Abstract
Endovascular repair has been introduced to decrease the morbidity and mortality associated with open surgical repair of aortic arch pathology. We illustrate total percutaneous transfemoral approach with a 3-vessel inner branch stent-graft to treat aortic arch aneurysm. (Level of Difficulty: Advanced.)
Key Words: endovascular arch repair, patient-specific stent-graft, percutaneous arch repair, preloaded catheter, postdissection arch aneurysm, transcatheter arch repair
Abbreviations and Acronyms: CTA, computed tomographic angiography; IA, innominate artery; LCCA, left common carotid artery; LSA, left subclavian artery; RCCA, right common carotid artery
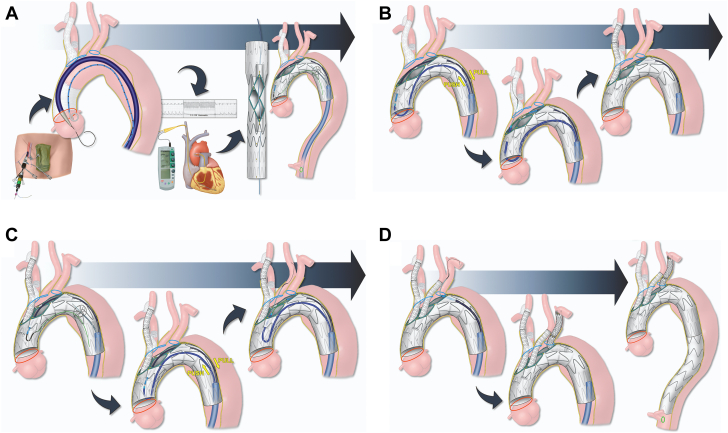
Graphical abstract
A 72-year-old man presented with an enlarging 6-cm arch and thoracic aortic aneurysm following earlier ascending aortic repair for type A dissection (A0,10). The patient was referred for consideration of possible total endovascular aortic repair. The patient consented to the publication of his case details and images.
Learning Objectives
-
•
To describe an innovative and inedited technique using a total percutaneous femoral approach to repair aortic arch pathologies.
Medical history
His medical history was notable for hypertension and tobacco use.
Investigations
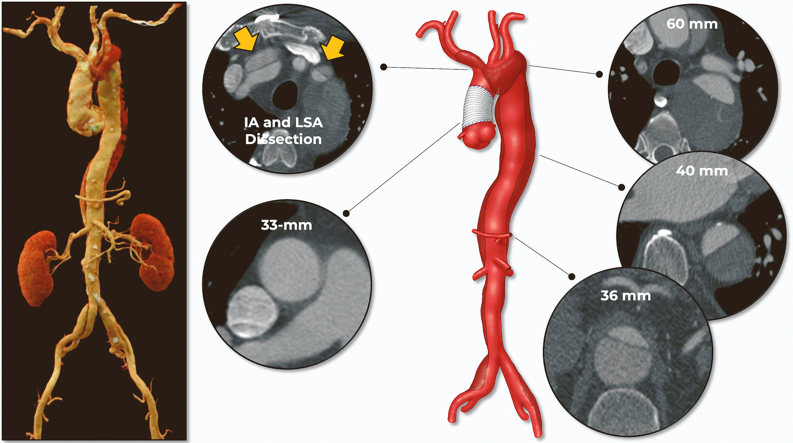
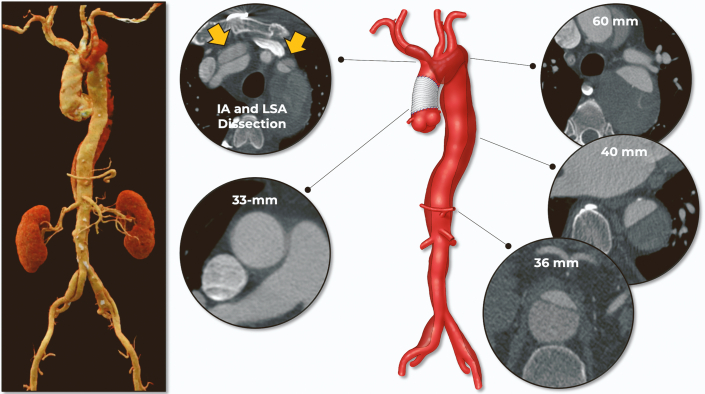
Computed tomographic angiography (CTA) demonstrated residual dissection involving the aortic arch, innominate artery (IA), and left subclavian artery (LSA) with distal extension to both common iliac arteries (Figure 1, Video 1). Of utmost importance, the IA was short (14.3 mm in length) with a diameter of 13.5 mm.
Figure 1.
Preoperative Measurements and Anatomy
Management
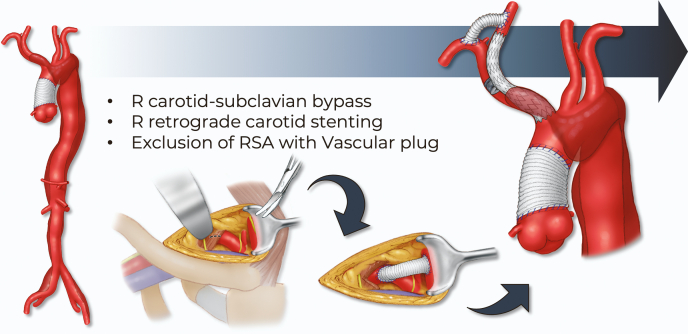
The patient was treated with staged approach. The first-stage procedure included a right carotid-subclavian artery bypass using 8-mm polyester graft, retrograde stenting of the IA and proximal common carotid artery using balloon-expandable stent-graft (8L mm × 59 mm and postdilation in the proximal portion of the IA with a 14 mm × 2 cm balloon), and exclusion of the right subclavian artery with the use of a vascular plug (Figure 2). The first step allowed the creation of an adequate sealing zone for the AI and preserved the flow to the right subclavian artery. The patient had an uncomplicated postoperative course, and a second-stage total endovascular aortic arch repair was planned.
Figure 2.
First Stage
Device design
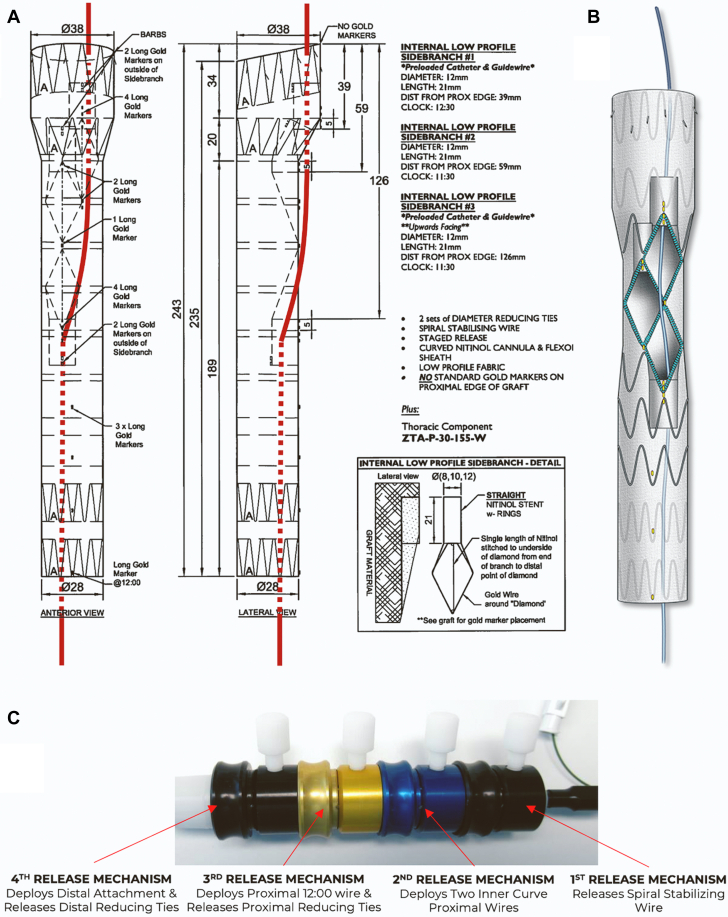
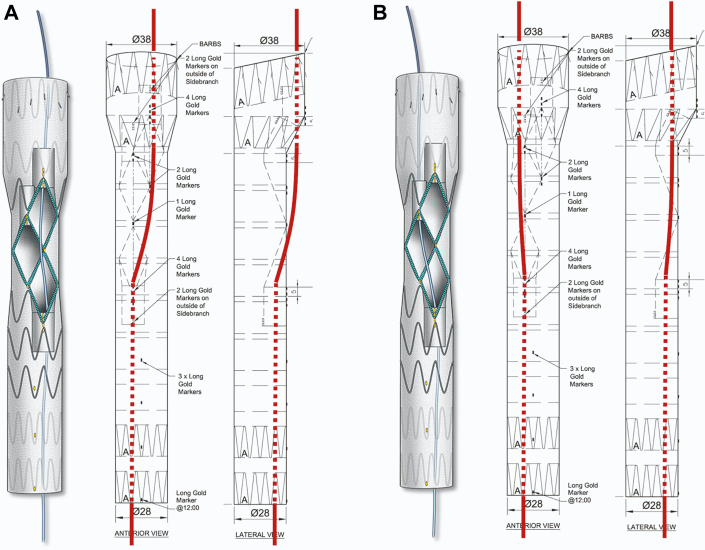
A patient-specific stent graft was designed with 2 antegrade and 1 retrograde inner branches for the IA, left common carotid artery (LCCA), and LSA. The device had 2 sealing stents with diameter tapering from 38 mm to 28 mm. A preloaded catheter was routed inside the main stent-graft lumen, outside via the LSA inner branch, and back inside the main aortic device via the IA inner branch (Figure 3). This modified arrangement allowed direct catheterization of IA from the femoral access. A 4-release wire mechanism was added for orientation, precise positioning, and deployment in the ascending aorta.
Figure 3.
Device Design
Implantation
The procedure was performed under general anesthesia with intraoperative electroencephalographic monitoring in a hybrid operating room with fixed imaging unit. Unilateral right percutaneous femoral approach was established using a preclosure technique. Onlay fusion of the CTA was used to assist with device implantation. The 3-vessel inner branch stent-graft was flushed with CO2, and the CO2 was flushed out with >200 mL heparinized saline solution. A Lundquist wire was carefully positioned in the left ventricle. The stent-graft was introduced via the right femoral access over the Lundquist wire and positioned in the ascending aorta. The onlay fusion was calibrated with angiography and the delivery system was advanced across the aortic valve into the left ventricle. Using temporary rapid ventricular pacing, the 3-vessel inner branch stent-graft was deployed precisely distal to the sinotubular junction. All trigger wires were rapidly removed, and the nose-cone of the delivery system was removed from across the aortic valve and positioned at the distal aspect of the stent-graft. A transesophageal echocardiogram was obtained, which revealed no evidence of injury in the aortic valve (Video 2, Figure 4A). A second Lunderquist wire was advanced via the preloaded catheter and positioned in the ascending aorta. The delivery system of the device was removed along with the initial Lunderquist wire, and a 22-F × 65 cm DrySeal sheath was advanced via the second Lunderquist wire. A 6-F × 110 cm shuttle sheath was advanced into the IA branch. By means of a VS1 catheter, a 0.014-inch wire was advanced and snared via the same right femoral sheath, establishing through-and-through right femoral access via the IA branch. The 6-F shuttle sheath was advanced via the IA branch and married into the dilator of with an 8.5-F × 90 cm steerable sheath. Using a “push and pull” maneuver, the 8.5-F × 90 cm steerable sheath was advanced and positioned inside the IA branch. The IA and right common carotid artery (RCCA) were selectively catheterized with a Glidewire, which was exchanged for an Amplatz wire. The repair was extended into the RCCA with the use of 2 8-L mm × 79 mm balloon-expandable stent-grafts, which were postdilated proximally to 14 mm. Selective RCCA angiography revealed widely patent stent with no endoleak or embolization (Video 3, Figure 4B). The through-and-through wire was removed and the 6-F shuttle sheath was used to catheterize the LCCA branch, and the same steps were repeated with placement of 8L mm × 79 mm balloon-expandable stent-graft in the LCCA, which was postdilated proximally to 14 mm. Completion LCCA angiography revealed widely patent stent with no endoleak or dissection (Video 4, Figure 4C). Finally, a Lunderquist wire was advanced via the 6-F shuttle sheath, which was exchanged for a 12-F Flexor Ansel guiding sheath. The LSA was selectively catheterized with a Glidewire and Kumpe catheter, which was exchanged for Amplatz wire. Stenting from the retrograde LSA branch to the proximal LSA was performed using 2 13 × 50 mm overlapping self-expandable stent-grafts. Completion LSA angiography revealed widely patent stent with no endoleak or dissection and with patent left vertebral artery (Video 5). The repair was extended distally into zone 5 in the thoracic aorta with the use of a 30-30-200-mm Alpha thoracic stent-graft. The stent attachments were dilated with the use of Tri-Lobe balloon catheter (Video 6, Figure 4D). Cone-beam computed tomography with rotational digital subtraction angiography was performed, which revealed no evidence of type IA endoleak, dissection or embolization (Video 7). Total endovascular time was 193 minutes with a fluoroscopy time of 92 minutes. Total contrast volume used was 225 mL. Postoperative course was unremarkable and the patient was discharged home on postoperative day 4 (Figure 5).
Figure 4.
The Sequence of the Device Implantation
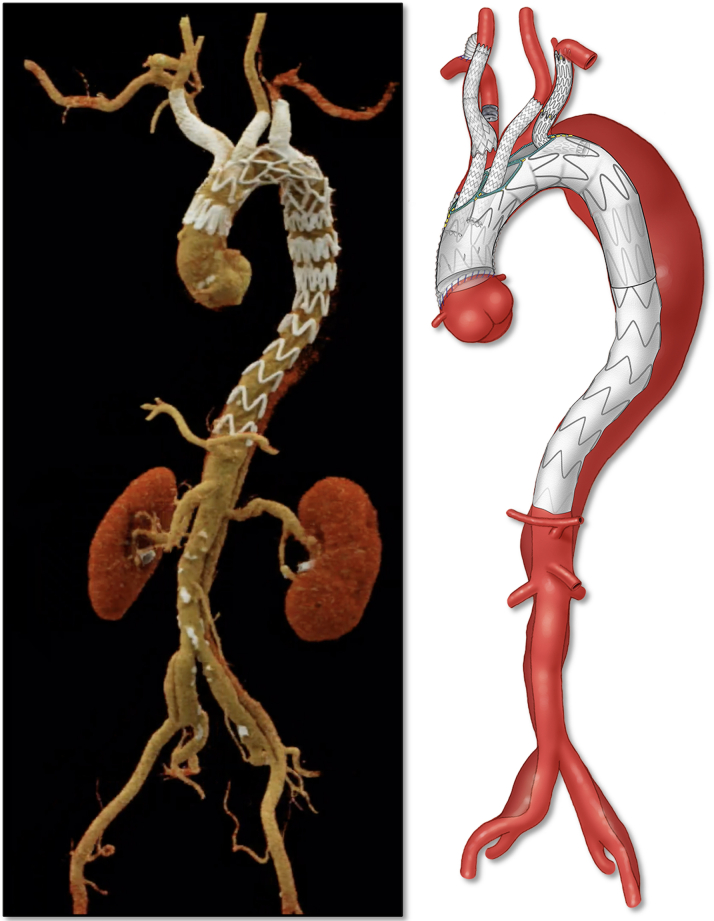
Figure 5.
Postoperative 3D Computed Tomographic Angiography Reconstruction
Discussion
Total endovascular arch repair has been used in selected centers worldwide with favorable results compared with the initial clinical experience, especially among higher-risk patients or those who had prior median sternotomy. Traditionally, bilateral cervical incisions were recommended for access and sequential clamping of the carotid arteries to prevent distal embolization during catheter manipulations and stenting of inner branches. However, a report of the early feasibility study indicated that cervical access complications, along with target vessel endoleaks, were the most common reasons for early secondary interventions.1,2 The approach herein described aims to reduce the risk of these complications by creating a suitable landing zone in the supra-aortic trunks and avoiding the cervical incision.
Mougin et al3 described the first total percutaneous aortic arch 3-vessel repair using axillary access for placement of the IA stent. They used a similar stent design, but routed the preloaded catheter from the LSA into the LCCA branch instead of the IA branch. This modification allowed the incorporation of the LCCA and LSA from the femoral approach, whereas the IA was incorporated by means of the right axillary artery access. Unlike our case, all of the 3 supra-aortic trunks were suitable for stent placement. Our patient had dissection and aneurysm involvement of the IA, which precluded stent placement and required a prior staging procedure with right carotid-subclavian bypass and exclusion of the right subclavian artery (Figure 6). Furthermore, transaxillary percutaneous access has been associated with considerable rates of open conversion and adjunctive endovascular procedures.4
Figure 6.
Device Designs for Total Femoral Approach and Axillary Approach
(A) Femoral; (B) axillary.
Beyond the unique aspects of the device design used in our case, an important technical pitfall is the use of 2 coaxial systems in parallel (6-F × 90 cm shuttle and 8.5-F × 90 cm steerable sheaths) via a 22-F × 65 cm DrySeal sheath to provide support and stability in the proximal thoracic aorta. The “push and pull” maneuver allows advancement of the sheath without undue stress in the inner branch or risk of stent dislodgement.
Aside from the controversy of which patients benefit from endovascular arch repair, the questions remain of which patients should be selected for total percutaneous vs open cervical access techniques during endovascular repair, and how we can prevent emboli using percutaneous approach. Currently, it seems prudent to select patients based on the underlying pathology along with the quality of the aortic arch and supra-aortic trunks. The ideal candidate has prior replacement of the ascending aorta and no evidence of any atheromatous disease in the arch. Other technologies, such as filters specific to aortic arch stent-grafts, are under development and may be used as adjuncts, particularly in these total percutaneous cases.
Follow-Up
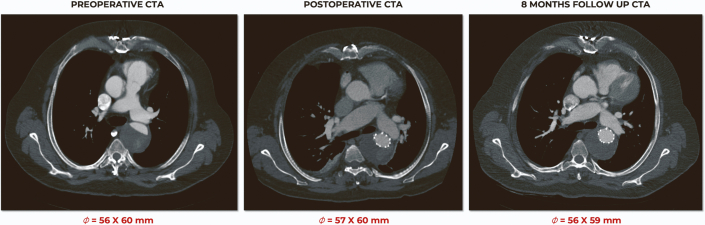
At 8 months, the patient had remained asymptomatic. CTA showed a widely patent arch branch stent-graft with no endoleak and stable aneurysm sac (Figure 7). In addition, the patient had no major adverse events during that follow-up period.
Figure 7.
Follow-Up Computed Tomographic Angiography
Conclusions
Total endovascular arch repair provides a valuable alternative option for patients who are poor surgical candidates, and the use of a total femoral approach can potentially reduce the need for reintervention due to cervical access site complications.
Funding Support and Author Disclosures
Dr Oderich has received consulting fees and grants from Cook Medical, W.L. Gore, Centerline Biomedical, and GE Healthcare (all paid to Mayo Clinic and the University of Texas Health Science at Houston with no personal income). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Preoperative Computed Tomographic Angiography
Device Deployment
Right Common Carotid Artery Branch Construction
Left Common Carotid Artery Branch Construction
Left Subclavian Artery Branch Construction
Distal Extension Thoracic Endovascular Repair
Intraoperative Technical Assessment With Cone-Beam Computed Tomography
References
- 1.Tenorio E.R., Oderich G.S., Kölbel T., Dias N.V., et al. Multicenter global early feasibility study to evaluate total endovascular arch repair using three-vessel inner branch stent-grafts for aneurysms and dissections. J Vasc Surg. 2021;74(4):1055–1065.e4. doi: 10.1016/j.jvs.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 2.Verscheure D., Haulon S., Tsilimparis N., et al. Endovascular treatment of post type A chronic aortic arch dissection with a branched endograft: early results from a retrospective international multicenter study. Ann Surg. 2021;273(5):997–1003. doi: 10.1097/SLA.0000000000003310. [DOI] [PubMed] [Google Scholar]
- 3.Mougin J., Azogui R., Guihaire J., Tyrrell M.R., Oderich G.S., Fabre D., et al. “First in man” total percutaneous aortic arch repair with 3-inner-branch endografts: a report of two cases. Ann Surg. 2021;274(6):e652–e657. doi: 10.1097/SLA.0000000000005167. [DOI] [PubMed] [Google Scholar]
- 4.Bertoglio L., Conradi L., Howard D.P.J., Kaki A., van den Eynde W., Rio J., et al. Percutaneous transaxillary access for endovascular aortic procedures in the multicenter international PAXA registry. J Vasc Surg. 2022;75(3):868–876.e3. doi: 10.1016/j.jvs.2021.08.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preoperative Computed Tomographic Angiography
Device Deployment
Right Common Carotid Artery Branch Construction
Left Common Carotid Artery Branch Construction
Left Subclavian Artery Branch Construction
Distal Extension Thoracic Endovascular Repair
Intraoperative Technical Assessment With Cone-Beam Computed Tomography