Abstract
Introduction
Microvascular changes in eye and kidney shares some common factors in diabetes mellitus (DM). The purpose was to evaluate choroidal thickness (CT) and choriocapillaris (CC) density in patients with type 2 diabetes (T2D) and their association with diabetic kidney disease (DKD) using swept-source optical coherence tomography (SS-OCT).
Research design and methods
A cross-sectional study was conducted with patients with T2D with mild or no diabetic retinopathy (DR) and non-diabetic controls. CT was measured with SS-OCT, and CC vascular density was measured with OCT angiography. These parameters were compared with inner retinal layers thickness in patients with and without DKD and non-diabetic controls.
Results
Ninety-three eyes from patients with T2D and 34 eyes from controls volunteers were included. Within the T2D group, 56 eyes with DKD and 37 eyes from patients with no diabetic kidney disease were examined. A statistically significant reduction of CT was observed in patients with DKD compared with controls, with no difference in CC density. There was an association between ganglion cell layer and central choroidal thickness reduction in the DKD group.
Conclusions
Patients with T2D with DKD showed a decrease in CT with no difference in CC density compared with non-diabetic controls. This thinning might be related to vascular changes of choroidal layers such as Haller’s and Sattler’s with preservation of CC density, which is crucial for outer retina and retinal pigment epithelium health. Longitudinal studies are warranted to determine the association of choroidal changes with the pathogenesis of diabetes, and its association with early DKD and progression to more severe DR.
Keywords: diabetes mellitus, type 2; kidney diseases; diabetic retinopathy
What is already known on this topic
Several authors have described choroidal thickness in patients with diabetes without retinopathy in association with kidney disease, the relationship between diabetic retinopathy and diabetic choroidopathy as well as CT and renal function remains unclear.
What this study adds
Our study shows that choroid might be affected by diabetes even before clinical signs of diabetic retinopathy are present, mainly in patients with some grade of diabetic kidney disease and choriocapillaris may be preserved in early stages.
How this study might affect research, practice or policy
Our findings might be a new imaging biomarker useful for research and clinical follow-up of patients with diabetes.
Introduction
Diabetes mellitus (DM) is a complex, progressive disease associated with multiple pathophysiological changes that can result in macrovascular and microvascular complications such as nephropathy, retinopathy and neuropathy. These complications may lead to tissue and organ damage in approximately one-third to one-half of people with diabetes.1
Diabetic retinopathy (DR) is the leading cause of vision loss and the most important ocular complication of DM.2 According to the International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales, DR is classified clinically into a severity scale based on the presence of visible microvascular changes as microaneurysms, hemorrhages, venous beading, intraretinal microvascular abnormalities and neovascularization.3 Despite the gold standard to DR diagnosis and classification still being funduscopy examination, optical coherence tomography (OCT) technology can detect early changes in retinal and vascular morphology in patients without DR.4 5
The choroid is a highly vascularized structure that plays an important role in the regulation of ocular metabolism; it is responsible for supplying blood to the retinal epithelium, outer retina and optic nerve, and is the only source of metabolic exchange for the avascular fovea.6 Several choroidal changes have been described in patients with diabetes, including increased and decreased thickness, in efforts to better define choroidal findings as a predictive factor for DR progression and treatment response.7
The glomerular vascular network and choroidal circulation share some structural analogy and similar pathways as well as glomerular filtration barrier and inner blood-retinal barrier.8 DM is known to be one of the main causes of chronic kidney disease, alongside hypertension.9
Swept-source OCT (SS-OCT) has improved image penetration compared with conventional OCT, by using a longer laser wavelength (1050 nm) that helps minimize dispersion caused by the retinal pigment epithelium (RPE), higher imaging speeds (axial scan rate of at least 100 000 scans per second) and higher detection efficiency.5 It provides high-resolution images and data about retinal and choroid thickness and vascular structure of the posterior pole.10 SS-OCT is also capable of performing concomitant OCT angiography (OCT-A), using an eye-tracking system that allows to decrease motion artifacts.11
Compared to kidney, morphological changes on retino-choroidal microcirculatory system is more accessible to clinical evaluation in a repeatable and non-invasive manner, offering a good opportunity to observe the vascular and neurological structures when affected by factors such as systemic diseases and diurnal variation.7 12 13
Structural and metabolic alterations of choroid as involving CT and CC density may lead to metabolic disorders of photoreceptors and RPE, making CT an imaging biomarker that could be used to evaluate the pathophysiology of choroidal and retinal diseases, including DR.14
Therefore, the aim of this study was to detect choroidal thickness and choriocapillaris (CC) vascular density changes in patient with type 2 diabetes (T2D), with or without kidney disease (DKD), using SS-OCT and OCT-A.
Methods
Subjects
This study was a cross-sectional study conducted at a Hospital de Clinicas de Porto Alegre between July 2018 and July 2019. The research design and methodological procedures, including inclusion/exclusion criteria, were previously published by our group.15
Inclusion criteria: patients with T2D referred from Endocrinology Unit of Hospital de Clinicas de Porto Alegre were included in this study, and control group was composed of volunteers without medical history of DM or kidney disease (last 3 months laboratory exams). Patients with diabetes were submitted to biochemical exams to measure glycated hemoglobin (HbA1c), urinary albumin excretion (UAE) and creatinine/estimated glomerular filtration rate. Exclusion criteria follow our published protocol.15 Images with low quality (TopQ Image Quality <40), significant artefacts or media opacities, surgeries, spherical equivalent outside±3 D were considered as screening failure. Only the right eye (OD) was included in the study statistical analysis. To avoid bias regarding diurnal variation of choroidal thickness, SS-OCT/OCT-A were carried out always in the morning.16
During study visit, all patients and controls answered a demographic and medical history questionary, performed complete ophthalmological examination, best-corrected visual acuity (BCVA), SS-OCT (Triton system, Topcon, New Jersey, USA) and OCT-A. DR was graded by a masked ophthalmologist based on the international clinical DR and diabetic macular edema disease severity scales.3
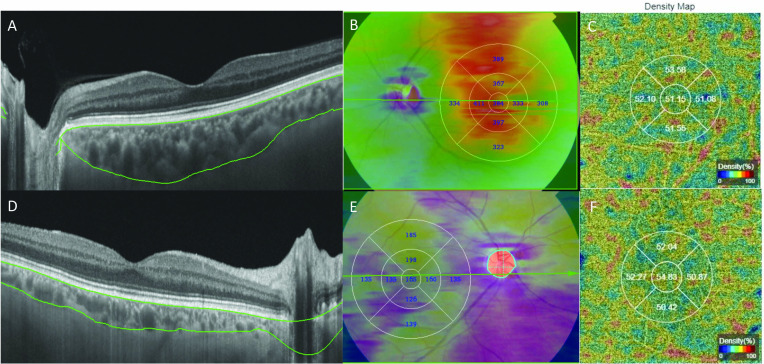
SS-OCT/OCT-A images were obtained using the 3D(H) wide (12 mm×9 mm) macula+line protocol with Early Treatment Diagnostic Retinopathy Study (ETDRS) grid centered at the fovea using automated segmentation. A trained masked investigator revised choroidal segmentation and performed manual corrections when necessary. Automated ganglion cell layer plus (GCL+) complex was also obtained to evaluate correlation between inner retina and CT. GCL+ is measured from the inner nerve fiber layer interface to the inner plexiform layer/inner nuclear layer. OCT-A algorithm (OCTARA) uses a graduation of dark/bright pixels to derive measure of capillary density. The CC automated segmentation was defined from Bruch’s membrane (BM) (0 µm offset) to 10.4 μm below, using native ImageNet 6 software. Figure 1 shows representative cases of automated segmentation of BM and choroidoscleral junction for no diabetic kidney disease (nDKD) and DKD groups and choroidal thickness maps.
Figure 1.
Swept-source optical coherence tomography structural image for a patient with type 2 diabetes (T2D) with no diabetic kidney disease (nDKD) (A) showing segmentation lines for Bruch’s membrane and scleral-choroidal junction (green) with automated choroidal thickness map (B) and density map for choriocapillaris from OCT angiography measurements (C). Second row shows representative case of a patient with T2D with DKD that presented with decreased choroidal thickness as shown on the structural (D) scan and choroidal thickness map (E), however, there was no detectable decrease in choriocapillaris density (F).
Statistics
Statistical analysis was performed using IBM SPSS software (V.26.0). Nominal variables comparisons were carried out with a χ2 test and independent t-test was used for continuous variables. Continuous variables were presented as the mean±SD; categorical variables were presented as percentages (% of each group).
Shapiro-Wilk test was performed to verify the normality of distribution. Generalized estimating equations, adjusted by age, was carried out with exclusion of missing values. Relationships between pairs of continuous variables were evaluated by Spearman’s correlation analysis. All tests were two-tailed with α=0.05.
Results
Table 1 summarizes demographics and clinical characteristics of patients with T2D versus controls and subjects with T2D with nDKD versus those with DKD. Ninety-three eyes from patients with T2D were included in the final analysis: 37 eyes from patients with nDKD and 56 eyes from patients with DKD, as well as 34 eyes from controls volunteers. Patients with diabetes were older (61±8.2 vs 56±7.5; p<0.001), with more prevalent hypertension (92.1% vs 33.3%; p<0.0001). The mean DM duration was 14.3 years. Among subjects with diabetes, we excluded 16 eyes due to moderate DR, 2 eyes with proliferative DR, 2 eyes with high opacity, 2 myopic eyes, 10 type 1 diabetes eyes and 2 MODY (maturity onset diabetes of the young) eyes.
Table 1.
Demographic and clinical characteristics of patients with T2D with no or mild diabetic retinopathy versus controls and patients with no diabetic kidney disease (nDKD) versus patients with diabetic kidney disease (DKD)
| Variable | T2D group (n=93 eyes) |
Control group (n=34 eyes) |
P value | nDKD (n=37 eyes) |
DKD (n=56 eyes) |
P value |
| Gender, n (%)* | ||||||
| Male | 35 (37.6%) | 14 (41.2%) | 0.717 | 12 (32.4%) | 23 (41.1%) | 0.659 |
| Female | 58 (62.4%) | 20 (58.8%) | 25 (67.6%) | 33 (58.9%) | ||
| Age in years, mean (SD) | 61.0 (±8.2) | 56.1 (±7.5) | <0.001 | 62 (±7.9) | 60.4 (±8.4) | 0.215 |
| Ethnicity, n (%)* | ||||||
| Caucasian | 79 (85.9%) | 11 (78.6%) | 0.547 | 33 (89.2%) | 46 (83.6%) | 0.151 |
| African descent/mixed | 13 (14.1%) | 3 (21.4%) | 4 (10.8%) | 10 (16.4%) | ||
| Diabetic retinopathy stage, n (%) | ||||||
| No DR | n/a | n/a | n/a | 97 (83.8%) | 58 (75.0%) | 0.007 |
| Mild NPDR | n/a | n/a | n/a | 17 (16.2%) | 18 (25.0%) | |
| DM duration in years, mean (SD) | 14.3 (±6.9) | n/a | n/a | 14 (±6.3) | 14.5 (±7.3) | 0.693 |
| HbA1c, mean (SD) | 8.3 (±1.7) | 5.5 (±0.3) | <0.001 | 8.1 (±1.6) | 8.5 (±1.7) | 0.155 |
| BCVA, mean (SD) | 44.8 (±10.8) | 50.9 (±10.7) | 0.051 | 47.5 (±8.2) | 43.4 (±11.8) | 0.144 |
| Hypertension, n (%)* | 82 (92.1%) | 5 (33.3%) | <0.001 | 31 (91.2%) | 51 (92.7%) | 0.725 |
| UAE (SD) | n/a | n/a | n/a | 6.6 (±3.4) | 190.4 (± 418.1) | <0.001 |
| eGFR (SD) | n/a | n/a | n/a | 88.3 (±14.6) | 78.5 (± 26.8) | 0.004 |
Significant values in bold.
*Subjects.
BCVA, best-corrected visual acuity; DM, diabetes mellitus; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; n/a, not available; NPDR, Non Proliferative Diabetic Retinopathy; T2D, type 2 diabetes; UAE, urinary albumin concentration.
Comparison between subjects with T2D with nDKD and those with DKD showed no difference in HbA1c (8.1%±1.6% vs 8.5%±1.7%, p=0.155), age (62±7.9 vs 60.4±8.4, p=0.215), DM duration (14±6.3 vs 14.5±7.3; p=0.693) or BCVA (47.5±8.2 vs 43.4±11.8; p=0.144). Patients with diabetes with DKD presented prevalence of mild DR (25.0% vs 16.2%, p=0.007), lower eGFR values (78.5±26.8 mL/min/1.73 m2, p=0.004) and higher UAE values (190.4±418.1 mg/L, p<0.001) (table 1).
Choroidal layers in patients with T2D were thinner compared with controls in all ETDRS quadrants, including average thickness and total volume (table 2). CC density values showed no difference between patients with T2D and controls (table 3). Patients with DKD presented significantly thinner CT in the following quadrants compared with controls: inner superior (95% CI 8.2 to 90.1; p=0.012), outer superior (95% CI 13.6 to 90.6; p=0.004), outer nasal (95% CI 0.4 to 82.2; p=0.046) and outer inferior (95% CI 8.7 to 86.7; p=0.010).
Table 2.
Mean choroidal layer thickness in subjects with T2D and controls
| Choroidal thickness, ETDRS (μm) | T2D (n=93 eyes) | Controls (n=34 eyes) | 95% CI | P value |
| MD (µm)±SD | MD (µm)±SD | |||
| Center thickness | 241.3±9.1 | 289.0±15.6 | −83.3 to −12.0 | 0.009 |
| Total volume | 6.2±0.2 | 7.3±0.3 | −2.0 to −0.4 | 0.003 |
| Inner temporal | 235.9±8.2 | 274.8±13.6 | −70.5 to −7.3 | 0.016 |
| Inner superior | 251.3±7.5 | 296.8±14.2 | −76.6 to −14.5 | 0.004 |
| Inner nasal | 229.0±8.3 | 269.6±8.3 | −74.9 to −6.2 | 0.021 |
| Inner inferior | 227.9±9.3 | 270.4±15.4 | −77.9 to −7.1 | 0.019 |
| Outer temporal | 217.1±7.0 | 252.2±11.4 | −61.2 to −9.0 | 0.008 |
| Outer superior | 243.5±7.3 | 292.2±13.5 | −78.0 to −19.3 | 0.001 |
| Outer nasal | 185.1±7.7 | 220.5±14.2 | −66.6 to −4.0 | 0.027 |
| Outer inferior | 208.8±8.5 | 255.0±11.8 | −74.6 to −17.6 | 0.002 |
| Average thickness | 219.3±7.3 | 260.8±12.2 | −69.0 to −13.9 | 0.003 |
Significant values in bold.
ETDRS, Early Treatment Diagnostic Retinopathy Study; MD, Average; T2D, type 2 diabetes.
Table 3.
Meanchoroidal layers density in subjects with T2D versus controls
| Choriocapillaris density | T2D (n=93 eyes) | Controls (n=34 eyes) | 95% CI | P value |
| MD (µm)±SD | MD (µm)±SD | |||
| Central density | 48.8±0.5 | 41.0±0.8 | −4.5 to 0.1 | 0.060 |
| Superior | 53.1±0.2 | 53.0±0.5 | −1.1 to 1.2 | 0.911 |
| Nasal | 53.3±0.3 | 54.0±0.5 | −1.9 to 0.5 | 0.267 |
| Inferior | 53.0±0.3 | 53.5±0.6 | −1.9 to 0.8 | 0.441 |
| Temporal | 53.1±0.3 | 53.1±0.4 | −1.0 to 1.0 | 0.910 |
MD, Average; T2D, type 2 diabetes.
Patients with DKD also showed lower choroidal total volume (95% CI 0.2 to 2.1; p=0.013) and average thickness (95% CI 6.6 to 76.2; p=0.016) (table 4). CC density values showed no difference between patients with T2D with nDKD compared with controls (table 5). GCL+ and CT showed a significant positive association in the DKD group (r=0.3; p=0.005); however, no association was found in the nDKD or control groups.
Table 4.
Choroidal thickness: statistical differences between nDKD and DKD subgroups versus control group
| Choroidal layers thickness | Controls (n=34 eyes) |
nDKD (n=37 eyes) |
DKD (n=56 eyes) |
||||
| MD (µm)±SD | MD (µm)±SD | 95% CI | P value | MD (µm)±SD | 95% CI | P value | |
| Center thickness | 288.9±15.6 | 243.3±13.9 | −5.1 to 96.2 | 0.094 | 240.0±11.9 | 2.0 to 95.9 | 0.038 |
| Total volume | 7.4±0.3 | 6.3±0.3 | −0.05 to 2.2 | 0.067 | 6.1±0.3 | 0.2 to 2.3 | 0.013 |
| Inner temporal | 274.9±13.7 | 231.8±12.8 | −2.2 to 88.5 | 0.069 | 238.6±10.5 | −5.3 to 78.0 | 0.110 |
| Inner superior | 296.7±14.2 | 257.0±11.7 | −3.9 to 83.2 | 0.087 | 247.5±9.9 | 8.2 to 90.1 | 0.012 |
| Inner nasal | 269.4±15.5 | 235.2±13.4 | −14.9 to 83.4 | 0.287 | 225.0±10.7 | −0.3 to 89.2 | 0.053 |
| Inner inferior | 270.4±15.4 | 226.3±14.8 | −7.6 to 95.8 | 0.124 | 228.9±11.9 | −5.0 to 88.1 | 0.098 |
| Outer temporal | 252.2±11.4 | 214.5±10.9 | −0.03 to 75.5 | 0.050 | 218.7±9.1 | −1.2 to 68.2 | 0.063 |
| Outer superior | 292.1±13.5 | 248.9±12.0 | 0.5 to 85.8 | 0.046 | 240.0±9.5 | 13.6 to 90.6 | 0.004 |
| Outer nasal | 220.2±14.2 | 194.8±12.4 | −19.3 to 70.3 | 0.521 | 178.9±9.7 | 0.4 to 82.2 | 0.046 |
| Outer inferior | 254.9±11.8 | 211.3±12.8 | 1.6 to 85.5 | 0.039 | 207.2±11.4 | 8.7 to 86.7 | 0.010 |
| Average thickness | 260.8±12.2 | 222.5±11.3 | −1.8 to 78.3 | 0.066 | 217.3±9.5 | 6.8 to 80.1 | 0.013 |
Significant values in bold.
DKD, diabetic kidney disease; nDKD, no diabetic kidney disease.
Table 5.
Choriocapillaris density: statistical differences between nDKD and DKD subgroups versus control group
| Choriocapillaris density | Controls (n=34 eyes) |
nDKD (n=37 eyes) |
DKD (n=56 eyes) |
||||
| MD±SD | MD±SD | 95% CI | P value | MD±SD | 95% CI | P value | |
| Central | 51.0±1.0 | 48.5±0.9 | −0.8 to 5.8 | 0.198 | 49.0±0.6 | −0.9 to 4.9 | 0.299 |
| Superior | 53.0±0.4 | 53.4±0.3 | −2.0 to 1.3 | 1.000 | 53.4±0.3 | −1.4 to 1.6 | 1.000 |
| Nasal | 54.0±0.5 | 53.1±0.3 | −0.7 to 2.4 | 0.601 | 53.4±0.4 | −1.0 to 2.2 | 1.000 |
| Inferior | 53.5±0.3 | 52.9±0.4 | −1.2 to 2.5 | 1.000 | 53.0±0.3 | −1.3 to 2.5 | 1.000 |
| Temporal | 53.1±0.4 | 53.7±0.4 | −2.0 to 0.8 | 0.953 | 52.8±0.4 | −1.2 to 1.7 | 1.000 |
DKD, diabetic kidney disease; nDKD, no diabetic kidney disease.
Discussion
In this study, we used SS-OCT to analyze CT and CC vessel density in patients with T2D with no or mild DR, according to presence of DKD, compared with a non-diabetic control group and adjusted by age. We showed that DKD was associated with sectorial and average choroidal thinning in patients with T2D compared with controls.
The absence of differences in CC density between T2D versus control groups could indicate that Sattler’s and Haller’s vessels may be affected before CC microvessels in the early pathogenesis of diabetic choroidopathy. Borrelli et al described a strong relationship between CC perfusion and photoreceptor health measured by ellipsoid zone reflectivity in patients with NPDR compared with healthy controls.17 Therefore, a possible preservation of CC prefusion could have a protective effect to photoreceptors and the RPE.
These findings are in consonance with those of Foo et al, who evaluated the choroidal vascular index (CVI) of Haller’s and Sattler’s layers in patients with T2D compared with non-diabetic controls and found that diabetic eyes with no DR have a lower macular CVI of Haller’s layer compared with control eyes, with no significant differences in CT or volume between these two groups.18 Unlike Foo et al, who consider that retinal layers are relatively preserved in early stage DR, we observed that central CT is slightly correlated with GCL+ thickness in patients with DKD, confirming our previous results.15 19
We found that patients with nDKD showed no changes on choroidal thickness compared with controls, except in outer temporal, superior and inferior quadrants. As other authors suggest that microvasculopathy in the retina, choroid and kidney may share pathogenic mechanisms related to metabolic changes, inflammation and endothelial dysfunction characteristic of diabetes.18 20 21
Chen et al, in an 8-year prospective study, concluded that microalbuminuria has a greater impact on predicting the development and progression of DR compared with moderate decline in GFR among patients with T2D.22 Nevertheless, the relationship between choroidal alterations and DR is already unclear. Our results indicate that diabetic choroidopathy, observed in patients with T2D with DKD, also may be related to microvascular chronic complications of diabetes independent of DR.22
Alterations in choroid is largely reported in patients with diabetes without DR or in the initial stages of the disease with different and even antagonistic results. In a study with a large sample of diabetic population, using SS-OCT, Wang et al found that CT increased in the early stage of DR and further decreased with DR progression.23 However, this study had no non-diabetic control group. Oliveira-Ferreira et al compared patients with T2D with normoalbuminuria or microalbuminuria, without DR, with healthy controls, and also found an increase of mean subfoveal CT in diabetics as well as thicker subfoveal CT and temporal CT in patients with T2D with microalbuminuria compared both with controls as normoalbuminuric patients.24
In contrast, a recent meta-analysis suggested that subfoveal choroidal thickness is thinner in diabetic eyes compared with controls, and that the ratio of luminal areas (corresponding to the choroidal vascular lumens) is lower in diabetic eyes of patients without clinically detectable DR compared with control eyes.25 26 Likewise, Ashour et al, in a study whose aim was to understand the effect of microalbuminuria on diabetic choroid, had found that patients with T2D have thinner CT compared with non-diabetic controls.27 Authors added that conflicting results may be related to involvement of different pathological mechanisms.28 We have shown in previous studies using spectral domain OCT and SS-OCT that both retina and choroidal layers presented decreased thickness in early stages of DR (no or mild DR), especially in patients with some degree of kidney damage.15 28 According to Liu et al, conflicting results about CT in diabetic eyes can be explained by different protocol designs, patient profiles, adjustment for confounding factors and different devices.29
The strength of our study is the use of SS-OCT technology, which enables more precise identification of the choroidal-scleral edge and, consequently, more reliable CT and CC density measurements. The main limitations are its cross-sectional design, relatively small sample and limiting sample factors such as the high prevalence of white women and not age-matched controls. These factors were included in the statistical models to decrease the chances of bias.
In conclusion, we observed a decrease in CT in patients with T2D, with no differences in CC density compared with non-diabetic controls. This thinning might be related to choroidal layers such as Haller’s and Sattler’s with preservation of CC density, which is crucial for outer retina and RPE health. Longitudinal studies are warranted to further determine the association of choroidal changes with the pathogenesis of DM and early kidney disease, as well as with progression to more severe DR.
Acknowledgments
We thank the Ophthalmology and Endocrinology Unit of Hospital de Clinicas de Porto Alegre for their support and input for study recruitment; clinical research assistant Elisangela Soares for her valuable support in constructing the OCT-A tables and Vania Naomi Hirakata for her assistance with biostatistics.
Footnotes
Contributors: MOS contributed to the volunteers selection, recording, data analysis and paper writing and is also responsible for the overall content as the guarantor. AECdCC and FL contributed in performing patients’ examinations and data review. GCG contributed with data collection. BDS contributed with study protocol and paper review. DL contributed with study protocol, data analysis and paper writing.
Funding: This project was partially supported by a research investment grant from the Hospital de Clínicas de Porto Alegre Research and Event Investment Fund (FIPE). The funder had no role in the design, conduct, analysis or reporting of the study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Patient data are registered in patient medical records and on the OCT device. According to Brazilian legislation, data can only be accessed with express permission from patients or their legal representatives.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Hospital de Clinicas de Porto Alegre Ethics Committee (protocol registration number CAEE 87894418800005327), and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
References
- 1.Uk prospective diabetes study (UKPDS) VIII . Study design, progress and performance. Diabetologia 1991;34:877–90. [PubMed] [Google Scholar]
- 2.Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35:556–64. 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkinson CP, Ferris FL, Klein RE, et al. Proposed International clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003;110:1677–82. 10.1016/S0161-6420(03)00475-5 [DOI] [PubMed] [Google Scholar]
- 4.Vujosevic S, Muraca A, Alkabes M, et al. Early microvascular and neural changes in patients with type 1 and type 2 diabetes mellitus without clinical signs of diabetic retinopathy. Retina 2019;39:435–45. 10.1097/IAE.0000000000001990 [DOI] [PubMed] [Google Scholar]
- 5.Lavinsky F, Lavinsky D. Novel perspectives on swept-source optical coherence tomography. Int J Retina Vitreous 2016;2:25. 10.1186/s40942-016-0050-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mrejen S, Spaide RF. Optical coherence tomography: imaging of the choroid and beyond. Surv Ophthalmol 2013;58:387–429. 10.1016/j.survophthal.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Melancia D, Vicente A, Cunha JP, et al. Diabetic choroidopathy: a review of the current literature. Graefes Arch Clin Exp Ophthalmol 2016;254:1453–61. 10.1007/s00417-016-3360-8 [DOI] [PubMed] [Google Scholar]
- 8.Vadalà M, Castellucci M, Guarrasi G, et al. Retinal and choroidal vasculature changes associated with chronic kidney disease. Graefes Arch Clin Exp Ophthalmol 2019;257:1687–98. 10.1007/s00417-019-04358-3 [DOI] [PubMed] [Google Scholar]
- 9.KDOQI . KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49:S12–154. 10.1053/j.ajkd.2006.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Ferrara D, Waheed NK, Duker JS. Investigating the choriocapillaris and choroidal vasculature with new optical coherence tomography technologies. Prog Retin Eye Res 2016;52:130–55. 10.1016/j.preteyeres.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina 2014;45:382–9. 10.3928/23258160-20140909-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong CW, Wong TY, Cheng C-Y, et al. Kidney and eye diseases: common risk factors, etiological mechanisms, and pathways. Kidney Int 2014;85:1290–302. 10.1038/ki.2013.491 [DOI] [PubMed] [Google Scholar]
- 13.Grunwald JE, Alexander J, Maguire M, et al. Prevalence of ocular fundus pathology in patients with chronic kidney disease. Clin J Am Soc Nephrol 2010;5:867–73. 10.2215/CJN.08271109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie R, Qiu B, Chhablani J, et al. Evaluation of choroidal thickness using optical coherent tomography: a review. Front Med 2021;8:783519. 10.3389/fmed.2021.783519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.da Silva MO, do Carmo Chaves AEC, Gobbato GC, et al. Early neurovascular retinal changes detected by swept-source OCT in type 2 diabetes and association with diabetic kidney disease. Int J Retina Vitreous 2021;7:73. 10.1186/s40942-021-00347-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan CS, Ouyang Y, Ruiz H, et al. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci 2012;53:261–6. 10.1167/iovs.11-8782 [DOI] [PubMed] [Google Scholar]
- 17.Borrelli E, Palmieri M, Viggiano P, et al. Photoreceptor damage in diabetic choroidopathy. Retina 2020;40:1062–9. 10.1097/IAE.0000000000002538 [DOI] [PubMed] [Google Scholar]
- 18.VHX F, Gupta P, Nguyen QD. Decrease in Choroidal Vascularity Index of Haller’s layer in diabetic eyes precedes retinopathy.. BMJ open diabetes Res care. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farias LB, Lavinsky D, Benfica CZ, et al. Microalbuminuria is associated with early retinal neurodegeneration in patients with type 2 diabetes. Ophthalmic Surg Lasers Imaging Retina 2018;49:e36–43. 10.3928/23258160-20180907-05 [DOI] [PubMed] [Google Scholar]
- 20.Garrido-Hermosilla AM, Méndez-Muros M, Gutiérrez-Sánchez E, et al. Renal function and choroidal thickness using swept-source optical coherence tomography in diabetic patients. Int J Ophthalmol 2019;12:985–9. 10.18240/ijo.2019.06.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balmforth C, van Bragt JJMH, Ruijs T, et al. Chorioretinal thinning in chronic kidney disease links to inflammation and endothelial dysfunction. JCI Insight 2016;1:e89173. 10.1172/jci.insight.89173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen H, Zheng Z, Huang Y, et al. A microalbuminuria threshold to predict the risk for the development of diabetic retinopathy in type 2 diabetes mellitus patients. PLoS One 2012;7:e36718. 10.1371/journal.pone.0036718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Liu S, Qiu Z, et al. Choroidal thickness in diabetes and diabetic retinopathy: a swept source OCT study. Invest Ophthalmol Vis Sci 2020;61:29. 10.1167/iovs.61.4.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira-Ferreira C, Leuzinger-Dias M, Tavares-Ferreira J, et al. Choroidal thickness and urinary albumin excretion in type 2 diabetic patients without retinopathy. J Ophthalmol 2020;2020:1–5. 10.1155/2020/3648941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endo H, Kase S, Saito M, et al. Choroidal thickness in diabetic patients without diabetic retinopathy: a meta-analysis. Am J Ophthalmol 2020;218:68–77. 10.1016/j.ajo.2020.05.036 [DOI] [PubMed] [Google Scholar]
- 26.Kase S, Endo H, Takahashi M, et al. Choroidal vascular structures in diabetic patients: a meta-analysis. Graefes Arch Clin Exp Ophthalmol 2021;259:3537–48. 10.1007/s00417-021-05292-z [DOI] [PubMed] [Google Scholar]
- 27.Ashour DM, El-Shazly AAE-F, Abdelgawad RHA, et al. Choroidal thickness in relation to urinary albumin excretion rate in type 2 diabetes mellitus without retinopathy. Int J Retina Vitreous 2021;7:1–7. 10.1186/s40942-021-00332-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farias LB, Lavinsky D, Benfica CZ, et al. Changes in choroidal thickness and volume are related to urinary albumin excretion in type 2 diabetic patients without retinopathy. Clin Ophthalmol 2018;12:1405–11. 10.2147/OPTH.S164195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Wang W, Tan Y, et al. Relationship between renal function and choroidal thickness in type 2 diabetic patients detected by Swept-Source optical coherence tomography. Transl Vis Sci Technol 2020;9:17. 10.1167/tvst.9.5.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Patient data are registered in patient medical records and on the OCT device. According to Brazilian legislation, data can only be accessed with express permission from patients or their legal representatives.