Abstract
Mycobacterium avium is an opportunistic pathogen in AIDS patients, who acquire the infection mainly through the gastrointestinal tract. Previous studies in vitro have shown that M. avium invades epithelial cells of both intestinal and laryngeal origin. In addition, M. avium enters the intestinal mucosa of healthy mice. Because M. avium invasion of the intestinal mucosa in vivo initially is not accompanied by significant influx of inflammatory cells, we sought to determine whether M. avium would trigger chemokine release upon entry into epithelial cells by using HT-29 intestinal and HEp-2 laryngeal epithelial cell lines. Chemokine synthesis was measured both by the presence of specific mRNA and protein secretion in the cell culture supernatant as determined by enzyme-linked immunosorbent assay. Infection of HT-29 intestinal cells with M. avium did not induce the release of interleukin-8 (IL-8) or RANTES for up to 7 days postinfection. However, infection of HEp-2 cells resulted in the release of IL-8 and RANTES at 72 h. Similar findings were observed with other AIDS M. avium isolates belonging to different serovars. Secretion of IL-8 by HEp-2 cells was dependent upon bacterial uptake. In addition, prior infection with M. avium suppressed IL-8 production by HT-29 cells infected with Salmonella typhimurium. Our results suggest that M. avium infection of epithelial cells is associated with a delay in IL-8 and RANTES production which, in the case of HT-29, is prolonged up to 1 week. These findings may explain the weak inflammatory response after intestinal mucosa invasion in mice and are probably related with the ability of the bacterium to evade the host’s immune response.
Infections caused by organisms of the Mycobacterium avium complex are the most common bacterial infection in patients with AIDS (11, 14). In AIDS patients, in contrast to individuals without AIDS, where the pulmonary infection is more frequent, the gastrointestinal (GI) tract appears to be the primary route of M. avium infection (7, 15, 22). Colonization of the intestine is usually observed months prior to the diagnosis of disseminated infection (7, 22, 26), and it is assumed to be a risk factor for the disseminated disease.
We have shown that M. avium is able to bind to and invade intestinal and laryngeal epithelial cells in vitro (3, 4). In addition, when given orally to mice, M. avium enters the intact intestinal mucosa and subsequently disseminates (2).
Epithelial cells of the intestinal mucosal surface form a major mechanical barrier that separates the host’s internal milieu from the external environment. Mucosal surfaces have a highly specialized immune system, and mucosal epithelial cells produce an array of cytokines and chemokines in response to different stimuli. The existence of mucosal cytokine response to infection was first shown to occur in mice as well as in patients after urinary tract colonization by Escherichia coli (10). In addition, secretion of interleukin-6 (IL-6), IL-1β, and IL-8 have been demonstrated in a number of studies in vitro and in vivo after infection of mucosal epithelial cells with E. coli (9), Helicobacter pylori (6, 13, 24), Salmonella spp. (16, 27), and Shigella spp. (16), as well as other microorganisms (23).
In mice, infection of the intestinal mucosa by M. avium is followed by little inflammatory response, with neutrophil infiltration of the Peyer’s patches being observed approximately 1 week after infection and migration of mononuclear cells to the site of infection thereafter (17). Because the release of chemokines, such as IL-8 (a potent T-cell and neutrophil recruitment factor) has been reported in a number of inflammatory conditions of the mucosal layer, we investigated, using two different cell lines, whether M. avium infection of epithelial cells results in the induction of chemokine secretion.
MATERIALS AND METHODS
Bacteria.
M. avium strains 101 (serovar 1), 104 (serovar 1), and 100 (serovar 8) were isolated from the blood of AIDS patients. Mycobacterium smegmatis 11727 and Salmonella typhimurium 15277 were purchased from The American Type Culture Collection (ATCC), Rockville, Md. E. coli DH5α was a gift from Raul Barletta (University of Nebraska, Lincoln, Nebr.).
M. avium strains were cultured in Middlebrook 7H10 agar supplemented with oleic acid, albumin, dextrose, and catalase (Difco Laboratories, Detroit, Mich.) for 10 days and harvested in phosphate-buffered saline (pH 7.4), whereas M. smegmatis was harvested after 3 days in culture as described previously (3). Only colonies of M. avium expressing the transparent morphotype were used in the experiments. S. typhimurium and E. coli were cultured in Luria-Bertani broth for 24 h under static conditions.
Because of the tendency of mycobacteria to form clumps, the inoculum was carefully prepared to ensure a dispersed preparation as described earlier (1). The viability of the inoculum was determined by both plating onto 7H10 agar and by visualization under fluorescent microscopy by using the LIVE-DEAD assay (Molecular Probes, Eugene, Oreg.) as previously reported (1).
Cell cultures.
The human intestinal epithelial cell line HT-29 was obtained from the ATCC. Cells were grown in a medium consisting of modified McCoy-5A medium (Difco) with 10% of galactose (Sigma Chemicals, St. Louis, Mo.) and 10% fetal bovine serum (Sigma). The HEp-2 laryngeal cell line was also obtained from the ATCC, and it was maintained in RPMI 1640 supplemented with 5% fetal bovine serum as described previously (4).
Cell lines were used between passages 15 and 20. HT-29 and HEp-2 cells were seeded in 24-well or 12-well tissue culture plates (Costar, Cambridge, Mass.) at 1 × 105 or 2 × 105 cells per well, respectively, in a volume of 1 ml, and then cultured to 90 to 100% confluence. HT-29 cells were also seeded on transwell membrane (3-μm pore size) and allowed to achieve confluence (ca. 1 week). The integrity of the polarized monolayers on transwell was determined by measuring the transmembrane resistance. Monolayers were used when the resistance reached approximately 400 Ω/cm2.
Monolayers were infected with bacteria by removing the medium and replacing it with 1 ml of medium containing 106 organisms to each well in the 24-well tissue culture plate and transwell monolayers or 2 × 106 in each well in the 12-well plates.
Invasion assay and intracellular growth.
The invasion assay was performed as previously described (3, 4), with the modification that bacteria were allowed to invade for 1 h at 37°C instead of 2 h as originally described (4). After 1 h, cells were washed to remove unattached bacteria and incubated with 200 μg of amikacin per ml for 2 h (4). Then, cells were lysed by adding 1% Triton X-100 (Sigma), and the lysate was subsequently diluted and plated onto 7H10 agar to quantitate the viable intracellular bacteria. Results were reported as a percentage of the initial inoculum that entered both HT-29 and HEp-2 cells. The viability of the infected monolayers versus uninfected control was monitored during the experiments by trypan blue exclusion and did not show any differential cytotoxic effect related to the presence of the bacteria.
Infected transwell monolayers were maintained for up to 1 week, and the integrity of the monolayer was verified daily by measuring the resistance between both membranes.
Coinfection experiments.
To determine whether M. avium infection of HT-29 cells would suppress IL-8 production by the cells after uptake of S. typhimurium, we infected a 100% confluent HT-29 cell monolayer with 107 or 108 M. avium 101 for 1 h; this was then washed and treated with amikacin for 2 h as described above. After 18 h the monolayers were infected with S. typhimurium (105 cells) for 2 h and then treated with gentamicin (20 μg/ml) for 1 h. Monolayers were then washed, and 24 h later the supernatants were obtained to measure IL-8 concentration. Controls of cells without infection, infected with M. avium or Salmonella were run in parallel.
To determine whether M. avium and Salmonella were coinfecting the same cells, we performed similar assays in LabTek chamber slides as reported (1, 4).
After M. avium and/or Salmonella infection and treatment with antibiotics, the monolayers were fixed with 2% paraformaldehyde for 30 min at room temperature. After being washed with Hank’s balanced salt solution (HBSS), monolayers were incubated with Triton X-100 (Sigma) for 10 min and then washed three more times with HBSS. Rabbit anti-M. avium antibody (1:40) and fluorescein isothiocyanate (FITC)-labelled anti-rabbit antibody (1:400), as well as mouse anti-S. typhimurium (1:30) antibody and Texas Red anti-mouse antibody (1:800), were used to identify the bacteria. After a washing, the slides were mounted and observed by using a light microscope (Nikkon). Intracellular bacteria in 100 cells per field in 10 fields were numbered by using a source of UV light and specific filters for FITC and Texas Red.
Assays for chemokine release.
Monolayers were infected with bacteria or incubated with lipopolysaccharide (LPS). Infection was allowed to occur for 1 h at 37°C, and then the wells were washed with HBSS to remove noninternalized bacteria. Fresh medium was then added, and at 4, 24, 48, and 72 h after infection, supernatants were obtained, filtered through a 0.22-pore-size-μm filter to remove residual bacteria and cell debris, and frozen at −20°C.
Concentration of human IL-8 and RANTES, as well as IL-1α, IL-1β, transforming growth factor β (TGF-β), and IL-6 were measured in the supernatant by enzyme-linked immunosorbent assay (ELISA) by using kits purchased from R & D Systems, Minneapolis, Minn., and BioSource, Camarillo, Calif. The assays were carried out according to directions provided by the manufacturers.
RNA preparation and RT-PCR analysis.
To determine the relative level of IL-8 mRNA transcription, total RNA was obtained with the RNeasy Mini Kit (QIAGEN, Santa Clarita, CA), following the supplier’s instructions, from 12-well infected monolayers. It was then retrotranscribed with the Ready-To-Go T-Primed First-Strand Kit (Pharmacia, Piscataway, NJ), and quantitated by reverse transcriptase PCR (RT-PCR) with previously described primers (ATG ACT TCC AAG CTG GCC GTG GCT and TCT CAG CCC TCT TCA AAA ACT TCT C) for IL-8 and GADPH (glyceraldehyde-3-phosphate dehydrogenase) as a control (TGA AGG TCG GAG TCA ACG GAT TTG CT and CAT GTG GGC CAT GAG GTC CAC CAC) (16).
The samples were heated at 94°C for 4 min and then subjected to 30 cycles of denaturation at 94°C for 45 s, annealing at 60°C for 30 s, and extension at 72°C for 45 s in a GeneAmp PCR System 2400 (Perkin-Elmer, Foster City, Calif.). Then, 10 μl of the PCR products was separated on 1.5% agarose gels and visualized by staining with ethidium bromide.
Statistical analysis.
Each experiment was repeated at least three times, and the results were expressed as the mean ± the standard deviation. The significance of the differences between experimental groups was analyzed by the Student’s t test. A P value of <0.05 was considered significant.
RESULTS
Chemokine and cytokine production by uninfected cells.
HT-29 intestinal cells do not produce IL-1α, IL-1β, IL-6, or RANTES constitutively. However, IL-8 and TGF-β (ca. 800 and 700 pg, respectively) were observed in uninfected HT-29, confirming results shown by Eckmann and colleagues (8). Uninfected HEp-2 produced approximately 1,600 pg of IL-8 and 1,400 pg of RANTES per ml.
Chemokine and cytokine production after infection in vitro.
After infection with M. avium at a ratio of 10:1, HT-29 cells secreted IL-8 at similar levels of noninfected monolayer at 24, 48, 72, 96, and 168 h (Fig. 1). In addition, no production of other inflammatory cytokines (IL-1α, IL-1β, and IL-6) was observed (data not shown). In contrast, HT-29 cells infected with a 10:1 ratio of S. typhimurium produced 6,400 ± 150 pg of IL-8 by 24 h.
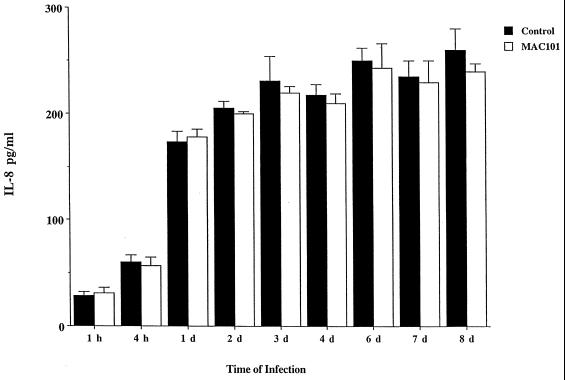
FIG. 1.
Production of IL-8 by infected HT-29 cells overtime. HT-29 cells (confluent monolayers) were infected with M. avium 101 (MAC101) (MOIs of 1, 10, or 100) for several days, and IL-8 production was measured in the supernatant after 1, 2, 3, 4, and 7 days. Results are shown for the assays by using an MOI of 10. Cells infected with S. typhimurium produced 343 ± 29 pg of IL-8 per ml after 24 h (data not shown).
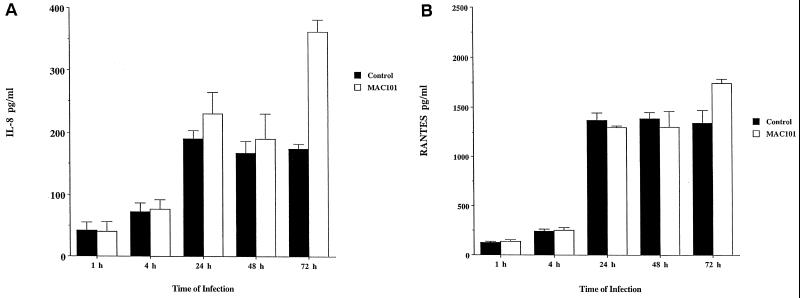
HEp-2 cells infected with M. avium did not synthesize increased amounts of chemokines (IL-8 and RANTES) for the first 48 h, but a significant increase of IL-8 and RANTES was observed at 72 h (Fig. 2). Greater ratios of infection than 10:1 (100:1) resulted in production of IL-8 after 24 h (data not shown).
FIG. 2.
Production of chemokines by HEp-2 cells infected with M. avium 101 (MAC101). (A) IL-8. (B) RANTES. Cells were infected with an MOI of 10. ∗, P < 0.05 compared with the uninfected control.
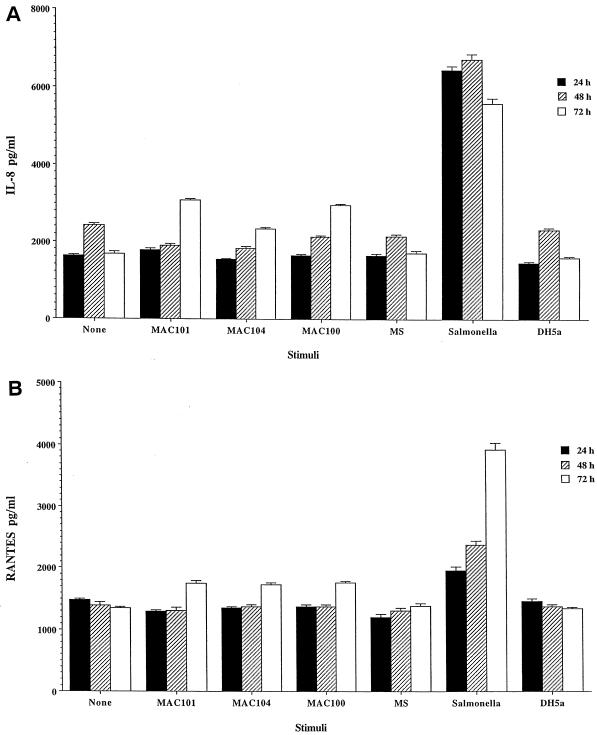
Production of IL-8 and RANTES did not change independently of the M. avium strains (100, 104, and 101) used in the experiment. As shown in Fig. 3, in contrast to M. avium and E. coli DH5α, a noninvasive strain of E. coli, infection of HEp-2 cells with S. typhimurium triggered a significant release of IL-8 after 24 h.
FIG. 3.
Production of chemokines by HEp-2 cells infected with different M. avium strains, M. smegmatis, Salmonella, and E. coli DH5α for 24 to 72 h. (A) IL-8. (B) RANTES. Monolayers were infected with an MOI of 10. For the 72-h time point of M. avium-infected cells compared with uninfected control and for Salmonella at the three time points, the P value is <0.05.
The viability of HEp-2 and HT-29 monolayers was not affected by M. avium and remained 96 ± 2% after M. avium infection.
M. avium infection of polarized HT-29 monolayer in transwell plates did not result in either IL-8 or RANTES production for up to 72 h after infection, a result similar to that observed in assays with cell monolayers on plastic. The concentration of chemokines was measured in both the apical and basal supernatants.
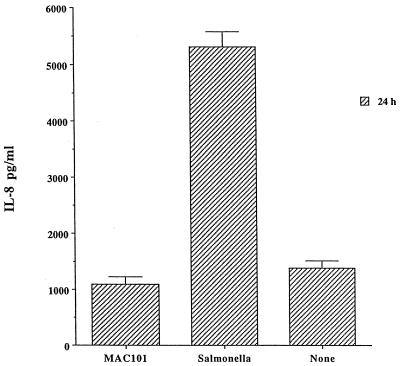
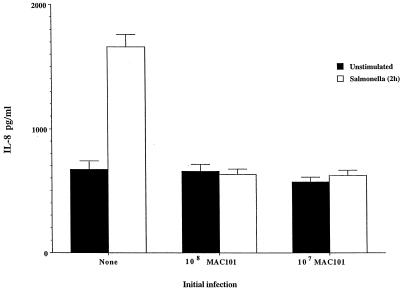
To confirm the level of infection of the monolayers, HEp-2 monolayers infected with bacterial strains were lysed, and the percentage of initial inoculum within cells was determined. Cells infected for 1 h with M. avium 101 had taken up 3.6% of the inoculum, while cells infected with M. avium strains 104 and 100 had taken up 3.2 and 1.8% of the inoculum, respectively. M. smegmatis had 0.04% of the inoculum taken up by HEp-2 cells. The uptake of E. coli DH5α was 0.0018%, and that for S. typhimurium was approximately 26% of the inoculum (Table 1). Because Salmonella invades HEp-2 cells more efficiently than M. avium, we repeated the assay with a 100:1 ratio of M. avium and a 10:1 ratio Salmonella per cell. After 2 h of uptake, the number of intracellular bacteria was determined and shown to be similar. We then measured IL-8 production by the monolayers after 24 h. As shown in Fig. 4, Salmonella still induced significantly more IL-8 production, while M. avium uptake did not induce IL-8 at 24 h, which can be explained by the shorter replication time of Salmonella compared with M. avium.
TABLE 1.
Bacterial uptake by HEp-2 and HT-29 cellsa
| Strain | Inoculum (106) | % Inoculum
|
|
|---|---|---|---|
| HEp-2 | HT-29 | ||
| M. avium 101 | 2.4 ± 0.3 | 3.6 | 2.9 |
| M. avium 104 | 4.1 ± 0.5 | 3.2 | 3.3 |
| M. avium 100 | 2.1 ± 0.3 | 1.8 | 1.6 |
| M. smegmatis | 4.7 ± 0.7 | 0.04 | 0.02 |
| S. typhimurium | 2.7 ± 0.3 | 26 | 28 |
| E. coli DH5α | 3.1 ± 0.2 | 0.0018 | 0.0012 |
HEp-2 and HT-29 cells were incubated with bacteria for 1 h, and then monolayers were washed to remove extracellular bacteria. Monolayers were then lysed to determine the number of intracellular bacteria as described in Materials and Methods.
FIG. 4.
Comparative production of IL-8 by HEp-2 cells after 24 h of infection with similar number of intracellular M. avium 101 (MAC101) and S. typhimurium. P values were <0.05 for the comparison between Salmonella and the control and <0.05 for the comparison between M. avium 101 and the control.
mRNA.
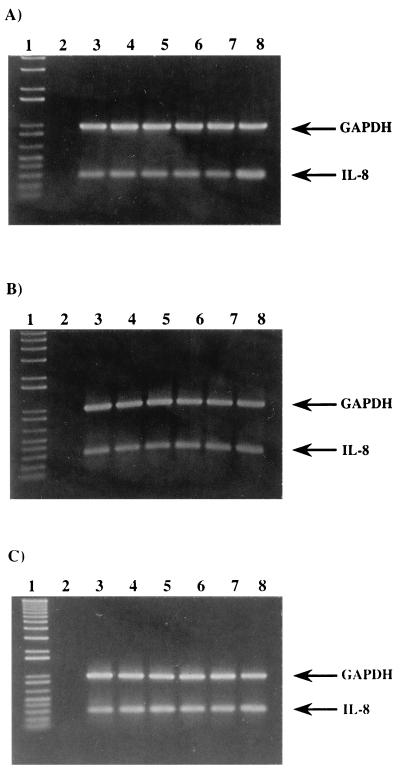
As shown in Fig. 5A and B, RT-PCR with total RNA extracted from HT-29 and HEp-2 cells confirmed the results shown by ELISA that M. avium does not trigger IL-8 production by epithelial cells.
FIG. 5.
IL-8 mRNA upon M. avium infection. (A) HT-29 cells infected with M. avium 101 at an MOI of 100. (B) HEp-2 cells infected with M. avium 101 at an MOI of 10. (C) HEp-2 cells infected with MAC101 at an MOI of 100. Lanes: 1, 1 kb plus molecular weight marker; 2, negative control; 3, uninfected control; 4, 4 h after infection; 5, 24 h after infection; 6, 48 h after infection; 7, 72 h after infection; 8, Salmonella-infected cells for 4 h. The amplified products are IL-8 (289 bp) and GADPH (983 bp).
Uptake is necessary to induce IL-8 production.
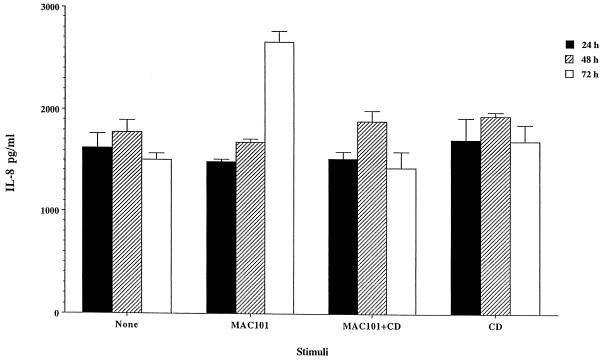
Previous studies have determined that M. avium entry into both HEp-2 and HT-29 cells was accompanied by cytoskeleton rearrangement and incubation of epithelial cells with cytochalasin prior to the exposure to the bacterium would block uptake (4). Cytochalasin D (5 μM, a concentration that blocks 90 ± 4% of M. avium uptake) was then used to examine whether bacterium uptake was necessary to induce IL-8 production by HEp-2 cells. Cytochalasin was added for 30 min and then removed, and then M. avium 101 was used to infect HEp-2 monolayers (at a multiplicity of infection [MOI] of 10). After 2 h, extracellular bacteria was removed by washing, and amikacin (200 μg/ml) was used for an additional 2 h to kill extracellular bacteria. As shown in Fig. 6, IL-8 production after 72 h of incubation did not occur if uptake was inhibited. Cytochalasin D, however, when added alone or 1 h after M. avium infection, did not have influence on chemokine production by HEp-2 cells. In addition, cytochalasin D does not block IL-8 production by HEp-2 cells stimulated with IL-1β (data not shown).
FIG. 6.
Production of IL-8 by HEp-2 cell requires M. avium uptake. Cells were treated or not treated with cytochalasin D (CD) for 30 min (known to prevent M. avium uptake) and then exposed to M. avium 101 (MAC101) (MOI 10) after 2 h; extracellular bacteria were removed by washing. Supernatants were obtained after 24, 48, and 72 h, and the IL-8 concentration was determined. Production of IL-8 was not observed on cytochalasin D-treated monolayers.
M. avium blocks IL-8 production in response to Salmonella invasion.
HT-29 monolayers were infected with either 107 or 108 (to maximize the number of infected cells) M. avium 101 and 18 h later infected with S. typhimurium (105/well) for 1 h. Preinfection with M. avium resulted in complete blockage of IL-8 release by 24 h after S. typhimurium infection (Fig. 7). To determine the percentage of cells that had the two bacteria, we stained intracellular bacteria with different colors and scored them. We found that 78 ± 4% of the cells had at least one M. avium bacillus, while 81 ± 6% of the cells had at least one Salmonella organism and that 72 ± 5% of the cells in the monolayer were coinfected with M. avium and Salmonella.
FIG. 7.
IL-8 production by HT-29 cells coinfected with M. avium 101 (MAC101) and S. typhimurium. HT-29 monolayers were infected with M. avium (107 or 108 bacteria) for 1 h and after 18 h were coinfected with S. typhimurium (105 bacteria). Approximately 70% of the cells in the monolayers were coinfected as evidenced by light microscopy as described in Materials and Methods. M. avium infection suppresses IL-8 production after Salmonella invasion of HT-29 cells.
DISCUSSION
Several studies have demonstrated that M. avium infection in AIDS patients is acquired primarily through the GI tract (7, 15, 22). When M. avium is given orally to healthy C57BL/6 mice, the bacterium invades the intact mucosa of the intestinal tract and secondarily causes disseminated infection (2). Epithelial cells are the first cell type to encounter bacteria at mucosal sites. It was proposed that epithelial cell cytokine-chemokine production could function as an “early warning system” of mucosal infection. In fact, a number of microorganisms have been shown to induce cytokine-chemokine synthesis upon contact with epithelial cells such as IL-8, MCP-1, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, IL-6, and IL-1α (6, 8, 16, 20).
In this study we show that M. avium uptake by both HT-29 intestinal and HEp-2 laryngeal cell lines does not result in the release of chemokines for several days. We found that the ability to delay IL-8 and RANTES production was shared among the three strains of M. avium (representing the three most common serovars isolated from AIDS patients). It is interesting that previous studies by Yuanguang et al. (28) and Rhoades et al. (21) had shown that Mycobacterium tuberculosis infection of the A549 lung epithelial cell line and macrophages, respectively, trigger the release of great amounts of chemokines, including IL-8. In fact, Yuanguang et al. also showed that M. avium infection of A549 cells did not induce the synthesis of IL-8 and MCP-1. Although these authors argued that M. avium’s failure to induce chemokine production was due to the inability to grow intracellularly, this is not the case with both HT-29 and HEp-2 cells, in which M. avium grows intracellularly (4). These findings suggest that the initial mechanisms of pathogenesis (i.e., contact with the epithelial layer) differ significantly between M. avium and M. tuberculosis. It is certainly plausible to hypothesize that, because of the decreased intrinsic virulence compared with M. tuberculosis, M. avium is required to prevent immune response early in the infection to survive. M. avium infection of the intestinal mucosa ultimately causes segmental necrosis of the intestinal villi followed by the influx of inflammatory cells (17). However, this is not observed until several weeks after infection, which suggests that M. avium infection of intestinal epithelial cells is a quiet process in its beginning.
While bacteria such as Salmonella trigger chemokine production soon after entering intestinal cells (27), it has been shown that organisms such as Chlamydia sp. also delay inflammation for several hours (20). Therefore, it may be important to some bacteria to maintain the immune system of the host “in check” while the infection is being established. Several of the cytokines and chemokines are potent chemoattractants and activators for neutrophils, monocytes, and T lymphocytes (8, 25). The fact that intestinal epithelial cells can participate in the network of cytokine-chemokine response and the observation that these cells can express human lymphocyte antigen class II molecules suggests a role for them in the mucosal defense against M. avium infection. Cytokines and chemokines in the intestinal mucosa provide bidirectional communication between inflammatory cells such as tissue macrophages, monocytes, T cells, and epithelial cells. Inhibition of chemokines production appears to be secondary to active interaction of the bacterium and epithelial cells. Although M. avium infection cannot inhibit RANTES and IL-8 production after LPS stimulation (data not shown), it significantly inhibits IL-8 production after stimulation with Salmonella, suggesting that the mechanisms of chemokine stimulation is probably different for LPS and Salmonella. This also implies that M. avium infection actively blocks some step the signal transduction pathway triggered by Salmonella and does not simply remain quiet in the host cell, thus resembling the case of Yersinia, which is able to interrupt host cell pathways through the secretion of Yop proteins (19).
The findings that M. avium triggers different responses in HT-29 intestinal and HEp-2 laryngeal cells could be relevant for the pathogenesis of the infection and is currently being investigated. However, it also suggests either that the signal transduction pathways for chemokine synthesis differ between the cells or that the mechanism of M. avium invasion of HT-29 cells is probably different from the mechanism of invasion of HEp-2 cells.
It is interesting to speculate that in human immunodeficiency virus type 1 (HIV-1)-infected individuals, infection of the intestinal mucosa with HIV-1 prior to M. avium may be associated with production of chemokines (18), although HIV-1 has been shown to enter the intestinal mucosa through M cells (5), whereas M. avium invades the intestinal mucosa primarily through enterocytes (12).
Our findings offer another perspective on M. avium infection of the host, with little initial inflammatory response followed, depending on the cell infected, by the release of inflammatory cytokines (17). However, it seems important for M. avium to be able to prevent cytokines and chemokine synthesis by infected cells, potentially creating a protective shield against the host immune response.
ACKNOWLEDGMENTS
We thank Karen Allen for preparing the manuscript.
This work was supported by contract N01-AI-25140 of the National Institute of Allergy and Infectious Diseases. F.J.S. was supported by a fellowship of the North Atlantic Treaty Organization.
REFERENCES
- 1.Bermudez L E, Parker A, Goodman J. Growth within macrophages increases the efficiency of Mycobacterium avium to invade other macrophages by complement receptor independent pathway. Infect Immun. 1997;65:1916–1922. doi: 10.1128/iai.65.5.1916-1925.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. An animal model of Mycobacterium avium complex disseminated infection after colonization of the intestinal tract. J Infect Dis. 1992;165:75–79. doi: 10.1093/infdis/165.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Bermudez L E, Shelton K, Young L S. Comparison of the ability of M. avium, M. smegmatis, and M. tuberculosis to invade and replicate within HEp-2 epithelial cells. Tubercle Lung Dis. 1995;76:240–247. doi: 10.1016/s0962-8479(05)80012-7. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez L E, Young L S. Factors affecting invasion of HT-29 and HEp-2 epithelial cells by organisms of the Mycobacterium avium complex. Infect Immun. 1994;62:2021–2026. doi: 10.1128/iai.62.5.2021-2026.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–46. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 6.Cole S P, Cirillo D, Kagnoff M F, Guiney D G, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damsker B, Bottone E J. Mycobacterium avium-Mycobacterium intracellulare from the intestinal tracts of patients with the acquired immunodeficiency syndrome: concepts regarding acquisition and pathogenesis. J Infect Dis. 1985;151:179–180. doi: 10.1093/infdis/151.1.179. [DOI] [PubMed] [Google Scholar]
- 8.Eckmann L, Kagnoff M F, Fierer J. Intestinal epithelial cells as watch dogs for the natural immune system. Trends Microbiol. 1995;3:118–120. doi: 10.1016/s0966-842x(00)88894-0. [DOI] [PubMed] [Google Scholar]
- 9.Hedges S R, Agace W W, Svanborg C. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 1995;3:266–270. doi: 10.1016/s0966-842x(00)88941-6. [DOI] [PubMed] [Google Scholar]
- 10.Hedges S R, Bjarnadotter M, Agace W, Hang L, Svanberg C. Immunoregulatory cytokines modify Escherichia coli induced uroepithelial cell IL-6 and IL-8 responses. Cytokine. 1996;8:686–697. doi: 10.1006/cyto.1996.0091. [DOI] [PubMed] [Google Scholar]
- 11.Horsburgh C R., Jr Mycobacterium avium complex in the acquired immunodeficiency syndrome (AIDS) N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 12.Hsu N, Goodman J R, Young L S, Bermudez L E. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Interaction between Mycobacterium avium complex and intestinal mucosal cells in vivo, abstr. B35; p. 26. [Google Scholar]
- 13.Huang J, O’Toole P W, Doig P, Trust T J. Stimulation of interleukin-8 production in epithelial cell lines by Helicobacter pylori. Infect Immun. 1995;63:1732–1738. doi: 10.1128/iai.63.5.1732-1738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson M A, Hopewell P C, Yajko D M, Hadley W K, Lazarus E, Mohanty P K, Modin G W, Feigal D W, Cusick P S, Sande M A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991;164:994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- 16.Jung H C, Eckman L, Yang S K, Panga A, Freier J, Morzychka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S Y, Goodman J R, Petrofsky M, Bermudez L E. Mycobacterium avium infection of the gut mucosa in mice is associated with later inflammatory response and ultimately results in areas of intestinal cell necrosis. J Med Microbiol. 1998;47:725–731. doi: 10.1099/00222615-47-8-725. [DOI] [PubMed] [Google Scholar]
- 18.McGowan I, Radford-Smith G, Jewell D P. Cytokine gene expression in HIV-infected intestinal mucosa. AIDS. 1994;8:1569–1575. doi: 10.1097/00002030-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Mecsas J, Raupach B, Falkow S. The Yersinia yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol Microbiol. 1998;28:1269–1281. doi: 10.1046/j.1365-2958.1998.00891.x. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y X, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Invest. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoades E R, Cooper A M, Orme I M. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63:3871–3877. doi: 10.1128/iai.63.10.3871-3877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth R I, Owen R L, Keren D F, Volberding P A. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple’s disease. Dig Dis Sci. 1985;30:497–504. doi: 10.1007/BF01318186. [DOI] [PubMed] [Google Scholar]
- 23.Schuerer-Maly C C, Eckmann L, Kagnoff M F, Falco M T, Maly F E. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994;81:85–91. [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma S A, Tummuru M K R, Miller G B, Blaser M J. Interleukin-8 response of gastric epithelial cell lines to Helicobacter pylori stimulation in vitro. Infect Immun. 1995;63:1681–1687. doi: 10.1128/iai.63.5.1681-1687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stadnyk A W. Cytokine production by epithelial cells. FASEB J. 1994;8:1041–1047. doi: 10.1096/fasebj.8.13.7926369. [DOI] [PubMed] [Google Scholar]
- 26.Torriani F, Maslow J N, Kornbluth R, Arbeit R D, McCutchan J A, Hasegawa P, Keays L, Havlir D. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Analysis of Mycobacterium avium complex isolates infecting multiple organs of AIDS patients using pulsed field gel electrophoresis, abstr. I91; p. 221. [Google Scholar]
- 27.Weinstein D L, O’Neill B L, Metcalf E S. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997;65:395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuanguang L, Zhang M, Barnes P. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998;66:1121–1126. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]