Introduction
The management of pelvic recurrence after hysterectomy depends on whether the patient has previously received pelvic irradiation and whether the recurrence site is pelvic central or pelvic sidewall. The 2022 National Comprehensive Cancer Network guidelines for uterine cervical cancer recommend that patients with pelvic sidewall recurrence without a prior history of irradiation are treated primarily with surgical resection, external beam radiation therapy (EBRT), or chemotherapy.1 However, pelvic sidewall recurrence has a worse prognosis than central recurrence,2, 3, 4 and EBRT do not provide a sufficient dosage for the recurrent tumor, making eradication difficult. On the other hand, interstitial brachytherapy (ISBT) can prescribe large doses to pelvic sidewall tumors and is a useful tool for tumor control, and this modality is effective in reducing the dose to the surrounding normal organs (organs at risk [OARs]) due to its steep dose gradient. However, when OARs are in close proximity to the tumor, it is difficult to prescribe a sufficient dose to the tumor while keeping the dose to the OARs low, even with brachytherapy. Here, we report a postoperative patient with recurrent uterine cervical adenocarcinoma in the pelvis who had sigmoid colon adhesion right above the recurrent tumor and underwent brachytherapy after a laparotomy to directly insert a spacer between the tumor and the sigmoid colon, which led to adequate dose delivery with ISBT and local control with no serious side effects.
Methods and Materials
A 44-year-old Japanese woman who had undergone abdominal radical hysterectomy for uterine cervical adenocarcinoma with stage IB1 based on the 2009 edition of the International Federation of Gynecology and Obstetrics stage and was followed up for 1 year and 4 months without any adjuvant treatment received a diagnosis of left pelvic sidewall recurrence. Due to the tumor's adhesion to the pelvic wall, salvage surgery was judged to be difficult, and radical radiation therapy (RT) was decided to be performed. However, there was a possibility of adhesion between the tumor and the sigmoid colon, which meant a risk that the radiation dose to the sigmoid colon would exceed its tolerable dose when a tumoricidal dose was delivered to the recurrent tumor (Fig. 1). Therefore, before salvage RT, surgery to insert a spacer in the pelvic floor under direct visual guidance was performed to create space between the bowel and the recurrent tumor. The details of the surgery are as follows: the sigmoid colon's mesentery and the left pelvic wall as well as the sigmoid colon's mesentery and the left common iliac artery and vein were found to be mildly adherent to the recurrent tumor at the time of laparotomy. Additionally, a recurrent tumor without mobility was palpable on the left side of the pelvic floor in the retroperitoneum. Next, a polypropylene mesh implant 150 × 150 mm in size for the treatment of intestinal hernia (Bard Soft Mesh; Becton, Dickinson and Company, Franklin Lakes, NJ) was folded 6 times and stitched together with sutures (Vicryl 3-0; Ethicon, Inc; Johnson & Johnson, Somerville, NJ), which was inserted into the pelvic floor as a spacer. After confirming that the sigmoid colon had separated from the pelvic wall, the spacer was fixed with sutures (Prolene 3-0 stitches; Ethicon, Inc; Vicryl 3-0; Ethicon, Inc) and the surgery was completed (Fig. 2). The subsequent computed tomography (CT) images revealed an approximately 10-mm-thick spacer between the tumor and the adjacent sigmoid colon (Fig. 3). Then, salvage radical RT was started, which consisted of a combination of EBRT and high-dose-rate ISBT (HDR-ISBT). EBRT was performed at 50 Gy in 25 fractions with the 4-fields box irradiation technique of 0°, 90°, 180°, and 270° portals, using high-energy, 15-MV x-ray photons from a linear accelerator (Fig. 4). The following is a detailed description of the HDR-ISBT treatment procedure: With the patient in the lithotomy position under general and epidural anesthesia, a total of 14 intersitital needle applicators were inserted transperineally freehand, parallel and evenly spaced at 5- to 10-mm interval, guided by a transrectal ultrasound to cover the 30 x 30 x 50 mm sized tumor. Simultaneous CT scanning was performed using a large-bore CT scanner (Aquilion LB; Canon, Tokyo, Japan), which was capable of imaging patients lying in the lithotomy position with applicators in place without moving them, and image-guided brachytherapy planning was performed using those CT images with a slice interval of 2 mm. Dose calculation was performed by the planning system (PLATO, Nucletron, Veenendaal, The Netherlands), and irradiation was performed with MicroSelectron HDR (Nucletron). The clinical target volume (CTV) was defined as the gross tumor volume using CT scans taken immediately after the needle was inserted. On the surface of the CTV, reference points were marked and a dose calculation was performed so that each point received 6 Gy per fractionation, up to a total of 24 Gy in 4 fractions. Dose calculation was performed by a manual graphical modification to completely cover the CTV with the 100% prescribed isodose line of 6 Gy while minimizing dose to OARs (Fig. 5). Needle applicators were fixed and treated twice daily, every 6 hours (Fig. E1). CT scanning was used to evaluate and correct for needle displacement before each morning session, and if needle displacement was greater than 5 mm, the needles were relocated and CT scanning was performed before the evening session as well. The combined dose of EBRT and HDR-ISBT was calculated using the linear-quadratic dose-effect model of the equivalent dose in 2-Gy fractions (EQD2).5, 6, 7
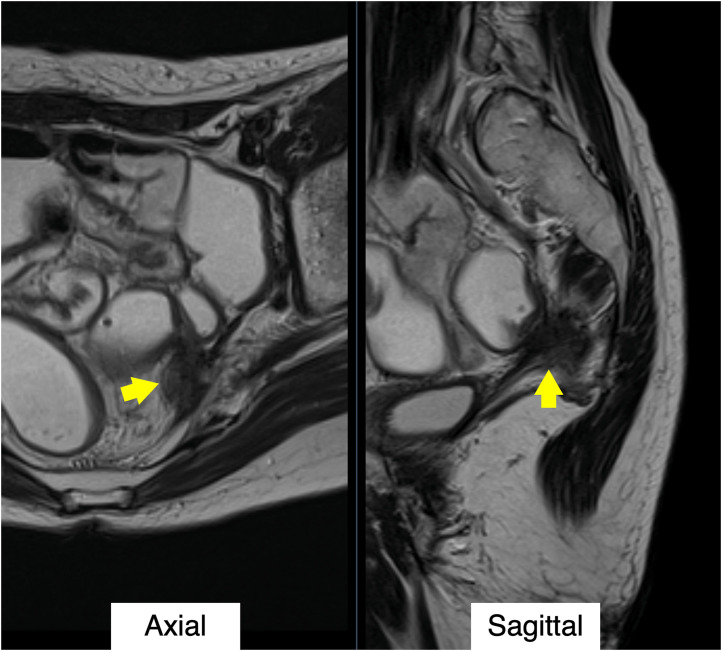
Figure 1.
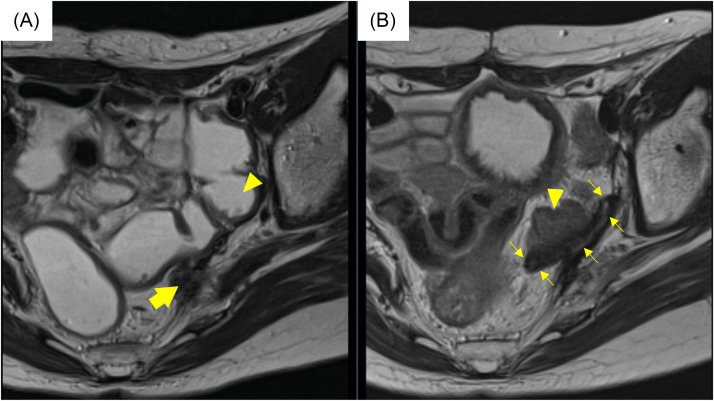
T2-weighted magnetic resonance images before the salvage treatment. The recurrence tumor (arrows) was adjacent to a sigmoid colon. The possibility of adhesions between the tumor and the sigmoid colon was suspected.
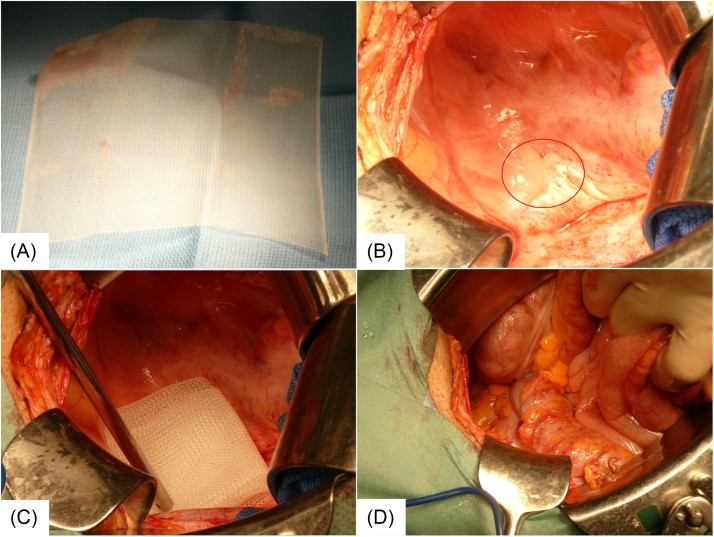
Figure 2.
The procedure of inserting a spacer into the pelvic floor. (A) A polypropylene mesh implant 150 × 150 mm in size for the treatment of intestinal hernia was used. (B) After inverting the intestine, the lower portion of the abdominal cavity was examined; the sigmoid colon was mildly adherent to the retroperitoneum, but there was no direct adhesion to the tumor (red circle). (C) The mesh implant, folded 6 times and stitched together with sutures, was inserted into the pelvic floor as a spacer. (D) The inverted intestine was placed back on the spacer, and the operation was completed.
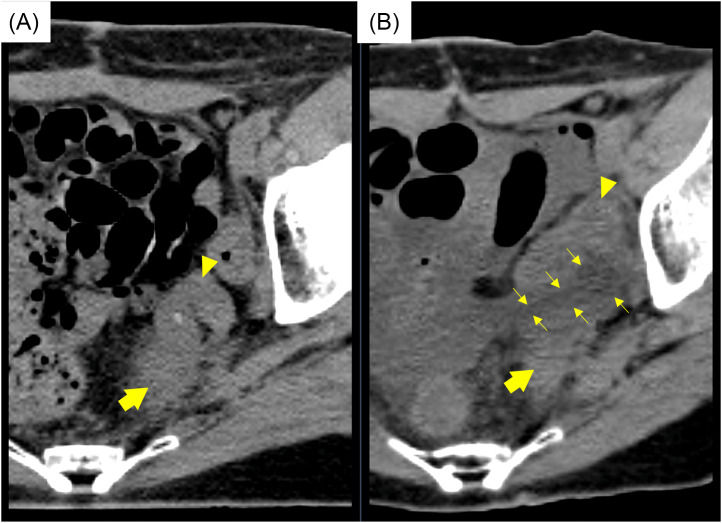
Figure 3.
Comparison of computed tomography images between (A) before treatment and (B) after spacer insertion. The tumor (thick arrows) was separated from the sigmoid colon (arrow heads) due to a spacer (thin arrows).
Figure 4.
A dose distribution of external beam radiation therapy with the 4-field box irradiation technique of 0°, 90°, 180°, and 270° portals. This patient was prescribed a dose of 50 Gy in 25 fractions.
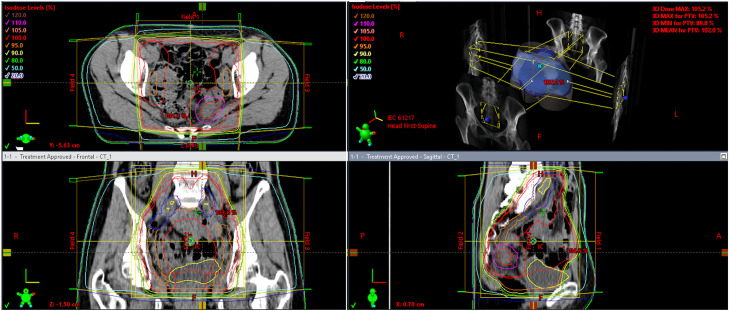
Figure 5.
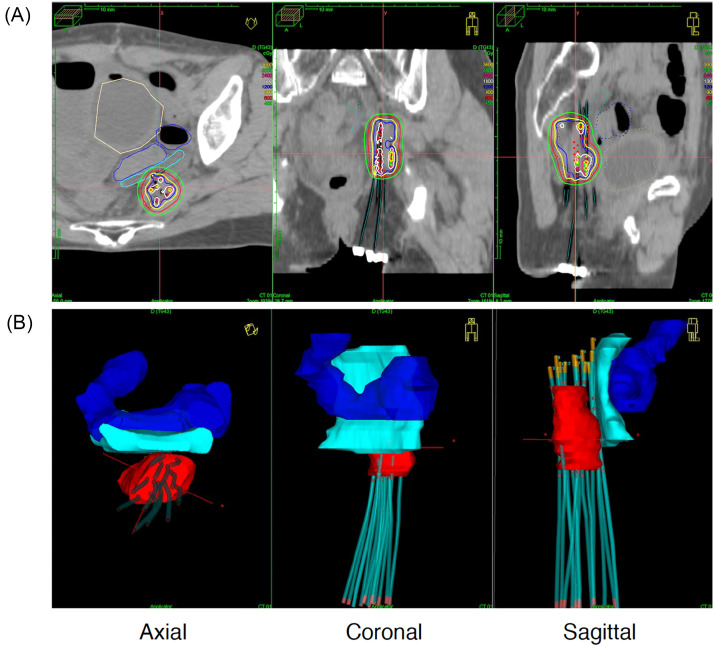
Brachytherapy treatment planning calculated by PLATO and shown in Oncentra (Nucletron, Veenendaal, The Netherlands). (A) The upper panel shows the dose distribution of brachytherapy. (B) The lower panel shows a 3-dimensional reconstruction of the tumor (red), the needle applicators, the sigmoid colon (blue), and the spacer (cyan).
Results
In this case, the minimum dose of EQD2 that covered 90% of the CTV was 94.5 Gy (α/β = 10 Gy). The doses delivered to the rectum, bladder, and sigmoid colon's most exposed 2.0 cm3 were 57.8, 60.6, and 60.1 Gy in EQD2, respectively (α/β = 3 Gy). Three weeks after the end of treatment, the tumor was no longer palpable on internal and rectal examinations, and CT scans and magnetic resonance images 3 months later confirmed the tumor's disappearance (Fig. 6). However, 5 months after tumor disappearance was confirmed (8 months after the end of treatment), CT scans revealed multiple lung metastases. Subsequent chemotherapy slowed tumor progression, but 2 years and 1 month after the end of the salvage HDR-ISBT, she died of respiratory failure due to multiple lung metastases without pelvic failure. Neither the radiation therapy nor the placement of the spacer caused any adverse events while the patient was still alive.
Figure 6.
Comparison of computed tomography images between (A) before treatment and (B) 2 years after the salvage treatment. The tumor (thick arrow), adjacent to the sigmoid colon (arrow heads), was disappeared. The spacer (thin arrows) was a nonabsorbable material and still remained in the pelvis.
Discussion
We used intraperitoneal spacers to protect her sigmoid colon from RT, especially brachytherapy. One of the characteristics of brachytherapy is its steep dose gradient; it delivers a high dose intensively to the tumor, and the dose rapidly decreases as the inverse square of the distance from the radiation source increases. Therefore, if there is an organ for which we want to reduce radiation dose, we can reduce the dose by simply creating space between the organ and the radiation source. Without the spacer, the irradiated dose to the sigmoid colon would have been much higher. This strategy, although it requires surgery, is theoretically reliable and has the advantage of reproducibility in creating the distance between the recurrent tumor and the sigmoid colon at each brachytherapy session. Artificial ascites8,9 and hyaluronic acid gel injected into the vesicovaginal and rectovaginal spaces10, 11, 12, 13, 14 are also used as other means of dose reduction for OARs in pelvic brachytherapy. However, these methods cannot ensure reproducibility for each treatment due to inconsistent injection volume and position, as well as the volume of artificial ascites changing over time because of absorption. Additionally, these methods are unable to separate the distance between OARs and tumors that are in close proximity due to adhesion. Of course, these methods are relatively less invasive to perform as they require no surgery, and they are effective at reducing the dose of OARs. With adequate patient understanding and consent, intraperitoneal spacer insertion can be an extremely effective and reliable way to accomplish this goal.
Regarding the spacer placement method, Dalwadi et al reported a case with a pelvic central recurrence of endometrial cancer by laparoscopic insertion of a spacer into the peritoneal cavity just above the tumor, followed by EBRT and brachytherapy.15 They reported that they were able to prescribe up to 79.7 Gy in EQD2 for CTV and that the patient achieved a complete response for more than 1 year without any serious adverse events. In the current case, laparotomy was performed due to organ adhesions, but less invasive intraperitoneal spacer placement might have been considered if possible.
The following are some limitations and future considerations: (1) insert spacers using laparoscopy or robotic surgery, which is less invasive than open laparotomy; (2) perform a long-term follow-up for similar situations and investigate whether the nonabsorbable spacers, which have the risk of abdominal infection caused by residual foreign material or intestinal perforation caused by spacers’ rigidity or size associated with prolonged use16, are safe; and (3) assess whether the nonabsorbable spacers can be replaced with nonsurgically removable spacers under development17 or bioresorbable spacers such as polyglycolic acid spacers (Neskeep; Alfresa Pharma Co, Osaka, Japan) used in carbon ion radiation therapy.18,19
Conclusion
Intraperitoneal spacers are beneficial not only for protecting OARs during HDR-ISBT but also for good local control.
Acknowledgments
We are grateful to Dr Koji Inaba, Dr Kana Takahashi, Dr Tomoya Kaneda, Dr Tairo Kashihara, Dr Ayaka Takahashi, Dr Yuri Shimizu, Dr Kanako Kojima, Dr Madoka Sakuramachi, Dr Tomomi Aoshika, Dr Kousuke Morishima, and all of our colleagues who helped us with treatment.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: Dr Igaki received a research grant from HekaBio, Cancer Intelligence Care Systems, Inc., and Elekta KK, consulting fees from HekaBio, and lecture fees from Varian, Itochu, Cancer Intelligence Care Systems, Inc., and Himedic. Dr Itami received a research grant from Elekta and consulting fees from AlphaTAU, HekaBio, and Palette Science. No other disclosures were reported.
All data generated and analyzed during this study are included in this published article.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2022.101118.
Appendix. Supplementary materials
References
- 1.National Comprehensive Cancer Network (NCCN) National Comprehensive Cancer Network; 2022. NCCN Guidelines for Cervical Cancer V.1.2022.Available at: https://www.nccn.org/guidelines/category_1 Accessed February 9, 2022. [Google Scholar]
- 2.Deutsch M, Parsons JA. Radiotherapy for carcinoma of the cervix recurrent after surgery. Cancer. 1974;34:2051–2055. doi: 10.1002/1097-0142(197412)34:6<2051::aid-cncr2820340625>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Larson DM, Copeland LJ, Stringer CA, Gershenson DM, Malone JM, Jr, Edwards CL. Recurrent cervical carcinoma after radical hysterectomy. Gynecol Oncol. 1988;30:381–387. doi: 10.1016/0090-8258(88)90252-1. [DOI] [PubMed] [Google Scholar]
- 4.Jobsen JJ, Leer JW, Cleton FJ, Hermans J. Treatment of locoregional recurrence of carcinoma of the cervix by radiotherapy after primary surgery. Gynecol Oncol. 1989;33:368–371. doi: 10.1016/0090-8258(89)90529-5. [DOI] [PubMed] [Google Scholar]
- 5.Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515–528. doi: 10.1259/0007-1285-58-690-515. [DOI] [PubMed] [Google Scholar]
- 6.Fowler JF. Sensitivity analysis of parameters in linear-quadratic radiobiologic modeling. Int J Radiat Oncol Biol Phys. 2009;73:1532–1537. doi: 10.1016/j.ijrobp.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Bentzen SM, Dörr W, Gahbauer R, et al. Bioeffect modeling and equieffective dose concepts in radiation oncology—Terminology, quantities and units. Radiother Oncol. 2012;105:266–268. doi: 10.1016/j.radonc.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Murakami N, Shima S, Okuma K, et al. Artificial ascites for organs at risk sparing in intrapelvic brachytherapy: A case report of recurrent uterine cervical carcinoma adjacent to the bowel. BJR Case Rep. 2019;5 doi: 10.1259/bjrcr.20180067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karube M, Murakami N, Okamoto H, et al. Transvaginal artificial ascites infusion as a spacer in gynecological brachytherapy: A novel technique. J Contemp Brachytherapy. 2020;12:487–491. doi: 10.5114/jcb.2020.100382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kishi K, Sonomura T, Shirai S, Sato M, Tanaka K. Critical organ preservation in reirradiation brachytherapy by injectable spacer. Int J Radiat Oncol Biol Phys. 2009;75:587–594. doi: 10.1016/j.ijrobp.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 11.Kashihara T, Murakami N, Tselis N, et al. Hyaluronate gel injection for rectum dose reduction in gynecologic high-dose-rate brachytherapy: Initial Japanese experience. J Radiat Res. 2019;60:501–508. doi: 10.1093/jrr/rrz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami N, Shima S, Kashihara T, et al. Hyaluronic gel injection into the vesicovaginal septum for high-dose-rate brachytherapy of uterine cervical cancer: An effective approach for bladder dose reduction. J Contemp Brachytherapy. 2019;11:1–7. doi: 10.5114/jcb.2019.82612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murakami N, Nakamura S, Kashihara T, et al. Hyaluronic acid gel injection in rectovaginal septum reduced incidence of rectal bleeding in brachytherapy for gynecological malignancies. Brachytherapy. 2020;19:154–161. doi: 10.1016/j.brachy.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Iijima K, Murakami N, Nakamura S, et al. Configuration analysis of the injection position and shape of the gel spacer in gynecologic brachytherapy. Brachytherapy. 2021;20:95–103. doi: 10.1016/j.brachy.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 15.Dalwadi S, Suri A, Kamat A, Butler EB, Farach AM. Laparoscopic allograft spacer placement to minimize bowel dose during re-irradiation with interstitial brachytherapy. Cureus. 2019;11:e5958. doi: 10.7759/cureus.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schörghofer A, Drerup M, Kunit T, et al. Rectum-spacer related acute toxicity - Endoscopy results of 403 prostate cancer patients after implantation of gel or balloon spacers. Radiat Oncol. 2019;14:47. doi: 10.1186/s13014-019-1248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo N, Yokobori T, Takahashi R, et al. An abdominal spacer that does not require surgical removal and allows drainage of abdominal fluids in patients undergoing carbon ion radiotherapy. PLoS One. 2020;15 doi: 10.1371/journal.pone.0234471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akasaka H, Sasaki R, Miyawaki D, et al. Preclinical evaluation of bioabsorbable polyglycolic acid spacer for particle therapy. Int J Radiat Oncol Biol Phys. 2014;90:1177–1185. doi: 10.1016/j.ijrobp.2014.07.048. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki R, Demizu Y, Yamashita T, et al. First-in-human phase 1 study of a nonwoven fabric bioabsorbable spacer for particle therapy: Space-making particle therapy (SMPT) Adv Radiat Oncol. 2019;4:729–737. doi: 10.1016/j.adro.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.