Abstract
We present a case of a patient with metastatic lung adenocarcinoma who developed severe right lower limb radicular pain in a L5-S1 dermatomal distribution 5 months into treatment with carboplatin, pemetrexed and pembrolizumab. MRI of the lumbar spine demonstrated contrast enhancement of the right L5 nerve root consistent with neuritis. The patient was treated with intravenous methylprednisolone 2 mg/kg/day for 3 days, followed by oral prednisolone 1 mg/kg/day with a slow wean over 6 weeks. There was no improvement and their performance status deteriorated to an Eastern Cooperative Oncology Group (ECOG) score of 3, representing capability of only limited self-care. We commenced induction therapy with intravenous immunoglobulin 2 g/kg over 5 days, which resulted in complete resolution of pain sustained for 3 weeks before recurrence of symptoms. We continued maintenance therapy with intravenous immunoglobulin 0.4 g/kg over 2 days at 4–5 weekly intervals, which led to resolution of symptoms and ECOG score to 1.
Keywords: Neurooncology, Peripheral nerve disease, Lung cancer (oncology), Unwanted effects / adverse reactions
Background
Immune checkpoint inhibitors (ICIs) are a first-line standard-of-care option in combination with chemotherapy for metastatic non-small cell lung cancer without oncogene driver mutations. Immunotherapy has a different mechanism of action to traditional chemotherapy, with unique immune-related adverse effects (irAEs).
One key signal pathway targeted by current immunotherapy is between programmed cell death 1 receptor (PD-1) and programmed cell death receptor ligand 1 (PDL-1), which are expressed on T cells and tumour cells, respectively. The interaction between PD-1 and its ligand PDL-1 helps facilitate immune evasion through negative modulation of T cell migration and function, thus promoting tumour survival.1 Pembrolizumab is a monoclonal antibody that binds to and inhibits PD-1, blocking its interaction with its ligand PDL-1 and thus preventing downregulation of the host immune response.1
Parallel to the success of ICI therapy in improving outcomes in many solid-organ malignancies, there is increasing recognition of irAEs that occur at any stage of treatment, including after completion. While irAEs can affect any system, neurological irAEs are comparatively rare with a reported frequency of 1 to 5% for all grades of severity combined.2
Case presentation
We present the case of a patient who was diagnosed with a left upper lobe lung mass and upfront metastatic lung adenocarcinoma, proven to be (anaplastic lymphoma kinase negative, ROS proto-oncogene 1 receptor tyrosine kinase (ROS1) negative, epidermal growth factor receptor negative and programmed death-ligand 1 (PDL-1) score of 0%) on CT-guided biopsy. The patient had a good premorbid functional baseline with an Eastern Cooperative Oncology Group (ECOG) performance status scale score of 0, reflective of fully independent function. The ECOG scale ranges between 0 and 5 based on a patient’s ability to undertake tasks of daily living, with an increasing score indicative of an increasing degree of functional impairment. An initial FDG PET scan showed bilateral moderately intense FDG-avid lung lesions in addition to a moderately intense FDG-avid L5 vertebral body metastasis. The patient was commenced on combination chemoimmunotherapy with carboplatin, pemetrexed and pembrolizumab.
Five months into treatment, the patient reported progressive worsening of right lumbar radicular pain. At that time, the patient’s physical examination revealed an antalgic gait on the right side with use of a single point stick for mobility. Hip flexion and extension were significantly limited on the right secondary to severe radicular pain described in a typical L5-S1 dermatomal distribution. Weakness of right great toe dorsiflexion was also apparent without any significant sensory deficit. With an initial provisional diagnosis of right hip bursitis, a short course of oral corticosteroids in addition to a bursal steroid injection was trialled without effect. The patient had constant excruciating pain that significantly limited their mobility and was subsequently admitted to hospital for further investigation.
Investigations
During the admission, an MRI of the lumbar spine and pelvis was performed. The T2-based fluid-sensitive neurography sequence (figure 1) best illustrates the markedly abnormal thickening, signal hyperintensity and enhancement of the right L5 nerve root consistent with neuritis, with the extent of enhancement far exceeding that which could reasonably be expected with nerve root impingement. This is supported by both the mid-sagittal T2 and axial T2 non-contrast sequences (figures 2 and 3) depicting only a mild posterior disc bulge at the L5/S1 level, which would not be expected to produce this extent of inflammation. A rounded L5 vertebral body metastatic deposit that was observed to have only a small epidural component is also depicted (figure 2). A postcontrast axial T1 fat-suppressed sequence also further highlights the significant L5 nerve root enhancement at the L5/S1 neural foramen level (figure 4).
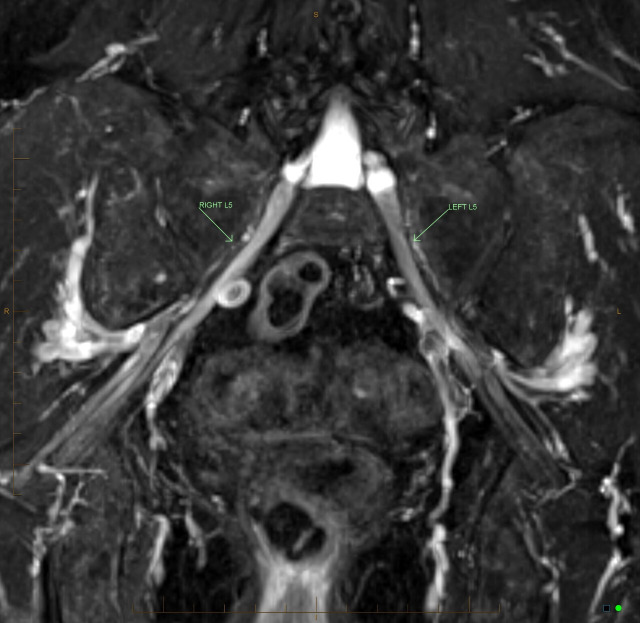
Figure 1.
Coronal T2-weighted Dixon turbo spin echo sequence without contrast which provides T2-weighted fat and water-separated images useful in depicting peripheral nerve pathologies. This image represents abnormal enhancement of the right L5 nerve root from immediately lateral to the neural foramina up to the level of the greater sciatic notch.
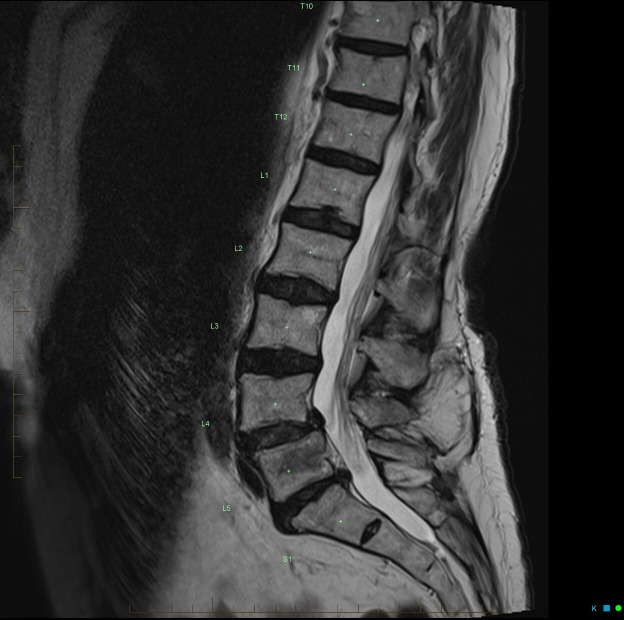
Figure 2.
Mid-sagittal T2 (non-contrast) sequence which depicts: mild (grade 1) degenerative anterolisthesis of the L4 over L5 vertebral body; mild posterior disc bulge at the L5/S1 level without significant compression on the central thecal sac; and a rounded L5 vertebral body metastatic deposit that was observed to have a small epidural component can also be seen.
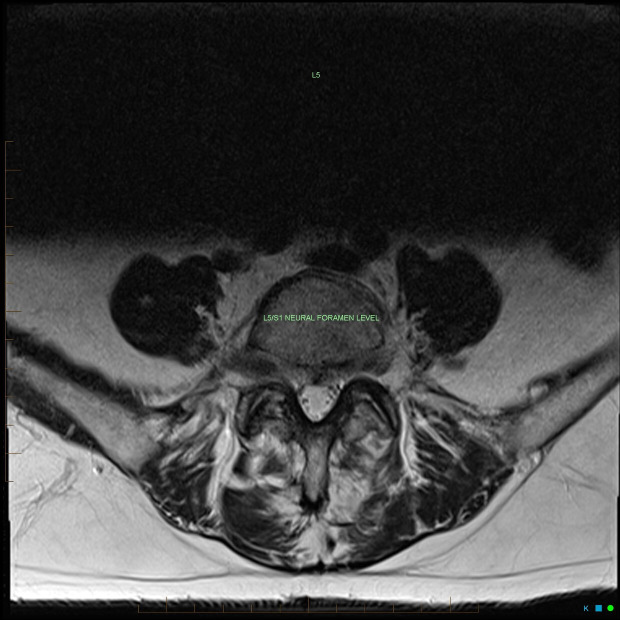
Figure 3.
Axial T2 (non-contrast) sequence further depicting a mild posterior disc bulge at the L5/S1 level without significant neural compression, which would not be sufficient to explain the degree of enhancement of the right L5 nerve root depicted in figures 1 and 4.
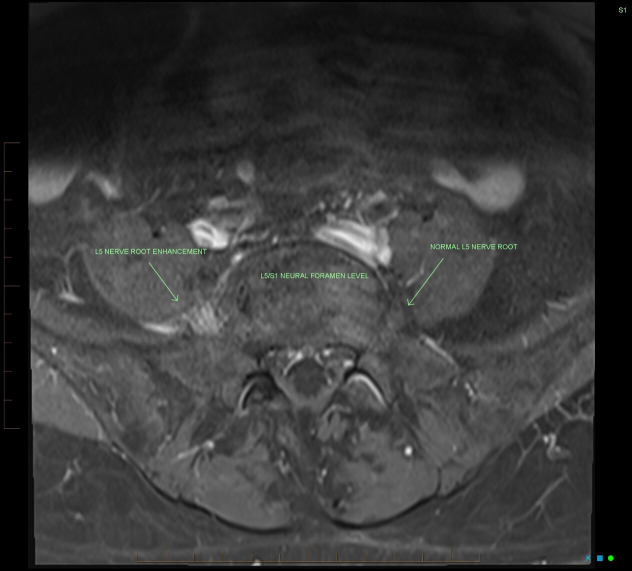
Figure 4.
Axial T1 fat-suppressed postcontrast sequence which also highlights marked postcontrast enhancement of the right L5 nerve root at the L5/S1 neural foramen level.
Treatment
The working diagnosis was a grade 3 neurological toxicity from suspected immunotherapy-induced neuritis. Pembrolizumab was ceased and the patient was commenced on intravenous methylprednisolone 2 mg/kg/day for 3 days, followed by oral prednisolone 1 mg/kg/day per guidelines. Steroids were weaned over a period of 6 weeks with concurrent up-titration of Pregabalin to a dose of 200 mg daily for neuropathic pain. Unfortunately, the patient developed significant functional deterioration in their right lower limb rendering them unable to extend their leg or sit due to severe pain, with an ECOG deterioration to 3. After a neurology consultation, induction therapy with intravenous immunoglobulin (IVIg) was commenced (2 g/kg spread over 5 days) resulting in a significant clinical response and the patient was able to subsequently mobilise independently, free from pain or discomfort. The degree of L5 nerve root enhancement and inflammation seen on the T2-based fluid-sensitive sequence (figure 1) exceeded that which could reasonably be expected with L5 nerve root impingement, and the lack of response to first-line therapy and prompt symptom resolution with IVIg further supported the diagnosis of immunotherapy-induced neuritis, as compressive neuropathies do not respond to intravenous immunoglobulins. Therapeutic benefit was maintained for 3 weeks before gradual recurrence and worsening of symptoms. Maintenance therapy with IVIg was then continued (0.4 g/kg over 2 days) at 4–5 weekly intervals with a sustained clinical response. During the peak of a COVID-19 pandemic wave there was a 3-month treatment pause, which resulted in recurrence of severe radicular pain. We reinstituted full induction with IVIg, which again resulted in reversal of symptoms and improvement of ECOG score to 1. IVIg was then continued as 5 weekly maintenance therapy with sustained benefit and no wearing-off phenomena.
Outcome and follow-up
There was consistent improvement in the patient’s right lumbar radicular pain for several months with maintenance IVIg therapy. Ongoing benefit was limited by subsequent disease progression with an increase in size of the L5 vertebral body metastasis with soft tissue extension causing nerve root impingement necessitating palliative radiation therapy. The patient was treated with further lines of systemic therapy and passed away 2 years later.
Discussion
Given the propensity for irAEs to affect any organ system, a high degree of suspicion is essential once a patient is commenced on immunotherapy. The therapeutic advantage of sustained altered host immune regulation that allows the effects of immunotherapy to last for many months even after cessation also reflects the fact that irAEs have been observed both during treatment and well beyond completion.
The specific mechanisms of immune-related toxicity are not completely understood but are felt to represent an extensive inflammatory process with immune dysregulation and abnormal CD4+ and CD8+ T cell-mediated responses against self-antigens.3 Altered host autoimmunity as a likely mechanism is supported by the general exclusion of patients with pre-existing significant autoimmune disease such as myasthenia gravis, systemic lupus erythematosus or inflammatory bowel disease in IO clinical trials, due to the risk of disease flare and serious AEs.
Despite the heterogeneity of irAEs, neurological toxicity represents a broad clinical phenotype with central, peripheral and autonomic nervous system toxicity as well as neuromuscular junction toxicity reported largely through case reports and case series.4 Based on this data, neurological irAEs have an estimated incidence of 1%–5% for all toxicity severities with an increased risk observed with combined immune checkpoint blockade (eg, PD-1 and CTLA-4 coinhibition).2 The diverse presentation of neurological toxicities often makes them difficult to recognise, as presenting symptoms can be nonspecific such as fatigue and there can be a mixture of lower and upper motor neuron signs depending on the specific pathology.
With regard to peripheral nervous system toxicity, pembrolizumab has been reported to be associated with the development of cranial neuropathies, isolated optic neuritis, acute inflammatory demyelinating polyradiculopathies and inflammatory myeloradiculitis.4–7 Neuromuscular toxicity has also been described with PD-1 inhibition including myasthenia gravis, as well as neuropathy and myopathy, often with overlapping features.8
Management of irAEs is fundamentally based on an accurate assessment of the grade of severity of toxicity. Severity of neurological toxicity is graded 1–4 based on the impact of symptoms on the patient’s ability to complete instrumental activities of daily living. Grade 2 toxicity, which involves some functional limitation, is generally managed by withholding the immunotherapy and initiating oral corticosteroid therapy at a dose of 1 mg/kg/day. For patients that do not respond to oral corticosteroid therapy, input is often sought from a neurologist for consideration of escalation of therapy. As evidenced in our case, response to an initial oral corticosteroid course is variable and risk factors for identifying patients who are not likely to respond early is poorly elucidated.
Mechanistically, steroid-ineffectiveness in some patients is proposed to result from a lack of specificity of steroids for targeting the implicated mediator driving the immune-mediated toxicity and perhaps highlights why IVIg, which is known to inactivate auto-reactive T-cells, was a highly effective treatment in our case.9 10 Despite discontinuation of immunotherapy, relapse of immune-mediated toxicities can occur with weaning of immunosuppression and some patients require maintenance therapy to prevent long-term disability.
Our case represents a unique presentation of acute immune-mediated L5 neuritis secondary to pembrolizumab that was steroid-unresponsive and required maintenance IVIg therapy for sustained symptom relief. The specific location of our patient’s L5 vertebral body metastasis in relation to their symptoms and response to treatment in this case also highlights the importance of considering immune-mediated toxicity as a differential for patients presenting with similar features during treatment with ICIs.
Learning points.
Pembrolizumab is a selective monoclonal antibody that targets PD-1 on tumour-specific T-cells and is increasingly being used widely in different oncological treatment regimens.
Neurological immune-related adverse effects (irAEs) are rare, but a high-degree of vigilance needs to be maintained during treatment given the non-specific signs and symptoms that may be reported at onset.
Treatment of neurological irAEs is dependent on the type of toxicity, but for grade 2 toxicity treatment often initially involves stopping immunotherapy and commencement of oral corticosteroids with slow weaning.
Intravenous immunoglobulins are required in steroid resistance and can lead to sustained reversal of debilitating side effects.
Footnotes
Contributors: LF performed the literature review and case report write-up with significant contributions to the completed case report by both ZL and CG who were directly involved in the patient’s care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained from next of kin.
References
- 1.Fasano G, Pabon IM, Longhitano Y, et al. Pembrolizumab-Related side effects: acute renal failure and severe neurological toxicity. Medicina 2022;58:209. 10.3390/medicina58020209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf 2020;19:479–88. 10.1080/14740338.2020.1738382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Nebhan CA, Moslehi JJ, et al. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol 2022;19:254–67. 10.1038/s41571-022-00600-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng S, Coward J, McCaffrey E, et al. Pembrolizumab-Induced encephalopathy: a review of neurological toxicities with immune checkpoint inhibitors. J Thorac Oncol 2017;12:1626–35. 10.1016/j.jtho.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 5.Makri OE, Dimitrakopoulos F-I, Tsapardoni F, et al. Isolated optic neuritis after pembrolizumab administration for non-small-cell lung carcinoma. Int J Neurosci 2022;132:643–8. 10.1080/00207454.2020.1831489 [DOI] [PubMed] [Google Scholar]
- 6.Zimmer L, Goldinger SM, Hofmann L, et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer 2016;60:210–25. 10.1016/j.ejca.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 7.Vickers ML, Seidl B, Bigby K, et al. Inflammatory myeloradiculitis secondary to pembrolizumab: a case report and literature review. Case Rep Oncol Med 2020;2020:1–5. 10.1155/2020/8819296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen A, Christensen SJ, Scheie D, et al. Neuromuscular adverse events associated with anti-PD-1 monoclonal antibodies: systematic review. Neurology 2019;92:663–74. 10.1212/WNL.0000000000007235 [DOI] [PubMed] [Google Scholar]
- 9.Wang A, Xu Y, Fei Y, et al. The role of immunosuppressive agents in the management of severe and refractory immune-related adverse events. Asia Pac J Clin Oncol 2020;16:201–10. 10.1111/ajco.13332 [DOI] [PubMed] [Google Scholar]
- 10.Hartung H-P. Advances in the understanding of the mechanism of action of IVIg. J Neurol 2008;255 Suppl 3:3–6. 10.1007/s00415-008-3002-0 [DOI] [PubMed] [Google Scholar]