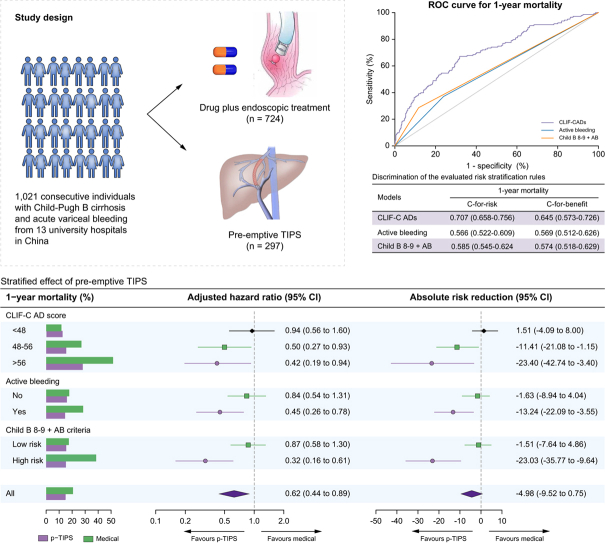
Abstract
Background & Aims
Among individuals with Child-Pugh B cirrhosis and acute variceal bleeding (AVB), the Baveno VII workshop recommended pre-emptive TIPS in those with a Child-Pugh score of 8-9 and active bleeding at initial endoscopy (Child B8-9 + AB criteria). Nevertheless, whether this criterion is superior to the CLIF-Consortium acute decompensation score (CLIF-C ADs) remains unclear.
Methods
Data on 1,021 consecutive individuals with Child-Pugh B cirrhosis and AVB from 13 university hospitals in China who were treated with pre-emptive TIPS (n = 297) or drug plus endoscopic treatment (n = 724) between 2010 to 2019 were retrospectively analysed. A competing risk regression model was used to compare the outcomes between the two groups after adjusting for confounders. The concordance-statistic for benefit (c-for-benefit) was used to evaluate a models’ ability to predict treatment benefit (risk difference between treatment groups).
Results
Pre-emptive TIPS was associated with reduced mortality compared to drug plus endoscopic treatment (adjusted hazard ratio 0.62, 95% CI 0.44 to 0.88). A higher baseline CLIF-C AD score was associated with greater survival benefit (i.e., larger absolute mortality risk reduction). After adjusting for confounders, a survival benefit was observed in individuals with CLIF-C ADs ≥48 or Child-Pugh B8-9 with active bleeding, but not in those with CILF-C ADs <48, no active bleeding or Child-Pugh B7 with active bleeding. The c-for-benefit of CILF-C ADs for predicting survival benefit was higher than that of Child B8-9+AB criteria.
Conclusions
In individuals with Child-Pugh B cirrhosis and AVB, CLIF-C ADs predicts survival benefit from pre-emptive TIPS and outperforms the Child B8-9+AB criteria. Prospective validation should be performed to confirm this result, especially for other aetiologies of cirrhosis.
Impact and implications
In this study, among individuals with Child-Pugh B cirrhosis and acute variceal bleeding, the CLIF-Consortium acute decompensation (CLIF-C AD) score could predict the survival benefit from pre-emptive TIPS, with patients with higher CLIF-C AD scores benefiting more from pre-emptive TIPS. Furthermore, the CLIF-C AD score outperformed the Child B8-9 plus active bleeding criteria in terms of discriminating between those who obtained more benefit vs. less benefit from pre-emptive TIPS. Depending on prospective validation, the CLIF-C AD score could be used as the model of choice to determine who should undergo pre-emptive TIPS.
Keywords: Cirrhosis, Variceal bleeding, Portal hypertension, Transjugular intrahepatic portosystemic shunt, Risk stratification
Abbreviations: AB, active bleeding; AVB, acute variceal bleeding; ACLF, acute-on-chronic liver failure; CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; HR, hazard ratio; MELD, model for end-stage liver disease; INR, international normalised ratio; TIPS, transjugular intrahepatic portosystemic shunt
Graphical abstract
Highlights
-
•
In those with Child-Pugh B cirrhosis and acute variceal bleeding, CLIF-C AD score could predict survival benefit from pre-emptive TIPS.
-
•
Active bleeding at endoscopy was associated with increased mortality in individuals with Child-Pugh B8-9 (but not B7) cirrhosis.
-
•
Pre-emptive TIPS improves survival in individuals with Child-Pugh B8-9 cirrhosis and active bleeding at endoscopy.
-
•
CLIF-C AD score is superior to active bleeding + Child-Pugh B8-9 to predict survival benefit with preemptive TIPS.
Introduction
Acute variceal bleeding (AVB) is the most life-threatening complication of cirrhosis.1 Despite improvements in the management of AVB in the past three decades, it is still associated with significant mortality. Studies have suggested that the risk of mortality varies widely among individuals with AVB. Thus, risk stratification and tailoring the therapeutic strategy to the expected risk is a reasonable approach.[1], [2], [3], [4] This approach is especially relevant now that several studies have demonstrated that “early” use of transjugular intrahepatic portosystemic shunt (TIPS) as a “pre-emptive strategy” leads to a significant improvement in survival of individuals with AVB at high risk of treatment failure.[5], [6], [7], [8] However, while the survival benefit associated with pre-emptive TIPS is clear in patients with Child-Pugh C (<14 points) cirrhosis,7,9,10 its role in those with Child-Pugh B cirrhosis remains a matter of debate.[9], [10], [11], [12], [13]
Currently, the only available risk stratification tool for Child-Pugh B cirrhosis is active bleeding at endoscopy despite intravenous vasoactive drug therapy. However, active bleeding at endoscopy has been criticised for overestimating the risk of death in individuals with Child-Pugh B cirrhosis and because of its subjectivity[14], [15], [16]. A recent meta-analysis of individual patient data suggested that only individuals with Child-Pugh scores of 8-9 and active bleeding at endoscopy (Child B8-9+AB criteria) are at high risk of death and can derive survival benefit from pre-emptive TIPS.7 Based on this finding, the Baveno VII workshop recommend use of pre-emptive TIPS in those with Child-Pugh 8-9 cirrhosis and active bleeding at initial endoscopy.17 On the other hand, a study from our team showed that the Chronic Liver Failure-Consortium acute decompensation score (CLIF-C ADs) was more accurate than active bleeding at endoscopy for predicting short-term and long-term mortality in individuals with Child-Pugh B cirrhosis and AVB.18 Furthermore, risk stratification using CLIF-C ADs with cut-off values of 48 and 56 identifies a subgroup with high risk of death that may benefit from pre-emptive TIPS.18 Nonetheless, it remains unclear whether Child B8-9+AB criteria is superior to CLIF-C ADs for predicting the treatment benefit from pre-emptive TIPS.
Therefore, we performed the present analysis to compare the CLIF-C ADs with Child B8-9+AB criteria and active bleeding at endoscopy for predicting the treatment benefit from pre-emptive TIPS.
Patients and methods
Study design and patients
We retrospectively included consecutive patients with cirrhosis who were admitted for AVB at six tertiary academic hospitals in China (Xijing Hospital, First Affiliated Hospital of Nanchang University, First Affiliated Hospital of Xi'an Jiaotong University, Nanfang Hospital of Southern Medical University, First Affiliated Hospital of Zhejiang University and First Affiliated Hospital of Soochow University) from June 2016 to December 2019. To increase the statistical power and effect size, the already collected individual data from two published studies were combined with current cohort data. The first study9 was a multicentre observational study in which consecutive patients with cirrhosis and AVB were treated with drug plus endoscopic treatment or (early) pre-emptive TIPS at 12 tertiary academic hospitals in China from December 2010 to June 2016. The second study6 was a randomised controlled trial (RCT, NCT01370161), in which eligible individuals with cirrhosis and AVB were randomly assigned (2:1) to receive (early) pre-emptive TIPS or drug plus endoscopic treatment between June 2011 and September 2017. The ethics committees of all participating hospitals approved the study protocol and all participants included in the study provided written informed consent.
The inclusion criteria for the present study were: (i) diagnosis of cirrhosis (based on clinical signs, laboratory, and imaging tests or liver biopsy); (ii) admission due to AVB confirmed by endoscopy according to Baveno VI definitions;3 (iii) with a liver function of Child-Pugh B class (7-9 points). Patients were excluded if they fulfilled any of the exclusion criteria of the early TIPS trial:5 age more than 75 years, hepatocellular carcinoma that did not meet the Milan criteria, a creatinine level ≥3 mg/dl (265 μmol/L), bleeding from isolated gastric or ectopic varices, complete portal vein thrombosis, recurrent hepatic encephalopathy, heart failure, previous liver transplantation and previous TIPS.
Pre-emptive TIPS was defined as placement of covered TIPS within 72 h of admission as preventive therapy prior to recurrent bleeding after a combination of vasoactive drugs, prophylactic antibiotics and endoscopic band ligation (EBL).5,6,19 Rescue TIPS was defined as TIPS placement as a salvage therapy for uncontrolled bleeding despite the use of a combination of intensive pharmacologic and endoscopic treatment.5,6,19
Therapeutic interventions
All patients were initially treated with a combination of vasoactive drugs and prophylactic antibiotics and EBL. Afterwards, patients received either pre-emptive TIPS (performed within 72 h after admission [pre-emptive TIPS group]) or drug plus endoscopic treatment (vasoactive drugs continued to day 5, followed by non-selective beta-blockers [NSBBs] plus EBL for the prevention of rebleeding, with TIPS as rescue therapy when needed [medical group]). In the previous observational study9 and the current cohort, whether to offer pre-emptive TIPS or drug plus endoscopic treatment was based on the individual centre policy and the treating physician’s opinion. In the RCT,6 the treatment of pre-emptive TIPS or drug plus endoscopic therapy for a patient was randomly allocated based on a secure web-based randomisation system.
All patients were followed-up to death, liver transplantation, until 1 year, or the end of study (December 30, 2021). The time of admission to hospital was considered the time zero for the follow-up. All prognostic variables were recorded and calculated within the first 24 h after admission.
Outcome and definitions
The primary endpoint for the analysis was 1-year mortality. The secondary endpoints were further bleeding (a composite outcome of failure to control acute bleeding or rebleeding defined according to the Baveno VI consensus,3 and the development of overt hepatic encephalopathy (OHE, diagnosed and graded according to the West-Haven criteria20) at 1 year.
Risk-stratification systems
We evaluated the effect of pre-emptive TIPS vs. drug plus endoscopic therapy on outcomes by stratifying individuals with Child-Pugh B cirrhosis based on (i) CLIF-C ADs 48-56 (low risk/intermediate risk/high risk: CLIF-C ADs <48/48-56/>56),18 (ii) presence vs. absence of active bleeding, and (iii) Child B8-9+AB criteria (low risk: Child-Pugh B without active bleeding [Child B + no AB] and Child-Pugh B7 with active bleeding [Child B7+AB]; high risk: Child-Pugh B8-9 with active bleeding [Child B8-9+AB]).7
Statistical analysis
All statistical analyses were performed using the R 3.6.1 (http://www.r-project.org/) with the add-on packages Hmisc, rms, riskRegression, pec, prodlim and Matchit. For all analyses, missing data of the covariates were imputed with multiple imputations methods (detailed in the supplementary methods). Statistical significance was set at p <0.05 (2-sided).
Data are presented as frequencies (percentage), mean ± SD or median (IQR) as appropriate. Comparisons of variables between groups were performed using Student’s t test, non-parametric Mann-Whitney U test, chi-squared test or Fisher’s exact test as appropriate. Cumulative incidence curves were constructed using Fine-Gray competing risks analysis to estimate the risk of death over time, considering liver transplantation as a competing event. When the cumulative incidence of further bleeding and OHE were estimated, death and liver transplantation were considered as competing risks.
The non-linear relationships between the CLIF-C ADs and the risk of the evaluated outcomes were visualised using restricted cubic splines by entering the CLIF-C ADs as a continuous variable into the logistic regression analysis. We evaluated the association between the treatment group (pre-emptive TIPS vs. medical) and clinical outcomes in three competing risk regression models, which provided unadjusted and confounder-adjusted estimates of hazards ratios (HRs) and absolute risk reduction with 95% CI: 1) unadjusted. 2) adjusted for severity of liver disease and other potential confounders. Potential confounders were variables that were significantly associated with the outcome in univariate analysis at a level of 10% or previously reported as potentially influencing the outcomes (regardless of the p value in univariate analysis). Within each risk category, the components (age, white blood cell, creatinine, international normalised ratio [INR], and sodium) instead of the overall CLIF-C ADs were included in the models to allow for fine-tuning of the models. 3) adjusted for propensity score that was constructed with logistic regression modelling the probability of a patient undergoing pre-emptive TIPS or medical treatment (detailed in the supplementary methods). Interaction testing was performed to determine if the relative or absolute treatment effects of pre-emptive TIPS vs. medical therapy varied across the risk categories. To verify the robustness of our results, we performed sensitivity analyses: (1) with propensity score matching (detailed in the supplementary methods) and (2) excluding patients with previous bleeding.
The discriminative performance of CLIF-C ADs in predicting the 6-week and 1-year mortality risk was compared with model for end-stage liver disease (MELD) score, active bleeding at endoscopy and Child B8-9+AB criteria using the concordance index (C-index), which is the area under the receiver-operating curve for survival time in the presence of censored data. The discrimination of treatment effect (defined as the difference between the probability of death between pre-emptive TIPS and medical groups) was assessed by the concordance index for benefit (c-for-benefit),21 which is a variant of the common C-index designed to specifically calculate the ability for a model to discriminate between people obtaining more benefit vs. less benefit from a treatment (rather than just higher vs. lower overall death risk).
Results
Baseline characteristics of patients
The assembled cohort yield 3,077 individuals with cirrhosis and AVB from 13 university hospitals in China, of whom 1,473 had Child-Pugh B cirrhosis. After excluding 452 individuals for the reasons shown in the Fig. S1, a total of 1,021 eligible participants were eventually included. The number of patients from each participating centre was shown in Table S1. Among the included patients, 724 (77.9%) received drug plus endoscopic therapy (medical group), and 297 (22.1%) received pre-emptive TIPS (pre-emptive TIPS group). The baseline characteristics of the patients are summarised in Table 1. The mean age was 52.8 ± 7.5 years, with a mean MELD score of 12.2 ± 3.4 and a CLIF-C ADs of 45.8 ± 8.9. HBV was the most common aetiology of cirrhosis (61.3%) and a total of 195 patients were in viral remission at bleeding presentation. Patients in the pre-emptive TIPS group had more severe liver disease (as reflected by higher Child-Pugh scores) and were more likely to have active bleeding at endoscopy, but they had a lower likelihood of bacterial infection and detectable HBV DNA at admission.
Table 1.
Baseline characteristics and outcomes of participants (N = 1,021).
| Variable | Medical (n = 724) | p-TIPS (n = 297) | p value |
|---|---|---|---|
| Age (years) | 52.6 ± 12.4 | 53.4 ± 12.1 | 0.364# |
| Female sex, n (%) | 207 (28.6%) | 115 (38.9%) | <0.001$ |
| Aetiology of cirrhosis, n (%) | <0.001$ | ||
| Chronic HBV infection | 438 (60.5%) | 188 (63.3%) | |
| Chronic HCV infection | 28 (3.9%) | 20 (6.7%) | |
| Alcohol | 75 (10.4%) | 12 (4.0%) | |
| Others | 72 (9.9%) | 33 (11.1%) | |
| Miscellaneous | 74 (10.2%) | 40 (13.5%) | |
| Cryptogenic | 37 (5.1%) | 4 (1.3%) | |
| HBV DNA detectable, n (%) | 324 (44.8%) | 107 (36.0%) | 0.012$ |
| Child-Pugh score | 7.6 ± 0.7 | 7.8 ± 0.8 | 0.015# |
| 7 | 347 (47.9%) | 119 (40.1%) | |
| 8 | 258 (35.6%) | 109 (36.7%) | |
| 9 | 119 (16.4%) | 69 (23.2%) | |
| MELD score | 12.1 ± 3.4 | 12.5 ± 3.2 | 0.110# |
| CLIF-C AD score | 45.8 ± 8.9 | 45.7 ± 8.9 | 0.839# |
| CLIF-C ADs 48-56 | 0.966$ | ||
| <48 | 432 (59.7%) | 175 (58.9%) | |
| 48-56 | 213 (29.4%) | 89 (30.0%) | |
| >56 | 79 (10.9%) | 33 (11.1%) | |
| Child B 8-9+AB criteria¶ | <0.001$ | ||
| Low risk | 615 (84.9%) | 212 (71.6%) | |
| High risk | 109 (15.1%) | 84 (28.4%) | |
| Active bleeding at endoscopy†, n (%) | 192 (26.5%) | 131 (44.1%) | <0.001$ |
| Location of varices at index gastroscopy, n (%) | 0.092$ | ||
| Oesophageal varices only | 508 (70.2%) | 192 (64.6%) | |
| Oesophageal and gastric varices | 216 (29.8%) | 105 (35.4%) | |
| Size of varices (large), n (%) | 678 (93.6%) | 274 (92.3%) | 0.496$ |
| Previous variceal bleeding, n (%) | 392 (54.1%) | 201 (67.7%) | <0.001$ |
| Previous hepatic encephalopathy, n (%) | 20 (2.8%) | 12 (4.0%) | 0.381$ |
| Previous ascites, n (%) | 151 (20.9%) | 100 (33.7%) | <0.001$ |
| Hepatic encephalopathy at admission, n (%) | 18 (2.5%) | 15 (5.1%) | 0.055$ |
| Ascites at admission, n (%) | <0.001$ | ||
| Mild | 306 (42.3%) | 131 (44.1%) | |
| Moderate | 124 (17.1%) | 67 (22.6%) | |
| Massive | 41 (5.7%) | 24 (8.1%) | |
| White blood cell ( × 109 cell/L) | 7.1 ± 5.0 | 6.2 ± 4.3 | 0.013# |
| Haemoglobin (g/L) | 76.4 ± 21.5 | 74.4 ± 19.1 | 0.158# |
| Platelet count ( × 109/L) | 81.4 ± 57.2 | 74.3 ± 58 | 0.073# |
| International normalised ratio | 1.4 ± 0.3 | 1.5 ± 0.5 | <0.001# |
| Albumin (g/L) | 28 ± 4.9 | 29.4 ± 4.7 | <0.001# |
| Bilirubin (mg/dl) | 1.6 ± 1.4 | 1.6 ± 1.1 | 0.911# |
| Creatinine (mg/dl) | 0.9 ± 0.4 | 0.8 ± 0.3 | 0.110# |
| Sodium (mmol/L) | 138.6 ± 5 | 137.7 ± 5.8 | 0.011$ |
| Comorbidities‡, n (%) | 172 (23.8%) | 70 (23.6%) | 1.000$ |
| Hepatocellular carcinoma, n (%) | 23 (3.2%) | 11 (3.7%) | 0.808$ |
| Portal vein thrombosis, n (%) | 110 (15.2%) | 51 (17.2%) | 0.475$ |
| Heart rate at admission (beats/min) | 84.7 ± 16.1 | 83.9 ± 15.6 | 0.502# |
| Systolic blood pressure at admission (mm Hg) | 112 ± 17 | 111.8 ± 14.7 | 0.875# |
| Diastolic blood pressure at admission (mm Hg) | 66.8 ± 10.9 | 66.9 ± 10.1 | 0.885# |
| Infection at admission, n (%) | 66 (9.1%) | 15 (5.1%) | 0.041$ |
| Shock at admission∗, n (%) | 135 (18.6%) | 51 (17.2%) | 0.658$ |
| Blood transfusion, n (%) | 438 (60.5%) | 167 (56.2%) | 0.257$ |
| Vasoactive drugs, n (%) | 0.340$ | ||
| Octreotide | 555 (76.7%) | 225 (75.8%) | |
| Somatostatin | 124 (17.1%) | 59 (19.9%) | |
| Terlipressin | 45 (6.2%) | 13 (4.3%) | |
| Duration of vasoactive drugs, (days) | 4.6 ± 1.8 | 1.8 ± 0.7 | <0.001# |
Data presented as mean ± SD or number of patients (percentage) where appropriate.
CLIF-C AD score, Chronic Liver Failure-Consortium acute decompensation score; MELD, model for end-stage liver disease; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt.
Commodities include hypertension, coronary artery disease, and diabetes.
Child B8-9+AB criteria: Low risk: Child-Pugh B without active bleeding and Child-Pugh B7 with active bleeding; High risk: Child-Pugh B8-9 with active bleeding at endoscopy.
Active bleeding was defined as the presence of spurting or oozing from varices on endoscopy despite being on vasoactive drugs.
Hypovolemic shock was defined as systolic blood pressure <100 mmHg and heart rate >100 beats per minute.
Comparisons between groups of variables were done with the Student’s t test.
Comparisons between groups of variables were done with the chi-squared test.
Risk-stratified effects of pre-emptive TIPS on mortality
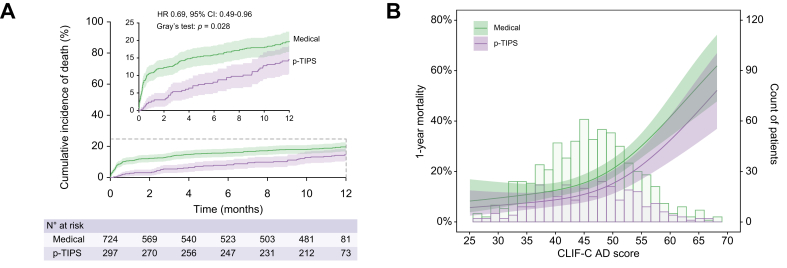
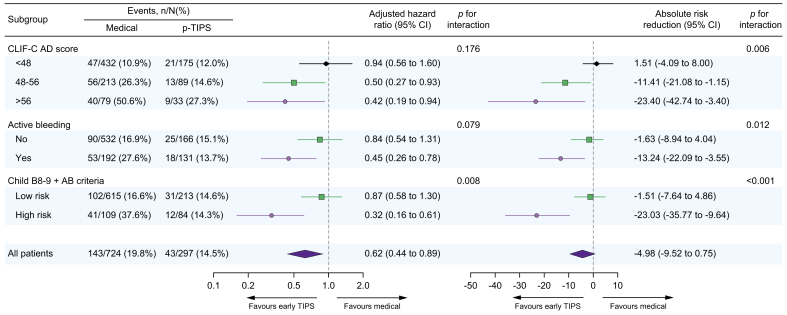
In the entire cohort, 95 patients (9.3%) died within 6 weeks and 186 patients (18.2%) died within 1 year. Seventeen patients (1.7%) and 63 patients (6.2%) received liver transplantation at 6 weeks and 1 year, respectively. The causes of death are summarised in Table 2. The cumulative incidences of death were significantly lower in the pre-emptive TIPS group (3.0% vs. 11.6% at 6 weeks, 14.5% vs. 19.8% at 1 year, p = 0.028) compared with the medical group (Fig. 1A). After adjustment for potential confounders (CLIF-C ADs, active bleeding, serum albumin, shock at admission and commodities, Table 3), pre-emptive TIPS was associated with a 38% relative risk reduction of death (adjusted HR 0.62; 95% CI 0.44 to 0.88; p = 0.008) at 1 year. This protective effect was consistent across the spectrum of CLIF-C ADs, but the absolute risk reduction was more pronounced in patients with high-risk profiles (Fig. 1B).
Table 2.
Summary of outcome measurements (N = 1,021).
| Variable | Medical (n = 724) | p-TIPS (n = 297) | p value |
|---|---|---|---|
| Rescue TIPS | 71 (9.8%) | 0 (0.0%) | <0.001 |
| Mortality, n (%) | 143 (19.8%) | 43 (14.5%) | 0.061 |
| Cause of death, n (%) | |||
| Liver failure | 41 (5.7%) | 19 (6.4%) | |
| Gastrointestinal bleeding | 60 (8.3%) | 7 (2.4%) | |
| Sepsis/pneumonia | 7 (1.0%) | 2 (0.7%) | |
| Hepatocellular carcinoma | 4 (0.6%) | 3 (1.0%) | |
| Multiorgan failure | 8 (1.1%) | 4 (1.3%) | |
| Unrelated with liver disease | 7 (1.0%) | 6 (2.0%) | |
| Unknown | 16 (2.2%) | 2 (0.7%) | |
| Liver transplantation, n (%) | 49 (6.8%) | 14 (4.7%) | 0.307 |
| Further bleeding, n (%) | 282 (39.0%) | 39 (13.1%) | <0.001 |
| Overt hepatic encephalopathy, n (%) | 212 (29.3%) | 89 (30.0%) | 0.862 |
Data presented as number of patients (percentage).
Comparisons between groups of variables were done with the chi-squared test.
p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt.
Fig. 1.
Competing risks analyses for all-cause mortality in entire cohort and the relationship between the mortality risk and CLIF-C AD scores stratified by treatment groups.
(A) Cumulative incidence of death in pre-emptive TIPS vs. medical groups (p = 0.028, Gray’s test) based on competing risk approach (the Fine and Gray method) with liver transplantation being the competing event. (B) Probability of death within 1 year and patient distribution in relation to CLIF-C AD score stratified by treatment groups (p = 0.042, logistic regression analysis). Restricted cubic splines were generated using logistic regression models after adjusting for active bleeding at endoscopy (yes vs. no), serum albumin (per g/L increase), platelet count (per 1 × 109/L increase), comorbidities (yes vs. no), infection (yes vs. no), and shock (yes vs. no). The coloured bands indicate 95% CIs. CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; HR, hazard ratio; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt.
Table 3.
Univariable and multivariable analysis of factors associated with 1-year mortality in the entire cohort (N = 1,021).
| Variable | Univariable analysis |
Multivariable analysis (model 1) |
Multivariable analysis (model 2) |
Multivariable analysis (model 3) |
||||
|---|---|---|---|---|---|---|---|---|
| HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | |
| Treatment (p-TIPS vs. medical) | 0.67 (0.48 to 0.94) | 0.021 | 0.59 (0.41 to 0.84) | 0.003 | 0.62 (0.44 to 0.88) | 0.008 | 0.62 (0.44 to 0.89) | 0.010 |
| Age (per year increase) | 1.04 (1.03 to 1.05) | <0.001 | 1.04 (1.03 to 1.05) | <0.001 | 1.04 (1.03 to 1.05) | <0.001 | ||
| MELD score (per point increase) | 1.12 (1.08 to 1.16) | <0.001 | 1.14 (1.10 to 1.18) | <0.001 | ||||
| CLIF-C ADs (per point increase) | 1.07 (1.05 to 1.08) | <0.001 | 1.06 (1.05 to 1.08) | <0.001 | ||||
| Active bleeding (yes vs. no) | 1.38 (1.02 to 1.85) | 0.034 | 1.55 (1.14 to 2.10) | 0.005 | 1.48 (1.09 to 2.00) | 0.012 | 1.52 (1.12 to 2.08) | 0.008 |
| WBC (per 1 × 109cell/L increase) | 1.04 (1.02 to 1.07) | <0.001 | 1.00 (0.96 to 1.03) | 0.838 | 1.00 (0.97 to 1.04) | 0.862 | ||
| INR (per unit increase) | 1.39 (1.12 to 1.72) | 0.003 | 1.47 (1.18 to 1.83) | 0.001 | ||||
| Bilirubin (per mg/dl increase) | 1.09 (1.02 to 1.17) | 0.012 | 1.14 (1.07 to 1.22) | <0.001 | ||||
| Serum albumin (per g/L increase) | 0.96 (0.93 to 0.98) | 0.002 | 0.96 (0.93 to 0.99) | 0.016 | 0.98 (0.95 to 1.01) | 0.135 | 0.96 (0.93 to 0.99) | 0.011 |
| Creatinine (per mg/dl increase) | 1.5 (1.23 to 1.83) | <0.001 | 1.71 (1.36 to 2.15) | <0.001 | ||||
| Platelet count (per 1 × 109/L increase) | 1 (1.00 to 1.00) | 0.023 | 1.00 (1.00 to 1.01) | 0.007 | 1.00 (1.00 to 1.00) | 0.315 | 1.00 (1.00 to 1.01) | 0.024 |
| Sodium (per mmol/L increase) | 0.97 (0.95 to 1.00) | 0.041 | 0.97 (0.95 to 0.99) | 0.013 | 0.97 (0.95 to 0.99) | 0.012 | ||
| Comorbidities‡ (yes vs. no) | 1.81 (1.34 to 2.45) | <0.001 | 1.37 (0.99 to 1.88) | 0.056 | 1.59 (1.17 to 2.17) | 0.003 | 1.43 (1.04 to 1.98) | 0.028 |
| Infection (yes vs. no) | 1.9 (1.24 to 2.92) | 0.003 | 1.60 (0.92 to 2.80) | 0.098 | 0.92 (0.58 to 1.48) | 0.738 | 1.58 (0.90 to 2.76) | 0.109 |
| Shock at admission∗ (yes vs. no) | 1.42 (1.01 to 2.00) | 0.043 | 1.32 (0.93 to 1.88) | 0.119 | 1.23 (0.87 to 1.74) | 0.244 | 1.36 (0.95 to 1.94) | 0.096 |
Only variables with a p value <0.1 in the univariable analysis are shown. Variables selected into the Univariable analysis were sex, age, aetiology of cirrhosis, HBV DNA or HCV-RNA detectable, MELD score, location of varices at index gastroscopy, grade of oesophageal varices, ascites, hepatic encephalopathy, previous bleeding, haemoglobin, serum albumin, serum total bilirubin, INR, serum creatinine, commodities, portal vein thrombosis, hepatocellular carcinoma, heart rate at admission, infection at admission, shock at admission and blood transfusion requirement.
CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; HR, hazard ratio; INR, international normalised ratio; MELD, model for end-stage liver disease; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt; WBC, white blood cell.
Commodities include hypertension, coronary artery disease, and diabetes.
Hypovolemic shock was defined as systolic blood pressure <100 mmHg and heart rate >100 beat per minute.
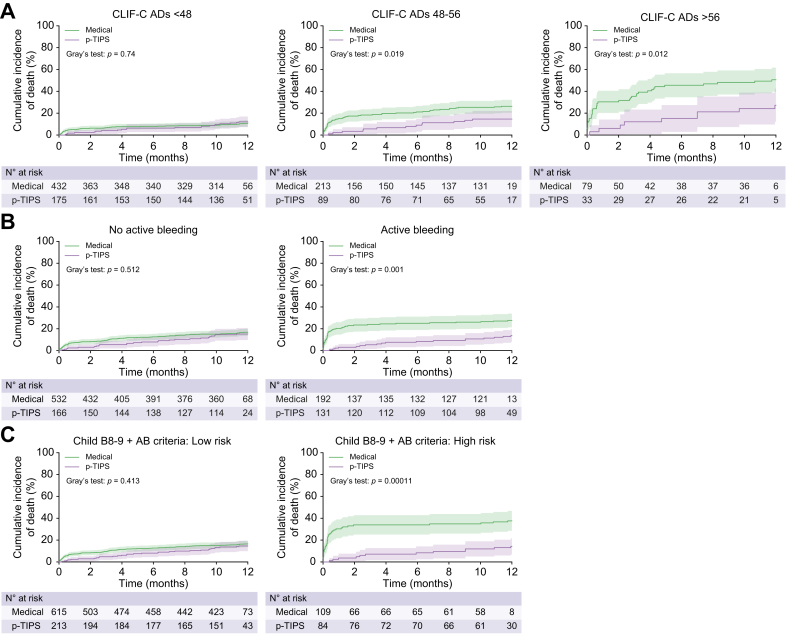
When stratified according to CLIF-C ADs of 48-56, the pre-emptive TIPS group had a significantly lower cumulative incidence of death in the CLIF-C ADs ≥56 category (6.1% vs. 30.4% at 6 weeks; 27.3% vs. 50.6% at 1 year; p = 0.012) and CLIF-C ADs 48-56 category (3.4% vs. 16.4% at 6 weeks; 14.6% vs. 26.3% at 1 year, p = 0.019) but not in CLIF-C ADs <48 category (2.3% vs. 5.8% at 6 weeks; 12.0% vs. 10.9% at 1 year, p = 0.740) compared with the medical group (Fig. 2A). This pattern persisted after adjusting for potential confounders, with the adjusted HRs (95% CI) being 1.10 (0.63 to 1.90), 0.48 (0.26 to 0.88), 0.41 (0.18 to 0.91) for CLIF-C ADs categories of <48/48-56/>56 at 1 year, respectively (Table 4). These results were confirmed by the analysis adjusting for propensity scores (Fig. 3). Although the relative risk reduction in mortality was homogeneous (p interaction =0.189), the 1-year absolute risk reduction of death with pre-emptive TIPS was greater in the CLIF-C ADs ≥56 category than the CLIF-C ADs 48-56 and CLIF-C ADs <48 categories (−23.4% vs. -11.4% vs. 1.5%, p interaction =0.006) after adjusting for propensity scores (Fig. 3). Similar results were observed when the cohort were stratified according to an individual CLIF-C ADs cut-off value of 48 (<48 or ≥48) (Fig. S2).
Fig. 2.
Effect of pre-emptive TIPS vs. drug plus endoscopic treatment on mortality by different risk stratification rules in those with Child-Pugh B cirrhosis.
(A) Cumulative incidence of death in pre-emptive TIPS group vs. medical group stratified by CLIF-C ADs of <48/48-56/>56 (p = 0.74, 0.019 and 0.012, respectively, Gary’s test) (B) active bleeding (p = 0.512 and 0.001, respectively, Gary’s test) and (C) Child B8-9 + AB criteria (p = 0.413 and 0.0011, respectively, Gary’s test) based on competing risk approach (the Fine and Gray method) with liver transplantation being the competing event. Child B8-9 + AB criteria: Low risk: Child-Pugh B without active bleeding and Child-Pugh B7 with active bleeding; High risk: Child-Pugh B8-9 with active bleeding at endoscopy. CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt.
Table 4.
Impact of pre-emptive TIPS (vs. drug plus endoscopic treatment) on 1-year mortality in individuals with Child-Pugh B cirrhosis according to different risk classification rules.
| Risk classification | No. of patients (%) | Univariable models Unadjusted estimates |
Multivariable models adjusted for the baseline predictive variables |
|||||
|---|---|---|---|---|---|---|---|---|
| HR∗ (95%CI) | p value | p for interaction | HR∗ (95%CI) | p value | p for interaction | Variables adjusted for‡ | ||
| CLIF-C ADs 48-56 | 0.064 | 0.176 | ||||||
| <48 | 607 (59.4%) | 1.05 (0.63 to 1.75) | 0.074 | 1.10 (0.63 to 1.90) | 0.740 | Age, WBC, bilirubin, albumin, creatinine, PLT, HCC | ||
| 48-56 | 302 (29.6%) | 0.49 (0.27 to 0.90) | 0.019 | 0.48 (0.26 to 0.88) | 0.018 | Age, active bleeding, bilirubin, PLT, comorbidities | ||
| >56 | 112 (11.0%) | 0.42 (0.20 to 0.86) | 0.012 | 0.41 (0.18 to 0.91) | 0.028 | Age, WBC, INR, bilirubin, albumin, creatinine, HCC | ||
| Active bleeding | 0.059 | 0.063 | ||||||
| No | 698 (68.4%) | 0.83 (0.54 to 1.30) | 0.512 | 0.80 (0.53 to 1.29) | 0.393 | Age, INR, bilirubin, albumin, creatinine, sodium | ||
| Yes | 323 (31.6%) | 0.43 (0.25 to 0.73) | 0.001 | 0.43 (0.25 to 0.75) | 0.003 | INR, bilirubin, creatinine, PLT, comorbidities, HCC | ||
| Child B 8-9+AB criteria¶ | 0.006 | 0.009 | ||||||
| Low risk | 828 (81.1%) | 0.82 (0.55 to 1.23) | 0.413 | 0.80 (0.53 to 1.21) | 0.292 | Age, INR, bilirubin, albumin, creatinine, comorbidities | ||
| High risk | 193 (18.9%) | 0.29 (0.15 to 0.56) | <0.001 | 0.36 (0.18 to 0.70) | 0.002 | Age, creatinine, PLT | ||
AB, active bleeding, CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; HCC, hepatocellular carcinoma; HR, hazard ratio; INR, international normalised ratio; PLT, platelet count; WBC, white blood cell.
HR for the effect of p-TIPS vs. drug plus endoscopic treatment (as reference).
We adjusted these variables based on their associations with the outcomes of interest or a change in effect estimates of more than 10%.
Child B8-9+AB criteria: Low risk: Child-Pugh B without active bleeding and Child-Pugh B7 with active bleeding; High risk: Child-Pugh B8-9 with active bleeding at endoscopy.
Fig. 3.
Forest plot showing the event rates, adjusted hazard ratios, and the absolute risk reduction for 1-year mortality stratified by different risk stratification rules in those with Child-Pugh B cirrhosis.
Adjusted hazard ratios and absolute risk reduction with 95% CIs indicate the effect of pre-emptive TIPS vs. drug plus endoscopic therapy (as reference) on 1-year mortality, which are derived from multivariable competing risk regression models, adjusted for propensity score within each risk category. p values for interaction were 0.176, 0.079 and 0.008, respectively, in multiplicative scale, and 0.006, 0.012 and <0.001 in additive scale (multivariable competing risk regression analysis). Child B8-9 + AB criteria: Low risk: Child-Pugh B without active bleeding and Child-Pugh B7 with active bleeding; High risk: Child-Pugh B8-9 with active bleeding at endoscopy. CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt.
When stratified according to active bleeding at endoscopy, a reduced risk of death with pre-emptive TIPS was only observed in patients with active bleeding (3.1% vs. 21.9% at 6 weeks and 13.7% vs. 27.6% at 1 year, adjusted HR 0.43, 95% CI 0.25–0.75; p = 0.003) but not in those without active bleeding (3.0% vs. 7.9% at 6 weeks and 15.1% vs. 16.9% at 1 year, adjusted HR 0.80, 95% CI 0.53–1.29; p = 0.393, Fig. 2B) after adjustment for potential cofounders (Table 4). Similar results were observed after adjusting for propensity scores (Fig. 3).
Similar patterns emerged when patients were stratified according to the Child B8-9+AB criteria. Pre-emptive TIPS was independently associated with a decreased risk of mortality in the high-risk category (6.1% vs. 29.3% at 6 weeks, 24.2% vs. 44.6% at 1 year, adjusted HR 0.36, 95%CI: 0.18 to 0.70, p = 0.002) but not in the low-risk category (2.4% vs. 3.9% at 6 weeks, 4.8% vs. 8.4% at 1 year, adjusted HR 0.80, 95% CI 0.53–1.21, p = 0.292) after adjusting for potential confounders (Fig. 2C and Table 4) or propensity score (Fig. 3).
When stratified according to each Child-Pugh score, active bleeding at endoscopy was associated with increased mortality only in those with Child-Pugh B8-9 but not B7 cirrhosis (Fig. S3). Furthermore, the survival benefit from pre-emptive TIPS was only observed in individuals with Child-Pugh B8-9 cirrhosis plus active bleeding but not in those with Child-Pugh B7 cirrhosis or Child-Pugh B8-9 cirrhosis without active bleeding (Fig. S4).
Risk-stratified effects of pre-emptive TIPS on further bleeding
The cumulative incidence of further bleeding was lower in the pre-emptive TIPS group (6.1% vs. 27.9% at 6 weeks; 13.1% vs. 39.0% at 1 year; p <0.001) compared with the medical group (Fig. S5). This pattern was not altered after adjusting for potential confounders (Table S2) and, more importantly, was homogeneous across the entire risk spectrum, based on different risk classification rules (Fig. S6,7 and Table S3).
Risk-stratified effects of pre-emptive TIPS on OHE
No significant differences in the cumulative incidence of OHE were observed between the two treatment groups (pre-emptive TIPS vs. medical: 17.2% vs. 19.3% at 6 weeks; 30.0% vs. 29.3% at 1 year, p = 0.996, Fig. S8). These results were not altered after adjusting for potential confounders (Table S4) and, more importantly, were consistent across risk strata, based on different risk classification rules (Fig. S9,10, and Table S5).
Sensitivity analysis
Similar risk-stratified effects of pre-emptive TIPS on outcomes, based on different risk classification rules, were obtained in the analysis after propensity score matching (Figs. S11-15) or after excluding individuals with previous bleeding (Figs. S16-19).
Comparison of different risk-stratification criteria
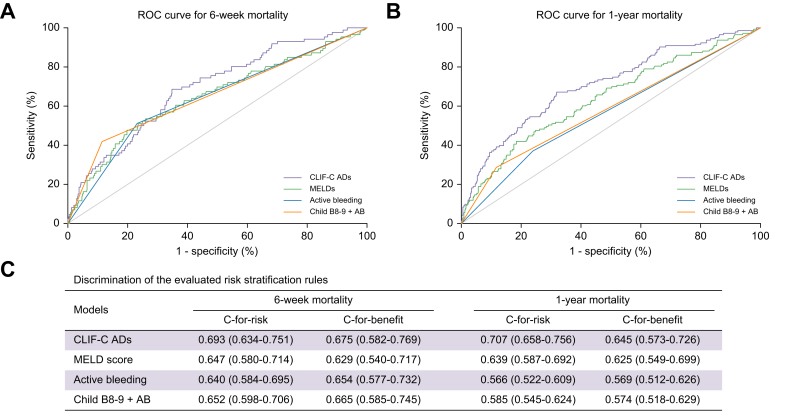
As shown in Fig. 4, the values of c-for-risk as well as c-for-benefit of CLIF-C ADs were significantly higher than those of MELD score, active bleeding at endoscopy and Child B8-9+AB criteria.
Fig. 4.
Comparison of different risk stratification rules.
The ROC of the CLIF-C AD score, MELD score, active bleeding at endoscopy and Child B8-9 + AB criteria for predicting (A) 6- week and (B) 1-year mortality. (C) Discrimination of the evaluated models. The concordance-statistic for benefit (c-for-benefit) is a variant of the conventional risk concordance-statistic (c-for-risk) designed to specifically calculate the ability for a model to discriminate between people obtaining more benefit vs. less benefit from a treatment. Note that the c-for-benefit statistic is in general much more conservative than the traditional concordance-statistic, which only assesses the ability of a model to detect higher vs. lower absolute mortality risk, not treatment effect. CLIF-C ADs, Chronic Liver Failure-Consortium acute decompensation score; MELDs, model for end-stage liver disease score; p-TIPS, pre-emptive transjugular intrahepatic portosystemic shunt; ROC, receiver-operating characteristic curve.
Discussion
In this observational study, we showed among individuals with Child-Pugh B cirrhosis and AVB that i) CLIF-C ADs identified a graded survival benefit from pre-emptive TIPS, ranging from no benefit in individuals with CLIF-C ADs <48 to a halving of 1-year mortality in CLIF-C ADs >56; ii) active bleeding at endoscopy was associated with increased mortality in those with Child-Pugh B8-9 but not B7 cirrhosis; iii) pre-emptive TIPS improves survival in individuals with Child-Pugh B8-9 cirrhosis plus active bleeding but not in those with Child-Pugh B7 cirrhosis or Child-Pugh B8-9 cirrhosis without active bleeding. iv) the c-for-benefit of CILF-C ADs for predicting survival benefit was higher than those of active bleeding at endoscopy and Child B8-9+AB criteria.
The strengths of the present study lie in: i) including a large sample size of individuals with Child-Pugh B cirrhosis regardless of active bleeding at endoscopy, which allowed us to generate estimates with narrow confidence intervals; ii) firstly and comprehensively evaluated the risk-stratified effect of pre-emptive TIPS in individuals with Child-Pugh B cirrhosis and AVB, iii) use of a survival-based primary endpoint, which is an objectively assessed and clinically relevant endpoint; and iv) use of various effective methods (multivariable adjustment, subgroup analyses and propensity score methods) to minimise and rule out potential confounding and selection bias.
When our entire Child-Pugh B cohort of 1,021 patients is considered, pre-emptive TIPS resulted in a statistically significant reduction in mortality with a HR of 0.62 (95% CI 0.44–0.88). After risk stratification by CLIF-C ADs 48-56 categories, the benefits of pre-emptive TIPS varied between the CLIF-C ADs categories; those with higher CLIF-C ADs benefitted more from pre-emptive TIPS. These results are in agreement with our and Trebicka et al.’s recent findings that the sickest patients (those with acute-on-chronic liver failure [ACLF] or high MELD score) are precisely those who benefit the most from pre-emptive TIPS.9,22,23 Specifically, in those with CLIF-C ADs >56, pre-emptive TIPS was associated with a marked mortality reduction, from 50.6% to 27.3% at 1 year. The number needed to treat to prevent one death was as low as four patients, suggesting that pre-emptive TIPS is likely to be most cost-effective in this subpopulation. In those with CLIF-C ADs <48, however, because the baseline risk of mortality was already low, the absolute risk reduction by pre-emptive TIPS was negligible. Alternatively, it can be suggested that drug plus endoscopic therapy is sufficient to control acute bleeding as well as prevent rebleeding in these patients, and consequently, pre-emptive TIPS may be not necessary.
The rationale of “pre-emptive TIPS” is to treat patients earlier in order to prevent early rebleeding and further deterioration and thereby improve survival.[24], [25], [26] In other words, a main therapeutic goal behind pre-emptive TIPS placement in AVB is to prevent the development of ACLF, which is characterised by rapid deterioration of organ function leading to multiple organ failure and high short-term mortality.[25], [26], [27] A recent large multicentre international study demonstrated that the presence of ACLF is the strongest predictor of rebleeding and mortality, and that pre-emptive TIPS improves outcomes in individuals with ACLF.22 Most individuals with Child-Pugh B cirrhosis and AVB did not have ACLF at admission.11,22 However, since variceal bleeding presents a frequent cause of acute decompensation in cirrhosis, they were still at high risk of progression to ACLF.[28], [29], [30] It is likely that pre-emptive TIPS improves survival in AVB and cirrhosis by preventing the inflammatory response induced by further bleeding and ascites through hemodynamic (reduction in portal pressure) and non-hemodynamic (reduction in gut bacterial translocation) mechanisms.11,25,[31], [32], [33] As patients with a higher CLIF-C ADs had a higher level of systemic inflammation (indicated by the white blood cell count) and a higher risk of ACLF development,[28], [29], [30] they benefit most from pre-emptive TIPS. On the other hand, pre-emptive TIPS significantly reduced the risk of further bleeding in all individuals with Child-Pugh B cirrhosis, even in those at low risk of death. This finding suggests that effective control of bleeding and prevention of rebleeding has a limited role in prolonging survival in patients at low risk of death and that pre-emptive TIPS is only able to reduce mortality when the patient is at risk of complications of cirrhosis other than bleeding.
A recent individual patient data meta-analysis7 showed a differential survival benefit from pre-emptive TIPS in those with Child-Pugh B8-9 vs. Child-Pugh B7 cirrhosis. However, the study excluded those with Child-Pugh class B cirrhosis without active bleeding. This prompted us to assess whether such a pattern could be observed in these patients. Our analysis confirmed that only those with Child-Pugh B8-9 cirrhosis and active bleeding should be considered a high-risk population for whom pre-emptive TIPS may elicit a survival benefit compared to drug plus endoscopic treatment. However, the definition of active bleeding is subjective; thus, one should be cautious when considering active bleeding as a risk-stratification tool for relevant therapeutic decisions in this setting.
Although CLIF-C ADs, active bleeding at endoscopy and Child B8-9+AB criteria can stratify patients into subgroups based on the survival benefit they are likely to derive from pre-emptive TIPS, our results showed that CLIF-C ADs presented with higher values of c-for-benefit compared to the other two criteria, suggesting CLIF-C ADs is better at predicting treatment benefit. The possible explanation is that, besides live function (creatinine, INR), the CLIF-C ADs considers systemic inflammatory response (indicated by the white blood cell count), which has been demonstrated to be significantly associated with progression of cirrhosis and related complications.18,[27], [28], [29], [30],34
Our study has several limitations. First, most data were retrospectively collected and hence the risk of selection bias is unavoidable. However, this risk has been minimised via the inclusion of all consecutive patients and a large cohort of individuals with Child-Pugh B cirrhosis and AVB. Second, whether to offer pre-emptive TIPS was entirely at the discretion of the treating physician in most cases, which may cause an indication bias. Nevertheless, a range of statistical analyses were performed to rule out this bias. Because individuals in two treatment groups were not similar in their baseline characteristics, we controlled confounders (both well-established predictors and those specific to the patient population) to adjust for the effects of pre-emptive TIPS on outcomes. Importantly, even after adjusting for all potential confounders, the conclusion was not changed. Furthermore, these results could be confirmed by propensity score matching analyses and thus had a high degree of consistency. Third, as most of the patients had viral cirrhosis, no definitive conclusion can be drawn for other types of chronic liver disease.
In conclusion, either CLIF-C ADs, active bleeding at endoscopy or Child B8-9+AB criteria can identify patients with differential survival benefit from pre-emptive TIPS, but CLIF-C ADs is superior to the others for predicting treatment benefit. These findings warrant further external validation in individuals with different aetiologies and in randomised controlled studies.
Financial support
This study was supported by grants from National Key Technology R&D Program (2015BAI13B07) for Prof. Daiming Fan and Boost Program of Xijing Hospital (XJZT18H02) for Prof. Guohong Han and China Postdoctoral Science Foundation (2019TQ0134) for Dr. Yong Lv.
Authors’ contributions
1. Study concept and design: Yong Lv, Guohong Han; 2. Acquisition of data: Yong Lv, Wei Bai, Xuan Zhu, Hui Xue, Jianbo Zhao, Yuzheng Zhuge, Junhui Sun, Chunqing Zhang, Pengxu Ding, Zaibo Jiang, Xiaoli Zhu, Weixin Ren, Yingchun Li, Kewei Zhang, Wenguang Zhang, Kai Li, Zhengyu Wang, Bohan Luo, Xiaomei Li, Zhiping Yang, Qiuhe Wang, Wengang Guo, DongDong Xia, Zhanxin Yin, Guohong Han; 3. Analysis and interpretation of data: Yong Lv, Guohong Han; 4. Drafting of the manuscript: Yong Lv; 5. Critical revision of the manuscript for important intellectual content: Changbing Yang, Yanglin Pan, Daiming Fan, Guohong Han; 6. Statistical analysis: Yong Lv; 7. Administrative and material support: Daiming Fan.
Data availability statement
All data, materials and methods in this study can be made available from the corresponding author upon request for non-commercial purposes and after approval of a study proposal through a signed data access agreement.
Conflict of interests
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgments
The authors thank Drs. Hongbo Zhang, Huahong Xie, Liping Yao, Jianhong Wang and Tao Li from Xijing Hospital of Digestive Disease for their treatment and follow-up of patients; Dr. Luo Zuo from Xijing Hospital of Digestive Disease, Dr. Jiawei Zhong from the First Affiliated Hospital of Nanchang University, Dr. Qifeng Peng from Nanfang Hospital of Southern Medical University, Dr. Fuquan Ma from the First Affiliated Hospital of Xi'an Jiaotong University, Dr. Junyang Luo from the Third Affiliated Hospital of Sun Yat-sen University, Dr. Ming Zhang from Affiliated Drum Tower Hospital of Nanjing University Medical School, Dr. Guangchuan Wang from Shandong Provincial Hospital affiliated to Shandong University, Dr. Minhuang Sun from the Second Affiliated Hospital of Kunming Medical University, and Dr. Junjiao Dong from Henan Provincial People's Hospital for their dedication in data collection; as well as Drs. Hui Chen and Enxin Wang from Xijing Hospital of Digestive Disease for their assistance in revising the manuscript.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100621.
Supplementary data
The following are the supplementary data to this article:
References
- 1.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G., Abraldes J.G., Berzigotti A., Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Tripathi D., Stanley A.J., Hayes P.C., Patch D., Millson C., Mehrzad H., et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut. 2015;64(11):1680–1704. doi: 10.1136/gutjnl-2015-309262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Pagan J.C., Caca K., Bureau C., Laleman W., Appenrodt B., Luca A., et al. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362(25):2370–2379. doi: 10.1056/NEJMoa0910102. [DOI] [PubMed] [Google Scholar]
- 6.Lv Y., Yang Z., Liu L., Li K., He C., Wang Z., et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4(8):587–598. doi: 10.1016/S2468-1253(19)30090-1. [DOI] [PubMed] [Google Scholar]
- 7.Nicoară-Farcău O., Han G., Rudler M., Angrisani D., Monescillo A., Torres F., et al. Effects of early placement of transjugular portosystemic shunts in patients with high-risk acute variceal bleeding: a meta-analysis of individual patient data. Gastroenterology. 2021;160(1):193–205. doi: 10.1053/j.gastro.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Pagan J.C., Di Pascoli M., Caca K., Laleman W., Bureau C., Appenrodt B., et al. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58(1):45–50. doi: 10.1016/j.jhep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Lv Y., Zuo L., Zhu X., Zhao J., Xue H., Jiang Z., et al. Identifying optimal candidates for early TIPS among patients with cirrhosis and acute variceal bleeding: a multicentre observational study. Gut. 2019;68(7):1297–1310. doi: 10.1136/gutjnl-2018-317057. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez-Gea V., Procopet B., Giraldez A., Amitrano L., Villanueva C., Thabut D., et al. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology. 2019;69(1):282–293. doi: 10.1002/hep.30182. [DOI] [PubMed] [Google Scholar]
- 11.Trebicka J. Emergency TIPS in a Child-Pugh B patient: when does the window of opportunity open and close? J Hepatol. 2017;66(2):442–450. doi: 10.1016/j.jhep.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 12.Dunne P.D.J., Sinha R., Stanley A.J., Lachlan N., Ireland H., Shams A., et al. Randomised clinical trial: standard of care versus early-transjugular intrahepatic porto-systemic shunt (TIPSS) in patients with cirrhosis and oesophageal variceal bleeding. Aliment Pharm Ther. 2020;52(1):98–106. doi: 10.1111/apt.15797. [DOI] [PubMed] [Google Scholar]
- 13.Thabut D., Pauwels A., Carbonell N., Remy A.J., Nahon P., Causse X., et al. Cirrhotic patients with portal hypertension-related bleeding and an indication for early-TIPS: a large multicentre audit with real-life results. J Hepatol. 2018;68:73–81. doi: 10.1016/j.jhep.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Rudler M., Bureau C., Carbonell N., Mathurin P., Saliba F., Mallat A., et al. Recalibrated MELD and hepatic encephalopathy are prognostic factors in cirrhotic patients with acute variceal bleeding. Liver Int. 2018;38(3):469–476. doi: 10.1111/liv.13632. [DOI] [PubMed] [Google Scholar]
- 15.Conejo I., Guardascione M.A., Tandon P., Cachero A., Castellote J., Abraldes J.G., et al. Multicenter external validation of risk stratification criteria for patients with variceal bleeding. Clin Gastroenterol Hepatol. 2018;16(1):132–139. doi: 10.1016/j.cgh.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Augustin S., Altamirano J., Gonzalez A., Dot J., Abu-Suboh M., Armengol J.R., et al. Effectiveness of combined pharmacologic and ligation therapy in high-risk patients with acute esophageal variceal bleeding. Am J Gastroenterol. 2011;106(10):1787–1795. doi: 10.1038/ajg.2011.173. [DOI] [PubMed] [Google Scholar]
- 17.de Franchis R., Bosch J., Garcia-Tsao G., Reiberger T., Ripoll C., Abraldes J.G., et al. Baveno VII – renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv Y., Wang Z., Li K., Wang Q., Bai W., Yuan X., et al. Risk stratification based on chronic liver failure consortium acute decompensation score in patients with child-pugh B cirrhosis and acute variceal bleeding. Hepatology. 2021;73(4):1478–1493. doi: 10.1002/hep.31478. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi D., Stanley A.J., Hayes P.C., Travis S., Armstrong M.J., Tsochatzis E.A., et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69(7):1173–1192. doi: 10.1136/gutjnl-2019-320221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vilstrup H., Amodio P., Bajaj J., Cordoba J., Ferenci P., Mullen K.D., et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European association for the study of the liver and the American association for the study of liver diseases. J Hepatol. 2014;61(3):642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 21.van Klaveren D., Steyerberg E.W., Serruys P.W., Kent D.M. The proposed ‘concordance-statistic for benefit’ provided a useful metric when modeling heterogeneous treatment effects. J Clin Epidemiol. 2018;94:59–68. doi: 10.1016/j.jclinepi.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trebicka J., Gu W., Ibáñez-Samaniego L., Hernández-Gea V., Pitarch C., Garcia E., et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73(5):1082–1091. doi: 10.1016/j.jhep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 23.Depaire M., Larrue H., Rudler M., Nault J.C., Bureau C. Futility criteria for preemptive TIPS in patients with cirrhosis and variceal bleeding are still missing in most severe patients. J Hepatol. 2021;74(4):997–999. doi: 10.1016/j.jhep.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Magaz M., Baiges A., Hernández-Gea V. Precision medicine in variceal bleeding: are we there yet? J Hepatol. 2020;72(4):774–784. doi: 10.1016/j.jhep.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Trebicka J. Does transjugular intrahepatic portosystemic shunt stent differentially improve survival in a subset of cirrhotic patients? Semin Liver Dis. 2018;38(1):87–96. doi: 10.1055/s-0038-1627457. [DOI] [PubMed] [Google Scholar]
- 26.García-Pagán J.C., Saffo S., Mandorfer M., Garcia-Tsao G. Where does TIPS fit in the management of patients with cirrhosis? JHEP Rep. 2020;2(4) doi: 10.1016/j.jhepr.2020.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jalan R., Saliba F., Pavesi M., Amoros A., Moreau R., Gines P., et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61(5):1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 28.Arroyo V., Angeli P., Moreau R., Jalan R., Clària J., Trebicka J., et al. The systemic inflammation hypothesis: towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J Hepatol. 2021;74(3):670–685. doi: 10.1016/j.jhep.2020.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097–1108. doi: 10.1016/j.jhep.2020.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Trebicka J., Fernandez J., Papp M., Caraceni P., Laleman W., Gambino C., et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842–854. doi: 10.1016/j.jhep.2020.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Trebicka J., Krag A., Gansweid S., Appenrodt B., Schiedermaier P., Sauerbruch T., et al. Endotoxin and tumor necrosis factor-receptor levels in portal and hepatic vein of patients with alcoholic liver cirrhosis receiving elective transjugular intrahepatic portosystemic shunt. Eur J Gastroenterol Hepatol. 2011;23(12):1218–1225. doi: 10.1097/MEG.0b013e32834a75dc. [DOI] [PubMed] [Google Scholar]
- 32.Xu W.H., Wu X.J., Li J.S. Influence of portal pressure change on intestinal permeability in patients with portal hypertension. Hepatobiliary Pancreat Dis Int. 2002;1(4):510–514. [PubMed] [Google Scholar]
- 33.Berres M.L., Asmacher S., Lehmann J., Jansen C., Gortzen J., Klein S., et al. CXCL9 is a prognostic marker in patients with liver cirrhosis receiving transjugular intrahepatic portosystemic shunt. J Hepatol. 2015;62(2):332–339. doi: 10.1016/j.jhep.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 34.Jalan R., Pavesi M., Saliba F., Amoros A., Fernandez J., Holland-Fischer P., et al. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62(4):831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, materials and methods in this study can be made available from the corresponding author upon request for non-commercial purposes and after approval of a study proposal through a signed data access agreement.