log RLU | NR |
| Noe, 2021, Germany | Retrospective cohort | 665 PWH | Median (IQR): 708 cells/mm3 (524–912) | 93.5% < 50 copies/mL | mRNA* (619), ChAdOx1 (31), Ad26.COV2.S (15) | Median (IQR): 49 d (35–63) | 97.3% | ≥34 BAU/mL | Median: 197 CD4 <200 cells/mm3, 1420 CD4 ≥200 cells/mm3 | IQR: 44.6–537.2 CD4 <200 cells/mm3, 687–2216 CD4 ≥200 cells/mm3 | BAU/mL | LIAISON SARS-CoV-2 Trimeric S IgG Assay; ≥34 BAU/mL | NR |
| Portillo, 2022, Switzerland | Observational open-label study | 131 PWH, 49 controls | 602 cells/mm3 (IQR 445.0–825.5) | 85.2% ≤20 copies/mL | mRNA-1273 (78); BNT162b2 (53) | 30 d | 100% | >1.1 IU/mL | GMT: 2372.0 PWH, 2815.6 controls | 95% CI: 2192.3–2566.4 PWH, 2677.9–2960.3 | IU/mL | Roche Elecsys; ≥1.1 IU/mL | NR |
| Ruddy (1), 2021, USA | Prospective observational cohort | 12 PWH | 2 <200 cells/mm3, 4 200–499 cells/mm3, 6 ≥500 cells/mm3 | 92% had an undetectable HIV VL |
mRNA-1273 (6); BNT162b2 (6) | Median (IQR): 21 d (17–27) after first dose | 100% | ≥0.8 U/mL | NR | Range: 2.1–250< | U/mL | Roche Elecsys Anti-SARS-CoV-2 S; >0.4 U/mL | NR |
| Ruddy (2), 2021, USA | Prospective observational cohort | 14 PWH | 2 CD4 <200 cells/mm3, 4 CD4 200–499 cells/mm3, 8 CD4 >500 cells/mm3 | 13 (93%) had an undetectable HIV VL | BNT162b2 (5), mRNA-1273 (9) | Median (IQR): 29 (28–32) d | 100% | ≥0.8 U/mL | >250 in 13 PWH, 239 in 1 PWH | NR | U/mL | Roche Elecsys Anti-SARS-CoV-2 S; >0.4 U/mL | NR |
| Speich, 2022, Switzerland | Open-label noninferiority RCT | 341 PWH | 98% CD4 >200 cells/mm3 | 5.7% HIV VL >50 copies/mL | mRNA-1273; BNT162b2 | 8 wk ±7 d | 100% | ≥0.8 U/mL | NR | NR | NR | Elecsys; ≥0.8 U/mL | NR |
| Spinelli, 2021, USA | Retrospective case–control observational | 100 PWH, 100 controls | Median (IQR): 511 cells/mm3 (351–796) | 95% ≤200 copies/mL | BNT162b2 (75 PWH, 75 controls); mRNA-1273 (25 PWH, 25 controls) | Median (IQR): 35 d (20–63) | 88% PWH, 95% controls | Anti-RBD IgG ≥10 relative fluorescent units | 0.57 | 95% CI: 0.36–0.88 | Geometric mean ratio (GMR), PWH vs controls | ET Health Pylon anti-RBD IgG assay; ≥10 relative fluorescent units | NR |
| Tuan, 2021, USA | Prospective observational | 39 PWH | 73.1% CD4 ≥500 cells/mm3 | 83% had undetectable VL | BNT162b2 | 2–3 wk | 97.5% | NR | NR | NR | NR | Healgen COVID-19 anti-S IgG/IgM rapid test; NR | NR |
| Woldemeskel, 2021, USA | Prospective cohort | 12 PWH, 17 controls | Median (range): 913 cells/mm3 (649–1678) | 75% <20 copies/mL | BNT162b2 | Range: 7–17 d | NR | NR | Median 8.84 | NR | Euroimmun relative units | Euroimmun Anti-SARS-CoV-2 IgG ELISA; ≥1.1 EU | D614G, B.1.1.7, B.1.351, P.1 |
| Xu, 2022, Sweden | Prospective open-label clinical trial | 79 PWH, 82 controls | Median (IQR): 565 cells/mm3 (280–723) | 86% <50 copies/mL | BNT162b2 | 14 d | 98.7% PWH, 100% controls | NR | Median: 1613 PWH, 2192 controls | IQR: 897–2643 PWH, 1398–3651 controls | U/mL | Roche Elecsys Anti-SARS-CoV-2 S | NR |
Abbreviations: A/CO, absorbance to cutoff; Ab, antibody; BAU, binding antibody units; GMT, geometric mean titer; HIV-negative individuals; IQR, interquartile ratio; ELISA, enzyme-linked immunosorbent assay; EU, ELISA units; IgG, immunoglobulin G; IU, international units; MIA, multiplexed immunoassay; NR, not reported; PWH, people with HIV; RBD, receptor binding domain; RLU, relative luminescence units; S/CO, signal/cutoff ratio; U, unit; VL, viral load.
Table 2.
Study Summary
| Author, Year, Location | Summary Findings |
|---|---|
| 1. González de Aledo, 2022, Spain | Following 2-dose vaccination with mRNA vaccines, all 100 PWH achieved detectable antispike IgG Ab, and only 3 had antibody levels <520 BAU/mL (1 of these had baseline CD4 count <200 cells/mm3). |
| 2. Antinori, 2022, Italy | Humoral and cell-mediated immune response against SARS-CoV-2 following 2 doses of mRNA vaccines was comparable between PWH with baseline CD4 counts >500 cells/mm3 and HIV-negative controls. Immune response was significantly poorer in PWH with baseline CD4 counts <200 cells/mm3 (P < .001). |
| 3. Brumme, 2022, Canada | After 2 doses of ChAdOx1 and/or mRNA vaccine, HIV status was not significantly associated with magnitude of anti-RBD response, and no significant correlation was observed between vaccine immunogenicity and the most recent or nadir CD4 cell counts. |
| 4. Feng, 2021, China | Similar immunologic response was found after 2-dose vaccination with Sinopharm BBIBP-CorV between PWH with high CD4/CD8 ratio and HIV-negative controls; PWH with low baseline CD4/CD8 ratios generated lower antibody responses compared with those with medium and high CD4/CD8 ratios (P < .01). |
| 5. Frater, 2021, UKa | No difference in magnitude or persistence of immunogenic response between PWH and HIV-negative controls after 2 doses of ChAdOx1. No correlation was found between the magnitude of the antispike IgG response and CD4 cell count. |
| 6. Ogbe, 2021, UKa | No difference in immunologic response between PWH and matched HIV-negative controls following 2 doses of ChAdOx1. SARS-CoV-2 spike-specific responses maintained for 6 mo in PWH were equivalent to HIV negative controls. |
| 7. Haidar, 2022, USA | Following 2 doses of mRNA vaccine, no difference in seropositivity was found between controls and PWH with CD4 counts >200 cells/mm3, but CD4 count <200 cells/mm3 was associated with lower odds for seropositivity (P < .001). |
| 8. Hassold, 2022, France | After 2 doses of mRNA vaccine or ChAdOx1, PWH with CD4 >500 cells/mm3 had a higher antibody response (P = .003) than those with lower CD4 cell counts, especially those with CD4 counts <200 cells/mm3 (P = .0017). 100% of patients with baseline CD4 counts >500 cells/mm3 were seropositive, compared with 95% and 78% of those with CD4 counts between 200 and 499 cells/mm3 or <200 cells/mm3, respectively. |
| 9. Huang, 2022, China | Immunogenicity of inactivated vaccines (Sinovac CoronaVac & Sinopharm BBIBP-CorV) was significantly lower in PWH than HIV-negative controls (P = .007), regardless of CD4 cell count or HIV viral load. |
| 10. Jedicke, 2021, Germany | Most PWH demonstrated robust immune response after complete BNT162b2 vaccination, but with significantly lower anti-S IgG response compared with HIV-negative controls (P < .0001). |
| 11. Khan, 2021, South Africa | Ad26.CoV2.S vaccination of PWH with well-controlled HIV led to noninferior immune response to SARS-CoV-2 Delta variant. |
| 12. Levy, 2021, Israelb | 97% of PWH developed immune response following second dose of BNT162b2; PWH had lower anti-RBD IgG levels than controls (P = .008). |
| 13. Rahav, 2021, Israelb | HIV patients showed a similar immunologic response to HIV-negative controls after 2 doses of BNT162b2. |
| 14. Liu, 2021, China | Two doses of Sinovac CoronaVac produced immunologic response in PWH, with significantly decreased anti-RBD-IgG titers in individuals with baseline CD4 <350 cells/mm3 (P = .023). |
| 15. Lombardi, 2022, Italy | PWH with well-controlled ART, stable viral suppression, and robust CD4+ T-cell count produced detectable humoral immune responses, similar to individuals without HIV infection, following 2 doses of mRNA-1273. |
| 16. Lv, 2021, China | Inactivated SARS-CoV-2 vaccines (Sinovac CoronaVac, Sinopharm BBIBP-CorV) had similar neutralizing antibody positivity among PWH and HIV-negative controls. However, the magnitude of immunogenicity was smaller in PWH than HIV-negative controls (P < .05). |
| 17. Madhi, 2021, South Africa | Patients immunized with 2 doses of ChAdOx1 showed a strong immunologic response, regardless of HIV status. |
| 18. Milano, 2022, Italy | 98.6% of PWH vaccinated with 2 doses of BNT162b2 mounted a detectable immune response; no significant correlation was observed between patient characteristics and immunologic response. |
| 19. Nault, 2022, Canadac | After the first dose of mRNA-based vaccine, anti-RBD IgG response was similar in PWH with CD4 counts >250 cells/mm3 and HIV-negative controls; PWH with low CD4 counts had a weaker immunologic response (P < .0001). |
| 20. Noe, 2021, Germany | PWH mounted a robust immune response after complete vaccination with BNT162b2, mRNA-1273, ChAdOx1, or Ad26.COV2.S vaccines. Higher anti-RBD IgG concentrations were observed in patients receiving mRNA-based vaccines and those with baseline CD4 >200 cells/mm3 (P < .001). Patients with CD4 <200 cells/mm3, in particular, mounted a significantly poorer immunologic response (P < .001). |
| 21. Portillo, 2022, Switzerland | All PWH elicited antibodies after 2 doses of mRNA vaccines, but antibody response was significantly lower (P < .001) compared with an unmatched group of HIV-negative controls, despite an overall high CD4 T-cell count. |
| 22. Ruddy (1), 2021, USAc | All participants developed anti-SARS-CoV-2 RBD antibodies after the first dose of mRNA vaccine. |
| 23. Ruddy (2), 2021, USA | All participants developed increased concentrations of anti-SARS-CoV-2 RBD antibodies following the second dose of mRNA vaccine. |
| 24. Speich, 2022, Switzerland | PWH developed a strong antibody response following 2 doses of mRNA vaccine, with no difference found between BNT162b2 and mRNA-1273. |
| 25. Spinelli, 2021, USA | Following 2 doses of mRNA vaccine, PWH demonstrated lower IgG response than HIV-negative controls (P = .01), which was most pronounced among those with lower baseline CD4 counts (P = .003); all 7 PWH with CD4 baselines <200 cells/mm3 failed to mount a pseudovirus neutralizing titer or IgG response. PWH vaccinated with mRNA-1273 had higher IgG titers than those vaccinated with BNT162b2 (P = .02). |
| 26. Tuan, 2021, USA | PWH with baseline CD4 <500 cells/mm3 were potentially less likely to seroconvert after a single dose of BNT162b2 (P = .07), but there was no CD4-related difference in immunologic response after 2 doses. 97.5% of PWH had a positive IgG response following the second dose. |
| 27. Woldemeskel, 2021, USA | Following 2 doses of BNT162b2 vaccine, PWH mounted an immunologic response similar to HIV-negative controls. |
| 28. Xu, 2022, Sweden | Despite high seroconversion rates in PWH following 2 doses of BNT162b2, PWH developed significantly lower levels of antispike IgG than HIV-negative controls 2 wk after the second dose (P = .0012), regardless of CD4 cell count. Patients with baseline HIV viral loads >50 copies/mL had particularly low levels of antispike IgG (P = .048). |
Abbreviations: ART, antiretroviral therapy; IgG, immunoglobulin G; PWH, people with HIV; RBD, receptor binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Ogbe was a follow-up study of the patients included in the Frater study.
Levy and Rahav recruited from the group of individuals, but the extent of overlap could not be determined.
Nault and Ruddy (1) reported results after the first dose of a 2-dose mRNA-based vaccine regimen.
Vaccine Types
Studies reported use of mRNA vaccines, namely BNT162b2 (Pfizer-BioNTech) [9, 11, 14, 18–21, 23, 25–34] and mRNA-1273 (Moderna) [9, 18–20, 22, 24–26, 28–32], nonreplicating viral vector AZD1222/ChAdOx1 nCoV-19 (AstraZeneca) [12, 13, 17, 20, 24, 28] or Ad26.COV2.S (Janssen) [9, 16, 24], and inactivated CoronaVac (Sinovac BioTech) [36–38] or BBIBP CorV (Sinopharm) vaccines [35, 36, 38]. Overall, 20 studies (69%) reported results from an mRNA vaccine platform. Nineteen (66%) studies reported results of BNT162b2, and 13 (45%) reported results of mRNA-1273; 12 reported on both. Seven studies reported results from ChAdOx1 (AstraZeneca), 4 studies reported results from BBIBP-CorV (Sinopharm) and/or Sinovac Coronavac, while 3 studies reported results from Janssen.
Study Reporting
Studies reporting anti-RBD IgG responses used various assay types, including manufactured and nonspecified assays against RBD. Five studies reported data on SARS-CoV-2 variants of concern (VOCs), with none reporting on the Omicron variant [16, 17, 21, 28, 37].
Risk of Bias
Overall, studies were considered to be at low to moderate risk of bias. The main concerns were lack of a control group, failure to identify and/or adjust for confounders, different lengths of patient follow-up, and difficulty in determining the external validity of reported findings. Representativeness of the study population was difficult to determine due to limited reporting of information about facilities and source populations.
Immunologic Responses
Overall, seroconversion rates and serologic titers in PWH were high after the final reported administered dose for completion of primary vaccination, with the exception of 1 study that used inactivated virus vaccine BBIBP [35].
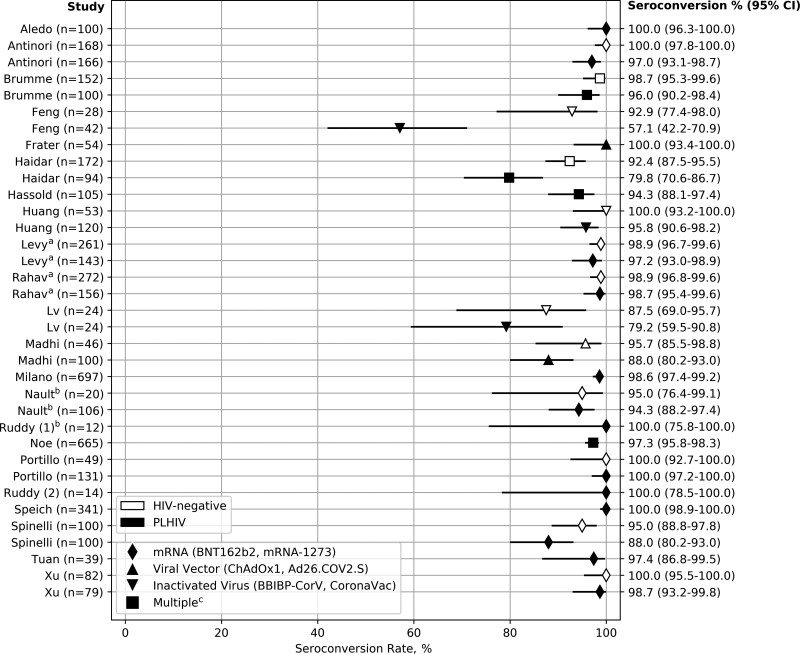
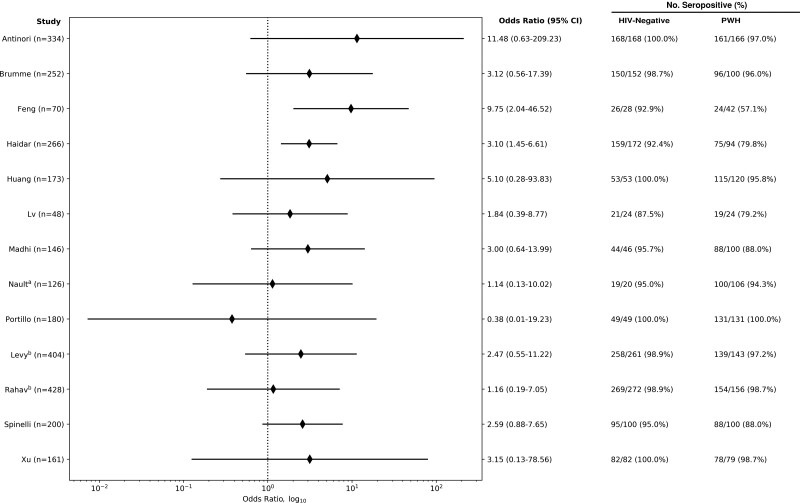
Of 22 (73%) studies that reported rates of seroconversion in PWH, 15 (68%) found seroconversion rates of 95% or higher, while only 3 (14%) found seroconversion rates below 85%. Thirteen (59%) of these studies also reported seroconversion rates in HIV-negative individuals, with all having rates >87.5%, of which 1 [25] found 100% seroconversion in both groups (Figure 1). Of the remaining 12 studies, 2 [9, 35] found significantly increased odds of failure to seroconvert in PWH compared with HIV-negative individuals (Figure 2).
Figure 1.
COVID-19 vaccine seroconversion rates in PWH and HIV-negative individuals by vaccine type. Solid diamonds: mRNA (BNT162b2, mRNA-1273). Solid upward triangles: Viral vector (ChAdOx1, Ad26.COV2.S). Solid downward triangles: Inactivated virus (BBIBP-CorV, CoronaVac). Solid squares: Multiplec. Hollow rectangles: HIV-negative. Solid rectangles: PWH. aThe Levy and Rahav studies may have overlap in the patients observed. Ogbe and Frater also had overlapping patients, but Ogbe did not report seroconversion rates and is therefore not included in this figure. bThe Nault and Ruddy (1) studies reported seroconversion rates only after the first dose of a 2-dose mRNA vaccine regimen. cIncludes studies using multiple vaccine types (eg, mRNA, viral vector): Brumme (BNT162b2, mRNA-1273, ChAdOx1), Haidar (BNT162b2, mRNA-1273, Ad26.COV2.S), Hassold (BNT162b2, mRNA-1273, ChAdOx1), Noe (BNT162b2, mRNA-1273, ChAdOx1, Ad26.COV2.S). Abbreviations: COVID-19, coronavirus disease 2019; PWH, people with HIV.
Figure 2.
Odds ratio between PWH and HIV-negative individuals for failing to seroconvert after COVID-19 vaccination. Odds ratio (log scale) for failure to seroconvert of papers that reported results for both PWH and HIV controls. OR calculation included a continuity correction for studies that found 0 seronegative cases in 1 or both groups. aNault reported results after only the first dose of a 2-dose mRNA vaccine regimen. bThe Levy and Rahav studies may have had an overlap of patients included. Abbreviations: COVID-19, coronavirus disease 2019; OR, odds ratio; PWH, people with HIV.
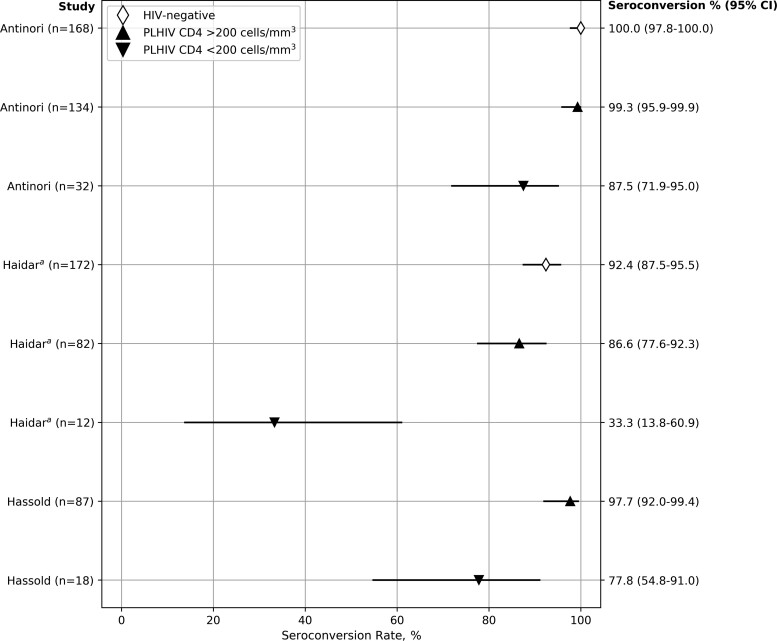
Two (6.8%) studies assessed serologic response after only 1 dose of a 2-dose vaccine [29, 30]. The majority of studies reported PWH with high baseline CD4 cell counts, with over half of the studies reporting mean or median baseline CD4 counts >500 cells/mm3 [11–13, 14, 21, 22, 25, 27, 28, 30, 32–34, 36]. Twenty-two (75.9%) studies reported HIV VL, with all reporting the majority of PWH having VL <1000 copies/mL, and often <50 copies/mL. Of 9 studies that compared either seroconversion rates or serologic titers by baseline CD4 cell count [9, 19, 20, 22, 24, 29–31, 37], 7 found a diminished immunologic response in PWH with lower CD4 counts [9, 19, 20, 24, 29, 30, 37]. All 3 studies that reported seroconversion rates stratified by baseline CD4 counts found decreased rates of seroconversion in PWH with lower CD4 cell counts [9, 19, 20] (Figure 3). Likewise, studies that reported results for individuals with lower CD4/CD8 ratios [35] or high HIV VL [27] also reported decreased responses.
Figure 3.
COVID-19 vaccine seroconversion rates in PWH by CD4 cell count. Hollow diamond symbols: HIV-negative. Solid upward triangles: PWH, CD4 >200 cells/mL3. Solid downward triangles: PWH, CD4 <200 cells/mL3. aHaidar result for PWH CD4 <200 (33.3% seropositivity) was not reported directly in the paper but was calculated from other reported values. Abbreviations: COVID-19, coronavirus disease 2019; PWH, people with HIV.
DISCUSSION
In this systematic review of 28 studies that reported on humoral immune responses to COVID-19 vaccination in PWH, seroconversion rates were overall very high in PWH. Several studies showed PWH failing to seroconvert; however, the numbers of PWH were very small. Adult PWH on established ART with suppressed HIV loads and higher baseline CD4 counts and CD4/CD8 ratios generally mounted robust humoral immune responses, especially following the final reported administered dose of the COVID-19 primary vaccination series, which was generally the second vaccine dose. PWH with diminished COVID-19 vaccine-induced antibody responses generally had greater baseline immunosuppression or advanced HIV disease or HIV VL, but further characterization of PWH vaccine nonresponders is needed. Study results underscore the need for establishing early ART, preserving immunologic status, maintaining HIV virologic control, and prioritizing SARS-CoV-2 vaccination as a key COVID-19 prevention strategy among PWH.
There are no standard correlates of protection for COVID-19 vaccination. Differences in seropositivity rates reported for the reviewed studies may have been attributable to variations in type and brand of serologic assay used, test characteristics, serologic assessment time points after COVID-19 vaccination, units of serologic titer reporting, cutoff levels selected for antibody positivity, definitions for serological response vs seropositivity, rates of prior SARS-CoV-2 infection, and duration of follow-up, among others.
The impact of underlying HIV infection and B- and T-cell impairment on humoral and cellular immunity, such as the effect of low CD4 counts on the immune response to COVID-19 vaccination, remains to be established. There is a need to understand better correlates of protection (eg, specific titers of anti-RBD antibodies or peak humoral response that correlate with neutralizing activity), vaccine induced T-cell responses, and factors that influence the durability and breadth of immune response in PWH. Additionally, a better understanding of the role of patient age across age groups from older adults to children, demographic background, levels of immunosuppression (including CD4 cell count strata), ART regimens, levels of HIV virologic control, and presence of comorbidities and coinfections (eg, tuberculosis) is needed. Global COVID-19 vaccine shortages may limit homologous vaccine use, so heterologous mix-and-match strategies need evaluation for maximum immunogenicity and effectiveness against challenging variants of concern and other subvariants, optimized dosing intervals, and boosting strategies (eg, additional doses in individuals with blunted responses or specific PWH subpopulations). Additionally, as SARS-CoV-2 breakthrough infections become more common, optimized COVID-19 vaccination strategies in individuals with prior infection are needed.
The strengths of this review include the searching of multiple databases using a broad search strategy and inclusion criteria that enabled the identification of a broad set of studies. Key steps of the review were conducted in duplicate to minimize errors, and reporting adhered to the PRISMA checklist, an evidence-based minimum set of items for reporting in systematic reviews. There are also several limitations to note. There is important heterogeneity of the body of evidence, as reflected by study design, methods, and reporting (vaccine types and combinations, sample sizes, inclusion of a control group and age-matched controls, interval between doses, postvaccination observation period, immune response measurement, and serologic titer units). Most reviewed studies had small sample sizes, were nonrandomized, and were insufficiently powered to compare vaccine efficacy, effectiveness, or durability of response. Of the few studies that reported results for both PWH and HIV-negative individuals, a handful suggested an increased risk of failure to seroconvert among PWH; however, the number of these comparison studies and participant volume were limited. Since the initial review was conducted, all preprints have been published in full, with no changes to the reported outcomes. We assessed the risk of bias using the modified Downs and Black system; however, the lack of applicability of criteria to all studies may have impacted scoring. At the time the studies were reviewed, vaccine regimens were either 1 or 2 doses depending on vaccine type. The review included SARS-CoV-2 variants in circulation; however, published and preprint data available on booster doses and variants and subvariants were limited, and no evaluated studies reported on the Omicron subvariant. Studies reviewed included only adult PWH who were relatively homogeneous for sex and race and generally had high baseline CD4 counts and excellent virologic control on ART, with limited characterization of the distribution of patient CD4 cell counts; these findings are therefore not representative of the entire PWH population. Importantly, there was very little information from low- and middle-income settings with a high burden of HIV, with only 2 studies from South Africa. Certain PWH subpopulations were under-represented or not studied, including children, adolescents, and PWH with coinfections and comorbidities (such as tuberculosis, diabetes, and hypertension, which may influence immunologic outcomes). The lack of longitudinal humoral response data limited the ability to characterize individuals' titer kinetics over time. There was also heterogeneity across studies in the definitions applied to advanced disease or immune status, and this may not have been well controlled in the studies that reported comparisons between PWH and HIV-negative individuals. Finally, it is important to note that the key outcomes of interest for COVID-19 vaccine effectiveness are breakthrough infections, progression to severe disease, and death; these outcomes are very poorly reported and remain an important area of future research.
We presented study data for the parameters related to anti-SARS-CoV-2 recombinant spike, RBD, or neutralizing IgG due to the paucity and greater heterogeneity of data of other immunologic parameters. Lack of international consensus on measures to determine immunogenicity and the immunological markers that predict protection against COVID-19 remains. Although antibody levels may be strongly correlated with anti-RBD titers, lack of immunoassay standardization across the studies reviewed and variation potentially due to underlying comorbidities or conditions may have contributed to the variation of results observed.
CONCLUSIONS
Long-lasting immune responses against SARS-CoV-2 are necessary for protection against severe COVID-19 disease. Notwithstanding important limitations of the current evidence base, the studies presented in this review of early immunity induced by currently EUL-approved COVID-19 vaccines in PWH provide reassuring data. In order to characterize and understand the associations of humoral and cellular immune responses to COVID-19 vaccines in PWH and clinical efficacy and effectiveness, future evaluations that include PWH of different ages, sexes, demographic backgrounds and settings, ART statuses, ART regimens, virologic suppression statuses, CD4 count strata, coinfections, and comorbidities will be important for developing strategies to maximize the durability of protection against SARS-CoV-2 variants and subvariants. While COVID-19 booster doses were not specifically assessed, waning antibody levels are anticipated, and booster doses for PWH, as for other persons who are immunocompromised, appear to be a reasonable recommendation. Potential reduced vaccine immunogenicity in PWH with more advanced disease and continued susceptibility to COVID-19 support additional doses, either as extended primary series or as additional booster doses. The current data available on COVID-19 disease in PWH suggest that the benefits of additional vaccine doses outweigh the risks, particularly among those with low CD4 counts. Future studies may provide data to help tailor vaccine regimens based on degree of immunocompromise or factors associated with poor COVID-19 coinfection outcomes.
COVID-19 vaccination remains a critical tool to prevent COVID-19 transmission and reduce death and severe illness among PWH. Continued prioritization of COVID-19 vaccination in PWH is needed, especially when supplies are limited. COVID-19 vaccination, coupled with comprehensive clinical management of HIV for virologic suppression and the prevention and management of comorbidities to improve COVID-19 outcomes, is paramount. Programs will need to identify strategies for COVID-19 vaccination integration and demand creation and uptake within the context of HIV and broader service delivery to ensure that evidence-based implementation goes hand in hand with the protection of individuals and communities.
Supplementary Material
Acknowledgments
We thank Joanna Taliano, MA, MLS, reference librarian at the Stephen B. Thacker CDC Library, for her literature search support.
Financial support. This work was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The funders of the review had no role in the review design, data collection, data analysis, writing of the report, or in the decision to submit results for publication.
Disclaimer. This publication has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies. Applicable federal laws for ethical review include 45 C.F.R. part 46.102(l)(2); 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Author contributions. The analysis was conceptualized by H.M.C., I.Z., K.M., E.A., A.R., S.D., A.W., and M.V. All authors were involved in the investigation. H.M.C., I.Z., and K.M. collected and curated the data. H.M.C., I.Z., E.A., K.M., M.V., and A.R. developed the methodology. H.M.C., I.Z., K.M., and E.A. conducted the formal analysis. H.M.C., I.Z., K.M., and E.A. wrote the original draft of the manuscript. All authors were involved in critically reviewing and editing the manuscript.
Patient consent. This review does not include factors necessitating patient consent.
Contributor Information
Helen M Chun, Division of Global HIV/TB, Center for Global Health, Atlanta, Georgia, USA.
Kyle Milligan, Division of Global HIV/TB, Center for Global Health, Atlanta, Georgia, USA; Peraton, Herndon, Virginia, USA.
Elfriede Agyemang, Division of Global HIV/TB, Center for Global Health, Atlanta, Georgia, USA.
Nathan Ford, Global HIV, Viral Hepatitis and Sexually Transmitted Infections Programmes, World Health Organization, Geneva, Switzerland.
Ajay Rangaraj, Global HIV, Viral Hepatitis and Sexually Transmitted Infections Programmes, World Health Organization, Geneva, Switzerland.
Shalini Desai, Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Annelies Wilder-Smith, Immunization, Vaccines and Biologicals, World Health Organization, Geneva, Switzerland.
Marco Vitoria, Global HIV, Viral Hepatitis and Sexually Transmitted Infections Programmes, World Health Organization, Geneva, Switzerland.
Isaac Zulu, Division of Global HIV/TB, Center for Global Health, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Johns Hopkins Coronavirus Resource Center . COVID-19 map. Available at: https://www.jhu.edu/. Accessed August 08, 2022.
- 2. Bertagnolio S, Thwin SS, Silva R, et al. . Clinical features of, and risk factors for, severe or fatal COVID-19 among people living with HIV admitted to hospital: analysis of data from the WHO Global Clinical Platform of COVID-19. Lancet HIV. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joint United Nations Programme on HIV/AIDS 2021 . Global HIV & AIDS statistics—2021 fact sheet. Available at: https://www.unaids.org/en/resources/fact-sheet. Accessed May 5, 2022.
- 4. Tesoriero JM, Swain CE, Pierce JL, et al. . COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York state. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singh R, Kang A, Luo X, et al. . COVID-19: current knowledge in clinical features, immunological responses, and vaccine development. FASEB J 2021; 35:e21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cooper T, Woodward B, Alom S, et al. . Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS patients: a systematic review. HIV Med 2020; 21:567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization . COVID-19 vaccines with WHO emergency use listing. Available at: https://extranet.who.int/pqweb/vaccines/vaccinescovid-19-vaccine-eul-issued. Accessed June 10, 2022.
- 8. González-Stegmaier R, Cereceda K, Briones JL, et al. . Seroconversion and abundance of IgG antibodies against S1-RBD of SARS-CoV-2 and neutralizing activity in the Chilean population. J Immunol Res 2021; 2021:6680337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haidar G, Agha M, Bilderback A, et al. . Prospective evaluation of COVID-19 vaccine responses across a broad spectrum of immunocompromising conditions: the COVICS study. Clin Infect Dis 2022; 75:e630–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frater J, Ewer KJ, Ogbe A, et al. ; Oxford COVID Vaccine Trial Group . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021; 8:e474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogbe A, Pace M, Bittaye M, et al. . Durability of ChAdOx1 nCoV-19 vaccination in people living with HIV. JCI Insight 2022; 7:e157031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levy I, Wieder-Finesod A, Litchevsky V, et al. . Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. Clin Microbiol Infect 2021; 27:1851–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahav G, Lustig Y, Lavee J, et al. . Bnt162b2 mRNA COVID-19 vaccination in immunocompromised patients: a prospective cohort study. EClinicalMedicine 2021; 41:101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomized and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Khan K, Lustig G, Bernstein M, et al. . Immunogenicity of SARS-CoV-2 infection and Ad26.CoV2.S vaccination in people living with HIV. Clin Infect Dis 2022; 75:e857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Madhi SA, Baillie V, Cutland CL, et al. . Efficacy of the ChAdOx1 nCoV-19 Covid-19 vaccine against the B.1.351 variant. N Engl J Med 2021; 384:1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Aledo M G, Cañizares A, Vázquez-Rodríguez P, et al. . Safety and immunogenicity of SARS-CoV-2 vaccines in people with HIV. AIDS 2022; 36:691–5. [DOI] [PubMed] [Google Scholar]
- 19. Antinori A, Cicalini S, Meschi S, et al. ; HIV-VAC Study Group . Humoral and cellular immune response elicited by mRNA vaccination against SARS-CoV-2 in people living with HIV (PLWH) receiving antiretroviral therapy (ART) according with current CD4 T-lymphocyte count. Clin Infect Dis 2022; 75:e552–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hassold N, Brichler S, Ouedraogo E, et al. . Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022; 36:F1–5. [DOI] [PubMed] [Google Scholar]
- 21. Jedicke N, Stankov MV, Cossmann A, et al. . Humoral immune response following prime and boost BNT162b2 vaccination in people living with HIV on antiretroviral therapy. HIV Med 2022; 23:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lombardi A, Butta GM, Donnici L, et al. . Anti-spike antibodies and neutralising antibody activity in people living with HIV vaccinated with COVID-19 mRNA-1273 vaccine: a prospective single-centre cohort study. Lancet Reg Health Eur 2022; 13:100287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Milano E, Ricciardi A, Casciaro R, et al. . Immunogenicity and safety of the BNT162b2 COVID-19 mRNA vaccine in PLWH: a monocentric study in Bari, Italy. J Med Virol 2022; 94:2230–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noe S, Ochana N, Wiese C, et al. . Humoral response to SARS-CoV-2 vaccines in people living with HIV. Infection 2022; 50:617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Portillo V, Fedeli C, Ustero Alonso P, et al. . Impact on HIV-1 RNA levels and antibody responses following SARS-CoV-2 vaccination in HIV-infected individuals. Front Immunol 2022; 12:820126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Speich B, Chammartin F, Abela IA, et al. ; Swiss HIV Cohort Study and the Swiss Transplant Cohort Study . Antibody response in immunocompromised patients after the administration of SARS-CoV-2 vaccine BNT162b2 or mRNA-1273: a randomised controlled trial. Clin Infect Dis 2022; 75:e585–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu X, Vesterbacka J, Aleman S, Nowak P; COVAXID Study Group . High seroconversion rate after vaccination with mRNA BNT162b2 vaccine against SARS-CoV-2 among people with HIV—but HIV viremia matters? AIDS 2022; 36:479–81. [DOI] [PubMed] [Google Scholar]
- 28. Brumme ZL, Mwimanzi F, Lapointe HR, et al. . Humoral immune responses to COVID-19 vaccination in people living with HIV receiving suppressive antiretroviral therapy. NPJ Vaccines 2022; 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nault L, Marchitto L, Goyette G, et al. . Covid-19 vaccine immunogenicity in people living with HIV-1. Vaccine 2022; 40:3633–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruddy JA, Boyarsky BJ, Werbel WA, et al. . Safety and antibody response to the first dose of severe acute respiratory syndrome coronavirus 2 messenger RNA vaccine in persons with HIV. AIDS 2021; 35:1872–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruddy JA, Boyarsky BJ, Bailey JR, et al. . Safety and antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in persons with HIV. AIDS 2021; 35:2399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spinelli MA, Peluso MJ, Lynch KL, et al. . Differences in post-mRNA vaccination SARS-CoV-2 IgG concentrations and surrogate virus neutralization test response by HIV status and type of vaccine: a matched case-control observational study. Clin Infect Dis 2022; 75:e916–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tuan JJ, Zapata H, Critch-Gilfillan T, et al. . Qualitative assessment of anti-SARS-CoV-2 spike protein immunogenicity (QUASI) after COVID-19 vaccination in older people living with HIV. HIV Med 2022; 23:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Woldemeskel BA, Karaba AH, Garliss CC, et al. . The BNT162b2 mRNA vaccine elicits robust humoral and cellular immune responses in people living with human immunodeficiency virus (HIV). Clin Infect Dis 2022; 74:1268–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng Y, Zhang Y, He Z, et al. . Immunogenicity of an inactivated SARS-CoV-2 vaccine in people living with HIV-1: a non-randomized cohort study. EClinicalMedicine 2022; 43:101226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang X, Yan Y, Su B, et al. . Comparing immune responses to inactivated vaccines against SARS-CoV-2 between people living with HIV and HIV-negative individuals: a cross-sectional study in China. Viruses 2022; 14:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu Y, Han J, Li X, et al. . COVID-19 vaccination in people living with HIV (PLWH) in China: a cross sectional study of vaccine hesitancy, safety, and immunogenicity. Vaccines (Basel) 2021; 9:1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lv Z, Li Q, Feng Z, et al. . Inactivated SARS-CoV-2 vaccines elicit immunogenicity and T-cell responses in people living with HIV. Int Immunopharmacol 2022; 102:108383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.