Abstract
Astragalus membranaceus Bunge is widely used in Traditional Chinese Medicine to treat various cancers. Astragaloside-IV (AS-IV) is one of the major compounds isolated from A. membranaceus Bunge and has been demonstrated to have antitumor effects by inhibiting cell proliferation, invasion and metastasis in various cancer types. Numerous studies have used in vitro cell culture and in vivo animal models of cancer to explore the antitumor activities of AS-IV. In the present study, the antitumor effects and mechanisms of AS-IV reported in studies recorded in the PubMed database were reviewed. First, the antitumor effects of AS-IV on proliferation, cell cycle, apoptosis, autophagy, invasion, migration, metastasis and epithelial-mesenchymal transition processes in cancer cells and the tumor microenvironment, including angiogenesis, tumor immunity and macrophage-related immune responses to cancer cells, were comprehensively discussed. Subsequently, the molecular mechanisms and related signaling pathways associated with antitumor effects of AS-IV as indicated by in vitro and in vivo studies were summarized, including the Wnt/AKT/GSK-3β (glycogen synthase kinase-3β)/β-catenin, TGF-β/PI3K/AKT/mTOR, PI3K/MAPK/mTOR, PI3K/AKT/NF-κB, Rac family small GTPase 1/RAS/MAPK/ERK, TNF-α/protein kinase C/ERK1/2-NF-κB and Tregs (T-regulatory cells)/IL-11/STAT3 signaling pathways. Of note, several novel mechanisms of Toll-like receptor 4 (TLR4)/NF-κB/STAT3, pSmad3C/3L, nuclear factor erythroid 2-related factor (NrF2)/heme oxygenase 1, circDLST/microRNA-489-3p/eukaryotic translation initiation factor 4A1 and macrophage-related high-mobility group box 1-TLR4 signaling pathways associated with the anticancer activity of AS-IV were also included. Finally, the limitations of current studies that must be addressed in future studies were pointed out to facilitate the establishment of AS-IV as a potent therapeutic drug in cancer treatment.
Keywords: astragaloside-IV, antitumor, apoptosis, proliferation, metastasis, migration, tumor microenvironment, signaling pathway
1. Introduction
Cancer is the second leading cause of death worldwide. It is estimated that 19.1 million new cancer cases are reported annually and 13 million cancer-related deaths will occur in 2030 (1). Due to the frequent cancer recurrence and metastasis, there is an urgent requirement to explore novel therapeutic agents and molecular mechanisms for precision-targeted cancer therapy (2). While cancer pathogenesis and the mechanisms of progression remain to be fully elucidated, it is known that the proliferation, invasion, migration and metastasis of cancer cells are associated with apoptosis, autophagy, epithelial-mesenchymal transition (EMT) and immune tolerance of cancer cells. Therefore, induction of apoptosis, suppression of invasion and metastasis and enhancing autophagy and the tumor microenvironment (TME) have been proposed as cancer treatment approaches (3,4).
Conventional cancer treatments include surgery, chemotherapy and radiation therapy. While these treatment options may kill or eradicate tumor cells, they damage surrounding normal cells, resulting in adverse effects on patients. Therefore, there is a requirement to develop effective anticancer treatments with fewer adverse effects on patients. Another treatment option is immunotherapy, which has advantages due to its use of specific antibodies or chimeric antigen receptor-engineered T-cells (CART-cell) to specifically target tumor antigens on the cancer cell surface. However, its use is limited to a small number of cancers, including blood cancers such as lymphoma (5).
Traditional Chinese Medicine (TCM) has long been used as an anticancer agent to inhibit tumor growth, regulate the TME and cancer immunity, and improve the efficacy of chemotherapeutic drugs such as cisplatin to reduce tumor recurrence and metastasis (6,7). TCM has the advantages of multiple targets and minimal side effects compared to pharmaceutical treatments. TCM decoctions, single TCM herbs and monomer TCM-derived compounds have exhibited anticancer effects in experimental animal tumor models and clinical trials (8,9). Numerous TCMs have been found to increase tumor cell apoptosis and autophagy, and inhibit their proliferation, angiogenesis, EMT, invasion, migration and metastasis (10,11). Certain TCM decoctions or their components have been indicated to improve the efficacy of cancer chemotherapy, radiation therapy or immunotherapy (12).
Astragalus membranaceus Bunge has been used in TCM for >2,000 years and it is growing mainly in Shanxi, Inner Mongolia, Gansu and other Chinese provinces of China, Korea and Mongolia. The dried root of A. membranaceus Bunge is known by its Chinese name Huangqi and is used in TCM herbal decoctions for treating various diseases. There are two botanical sources of A. membranaceus Bunge: A. membranaceus Bunge and A. membranaceus Var. mongholicus Bunge. Further details of these two botanical sources may be found in Plants of World Online (13). While the composition of A. membranaceus Bunge varies by region and country, its major components include astragalus polysaccharides, saponins and flavonoids. It also contains other constituents, such as amino acids, trace elements (e.g. Fe, Zn, Se, Mn, Co and Cu) and active small molecules such as riboflavin, chlorogenic acid, folic acid and sterols (14). These components have different biological activities, such as anti-inflammatory, antioxidant, antitumor, antiviral and improvement of cardiovascular functions (15,16).
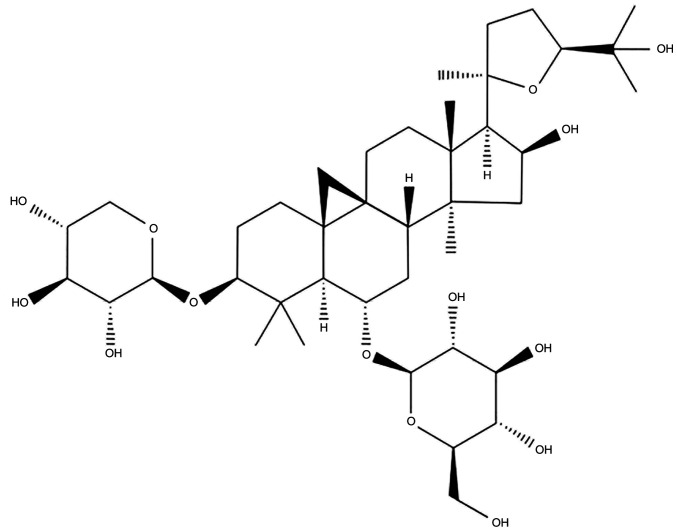
Studies have indicated that saponins are the primary constituents responsible for the antitumor activity of A. membranaceus Bunge (17,18). Astragaloside-IV (AS-IV; 3-O-β-D-xylopyranosyl-6-O-β-D-glucopyranosyl-cycloastragenol) is one saponin extracted from A. membranaceus Bunge. As a major active component of A. membranaceus Bunge, AS-IV has been found to have multiple biological functions, including antitumor, anti-oxidation, immune regulation and anti-inflammatory. The chemical structure of AS-IV is presented in Fig. 1 (19–21). The antitumor effects of A. membranaceus Bunge and its component AS-IV have been assessed in different cancer types, including lung, colon, liver, gastric and breast cancers, through in vitro cultured cancer cell lines and in vivo animal models with xenografted tumors (22,23). Accumulating evidence suggests that the antitumor effects of AS-IV are mainly conferred via mechanisms that increase apoptosis and autophagy, inhibit cell proliferation, invasion, migration and metastasis, and regulate the TME, including angiogenesis and innate and acquired immunity (10,23). The present review focuses on recent progress made regarding the antitumor effects in various cancer types and the antitumor mechanisms and related signaling pathways of AS-IV identified by in vitro and in vivo studies, and proposes potential applications of AS-IV in antitumor therapy.
Figure 1.
Chemical structure of AS-IV. AS-IV has the molecular formula C41H68O14 and molecular weight of 794.97 g/mol. AS-IV, astragaloside-IV.
2. Inhibitory effects of AS-IV on growth and proliferation of cancer cells
Apoptosis induction and cell cycle arrest in cancer cells
Cell proliferation and apoptosis are crucial cellular processes for cancer pathogenesis. Apoptosis is a continuous cascade to induce cell death that is usually regulated by extrinsic and intrinsic signaling pathways and characterized by morphological and molecular changes in the cancer cells (Fig. 2). In each cellular apoptotic pathway, different caspases are ubiquitously activated to induce apoptosis, of which caspase 3, 8 and 9 are the primary upregulated caspases. In addition, certain anti-apoptotic proteins, such as B-cell lymphoma 2 apoptosis regulator (BCL2) and B-cell lymphoma-extra large (BCL-xl) are downregulated (24,25).
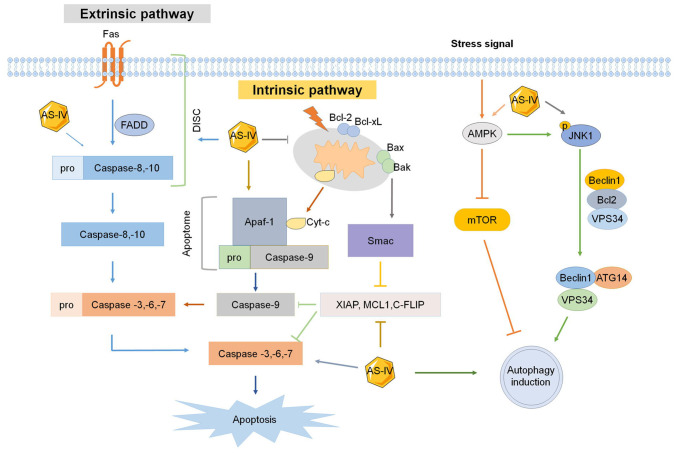
Figure 2.
Effect of AS-IV on apoptosis and autophagy. Extrinsic and intrinsic signaling pathways of apoptosis and autophagy are all affected by AS-IV. XIAP, X-linked mammalian inhibitor of apoptosis; MCL1, myeloid cell leukemia 1; c-FLIP, cellular FLICE-like inhibitory protein; SMAC, second mitochondria-derived activator of caspases; AS-IV, astragaloside-IV; Apaf-1, apoptotic peptidase activating factor 1; JNK1, c-Jun N-terminal kinase 1; FADD, Fas-associated protein with death domain; DISC, death-inducing signaling complex; BCL2, B-cell lymphoma 2 apoptosis regulator; BCL-xl, B-cell lymphoma-extra large; BAX, BCL2-associated X apoptosis regulator; Bak, BCL2 antagonist/killer 1; AMPK, adenosine monophosphate-activated protein kinase; VPS34, vacuolar protein sorting 34; ATG14, autophagy related 14.
The extrinsic apoptotic pathway is activated via dimerization of cell surface receptors such as Fas (CD95/Apo1), tumor necrosis factor receptors (TNFRs) and TNF-related apoptosis-inducing ligand receptors, which are bound by specific ligands. When cells are stimulated by death signals, the cytoplasmic domains of death receptors recruit death adaptor proteins, such as Fas-associated protein with death domain (FADD) and TNFR1-associated death domain, which then activate pro-caspase 8 and pro-caspase 10 and form death-inducing signaling complex (DISC) to initiate apoptosis. The activated pro-caspase 8 and pro-caspase 10 in the DISC are then directly cleaved to induce cleavage of pro-caspase 3, pro-caspase 6 and pro-caspase 7 to form caspase 3, caspase 6 and caspase 7, respectively, leading to cell apoptosis.
The intrinsic apoptotic pathway is initiated by inducing mitochondria to release cytochrome c into the cytoplasm. The apoptotic peptidase activating factor 1 and pro-caspase 9 are recruited to the cytoplasm to form the apoptosome with cytochrome c, which then activates apoptosis effector proteins caspase 3, 6, 7 and 9, resulting in apoptosis (Fig. 2). The intrinsic apoptotic pathway is usually regulated by the mitochondrial apoptotic proteins, including pro-apoptotic proteins such as BCL2-associated X apoptosis regulator (BAX), BCL2 antagonist/killer 1, BID, BCL2-interacting killer, BCL2-like 11 (BCL2L11; also known as BIM) and phorbol-12-myristate-13-acetate-induced protein 1 (also known as NOXA), as well as anti-apoptotic proteins such as BCL2, BCL-xL, BCL2L2 (also known as BCL-W), BCL2-related protein A1 (also known as BFL1), Diablo IAP-binding mitochondrial protein (also known as SMAC), X-linked inhibitor of apoptosis (XIAP), MCL1 apoptosis regulator BCL2 family member (MCL1) and caspase 8 and FADD-like apoptosis regulator (also known as CFLIP) (26,27). Therefore, inducing apoptosis in cancer cells via drugs targeting each apoptotic pathway is a promising approach in anticancer therapy.
AS-IV was indicated to suppress cell proliferation in three non-small cell lung cancer (NSCLC) cell lines, A549, NCIH1299 and HCC827. The expression of anti-apoptotic BCL2 was decreased in AS-IV-treated cells, while the expression of BAX and caspase 3 was increased, indicating that AS-IV induced apoptosis of NSCLC and osteosarcoma cells via the intrinsic pathway (28,29). In addition, when colorectal cancer (CRC) SW620 and HCT116 cell lines were treated with AS-IV, their growth was significantly inhibited. Flow cytometry indicated that these cells mainly exhibited G0/G1 phase arrest after being treated with AS-IV. The mRNA and protein levels of cyclin D1 and cyclin-dependent kinase 4 (CDK4) were markedly decreased during G0/G1 arrest (30). Another study determined that AS-IV caused cell cycle arrest in G0 phase with increased expression of the tumor suppressor CDK inhibitor 1A (also known as P21). An in vivo study suggested that AS-IV inhibited CRC cell proliferation and tumor growth in a xenograft CRC mouse model. Cytochrome c and HtrA serine peptidase 2 (also known as OMI) were released from mitochondria into the cytoplasm, promoted by AS-IV, suggesting that AS-IV has antitumor effects via the intrinsic apoptosis pathway. Further analysis indicated that the BAX/BCL2 ratio and expression levels of caspase 3 and caspase 9 were upregulated by AS-IV (Fig. 2) (23).
A recent study found the viability and proliferation of SK-Hep1 and Hep3B cells of hepatocellular carcinoma (HCC) cells to be significantly reduced after treatment with different doses of AS-IV. Western blots indicated that AS-IV activated the extrinsic and intrinsic apoptotic pathway proteins caspase 3, 8 and 9. In addition, the levels of the anti-apoptotic proteins XIAP, MCL1, CFLIP and baculoviral IAP repeat containing 5 (BIRC5/Survivin) were all reduced by AS-IV in both SK-Hep1 and Hep3B cells. This study suggested that AS-IV suppresses HCC cell proliferation and growth via both the extrinsic and intrinsic apoptotic pathways (Fig. 2) (31).
Suppressing tumor cell growth by activation of autophagy
Autophagy is the self-phagocytosis of cytoplasmic organelles to degrade misfolded proteins, damaged organelles or cancer cells. Autophagy frequently occurs through the fusion of the endoplasmic reticulum (ER) and mitochondria, and its activation is mainly through the adenosine monophosphate (AMP)-activated protein kinase (AMPK)-mammalian target of rapamycin (mTOR) pathway and mitogen/activated protein kinase 8 (MAPK8; also known as JNK1)-phosphatidylinositol 3-kinase (PI3K) catalytic subunit type 3 (PIK3C3; also known as VPS34) pathways (32,33). Several molecular complexes are involved in autophagic processes, including the JNK1, autophagy-related 13 like (ATG13L), ATG101, VPS34 complex-PI3K regulatory subunit 4 (PIK3R4; also known as VPS15), PIK3C3/VPS34, Beclin 1 (BECN1), ATG14 and ubiquitin-conjugation proteins-ATG5, ATG12 and ATG16L1 (Fig. 2) (34). Certain autophagy-related proteins such as sequestosome 1 (also known as P62), microtubule-associated protein 1 light chain 3 (LC3) unlipidated (LC3-I) and lipidated (LC3-II) forms, and BECN1 are usually affected in cancer cells. P62 is responsible for recognizing ubiquitin-labeled substrates targeted for autophagy. LC3 is a major effector of autophagy and its conversion from LC3-I to LC3-II may activate autophagy (35).
Bevacizumab inhibits tumor growth of multiple cancer types, including lung adenocarcinoma, but its therapeutic efficacy is markedly decreased by drug resistance. A recent study suggested that AS-IV was able to improve Bevacizumab's antitumor efficacy in treating lung adenocarcinoma. They observed that the levels of the autophagy-related proteins P62, LC3I and LC3II in cells treated with AS-IV + Bevacizumab were significantly higher than those in cells only treated with Bevacizumab, indicating that AS-IV reduced drug resistance and improved the antitumor efficacy of Bevacizumab (Fig. 2) (36). Another study combined AS-IV with α-solanine, neferine and 2,3,5,6-tetramethylpyrazine (named as SANT) to significantly inhibit cancer cell proliferation and migration and enhance autophagy flux in the cultured breast cancer cell line MDA-MB-231. SANT increased the levels of the autophagy proteins ATG16L1 and ATG4D and ATG9B to promote autophagic activity (37).
Cisplatin is a classical antitumor drug widely used to clinically treat cancer patients. It was found that cisplatin leads to upregulation of autophagy-related proteins, such as BECN1 and LC3-II/I, and the ER stress-related proteins heat shock protein family A member 5 (also known as GRP78) and eukaryotic translation initiation factor 2α kinase 3 (also known as PERK), indicating that cisplatin increases autophagy and ER stress in NSCLC cells. AS-IV was reported to significantly increase chemosensitivity to cisplatin in the NSCLC cell lines A549, HCC827 and NCI-H1299. Combined treatment with cisplatin and AS-IV increased cell apoptosis and suppressed cell proliferation in A549 and H1299 cells, suggesting that AS-IV acts synergistically with cisplatin to produce stronger antitumor effects in NSCLCs. AS-IV may also enhance chemosensitivity to cisplatin in HCC. After co-treatment of HepG2 cells with cisplatin and AS-IV, the antitumor effects of cisplatin on cell proliferation and tumor growth were significantly increased in HepG2 cells and H22 tumor-bearing mice, respectively (38–40).
Inhibiting cancer cell invasion, migration and metastasis
Cancer cells not only proliferate rapidly, but also invade surrounding cells or tissues in the TME or migrate to near and distant organs via direct invasion or the circulatory system. In a previous study, flow cytometry was used to analyze cell growth and migration of NSCLC A459 cells treated with AS-IV, indicating that, in addition to inhibiting cell proliferation and promoting apoptosis, AS-IV reduced cell migration (28). The inhibitory effect of AS-IV on cancer cell invasion was also observed in SiHa cervical cancer cells using the Transwell migration assay. Western blot analysis suggested that the protein levels of transforming growth factor β1 (TGF-β1), N-cadherin (also known as CDH2) and vimentin (VIM) were significantly decreased, but E-cadherin (also known as CDH1) protein levels were increased in AS-IV-treated cells compared to untreated cells. These results indicated that AS-IV suppressed SiHa cell invasion and migration by downregulating TGF-β1, CDH2 and VIM expression and upregulating CDH1 expression. Tumor growth and metastasis were also lower in AS-IV-treated cells than those in the untreated control cells (22).
In addition, a transwell invasion assay suggested that numbers of invading SK-Hep1 and Hep3B cells were significantly reduced by AS-IV (31). Assessment of the invasion of cervical cancer cells treated with AS-IV indicated that it significantly reduced cancer cell invasion of HeLa and SiHa cells. Furthermore, an in vivo study in which mouse models with xenografted human cervical cancer were treated with AS-IV suggested that after 35 days of treatment, tumor volumes in the AS-IV-treatment group (25 mg/kg/day) were significantly lower compared with those in the PBS-control group. These findings indicated that AS-IV was able to inhibit invasion of cultured cancer cells and in in vivo animals with cancer xenografts (41).
Suppressing EMT in cancer pathogenesis
EMT is one mechanism for transforming normal epithelial cells into mesenchymal cells and then malignant cancer cells. In tumorigenesis, EMT can be initiated by growth factors such as TGF-β1 or epidermal growth factor receptor (EGFR) (42). Therefore, drugs inhibiting EMT have therapeutic potential in cancer treatment. The glycogen synthase kinase 3β (GSK-3β) inhibitor 1-Az was found to attenuate the decrease in cell migration, invasion and EMT, indicating that GSK-3β activity is required for promoting migration, invasion and EMT in GBM cells via AKT/GSK-3β signaling (43–45). AS-IV was found to inhibit the EMT process of gastric cells and glioma U251 cells by inhibiting the TGF-β1-induced PI3K/AKT/nuclear factor κB (NF-κB) pathway, indicating that AS-IV may have therapeutic potential for treating gastric cancer (GC) (46,47). AS-IV was also found to suppress phosphorylation of P38, MAPK, PI3K, AKT and mTOR in cultured SiHa cervical cancer cells. Further analysis indicated that AS-IV suppressed the invasion and metastasis of cervical cancer by interfering with the EMT (Fig. 3) (22).
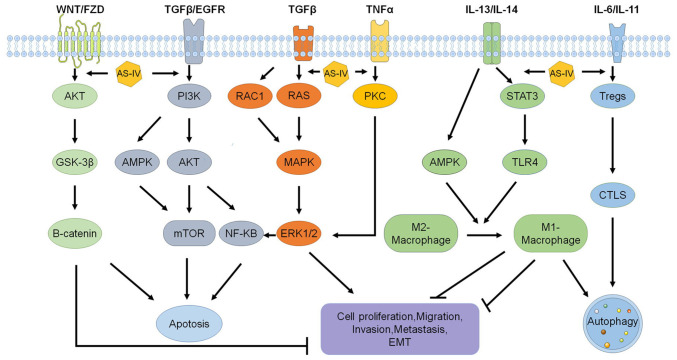
Figure 3.
Molecular mechanisms and signaling pathways of AS-IV's antitumor effects. AS-IV increases apoptosis and autophagy and inhibits cell proliferation, invasion, migration, metastasis and EMT via signaling pathways including WNT/AKT/GSK-3β/CTNNB1, TGF-β/PI3K/AKT/mTOR, PI3K/MAPK/mTOR, PI3K/AKT/NF-κB, RAC1/RAS/MAPK/ERK, TNF-α/PKC/ERK1-2/NF-κB, IL-13/AMPK/TLR4 and Tregs/IL-11/STAT3. AS-IV, astragaloside-IV; Tregs, regulatory T cells; CTLS, cytotoxic T lymphocytes; TLR4, Toll-like receptor 4; EMT, epithelial-mesenchymal transition; GSK, glycogen synthase kinase; PKC, protein kinase C; AMPK, adenosine monophosphate-activated protein kinase; RAC1, Rac family small GTPase 1.
3. Inhibiting tumor growth by improving the TME, tumor immunity and immunotherapy and decreasing tumor angiogenesis
Improving the TME to suppress tumor growth
The TME contributes to tumor immunity, angiogenesis, tumor-associated macrophages (TAMs) with an M2 phenotype, fibroblast growth and microRNAs (miRNAs) that surround tumor cells. Macrophages are the most abundant immune cells in the TME and their polarization has a key role in suppressing tumorigenesis. A recent study indicated that AS-IV has inhibitory effects on interleukin (IL)-4/IL-13-induced macrophage M2 polarization. AS-IV decreased M2 macrophages to inhibit cell proliferation, invasion and migration in ovarian cancer cells. Further analysis suggested that AS-IV attenuated the expression of high-mobility group box 1 (HMGB1) and Toll-like receptor 4 (TLR4) in macrophages co-cultured with ovarian cancer cells and decreased the expression of M2 markers of TGF-β, matrix metallopeptidase 9 (MMP-9) and IL-10. Of note, exogenous expression of HMGB1 reduced the inhibitory efficacy of AS-IV against the macrophage M2 polarization-induced malignant potential in ovarian cancer cells. Therefore, AS-IV may alleviate ovarian cancer progression by regulating macrophage M2 polarization against ovarian cancer cells within the TME (48). Tumor fibroblast cells typically have roles in increasing tumor growth. In one study GC-associated fibroblasts (GCAF) were isolated and treated with different AS-IV concentrations. They indicated that AS-IV treatment markedly inhibited GCAF-induced proliferation, migration and invasion of cancer cells. Further analysis suggested that AS-IV treatment significantly upregulated miR-214 expression and downregulated miR-301a expression in GCAFs. These findings indicated that AS-IV is able to inhibit the pathological functions of GCAFs, suggesting that regulating the TME is a potential therapeutic approach for cancer treatment (49).
Inducing the transformation of M2-macrophages into M1-macrophages
Innate and adaptive immunities in the TME have a modulatory role in recognizing and killing tumor cells. Therefore, immune escape of cancer cells may promote tumor progression, invasion and metastasis. One of the key innate immune cells is the macrophage, which may promote or inhibit the development and progression of tumor cells. Macrophages are usually divided into M1 and M2 subgroups, which exert different functions. Activated M1 macrophages mainly fight invading pathogens and inhibit tumor growth, while activated M2 macrophages promote tumor progression (50).
Since the M2 macrophages are found in most tumors, decreasing their numbers or transforming them into M1 macrophages is an important immunotherapeutic strategy for cancer treatment. Certain studies have used small molecules to inhibit different receptors, tyrosine kinases or other important transduction pathways in TAMs to induce the M2 to M1 transformation and suppress tumor growth (51–53). Another study used IL-1 and IL-4 to transform monocytes into M2 macrophages, collected the conditioned medium from M2 macrophages (M2-CM) and applied it to A549 and H1299 lung cancer cell lines treated with AS-IV. They observed that AS-IV significantly inhibited IL-13- and IL-4-induced production of M2 macrophages by reducing the expression of cluster of differentiation 206 (CD206) in M2 macrophages. AS-IV was also indicated to suppress the M2-CM-induced proliferation and invasion of A549 and H1299 cells (54). One study investigated the effect of AS-IV on CRC by subcutaneously injecting the CRC cell line CT26 into the mice to create a xenografted CRC mouse model. They found that after AS-IV was administered to mice with tumors, the proliferation of CT26 cell-derived tumors in vivo was suppressed by reducing M2 macrophages and increasing M1 macrophages. AS-IV was observed to induce apoptosis of CT26 cells in vitro and inhibit transplanted tumor growth and cell proliferation by inducing M2 macrophage transformation into M1 macrophages. AS-IV also decreased the production of anti-inflammatory factors such as TGF-β, IL-10 and vascular endothelial growth factor A (VEGF-A) and increased the production of pro-inflammatory factors such as interferon (IFN)-γ, IL-12 and TNF-α to exert its antitumor activity via related signaling pathways (Fig. 3) (55).
Activating cytotoxic T lymphocytes (CTLs)
The adaptive antitumor immunity is mainly mediated by CTLs and regulatory T cells (Tregs). Tregs are characterized by the expression of surface markers CD4 and CD25 and forkhead box P3 and have been proposed as a key component of acquired tolerance to tumors. CD8+ CTLs are activated by Tregs and immune-suppressive cytokines to kill tumor cells (56). It has been indicated that AS-IV is able to induce antitumor effects by regulating the activities of immune cells. A tryptophan-catabolizing enzyme, indoleamine 2,3-dioxygenase (IDO), was found to induce immune tolerance and be involved in tumor escape from host immune surveillance (57). Another study established a xenograft lung cancer model by implanting IDO-overexpressing murine lung carcinoma cells into C57BL/6 mice, which were treated with AS-IV, and their Tregs and CTLs in splenetic mononuclear cells were isolated for analysis. They found that AS-IV was able to downregulate Tregs and upregulate CD8+ CD28+ CTLs in vivo and in vitro by blocking IDO-induced immune escape and inhibiting tumor growth (58) (Fig. 3).
Improving immunotherapy by decreasing expression of programmed death-ligand 1 (PD-L1) and pro-inflammatory factors IFN-γ, IL-12 and TNF-α
AS-IV was found to improve immunotherapy and suppress angiogenesis. A study investigated the anticancer effects of AS-IV by assessing the proliferation, EMT and angiogenesis of the GC cell lines SGC7901 and MGC803 treated with AS-IV, transfected with an miR-195-5p inhibitor or a pcDNA3.1 vector expressing PD-L1. They found that AS-IV inhibited EMT and angiogenesis in GC cells and that PD-L1 was a potential target of miR-195-5p. Downregulation of miR-195-5p or upregulation of PD-L1 eliminated the inhibitory effect of AS-IV on EMT and angiogenesis on GC cells. Therefore, AS-IV inhibited EMT and angiogenesis in GC cells via upregulation of miR-195-5p and reduction of PD-L1 expression, indicating the therapeutic potential of AS-IV on GC cells via miR-195-5p-mediated regulation of PD-L1. AS-IV treatment was also observed to increase the chemosensitivity of CRC cells to cisplatin (12,59–61). One study explored the therapeutic mechanisms of AS-IV in CRC by injecting CT26 cancer cells into mice and assessing the effects of AS-IV on immunological functions based on macrophage markers, inflammatory factors and cytokines in tumors. They found that AS-IV induced apoptosis and decreased proliferation of CT26 cells in vitro and significantly decreased the transformation of M1 macrophages into M2 macrophages. In a mouse tumor model, AS-IV suppressed tumor growth and decreased anti-inflammatory factors such as TGF-β, IL-10 and VEGF-A, but promoted the infiltration of pro-inflammatory factors such as IFN-γ, IL-12 and TNF-α into transplanted tumor tissues. Of note, combined treatment of AS-IV and PD-1 produced a synergistic antitumor effect by inhibiting tumor growth and increasing T-cell infiltration. Therefore, in combination with immune checkpoint inhibitors, AS-IV may achieve improved antitumor effects in several cancer types (55).
Suppressing tumor angiogenesis to prevent tumor growth
Tumor angiogenesis is a growth abnormality in malignant tumors to maintain vascular supply and provide essential nutrients and oxygen to proliferating cancer cells. Due to the low-oxygen TME, angiogenesis is triggered by hypoxia to release multiple growth factors via hypoxia-induced factors by cancer cells, fibroblasts and macrophages, which are recruited to tumors (62). Therefore, blocking vascular supply or inhibiting the function of blood vessel receptors with pharmacological drugs has been proposed to promote tumor starvation and induce cell death in cancer treatment (63). A study indicated that AS-IV inhibited angiogenesis in mouse models with xenografted tumors treated with AS-IV by reducing protein levels of heparin-binding epidermal growth factor (EGF)-like growth factor, thrombospondin 2, amphiregulin, leptin, cellular communication network factor 3 (also known as insulin-like growth factor binding protein 9), EGF, coagulation factor III and the pro and active forms of MMP-9 in tumors, and increasing protein levels of serpin family E member 1 (also known as PAI-1) and platelet factor 4 (37). Another study indicated that AS-IV significantly reduced tumor weight and inhibited tumor microvessel formation in a nude mouse model of human HCC. They observed that the expression of angiogenesis-related factors such as fibroblast growth factor 2, VEGF, hepatocyte growth factor, transferrin and coagulation factor VII was decreased by AS-IV, indicating that the antitumor effects of AS-IV are mediated via decreased microvessel count and expression of angiogenic- and thrombosis-related factors (19).
The anticancer effects of AS-IV in different cancer cell lines in vitro and different cancer models in vivo are summarized in Tables I and II.
Table I.
Antitumor effects of AS-IV and related mechanisms based on in vitro studies.
| Author, year | Cancer type | Cell line | Concentration and duration | Anticancer effects | Molecular target/biomarker | (Refs.) |
|---|---|---|---|---|---|---|
| Zhang, 2019 | Cervical cancer | SiHa | 0.78125-800 µg/ml for 24 h | ↓Invasion, metastasis; ↓migration | TGF-β1/PI3K and TGF-β1/MAPK, p38 | (22) |
| Sun, 2019 | Colorectal cancer | HT29 and SW480 | 0–40 µg/ml for 24 h | ↓Growth, cell cycle arrest; ↑apoptosis | Bax/Bcl-2, Cyt C and Om, PARP, cleaved caspase-3 and cleaved caspase-9 | (23) |
| Jia, 2019 | Lung cancer | HCC827, A549 and NCI-H1299 | 0–24 ng/ml for 48 h | ↑Cell death; ↓migration | Bcl-2 and Bax, Akt/GSK-3β/β-catenin | (28) |
| Wang, 2018 | Colorectal cancer | SW620 and HCT116 | 50 and 100 ng/ml for 24,48 and 72 h | ↓Cell proliferation, G0/G1 cell cycle arrest | Cyclin D1 and CDK4, B7-H3 | (30) |
| Su, 2020 | Liver cancer | SK-Hep1 and Hep3B | 0–400 µM for 48 h | ↓Proliferation; ↑G1 phase arrest; ↑apoptosis; ↓invasion | Caspase-8,9, XIAP, MCL1, C-FLIP | (31) |
| Lai, 2020 | Lung cancer | A549, H1299 | 0–16 ng/ml for 48 h | ↑Endoplasmic reticulum stress; ↑autophagy | GRP78 and Beclin1 | (38) |
| Xia, 2020 | Cervical cancer | HeLa and SiHa cells | 25 µM for 12 h | ↓Invasion; ↑autophagy | DCP1A and TMSB4X, MGST3, AKR1C2 and ERLIN1 | (41) |
| Zhu, 2018 | Gastric cancer | BGC-823 and MKN-74 | 0–40 µg/ml for 24,48 h | ↓Viability, EMT, invasion, migration | PI3K/Akt/NF-κB pathway | (46) |
| Han, 2020 | Glioma | U251 | 20–80 µg/ml for 48 h | ↓EMT, migration, invasion, proliferation | Wnt/β-catenin pathway | (47) |
| Wang, 2021 | Ovarian cancer | SKOV3 | 0.5–100 µg/ml for 24 h | ↓Cell viability, invasion, migration | HMGB1-TLR4 signaling | (48) |
| Wang, 2017 | Gastric cancer | BGC-823 | 10–40 µmol/l for 48 h | ↓Proliferation, migration, invasion | SOX2 and NANOG | (49) |
| Xu, 2018 | Lung cancer | A549 and H1299 | 80 nM for 48 h | ↓Growth, invasion, migration, angiogenesis; ↑M2-macrophages | AMPK | (54) |
| Liu, 2020 | Colorectal cancer | CT26 | 10–100 nM for 48 h | ↓Proliferation; ↑apoptosis | Promoting M2 macrophage polarization to the M1 phenotype | (55) |
| Zhang, 2014 | Lung cancer | 3LL-luc-IDO | 160 µg/ml for 24, 48 and 72 h | ↑Immune response, regulatory T cells; ↑CTLs | CD8+CD28+, indoleamine 2,3-dioxygenase | (58) |
| Wang, 2017 | Hepatic cancer | Bel-7402/FU | 0–100 µM for 24 h | ↓Cell viability, AP-1 DNA binding activity; reverses drug resistance | JNK/c-Jun/AP-1 signaling pathway | (60) |
| Xie, 2016 | Colorectal cancer | HCT116, SW480 | 1–15 ng/ml | ↓Viability; ↑sensitivity to cisplatin | NOTCH3 | (61) |
| Qin, 2017 | Hepatocellular carcinoma | MHCC97-H and Huh7 | 0–100 µg/ml for 24, 48 and 72 h | ↓Migration, invasion, EMT | Akt/GSK-3β/β-catenin | (66) |
| Zhao, 2019 | Vulvar squamous cell carcinoma | SW962 | 0–800 µg/ml for 24, 48 and 72 h | ↓Cell viability, proliferation; cell-cycle arrest; ↑apoptosis | Bax, Bcl-xl, Bcl-2 and cleaved-caspase 3, Beclin-1, LC3-B and P62, TGF-β/Smad | (69) |
| He, 2021 | Prostate cancer | LNCap and PC-3 | 0–20 µM for 72 h | ↑Carboplatin sensitivity | AKT/NF-κB signaling pathway | (71) |
| Li, 2017 | Glioma | U251 | 10–80 µg/ml for 24 h | ↓Proliferation, migration, invasion | MAPK/ERK | (75) |
| Jiang, 2017 | Breast cancer | MDA-MB-231 | 0–40 µg/ml for 24 h | ↓Viability, invasion | Vav3 mediated Rac1/MAPK signaling | (76) |
| Cheng, 2014 | Lung cancer | A549 | 0–90 µM for 24 h | ↓Viability, adhesion, migration, invasion | PKC-α-ERK1/2-NF-κB pathway | (77) |
| Min, 2022 | Liver cancer | Huh-7 | 0–150 µM for 24 or 48 h | ↓Proliferation, migration, invasion | TLR4/NF-κB/STAT3 | (81) |
| Li, 2022 | Gastric cancer | HGC-27 and MKN-45 | 0–40 µg/ml for 24 or 48 h | ↓Viability, cell proliferation, metastasis | circDLST/miR-489-3p/EIF4A1 | (83) |
| Cui, 2020 | Liver cancer | SMMC-7721 and Huh7 | 0–200 µg/ml for 24 h | ↑Apoptosis | miR-150-5p/β-catenin | (85) |
| Li, 2022 | Lung cancer | A549 | 0–100 ng/ml for 24 h | Increase sensitivity of bevacizumab; ↓autophagy | Akt/mTOR | (36) |
↓ indicates suppression; ↑ indicates activation. AS-IV, astragaloside-IV; PARP, poly-ADP ribose polymerase; Cyt C, cytochrome C; GRP78, glucose regulated protein 78; EIF4A1, eukaryotic translation initiation factor 4A1; circDLST, circRNA dihydrolipoamide S-succinyltransferase; AMPK, adenosine monophosphate-activated protein kinase; SOX2, SRY-box transcription factor 2; RAC1, Rac family small GTPase 1; PKC, protein kinase C; XIAP, X-linked mammalian inhibitor of apoptosis; MCL1, myeloid cell leukemia 1; c-FLIP, cellular FLICE-like inhibitory protein; JNK, C-Jun N-terminal kinase; BCL2, B-cell lymphoma 2 apoptosis regulator; BAX, BCL2-associated X apoptosis regulator; HMGB1, high mobility group box 1; TLR4, Toll-like receptor 4; GSK, glycogen synthase kinase; RAC1, Rac family small GTPase 1; DCP1A, decapping mRNA 1A; TMSB4, thymosin beta 4 X-linked; MGST3, microsomal glutathione S-transferase 3; AKR1C2, aldo-keto reductase family 1 member C2; miR, microRNA.
Table II.
Antitumor effects and related mechanisms of AS-IV based on xenografted animal studies.
| Author, year | Cancer type | Xenografted animal model | Formulation | Dose and duration | Anticancer effect | Molecular target/biomarker | (Refs.) |
|---|---|---|---|---|---|---|---|
| Zhang, 2017 | Liver cancer | HepG2 cell-bearing mice | AS-IV, curcumin, cisplatin and AS-IV+curcumin | 20 mg/kg/day for 21 days | ↓Tumor growth | miR-122 and miR-221 | (19) |
| Zhang, 2019 | Cervical cancer | SiHa cell-bearing mice | AS-IV, cisplatin and cisplatin+AS-IV | 120 mg/kg/day for 21 days | ↓Tumor growth, ↓invasion | TGF-β1-mediated PI3K and MAPK pathways | (22) |
| Sun, 2019 | Colorectal cancer | HT29 cell-bearing mice | AS-IV | 20 mg/kg for 30 days | ↓Tumor growth | PARP, p21 | (23) |
| Hu, 2017 | Osteosarcoma | 143B cell-bearing mice | AS-IV, cisplatin and AS-IV+cisplatin | 20 mg/kg/day for 28 days | ↓Tumor growth | Fas/FasL signaling | (29) |
| Xia, 2020 | Cervical cancer | SiHa cell-bearing mice | AS-IV | 25 mg/kg/day for 35 days | ↓Invasion | DCP1A and TMSB4X | (41) |
| Xu, 2018 | Lung cancer | LLC cell-bearing mice | AS-IV | 40 mg/kg/day for 21 days | ↓Tumor growth, ↓invasion, ↓invasion, ↓migration, ↓angiogenesis | AMPK signaling pathway | (54) |
| Liu, 2020 | Breast cancer | CT26 cell-bearing mice | AS-IV, αPD1 and AS-IV + αPD1 | 15 mg/kg/3 days for 9 days | ↓Tumor growth, ↑T cell infiltration, ↑M2 macrophage polarization | TGF-β, IL-10, VEGF-A, IFN-γ, IL-12 and TNF-α | (55) |
| Zhang, 2014 | Lung cancer | 3LL cell-bearing mice | AS-IV, 1-MT, paclitaxel | 40 mg/kg until death | ↓Tumor growth | IDO | (58) |
| He, 2021 | Prostate cancer | PC-3 cell-bearing mice | AS-IV, carboplatin and AS-IV+carboplatin | 40 mg/kg/day for 24 days | ↑Carboplatin sensitivity | AKT/NF-κB signaling pathway | (71) |
| Li, 2017 | Glioma | U251 cell-bearing mice | AS-IV | 20 mg/kg/day for 7 days | ↓Tumor growth | MAPK/ERK signaling pathway | (75) |
| Min, 2022 | Liver cancer | Huh-7 cell-bearing mice | AS-IV | 20–100 mg/kg/3 days for 40 days | ↓Tumor growth | TLR4/NF-κB/STAT3 signaling pathway | (81) |
| Li, 2022 | Gastric cancer | HGC-27 cell-bearing mice | AS-IV, circDLST | 40 mg/kg/day for 21 days | ↓Tumor growth | circDLST/miR-489-3p/EIF4A1 | (83) |
| Cui, 2020 | Liver cancer | SMMC-772cell-bearing mice | AS-IV, miR-150-5p | Only treatment of cells | ↓Tumor growth | miR-150-5p/β-catenin | (85) |
↓ indicates suppression; ↑ indicates activation. AS-IV, astragaloside-IV; PARP, poly-ADP ribose polymerase; TMSB4X, thymosin beta 4 X-linked; circDLST, circRNA dihydrolipoamide S-succinyltransferase; EIF4A1, eukaryotic translation initiation factor 4A1; IDO, indoleamine 2,3-dioxygenase; Fas, Fas cell surface death receptor; FasL, Fas ligand; TLR4, Toll-like receptor 4; AMPK, adenosine monophosphate-activated protein kinase; DCP1A, decapping mRNA 1A; miR, microRNA.
4. Antitumor mechanisms and related signaling pathways
The antitumor effects of AS-IV are involved in various aspects of cancer pathogenesis, including cell proliferation, invasion, migration, metastasis, immune responses and apoptosis. Numerous studies have investigated the molecular mechanisms, including the signaling pathways and target genes, by which AS-IV inhibits tumor growth. The major signaling pathways involved in the antitumor effects of AS-IV are discussed below.
AKT/GSK-3β/β-catenin signaling pathway
Dysfunction of the AKT/GSK-3β/β-catenin (CTNNB1) signaling pathway affects the proliferation, invasion and migration of various cancer cells (64). Activated AKT induces phosphorylation of GSK-3β, inhibiting apoptosis and inducing the nuclear translocation of CTNNB1, increasing the expression of EMT-associated transcription factors such as Twist family BHLH transcription factor 1, zinc finger E-box binding homeobox 1 and Snail family transcriptional repressor 1. GSK-3β was found to promote tumor proliferation and growth via the AKT pathway in NSCLCs (65).
A study indicated that AS-IV suppressed the migration and invasion of HCC cells. In addition, the morphologies of HCC cells were altered after treatment with AS-IV. Furthermore, the levels of phosphorylated AKT (pAKT), GSK-3β and CTNNB1 were decreased in AS-IV-treated HCC cells, indicating that AS-IV suppressed the growth, invasion and migration of HCC cells by targeting the AKT/GSK-3β/CTNNB1 pathway (66). When the A549 NSCLC cells were treated with AS-IV at concentrations of 12 and 24 ng/ml for 48 h, the phosphorylation of AKT, GSK-3β and CTNNB1 was significantly inhibited, suggesting that AS-IV also suppressed lung cancer progression by regulating the AKT/GSK-3β/CTNNB1 pathway (Fig. 3) (28).
The TGF-β1-mediated PI3K/AKT/mTOR pathway and PI3K/MAPK pathway
The PI3K/AKT pathway is a common pathway regulating the proliferation, apoptosis, metastasis and EMT of cancer cells, which typically express high levels of PI3K and AKT and have high levels of pAKT. Therefore, inactivation or inhibition of the PI3K/AKT pathway may decrease cancer cell proliferation and invasion. Indeed, inhibition of the PI3K/AKT pathway increased apoptosis and inhibited metastasis of cancer cells (67). Several studies indicated that Astragalus radix and AS-IV components exert antitumor effects by suppressing the PI3K/AKT pathway (68). Another study investigated the mechanism of action of AS-IV in the BGC-823 and MKN-74 cell lines, examining the protein levels of pAKT, AKT, P65 and phosphorylated P65 (pP65) by western blot after treatment with AS-IV with or without 10 ng/ml of TGF-β1. They found that the pAKT/AKT and pP65/P65 ratios were increased by TGF-β1 in BGC-823 and MKN-74 cells. However, this upregulation was attenuated by AS-IV, indicating that AS-IV suppresses TGF-β1-induced activation of the PI3K/Akt/NF-κB signaling pathway, inhibiting GC cell proliferation and growth (46). In addition, AS-IV was also found to reduce the proliferation of SW962 cells of vulvar squamous cell carcinoma (VSCC) cells and induced cell-cycle arrest in G0/G1 phase by upregulating P53 and P21 expression, and downregulating cyclin D1 expression. Since TGF-β1 stimulated cell proliferation, AS-IV treatment decreased the expression of TGF-βRII and Smad4 in the SW962 cell line of VSCC. Thus, AS-IV may inhibit cell proliferation through the TGF-β/Smad signaling pathway in VSCC (69).
Another study investigated the effects of AS-IV on tumor growth in vivo using xenograft tumor models created by injecting the breast cancer cell line MCF7 subcutaneously into nude mice. After treating mice with Sanhuang decoction, whose main component is AS-IV, for four weeks, it was found to significantly inhibit grafted tumor growth. Furthermore, the expression of PI3K, AKT and mTOR was significantly decreased, indicating that Sanhuang decoction/AS-IV inhibited cancer growth via the PI3K/AKT/mTOR pathway (70). In addition, AS-IV was reported to increase chemosensitivity to carboplatin in the treatment of prostate cancer in cultured cells and tumor xenograft through interfering with AKT/NF-κB signaling to suppress EMT, which was induced by carboplatin (71). The TGF-β1-mediated PI3K/MAPK signaling pathway is also involved in the invasion and migration of cancer cells (72). One study assessed the effect of AS-IV on the PI3K and MAPK pathways in cultured SiHa cervical cancer cells, finding that AS-IV suppressed the phosphorylation of P38, MAPK, PI3K, AKT and mTOR. Therefore, AS-IV may inhibit the invasion of cancer cells, likely via the MAPK and PI3K pathways (Fig. 3) (22).
The MAPK/ERK/NF-κB signaling pathway
The MAPK/rat sarcoma virus (RAS)/ERK signaling pathway regulates cell proliferation, apoptosis and invasion by phosphorylating related targets in tumorigenesis. The RAS/MAPK kinase/ERK pathway is the most important signaling cascade of all MAPK pathways, regulating tumor cell survival and development (73,74). This pathway is interrupted in various cancers. Glioma is a common primary brain tumor aggressively invading the central nervous system. After glioma U251 cells were treated with AS-IV, their proliferation and invasion were decreased in a dose-dependent manner. In addition, a wound-healing assay indicated that U251 cell migration was significantly delayed by AS-IV (75). When they explored the underlying molecular mechanisms, they indicated that AS-IV significantly decreased the phosphorylation of MAPK and ERK, leading to the inactivation of downstream targets of the MAPK/ERK cascade, such as the MYC proto-oncogene BHLH transcription factor, which was inhibited by AS-IV, indicating that AS-IV inactivated the MAPK/ERK signaling pathway to prevent glioma cell invasion and migration (Fig. 3) (75).
The Rac family small GTPase 1 (RAC1)/MAPK/ERK signaling pathway
AS-IV was found to inhibit the proliferation and invasion of MDA-MB-231 cells and suppress breast tumor growth and metastasis to the lungs. Its antitumor activity reflected the inactivation of ERK1/2 and JNK, downregulation of MMP-2 and MMP-9, decreased levels of activated RAC1 and inhibition of RAC1/MAPK signaling, indicating the potential of AS-IV to treat metastatic breast cancer (76).
The protein kinase C (PKC)-α/ERK1-2/NF-κB signaling pathway
The migration and invasion ability of A549 lung cancer cells was suppressed by AS-IV under in vitro culture conditions. In addition, MMP-2, MMP-9 and integrin β1 expression was found to be significantly decreased, but CDG1 expression was significantly increased by AS-IV treatment. Further analysis indicated that AS-IV significantly decreased TGF-β1, TNF-α and IL-6 expression. Of note, the PKC pathway inhibitor AEB071, ERK inhibitor U0126 or NF-κB inhibitor PDTC were able to attenuate AS-IV suppression of cell invasion and migration, indicating that AS-IV inhibits migration and invasion of human lung cancer A549 cells by regulating the PKC-α/ERK1-2/NF-κB pathway (Fig. 3) (77).
Immune regulatory signaling pathways in cancer
Antitumor immunity is typically mediated by macrophages engulfing cancer cells or by CTLs or NK cells killing the cancer cells. Tumor growth and progression are typically suppressed by M1 macrophages, while M2-polarized macrophages have been reported to promote tumor invasion, migration and metastasis and can activate tumor-promoting genes in cancer cells (78). B7-H3 expression is upregulated in various cancers, inhibiting CTLs and inducing immune escape of cancers (79). After treatment with AS-IV, B7-H3 expression was decreased, suppressing the proliferation of colorectal cell lines SW620 and HCT116. Further analysis indicated that AS-IV was able to suppress CD276 (also known as B7-H3) expression by inducing the expression of miR-29c. These anticancer effects of AS-IV may be mediated by CTLs via the B7-H3/NF-κB/CCND1 signaling pathway (Fig. 3) (30).
AS-IV has also been shown to induce immunity against cancer as an adjunct (80). Treatment of the cancer cell lines A549 and H1299 with M2-CM promoted their migration ability, but M2-CM combined with AS-IV significantly reduced their migration in wound-healing assays, indicating that AS-IV inhibited cancer cell migration. After AS-IV was administered to a lung tumor mouse model, the number of M2 macrophages decreased in the tumor tissue. Further analysis indicated that AS-IV induced the transformation of M2 macrophages into M1 macrophages mainly by inhibiting IL-13 and IL-4 and suppressing AMPKα activation in M2 macrophages, since silencing AMPKα partially attenuated the inhibitory effect of AS-IV on cancer cell proliferation and invasion (Fig. 3) (54).
5. Other novel anticancer mechanisms and related pathways
Suppressing M2 macrophage polarization via the TLR4/NF-κB/signal transducer and activator of transcription 3 (STAT3) signaling pathway
Recently, a study reported that AS-IV suppressed HCC cell proliferation and invasion by inducing macrophage polarization. After macrophages were treated with AS-IV, CD206 levels in M2 macrophages were quantified by flow cytometry and the levels of pSTAT3, pNF-κB and TLR4 in macrophages were determined. AS-IV was found to decrease the M2 macrophage numbers and pSTAT3, pNF-κB and TLR4 levels, suppressing HCC cell proliferation and invasion by regulating the TLR4/NF-κB/STAT3 signaling pathway. In addition, small interfering RNA targeting TLR4 inhibited Huh-7 cell proliferation, indicating that AS-IV inhibited HCC tumor growth by suppressing M2 macrophage polarization via the TLR4/NF-κB/STAT3 signaling pathway (Fig. 3) (81).
Regulation of the pSMAD3C/3L and NFE2-like BZIP transcription factor 2 (NFE2L2)/heme oxygenase 1 (HO-1) pathways
Recently, a study indicated that AS-IV decreased the growth and multiplicity of primary liver cancer in mouse models. After 20 weeks of AS-IV treatment, it was observed that AS-IV acted on the TGF-β1/SMAD and NFE2L2 (also known as NRF2)/HO-1 pathways, increasing the levels of pSMAD3C and pNRF2, HO-1 and NAD(P)H quinone dehydrogenase 1 (NQO1) and decreasing the levels of pSMAD2C, pSMAD2L and pSMAD3L, PAI-1 and smooth muscle α-actin in mice with liver cancer. In vitro analysis also confirmed that AS-IV regulated the levels of pSMAD3C, pSMAD3L, HO-1 and NQO1 via the pSMAD3C/3L and NRF2/HO-1 pathways in HSC-T6 and HepG2 cells, which was activated by TGF-β1. These findings suggest that AS-IV promotes a switch in SMAD3 signaling from pSMAD3L-mediated oncogenesis to pSMAD3C-mediated tumor suppression, increasing levels of pSMAD3C, inhibiting pSMAD3L and pSMAD2C, and promoting NRF2 phosphorylation via TGF-β1 signal mediation-specific pSMAD3 (Fig. 4) (82).
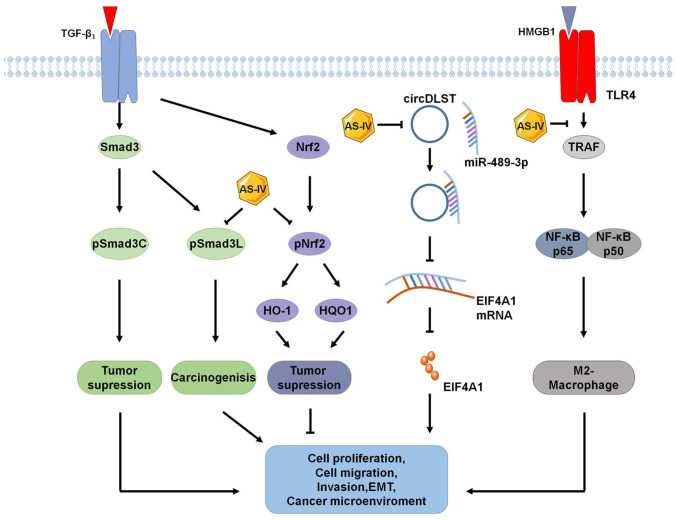
Figure 4.
Novel signaling pathways for the antitumor effects of AS-IV. Recently identified signaling pathways for the anticancer effects of AS-IV include pSMAD3C/3L, NRF2/HO-1, circDLST/miR-489-3p/EIF4A1 and HMGB1/TLR4, which inhibits M2-macrophage polarization. AS-IV, astragaloside-IV; TLR4, Toll-like receptor 4; HO-1, heme oxygenase 1; HQO-1, heme quinone oxidoreductase-1; EIF4A1, eukaryotic translation initiation factor 4A1; TRAF, tumor necrosis factor receptor-associated factor; pSMAD3C, phosphor-SMAD family member 3C; pSMAD3L, phosphor-SMAD family member 3L; HMGB1, high mobility group box 1; circDLST, circRNA dihydrolipoamide S-succinyltransferase.
Targeting the circDLST/miR-489-3p/eukaryotic translation initiation factor 4A1 (EIF4A1) pathway
Circular RNAs have been reported to have important roles in regulating malignant cancer progression. In most cancer cells, circRNA dihydrolipoamide S-succinyltransferase (circDLST) and EIF4A1 are highly expressed, while miR-489-3p expression is low. In the cultured GC cell lines HGC-27 and MKN-45 treated with AS-IV, it suppressed cell proliferation and metastasis by downregulating circDLST, increasing miR-489-3p expression and inhibiting EIF4A1 expression, preventing cancer progression. Conversely, inhibition of miR-489-3p decreased the antitumor activity of AS-IV. These findings indicated that AS-IV inhibits cancer cell growth and progression via a novel circDLST/miR-489-3p/EIF4A1 mechanism with circDLST-mediated downregulation of EIF4A1. AS-IV was also found to suppress the growth of HCC in vitro and in vivo by upregulating miR-150-5p and repressing β-catenin through a mechanism of miR-150-5p/β-catenin (83–85).
Suppressing the HMGB1/TLR4 signaling pathway to inhibit M2-macrophage polarization-induced cancer progression
AS-IV treatment suppressed M2 macrophage marker expression of CD206, C-C motif chemokine ligand 24 (CCL24), peroxisome proliferator-activated receptor γ (PPRAγ), arginase 1 and IL-10 in the ovarian cancer line SKOV3, indicating that AS-IV inhibits macrophage M2 polarization to improve the antitumor effect in the TME. In addition, AS-IV was indicated to inhibit the expression of HMGB1 and TLR4 and decrease the expression of M2 markers TGF-β, MMP-9 and IL-10 in co-cultures of ovarian cancer cells with macrophages. Blocking HMGB1 signaling suppressed M2 macrophage-induced ovarian cancer cell proliferation, invasion and migration, suggesting HMGB1 reversed the inhibitory effect of AS-IV on M2-macrophage polarization-induced malignant ovarian cancer cell progression. These findings indicate that AS-IV may protect against ovarian cancer cell progression by suppressing HMGB1/TLR4 signaling (Fig. 4) (48,86).
6. Conclusion and future perspectives
The antitumor effects of AS-IV have been demonstrated in vitro in cultured cancer cells and in vivo in animal cancer models at different stages of cancer progression. In each of these processes, AS-IV appears to increase tumor cell apoptosis and autophagy, inhibit tumor cell proliferation, invasion, migration and EMT, increase CTL immune activities and promote the transformation of M2 macrophages into M1 macrophages. The antitumor effects of AS-IV are mainly via upregulation or downregulation of different signaling pathways or various recently identified novel mechanisms. In addition, AS-IV increases the sensitivity of the cancer cells to cisplatin and other drugs, increasing chemotherapy and immunotherapy efficacy. These findings highlight the antitumor role of AS-IV and the potential of AS-IV as an antitumor drug or adjuvant for use in cancer therapy. However, current studies have their limitations and future priorities should be on developing and utilizing AS-IV as a new clinical drug. First, while several signaling pathways were found to be associated with the action of AS-IV, the direct targets and specific molecular networks of AS-IV for antitumor effects require to be further elucidated. In addition, the dose of AS-IV used in numerous studies varies greatly. Therefore, the safe and effective dose of AS-IV requires to be accurately established. Future studies should determine the effective doses of AS-IV to produce antitumor effects. Furthermore, to date, only a small number of studies have investigated the toxicity and the potential adverse effects of AS-IV in vivo and in vitro. Therefore, the toxicity and side effects of AS-IV should be studied to enable safer clinical applications.
Finally, while A. membranaceus Bunge or TCM decoctions containing A. membranaceus Bunge have been used in the clinic to treat cancer patients, no single AS-IV compound has been tested in the treatment of the patients. The main reason why a single compound AS-IV has not yet been investigated in clinical trials may be that the safe and effective dose, toxicity and potential adverse effects of AS-IV have not been determined accurately. This issue can probably be resolved by a biotech company to carefully design and perform in vitro and in vivo antitumor experiments of AS-IV based on the Food and Drug Administration guidelines for the registration of a new drug. Subsequently, clinical trials are required to further confirm the safety and efficacy of AS-IV in cancer patients.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the National Natural Science Foundation of China (grant nos. 81571241 and 82004402), Shandong Provincial Natural Science Foundation (grant no. ZR2019ZD39) and the Quancheng Talent Scholar Fund of Jinan City in Shandong of China (5150 Talent Plan, 2018 to FH).
Availability of data and materials
Not applicable.
Authors' contributions
FH conceived and designed this review. LZ, ML, ZC, JW, GZ and FH were major contributors in writing the manuscript and performed the literature search and selection. KC, JZ, LD, YL and CJ wrote sections of the manuscript and prepared the figures. All authors have read and approved the final manuscript. All authors are responsible for all aspects of the work and approved the submission in its current form. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Alexander M, Kim SY, Cheng H. Update 2020: Management of non-small cell lung cancer. Lung. 2020;198:897–907. doi: 10.1007/s00408-020-00407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghaznavi H, Shirvaliloo M, Zarebkohan A, Shams Z, Radnia F, Bahmanpour Z, Sargazi S, Saravani R, Shirvalilou S, Shahraki O, et al. An updated review on implications of autophagy and apoptosis in tumorigenesis: Possible alterations in autophagy through engineered nanomaterials and their importance in cancer therapy. Mol Pharmacol. 2021;100:119–143. doi: 10.1124/molpharm.121.000234. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Hu Y, Xiao W, Tian Z. Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol Immunol. 2021;18:2083–2100. doi: 10.1038/s41423-021-00732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ling CQ, Yue XQ, Ling C. Three advantages of using traditional Chinese medicine to prevent and treat tumor. J Integr Med. 2014;12:331–335. doi: 10.1016/S2095-4964(14)60038-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Lou Y, Wang J, Yu C, Shen W. Research status and molecular mechanism of the traditional Chinese medicine and antitumor therapy combined strategy based on tumor microenvironment. Front Immunol. 2021;11:609705. doi: 10.3389/fimmu.2020.609705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang K, Chen Q, Shao Y, Yin S, Liu C, Liu Y, Wang R, Wang T, Qiu Y, Yu H. Anticancer activities of TCM and their active components against tumor metastasis. Biomed Pharmacother. 2021;133:111044. doi: 10.1016/j.biopha.2020.111044. [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Zhong Z, Tan HY, Guo W, Zhang C, Tan CW, Li S, Wang N, Feng Y. Uncovering the anticancer mechanisms of Chinese herbal medicine formulas: Therapeutic alternatives for liver cancer. Front Pharmacol. 2020;11:293. doi: 10.3389/fphar.2020.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen T, Yang P, Jia Y. Molecular mechanisms of astragaloside-IV in cancer therapy (review) Int J Mol Med. 2021;47:13. doi: 10.3892/ijmm.2021.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu L, Zhao M, Liu Q, Cheng Z, Zou J, et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin Med. 2019;14:48. doi: 10.1186/s13020-019-0270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, Dai Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570. doi: 10.1016/j.biopha.2019.109570. [DOI] [PubMed] [Google Scholar]
- 13.Royal Botanic Gardens, Kew. https://mpns.science.kew.org/mpns-portal/?_ga=1.111763972.1427522246.1459077346 [Google Scholar]
- 14.Chang X, Chen X, Guo Y, Gong P, Pei S, Wang D, Wang P, Wang M, Chen F. Advances in chemical composition, extraction techniques, analytical methods, and biological activity of astragali radix. Molecules. 2022;27:1058. doi: 10.3390/molecules27031058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z, Lou Y, Kong M, Luo Q, Liu Z, Wu J. A systematic review of phytochemistry, pharmacology and pharmacokinetics on astragali radix: Implications for astragali radix as a personalized medicine. Int J Mol Sci. 2019;20:1463. doi: 10.3390/ijms20061463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang CH, Yang X, Wei JR, Chen NM, Xu JP, Bi YQ, Yang M, Gong X, Li ZY, Ren K, et al. Ethnopharmacology, phytochemistry, pharmacology, toxicology and clinical applications of radix astragali. Chin J Integr Med. 2021;27:229–240. doi: 10.1007/s11655-019-3032-8. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Qu L, Dong Y, Han L, Liu E, Fang S, Zhang Y, Wang T. A review of recent research progress on the astragalus genus. Molecules. 2014;19:18850–18880. doi: 10.3390/molecules191118850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong S, Ou S, Liu Y, Xie M, Mei T, Zhang Y, Zhang J, Wang Q, Yang B. Surface-enhanced raman spectroscopy analysis of astragalus saponins and identification of metabolites after oral administration in rats by ultrahigh-performance liquid chromatography/quadrupole time-of-flight mass spectrometry analysis. Front Pharmacol. 2022;13:828449. doi: 10.3389/fphar.2022.828449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Tang D, Zang W, Yin G, Dai J, Sun YU, Yang Z, Hoffman RM, Guo X. Synergistic inhibitory effect of traditional Chinese medicine astragaloside IV and curcumin on tumor growth and angiogenesis in an orthotopic nude-mouse model of human hepatocellular carcinoma. Anticancer Res. 2017;37:465–473. doi: 10.21873/anticanres.11338. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112. doi: 10.1016/bs.apha.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Gong AGW, Duan R, Wang HY, Kong XP, Dong TTX, Tsim KWK, Chan K. Evaluation of the pharmaceutical properties and value of astragali radix. Medicines (Basel) 2018;5:46. doi: 10.3390/medicines5020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Zhou J, Qin X, Huang H, Nie C. Astragaloside IV inhibits the invasion and metastasis of SiHa cervical cancer cells via the TGF-β1-mediated PI3K and MAPK pathways. Oncol Rep. 2019;41:2975–2986. doi: 10.3892/or.2019.7062. [DOI] [PubMed] [Google Scholar]
- 23.Sun P, Liu Y, Wang Q, Zhang B. Astragaloside IV inhibits human colorectal cancer cell growth. Front Biosci (Landmark Ed) 2019;24:597–606. doi: 10.2741/4738. [DOI] [PubMed] [Google Scholar]
- 24.Mariño G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: The interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Arcy MS. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43:582–592. doi: 10.1002/cbin.11137. [DOI] [PubMed] [Google Scholar]
- 26.Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: A review. Nutrients. 2018;10:1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hata AN, Engelman JA, Faber AC. The BCL2 family: Key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5:475–487. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia L, Lv D, Zhang S, Wang Z, Zhou B. Astragaloside IV inhibits the progression of non-small cell lung cancer through the Akt/GSK-3β/β-catenin pathway. Oncol Res. 2019;27:503–508. doi: 10.3727/096504018X15344989701565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu T, Fei Z, Wei N. Chemosensitive effects of astragaloside IV in osteosarcoma cells via induction of apoptosis and regulation of caspase-dependent Fas/FasL signaling. Pharmacol Rep. 2017;69:1159–1164. doi: 10.1016/j.pharep.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, Mou J, Cui L, Wang X, Zhang Z. Astragaloside IV inhibits cell proliferation of colorectal cancer cell lines through down-regulation of B7-H3. Biomed Pharmacother. 2018;102:1037–1044. doi: 10.1016/j.biopha.2018.03.127. [DOI] [PubMed] [Google Scholar]
- 31.Su CM, Wang HC, Hsu FT, Lu CH, Lai CK, Chung JG, Kuo YC. Astragaloside IV induces apoptosis, G1-phase arrest and inhibits anti-apoptotic signaling in hepatocellular carcinoma. In vivo. 2020;34:631–638. doi: 10.21873/invivo.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim MO, Lee HS, Chin YW, Moon DO, Ahn JS. Gartanin induces autophagy through JNK activation which extenuates caspase-dependent apoptosis. Oncol Rep. 2015;34:139–146. doi: 10.3892/or.2015.3948. [DOI] [PubMed] [Google Scholar]
- 33.Amaravadi RK, Kimmelman AC, Debnath J. Targeting autophagy in cancer: Recent advances and future directions. Cancer Discov. 2019;9:1167–1181. doi: 10.1158/2159-8290.CD-19-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell RC, Yuan HX, Guan KL. Autophagy regulation by nutrient signaling. Cell Res. 2014;24:42–57. doi: 10.1038/cr.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu WJ, Ye L, Huang WF, Guo LJ, Xu ZG, Wu HL, Yang C, Liu HF. p62 links the autophagy pathway and the ubiqutin-proteasome system upon ubiquitinated protein degradation. Cell Mol Biol Lett. 2016;21:29. doi: 10.1186/s11658-016-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Li G, Chen M, Cai R. Astragaloside IV enhances the sensibility of lung adenocarcinoma cells to bevacizumab by inhibiting autophagy. Drug Dev Res. 2022;83:461–469. doi: 10.1002/ddr.21878. [DOI] [PubMed] [Google Scholar]
- 37.Li QW, Zhang GL, Hao CX, Ma YF, Sun X, Zhang Y, Cao KX, Li BX, Yang GW, Wang XM. SANT, a novel Chinese herbal monomer combination, decreasing tumor growth and angiogenesis via modulating autophagy in heparanase overexpressed triple-negative breast cancer. J Ethnopharmacol. 2021;266:113430. doi: 10.1016/j.jep.2020.113430. [DOI] [PubMed] [Google Scholar]
- 38.Lai ST, Wang Y, Peng F. Astragaloside IV sensitizes non-small cell lung cancer cells to cisplatin by suppressing endoplasmic reticulum stress and autophagy. J Thorac Dis. 2020;12:3715–3724. doi: 10.21037/jtd-20-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang B, Yang N, Chen Y, Zhu M, Lian Y, Xiong Z, Wang B, Feng L, Jia X. An integrated strategy for effective-component discovery of astragali radix in the treatment of lung cancer. Front Pharmacol. 2021;11:580978. doi: 10.3389/fphar.2020.580978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu X, Gao H, Zhai J, Sun J, Tao L, Zhang Y, Song Y, Hu T. Astragaloside IV enhances cisplatin chemosensitivity in hepatocellular carcinoma by suppressing MRP2. Eur J Pharm Sci. 2020;148:105325. doi: 10.1016/j.ejps.2020.105325. [DOI] [PubMed] [Google Scholar]
- 41.Xia C, He Z, Cai Y. Quantitative proteomics analysis of differentially expressed proteins induced by astragaloside IV in cervical cancer cell invasion. Cell Mol Biol Lett. 2020;25:25. doi: 10.1186/s11658-020-00218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang X. EMT: New signals from the invasive front. Oral Oncol. 2011;47:686–687. doi: 10.1016/j.oraloncology.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7:re8. doi: 10.1126/scisignal.2005189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ribatti D. Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res. 2017;353:1–5. doi: 10.1016/j.yexcr.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 45.Kozak J, Forma A, Czeczelewski M, Kozyra P, Sitarz E, Radzikowska-Büchner E, Sitarz M, Baj J. Inhibition or reversal of the epithelial-mesenchymal transition in gastric cancer: Pharmacological approaches. Int J Mol Sci. 2020;22:277. doi: 10.3390/ijms22010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, Wen K. Astragaloside IV inhibits TGF-β1-induced epithelial-mesenchymal transition through inhibition of the PI3K/Akt/NF-κB pathway in gastric cancer cells. Phytother Res. 2018;32:1289–1296. doi: 10.1002/ptr.6057. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Shen X, Zhang Y, Wang S, Zhou L. Astragaloside IV suppresses transforming growth factor-β1-induced epithelial-mesenchymal transition through inhibition of Wnt/β-catenin pathway in glioma U251 cells. Biosci Biotechnol Biochem. 2020;84:1345–1352. doi: 10.1080/09168451.2020.1737502. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Gao S, Song L, Liu M, Sun Z, Liu J. Astragaloside IV antagonizes M2 phenotype macrophage polarization-evoked ovarian cancer cell malignant progression by suppressing the HMGB1-TLR4 axis. Mol Immunol. 2021;130:113–121. doi: 10.1016/j.molimm.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 49.Wang ZF, Ma DG, Zhu Z, Mu YP, Yang YY, Feng L, Yang H, Liang JQ, Liu YY, Liu L, Lu HW. Astragaloside IV inhibits pathological functions of gastric cancer-associated fibroblasts. World J Gastroenterol. 2017;23:8512–8525. doi: 10.3748/wjg.v23.i48.8512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mei J, Xiao Z, Guo C, Pu Q, Ma L, Liu C, Lin F, Liao H, You Z, Liu L. Prognostic impact of tumor-associated macrophage infiltration in non-small cell lung cancer: A systemic review and meta-analysis. Oncotarget. 2016;7:34217–34228. doi: 10.18632/oncotarget.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuccarese MF, Dubach JM, Pfirschke C, Engblom C, Garris C, Miller MA, Pittet MJ, Weissleder R. Heterogeneity of macrophage infiltration and therapeutic response in lung carcinoma revealed by 3D organ imaging. Nat Commun. 2017;8:14293. doi: 10.1038/ncomms14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sawa-Wejksza K, Dudek A, Lemieszek M, Kaławaj K, Kandefer-Szerszeń M. Colon cancer-derived conditioned medium induces differentiation of THP-1 monocytes into a mixed population of M1/M2 cells. Tumour Biol. 2018;40:1010428318797880. doi: 10.1177/1010428318797880. [DOI] [PubMed] [Google Scholar]
- 53.Li N, Qin J, Lan L, Zhang H, Liu F, Wu Z, Ni H, Wang Y. PTEN inhibits macrophage polarization from M1 to M2 through CCL2 and VEGF-A reduction and NHERF-1 synergism. Cancer Biol Ther. 2015;16:297–306. doi: 10.1080/15384047.2014.1002353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, Gong WY, Dong JC, Liu BJ. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37:207. doi: 10.1186/s13046-018-0878-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu F, Ran F, He H, Chen L. Astragaloside IV exerts anti-tumor effect on murine colorectal cancer by re-educating tumor-associated macrophage. Arch Immunol Ther Exp (Warsz) 2020;68:33. doi: 10.1007/s00005-020-00598-y. [DOI] [PubMed] [Google Scholar]
- 56.Chen J, Ye X, Pitmon E, Lu M, Wan J, Jellison ER, Adler AJ, Vella AT, Wang K. IL-17 inhibits CXCL9/10-mediated recruitment of CD8+ cytotoxic T cells and regulatory T cells to colorectal tumors. J Immunother Cancer. 2019;7:324. doi: 10.1186/s40425-019-0757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang LF, Yao YM, Li JF, Zhang SW, Li WX, Dong N, Yu Y, Sheng ZY. The effect of astragaloside IV on immune function of regulatory T cell mediated by high mobility group box 1 protein in vitro. Fitoterapia. 2012;83:1514–1522. doi: 10.1016/j.fitote.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 58.Zhang A, Zheng Y, Que Z, Zhang L, Lin S, Le V, Liu J, Tian J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J Cancer Res Clin Oncol. 2014;140:1883–1890. doi: 10.1007/s00432-014-1744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W, Chen H, Wang D. Protective role of astragaloside IV in gastric cancer through regulation of microRNA-195-5p-mediated PD-L1. Immunopharmacol Immunotoxicol. 2021;43:443–451. doi: 10.1080/08923973.2021.1936013. [DOI] [PubMed] [Google Scholar]
- 60.Wang PP, Luan JJ, Xu WK, Wang L, Xu DJ, Yang CY, Zhu YH, Wang YQ. Astragaloside IV downregulates the expression of MDR1 in Bel-7402/FU human hepatic cancer cells by inhibiting the JNK/c-Jun/AP-1 signaling pathway. Mol Med Rep. 2017;16:2761–2766. doi: 10.3892/mmr.2017.6924. [DOI] [PubMed] [Google Scholar]
- 61.Xie T, Li Y, Li SL, Luo HF. Astragaloside IV enhances cisplatin chemosensitivity in human colorectal cancer via regulating NOTCH3. Oncol Res. 2016;24:447–453. doi: 10.3727/096504016X14685034103590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dulloo I, Phang BH, Othman R, Tan SY, Vijayaraghavan A, Goh LK, Martin-Lopez M, Marques MM, Li CW, Wang de Y, et al. Hypoxia-inducible TAp73 supports tumorigenesis by regulating the angiogenic transcriptome. Nat Cell Biol. 2015;17:511–523. doi: 10.1038/ncb3130. [DOI] [PubMed] [Google Scholar]
- 63.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 64.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L, Zhou XM, Yang FF, Miao Y, Yin Y, Hu XJ, Hou G, Wang QY, Kang J. TRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3β/β-catenin signaling in non-small cell lung cancer. Oncotarget. 2017;8:62069–62080. doi: 10.18632/oncotarget.18911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qin CD, Ma DN, Ren ZG, Zhu XD, Wang CH, Wang YC, Ye BG, Cao MQ, Gao DM, Tang ZY. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol Rep. 2017;37:1725–1735. doi: 10.3892/or.2017.5389. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Liu C, Xie Z, Lu H. Knockdown of TRIM47 inhibits breast cancer tumorigenesis and progression through the inactivation of PI3K/Akt pathway. Chem Biol Interact. 2020;317:108960. doi: 10.1016/j.cbi.2020.108960. [DOI] [PubMed] [Google Scholar]
- 68.Li R, Song Y, Zhou L, Li W, Zhu X. Downregulation of RAGE inhibits cell proliferation and induces apoptosis via regulation of PI3K/AKT pathway in cervical squamous cell carcinoma. Onco Targets Ther. 2020;13:2385–2397. doi: 10.2147/OTT.S240378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, Wang L, Wang Y, Dong S, Yang S, Guan Y, Wu X. Astragaloside IV inhibits cell proliferation in vulvar squamous cell carcinoma through the TGF-β/Smad signaling pathway. Dermatol Ther. 2019;32:e12802. doi: 10.1111/dth.12802. [DOI] [PubMed] [Google Scholar]
- 70.Zhang XQ, Yao C, Bian WH, Chen X, Xue JX, Zhu ZY, Ying Y, Xu YL, Wang C. Effects of astragaloside IV on treatment of breast cancer cells execute possibly through regulation of Nrf2 via PI3K/AKT/mTOR signaling pathway. Food Sci Nutr. 2019;7:3403–3413. doi: 10.1002/fsn3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He Y, Zhang Q, Chen H, Guo Q, Zhang L, Zhang Z, Li Y. Astragaloside IV enhanced carboplatin sensitivity in prostate cancer by suppressing AKT/NF-κB signaling pathway. Biochem Cell Biol. 2021;99:214–222. doi: 10.1139/bcb-2020-0026. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Transforming growth factor-beta1-dependent urokinase up-regulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol Chem. 2004;279:8567–8576. doi: 10.1074/jbc.M309131200. [DOI] [PubMed] [Google Scholar]
- 73.Anfuso CD, Motta C, Giurdanella G, Arena V, Alberghina M, Lupo G. Endothelial PKCα-MAPK/ERK-phospholipase A2 pathway activation as a response of glioma in a triple culture model. A new role for pericytes? Biochimie. 2014;99:77–87. doi: 10.1016/j.biochi.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y, Hu LL. ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li B, Wang F, Liu N, Shen W, Huang T. Astragaloside IV inhibits progression of glioma via blocking MAPK/ERK signaling pathway. Biochem Biophys Res Commun. 2017;491:98–103. doi: 10.1016/j.bbrc.2017.05.102. [DOI] [PubMed] [Google Scholar]
- 76.Jiang K, Lu Q, Li Q, Ji Y, Chen W, Xue X. Astragaloside IV inhibits breast cancer cell invasion by suppressing Vav3 mediated Rac1/MAPK signaling. Int Immunopharmacol. 2017;42:195–202. doi: 10.1016/j.intimp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 77.Cheng X, Gu J, Zhang M, Yuan J, Zhao B, Jiang J, Jia X. Astragaloside IV inhibits migration and invasion in human lung cancer A549 cells via regulating PKC-α-ERK1/2-NF-κB pathway. Int Immunopharmacol. 2014;23:304–313. doi: 10.1016/j.intimp.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 78.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99:180–185. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H. Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-κB pathway. Sci Rep. 2016;6:27528. doi: 10.1038/srep27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong F, Xiao W, Ragupathi G, Lau CB, Leung PC, Yeung KS, George C, Cassileth B, Kennelly E, Livingston PO. The known immunologically active components of astragalus account for only a small proportion of the immunological adjuvant activity when combined with conjugate vaccines. Planta Med. 2011;77:817–824. doi: 10.1055/s-0030-1250574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Min L, Wang H, Qi H. Astragaloside IV inhibits the progression of liver cancer by modulating macrophage polarization through the TLR4/NF-κB/STAT3 signaling pathway. Am J Transl Res. 2022;14:1551–1566. [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang C, Li L, Hou S, Shi Z, Xu W, Wang Q, He Y, Gong Y, Fang Z, Yang Y. Astragaloside IV inhibits hepatocellular carcinoma by continually suppressing the development of fibrosis and regulating pSmad3C/3L and Nrf2/HO-1 pathways. J Ethnopharmacol. 2021;279:114350. doi: 10.1016/j.jep.2021.114350. [DOI] [PubMed] [Google Scholar]
- 83.Li F, Cao K, Wang M, Liu Y, Zhang Y. Astragaloside IV exhibits anti-tumor function in gastric cancer via targeting circRNA dihydrolipoamide S-succinyltransferase (circDLST)/miR-489-3p/eukaryotic translation initiation factor 4A1(EIF4A1) pathway. Bioengineered. 2022;13:10111–10122. doi: 10.1080/21655979.2022.2063664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang J, Hou L, Liang R, Chen X, Zhang R, Chen W, Zhu J. CircDLST promotes the tumorigenesis and metastasis of gastric cancer by sponging miR-502-5p and activating the NRAS/MEK1/ERK1/2 signaling. Mol Cancer. 2019;18:80. doi: 10.1186/s12943-019-1015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui X, Jiang X, Wei C, Xing Y, Tong G. Astragaloside IV suppresses development of hepatocellular carcinoma by regulating miR-150-5p/β-catenin axis. Environ Toxicol Pharmacol. 2020;78:103397. doi: 10.1016/j.etap.2020.103397. [DOI] [PubMed] [Google Scholar]
- 86.Wang L, Botchway BOA, Liu X. The repression of the HMGB1-TLR4-NF-κB signaling pathway by safflower yellow may improve spinal cord injury. Front Neurosci. 2021;15:803885. doi: 10.3389/fnins.2021.803885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.