Abstract
Preventing osteoporotic fractures is an issue requiring urgent attention to reduce mortality. However, unlike chronic kidney disease-mineral and bone disorder (CKD-MBD), osteoporosis is inadequately addressed in patients undergoing chronic dialysis. In fact, little is known about the proper use of anti-osteoporotic drugs for patients with CKD-MBD. A recent study showed that romosozumab, an anti-osteoporotic drug, increased bone mineral density in osteoporotic patients on hemodialysis without clinically significant adverse events. However, the efficacy and safety of coadministering romosozumab with a calcium-sensing receptor (CaSR) agonist, a pivotal drug used in the management of CKD-MBD, remain unclear. Here, we report the case of a postmenopausal woman undergoing chronic hemodialysis and treated with add-on romosozumab for osteoporosis to CaSR agonist for secondary hyperparathyroidism. After 1 year of treatment, her bone mineral density increased; however, hypocalcemia occurred during the treatment. These results suggest that the concomitant use of romosozumab with CaSR agonist may be a possible treatment option for severe osteoporosis in postmenopausal women receiving chronic hemodialysis with a high fracture risk, but serum calcium levels should be monitored closely and those at risk of ectopic calcification might not be ideal candidates for such treatment.
Keywords: Bone alkaline phosphatase, Bone mineral density, Calcimimetic, Calcium-sensing receptor agonist, Chronic kidney disease-mineral and bone disorder, Hemodialysis, Parathyroid hormone, Osteoporosis, Romosozumab, Secondary hyperparathyroidism, Tartrate-resistant acid phosphatase-5b
1. Introduction
The management of chronic kidney disease-mineral and bone disorder (CKD-MBD) is crucial in patients on dialysis, not only because of the risks for cardiovascular diseases and mortality, but also because of their increased risk for fractures when compared with non-CKD patients (Block et al., 2004; Vilaca et al., 2020). Moreover, in the aging population, preventing osteoporotic fractures needs to be urgently addressed to decrease mortality. Similarly, the population undergoing chronic hemodialysis is mostly aging; therefore, osteoporosis management should be considered to further decrease the risk of fractures.
Sclerostin, a glycoprotein encoded by the Sost gene and secreted primarily by osteocytes (Hernandez et al., 2014), has anti-anabolic effects on bone formation (Sutherland et al., 2004). Sclerostin is involved in the pathophysiology of CKD-MBD, with high serum levels of the molecule being found in patients with CKD (Thambiah et al., 2012). Romosozumab, a newly developed anti-osteoporotic drug, is an anti-sclerostin monoclonal antibody which has a dual mechanism, viz. promoting bone formation and inhibiting bone resorption (Chavassieux et al., 2019). In a previous phase II study, romosozumab, at a dose of 210 mg/month for 12 months, produced a significant increase in bone mineral density (BMD) at the lumbar spine, total hip, and femoral neck sites compared to placebo, alendronate, and teriparatide (McClung et al., 2014). A recent study by Sato et al. showed that romosozumab increased BMD in osteoporotic patients on hemodialysis without clinically significant adverse events (Sato et al., 2021). However, the efficacy and safety of coadministering romosozumab with a calcium-sensing receptor (CaSR) agonist (calcimimetic), a pivotal drug used in the management of secondary hyperparathyroidism, remain unclear. Here, we report the case of a postmenopausal woman undergoing chronic hemodialysis and treated with add-on romosozumab for osteoporosis and for possible worsening of osteoporosis in future cadaveric renal transplantation in addition to a CaSR agonist for secondary hyperparathyroidism.
2. Case report
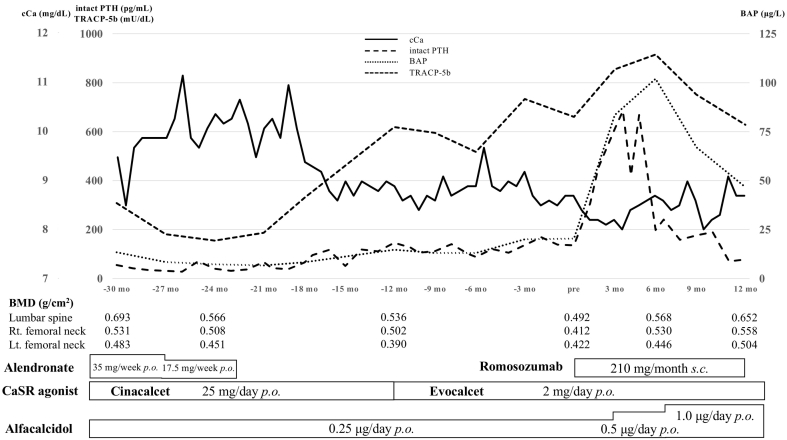
A 61-year-old woman had been on hemodialysis for 16 years due to end-stage renal disease caused by preeclampsia. Prior to romosozumab treatment, the patient was on alfacalcidol 0.25 μg/day and cinacalcet 25 mg/day (oral CaSR agonist) which was later replaced with evocalcet 2 mg/day (another oral CaSR agonist), for secondary hyperparathyroidism. Plasma intact parathyroid hormone (PTH) levels were controlled to less than 150 pg/mL (Fig. 1). Subsequently, romosozumab was administered (210 mg/month) for 12 months.
Fig. 1.
Trajectories of serum cCa, BAP, TRACP-5b, and plasma intact PTH levels, as well as BMD at the lumbar spine and the right and left femoral neck sites before and after romosozumab treatment.
Abbreviations: BAP, bone alkaline phosphatase; BMD, bone mineral density; CaSR, calcium-sensing receptor; cCa, corrected calcium; lt., left; mo, month; p.o., per os; PTH, parathyroid hormone; rt., right; s.c., subcutaneous; TRACP-5b, tartrate-resistant acid phosphatase-5.
Additionally, to increase BMD, the patient had received alendronate at a dose of 17.5 or 35 mg/week (half dose or standard dose in Japan) for osteoporosis depending on the measured BMD for approximately 3 years. BMD values obtained using dual-energy X-ray absorptiometry (DXA) scanning analysis (2018 Hologic Horizon WI Bone Densitometer, Hologic, Inc., Bedford, MA, USA) on starting alendronate were as follows; lumbar spine 0.693 g/cm2, right femoral neck 0.531 g/cm2, left femoral neck 0.483 g/cm2, and T score; lumbar spine −2.7SD, right femoral neck −2.9SD, left femoral neck −3.4SD. Alendronate was discontinued 2 years prior to romosozumab treatment. During this period, BMD declined after discontinuing alendronate to 0.492, 0.412, and 0.422 g/cm2 at the lumbar spine, right, and left femoral neck sites, respectively, as of starting romosozumab for possible glucocorticoid-induced worsening osteoporosis in future cadaveric renal transplantation, despite there being no history of fractures. Hemodialysis conditions did not change during romosozumab treatment.
As shown in Fig. 1, the serum bone alkaline phosphatase (BAP) level (normal range: 3.7–20.9 μg/L), a bone formation marker derived from osteoblasts, increased from 20.4 to as high as 102 μg/L over 6 months after the initiation of romosozumab. Meanwhile, the trend of serum tartrate-resistant acid phosphatase-5b (TRACP-5b) levels, a bone resorption marker derived from osteoclasts, varied from the discontinuation of alendronate treatment to the start of romosozumab treatment, and there was no significant difference between the rate of increase in serum TRACP-5b levels (normal range: 120–420 mU/dL) from 6 months (517 mU/dL) to 3 months (733 mU/dL) prior to the start of romosozumab treatment, and that from the start of romosozumab treatment (660 mU/dL) to 3 months (855 mU/dL) after romosozumab treatment. The rate of increase in serum TRACP-5b levels slowed down from 3 to 6 months (915 mU/dL) and serum TRACP-5b levels subsequently decreased from 6 to 12 months (628 mU/dL) after romosozumab treatment.
Of note, after one year of romosozumab treatment, the BMD of the patient substantially increased to 0.652 (ΔBMD +32.1 %/year), 0.558 (ΔBMD +34.9 %/year), and 0.504 g/cm2 (ΔBMD +19.2 %/year) at the lumbar spine, right, and left femoral neck sites, respectively. No cardio/cerebrovascular events or decreased cardiac function were observed throughout treatment with romosozumab, and chest computed tomography showed no coronary artery calcification after one year of treatment. However, albumin-corrected serum calcium (cCa) levels decreased from 8.6 to 7.9 mg/dL over 4 months after the initiation of romosozumab, and intact PTH levels increased from 136 to 685 pg/mL in response to the lower serum cCa levels. Hence, the alfacalcidol dosage was subsequently increased from 0.25 to 1.0 μg/day, since serum phosphorus levels were well-controlled.
3. Discussion
This is a suggestive case report in which romosozumab increased BMD in a postmenopausal woman with osteoporosis undergoing chronic hemodialysis and treated with a CaSR agonist. In the present case, BMD decreased due to increased bone resorption without increased bone formation after discontinuation of alendronate, and BMD increased due to induction of osteoanabolism by add-on administration of romosozumab alongside a CaSR agonist.
The effects of romosozumab are characterized by the promotion of bone formation and inhibition of bone resorption, resulting in increased BMD, with temporary increase in bone formation markers and sustained decrease in bone resorption markers in patients both on and not on hemodialysis with osteoporosis (Langdahl et al., 2017; Sato et al., 2021). In the present case, the trend of serum TRACP-5b levels appeared, at first glance, to be different from those in previous reports (Langdahl et al., 2017; Sato et al., 2021). However, there was no significant difference between the trend of serum TRACP-5b levels from the discontinuation of alendronate treatment to the start of romosozumab treatment and that from the start of romosozumab treatment to 3 months after the romosozumab treatment. In addition, from 6 to 12 months after the romosozumab treatment, serum TRACP-5b levels decreased to almost the same level as at the start of romosozumab treatment. Based on these changes in serum TRACP-5b levels, bone resorption was not accelerated, but was rather suppressed during the treatment with romosozumab in the present case.
Previous reports have shown that serum sclerostin levels negatively correlate with plasma PTH levels (Mirza et al., 2010) and PTH administration is associated with a decrease in serum sclerostin levels in postmenopausal women (Drake et al., 2010). In particular, PTH was found to decrease Sost messenger RNA levels in vitro (Keller and Kneissel, 2005), and continuous and intermittent administration of PTH decreased Sost messenger RNA levels in mice (Bellido et al., 2005). In addition, an ovariectomized rat study reported that intermittent PTH administration after prolonged alendronate treatment could substantially induce bone formation (Altman-Singles et al., 2017). Based on these reports, in addition to the effects of romosozumab itself, the significant elevation of plasma intact PTH levels in response to hypocalcemia might have suppressed the expression and secretion of sclerostin in osteocytes and additively promoted osteoblast differentiation in the present case.
In contrast, Moe et al. demonstrated in a CKD rat study that in the presence of high levels of PTH, serum Ca levels were lower than in the low PTH group independently of the class of anti-osteoporotic drugs, and the efficacy of romosozumab was reduced due to the activation of Wnt signaling by PTH that may bypass the LRP signaling (Moe et al., 2015). Two recent post hoc analyses investigated the efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate CKD (stages 1–3) (Miller et al., 2022; Miyauchi et al., 2022). Both studies demonstrated that BMD at the lumbar spine, total hip, and femoral neck, significantly increased as a result of treatment with romosozumab for 1 year. In addition, hypocalcemia as an adverse event rarely occurred. Although there was no information on the causes of mild-to-moderate CKD in the two studies, they appear to result from age-related decline in renal function. Therefore, it is expected that patients enrolled in the studies would have exhibited only mildly elevated PTH levels associated with decreased renal function. This may partly result in significant BMD increase in patients with mild-to-moderate CKD.
As previously reported, the use of romosozumab can cause hypocalcemia via Ca mobilization from blood to bone due to increased bone formation (McClung et al., 2014). In the present case, some factors may have caused the observed hypocalcemia. The Ca level at the start of romosozumab treatment in the present case was lower than that in the study by Sato et al. (2021). In addition, the dose of alfacalcidol (0.25 μg/day) which had been orally administered may have been smaller than required, leading to the hypocalcemia in the romosozumab treatment in the present case. A recent pharmacokinetics/pharmacodynamics study by Hsu et al. demonstrated that worsening hypocalcemia was found in patients on hemodialysis possibly due to the lack of compensatory increased PTH effect in the kidney, despite comparable levels of exposure to romosozumab between patients with normal renal function and those on hemodialysis (Hsu et al., 2022). Notably, in the present case on chronic hemodialysis, oral CaSR agonist was simultaneously used with romosozumab. CaSR agonists are commonly used to control secondary hyperparathyroidism in patients on chronic hemodialysis by inhibiting PTH secretion from the parathyroid glands. Therefore, PTH elevation in response to hypocalcemia may be relatively suppressed by CaSR agonist when compared with the expected level, although the absolute values of PTH were high.
In addition, Brown et al. reported that CaSR agonists may directly affect bone cells because CaSR is expressed on the bone surface (Brown and MacLeod, 2001). Previous studies have shown that the activation of CaSR (also by CaSR agonist) in osteocytes and a high extracellular Ca level appear to exert osteogenic effects by inducing osteoblast differentiation and osteoclast apoptosis (Hamdy, 2009; Kanatani et al., 1999). Based on these studies, although the oversuppression of PTH by CaSR agonists can be a risk for decreased BMD via suppression of osteoblast function, CaSR agonists may synergistically promote osteoanabolism with romosozumab, and result in hypocalcemia.
Romosozumab appears to be a good option because it not only possesses an osteoanabolic effect but may also attenuate skeletal PTH resistance (Massy and Drueke, 2017). However, Wnt signaling is involved not only in the bone but also in the blood vessels, and sclerostin has been reported to be essential for the maintenance of vascular normality (Koos et al., 2013). In this aspect, the administration of romosozumab to patients on dialysis prone to ectopic calcification associated with CKD-MBD may lead to vascular calcification and increase the risk of cardiovascular events.
In conclusion, romosozumab should not be used for patients with myocardial infarction and/or stroke (in the last 12 months), and the presence or absence of onset of cardiovascular events as well as serum cCa levels should be closely monitored during the treatment. However, the use of romosozumab may be worth considering in postmenopausal women receiving chronic hemodialysis who have a high risk for fracture and who are on a CaSR agonist.
Patient consent
We have obtained written consent from the patient for the publication of this case report and have listed it in her medical record.
Ethics approval
This study was conducted in accordance with the ethical standards of the Declaration of Helsinki. This article does not include any studies with human participants performed by any of the authors.
Declaration of competing interest
Naoto Tominaga received an honorarium from Astellas Pharmaceutical Inc. for being a speaker on HIF-PhD inhibitors which is not mentioned in this case report. The other authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgements
We would like to thank Editage (www.editage.com) for the English language editing.
Funding
This case report was not funded by any companies.
Data availability statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
References
- Altman-Singles A.R., Jeong Y., Tseng W.J., de Bakker C.M., Zhao H., Lott C., Robberts J., Qin L., Han L., Kim D.G., Liu X.S. Intermittent parathyroid hormone after prolonged alendronate treatment induces substantial new bone formation and increases bone tissue heterogeneity in ovariectomized rats. J. Bone Miner. Res. 2017;32:1703–1715. doi: 10.1002/jbmr.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T., Ali A.A., Gubrij I., et al. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J. Am. Soc. Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- Brown E.M., MacLeod R.J. Extracellular calcium sensing and extracellular calcium signaling. Physiol. Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Chavassieux P., Chapurlat R., Portero-Muzy N., Roux J.P., Garcia P., Brown J.P., Libanati C., Boyce R.W., Wang A., Grauer A. Bone-forming and antiresorptive effects of romosozumab in postmenopausal women with osteoporosis: bone histomorphometry and microcomputed tomography analysis after 2 and 12 months of treatment. J. Bone Miner. Res. 2019;34:1597–1608. doi: 10.1002/jbmr.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake M.T., Srinivasan B., Mödder U.I., Peterson J.M., McCready L.K., Riggs B.L., Dwyer D., Stolina M., Kostenuik P., Khosla S. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J. Clin. Endocrinol. Metab. 2010;95:5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdy N.A. Strontium ranelate improves bone microarchitecture in osteoporosis. Rheumatol. (Oxford) 2009;iv(Suppl. 4):9–13. doi: 10.1093/rheumatology/kep274. [DOI] [PubMed] [Google Scholar]
- Hernandez P., Whitty C., Wardale R., Henson F.M. New insights into the location and form of sclerostin. Biochem. Biophys. Res. Commun. 2014;446:1108–1113. doi: 10.1016/j.bbrc.2014.03.079. [DOI] [PubMed] [Google Scholar]
- Hsu C.P., Maddox J., Block G., Bartley Y., Yu Z. Influence of renal function on pharmacokinetics, pharmacodynamics, and safety of a single dose of romosozumab. J. Clin. Pharmacol. 2022;62(9):1132–1141. doi: 10.1002/jcph.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatani M., Sugimoto T., Kanzawa M., Yano S., Chihara K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem. Biophys. Res. Commun. 1999;261:144–148. doi: 10.1006/bbrc.1999.0932. [DOI] [PubMed] [Google Scholar]
- Keller H., Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Koos R., Brandenburg V., Mahnken A.H., Schneider R., Dohmen G., Autschbach R., Marx N., Kramann R. Sclerostin as a potential novel biomarker for aortic valve calcification: an in-vivo and ex-vivo study. J. Heart Valve Dis. 2013;22:317–325. [PubMed] [Google Scholar]
- Langdahl B.L., Libanati C., Crittenden D.B., Bolognese M.A., Brown J.P., Daizadeh N.S., Dokoupilova E., Engelke K., Finkelstein J.S., Genant H.K., Goemaere S. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: a randomised, open-label, phase 3 trial. Lancet. 2017;390:1585–1594. doi: 10.1016/S0140-6736(17)31613-6. [DOI] [PubMed] [Google Scholar]
- Massy Z., Drueke T. Adynamic bone disease is a predominant bone pattern in early stages of chronic kidney disease. J. Nephrol. 2017;30:629–634. doi: 10.1007/s40620-017-0397-7. [DOI] [PubMed] [Google Scholar]
- McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A., Langdahl B.L., Reginster J.Y., Zanchetta J.R., Wasserman S.M., Katz L. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- Miller P.D., Adachi J.D., Albergaria B.H., Cheung A.M., Chines A.A., Gielen E., Langdahl B.L., Miyauchi A., Oates M., Reid I.R., Santiago N.R. Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J. Bone Miner. Res. 2022;37:1437–1445. doi: 10.1002/jbmr.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza F.S., Padhi I.D., Raisz L.G., Lorenzo J.A. Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J. Clin. Endocrinol. Metab. 2010;95:1991–1997. doi: 10.1210/jc.2009-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi A., Hamaya E., Nishi K., Tolman C., Shimauchi J. Efficacy and safety of romosozumab among japanese postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. J. Bone Miner. Metab. 2022;40(4):677–687. doi: 10.1007/s00774-022-01332-8. [DOI] [PubMed] [Google Scholar]
- Moe S.M., Chen N.X., Newman C.L., Organ J.M., Kneissel M., Kramer I., Gattone V.H., Allen M.R. Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J. Bone Miner. Res. 2015;30:499–509. doi: 10.1002/jbmr.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Inaba M., Yamada S., Emoto M., Ohno Y., Tsujimoto Y. Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; an observational study. J. Bone Miner. Metab. 2021;39:1082–1090. doi: 10.1007/s00774-021-01253-y. [DOI] [PubMed] [Google Scholar]
- Sutherland M.K., Geoghegan J.C., Yu C., Turcott E., Skonier J.E., Winkler D.G., Latham J.A. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Thambiah S., Roplekar R., Manghat P., Fogelman I., Fraser W.D., Goldsmith D., Hampson G. Circulating sclerostin and Dickkopf-1 (DKK1) in predialysis chronic kidney disease (CKD): relationship with bone density and arterial stiffness. Calcif. Tissue Int. 2012;90:473–480. doi: 10.1007/s00223-012-9595-4. [DOI] [PubMed] [Google Scholar]
- Vilaca T., Salam S., Schini M., Harnan S., Sutton A., Poku E., Allen I.E., Cummings S.R., Eastell R. Risks of hip and nonvertebral fractures in patients with ckd g3a–g5d: a systematic review and meta-analysis. Am. J. Kidney Dis. 2020;76:521–532. doi: 10.1053/j.ajkd.2020.02.450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.