Abstract
Background
Studies on the pulmonary consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are impeded by limited access to pre–SARS-CoV-2 examinations.
Methods
We invited Copenhagen General Population Study participants with a confirmed SARS-CoV-2 polymerase chain reaction (PCR) test during the first and second coronavirus disease 2019 waves in Denmark for a repeat chest computed tomography (CT) scan. Paired CT scans were independently assessed for interstitial and noninterstitial abnormalities by 2 trained radiologists. A semiquantitative CT score (ranging from 0 to 20) was used to quantify the extent of interstitial abnormalities.
Results
Of 111 SARS-CoV-2–infected individuals, 102 (91.2%) experienced symptoms and 12 (11.2%) were hospitalized. Follow-up examination was performed at median of 5.4 (interquartile range, 4.1–7.8) months after a positive SARS-CoV-2 PCR test. Of 67 individuals with paired CT scans, ground glass opacities and reticulation were present in 31 (46.3%) individuals post–SARS-CoV-2 compared to 23 (34.1%) pre–SARS-CoV-2 (mean CT score, 3.0 vs 1.3; P = .011). Results were similar for nonhospitalized individuals. We did not detect development of bronchiectasis, emphysema, or nodules.
Conclusions
SARS-CoV-2 infection in predominantly nonhospitalized individuals with mild disease was associated with a small increase in only interstitial lung abnormalities.
Keywords: COVID-19, chest CT, lung abnormalities, lung function test, paired analysis
Even mild SARS-CoV-2 infection not requiring hospitalization was associated with the development/progression of interstitial lung abnormalities in individuals undergoing repeat chest computed tomography scans, indicating that even mild SARS-CoV-2 infections have a modest impact on radiographic changes.
Coronavirus disease 2019 (COVID-19), caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is most often associated with asymptomatic or mild upper respiratory tract symptoms but may progress to severe lower respiratory tract disease [1]. COVID-19 associated lung injury is radiographically characterized by peripheral ground glass opacity (GGO) and consolidation [2–5]. Up to 15%–30% of patients with COVID-19 maintain respiratory symptoms up to 12 weeks after acute infection, leading to concerns of persisting long-term lung damage [6–9]. Longitudinal lung function testing and computed tomography (CT) chest imaging have shown that lung diffusing impairment and fibrotic changes may persist in up to a third of hospitalized individuals for up to 6–12 months [5, 10–15]. However, precise estimates of changes in lung function and incident lung pathology caused by SARS-CoV-2 infection remain unclear as most studies have limited or no pre–SARS-CoV-2 assessments available. Furthermore, the majority of studies of lung function testing and CT imaging have focused on hospitalized patients, while studies that have investigated changes following mild COVID-19 are few even though the vast majority of infected adults experience no or mild symptoms [16]. We have recently shown that lung volumes, particularly forced vital capacity (FVC), declined more steeply in COVID-19 patients with predominantly mild disease compared to unaffected controls [17]. Here we extend these analyses and include findings from chest CT imaging in order to better understand the structural changes following predominantly mild COVID-19.
METHODS
Study Population and Ethics
Participants were recruited from the Copenhagen General Population Study (CGPS), an ongoing epidemiological study of the inhabitants of Copenhagen, Denmark [18, 19]. Only individuals from the CGPS who had performed a spirometry, undergone a chest CT scan before October 2019, and who had a positive real-time polymerase chain reaction (rt-PCR) SARS-CoV-2 test between 9 March 2020 and 16 January 2021 were invited to participate. The study was approved by the Regional Ethics Committee of Copenhagen (H-KF-01-144/0; H-20072296). Written informed consent was obtained from all participants.
Data Collection
Physical examination and spirometry prior to SARS-CoV-2 infection were performed between April 2010 and October 2018. Pre–SARS-CoV-2 chest CT scans were acquisitioned from September 2012 to October 2019. Post–SARS-CoV-2 examinations and chest CT scans were performed between 15 March 2021 and 9 June 2021. At the pre– and post–SARS-CoV-2 examinations, an identical questionnaire on comorbidity, risk factors, and self-reported dyspnea was completed. Smoking status was categorized as never smoker, former smoker, or current smoker. Cigarette consumption was assessed in pack-years. Dyspnea was reported according to the modified Medical Research Council dyspnea scale ranging from 0 to 4. A score >2 was interpreted as moderate dyspnea. Sputum was defined as daily productive cough with a minimum duration of 3 months per year. Questionnaires were reviewed and checked by an investigator at the day of examination. At the post–SARS-CoV-2 examination, additional questions related to COVID-19 disease course and symptoms were added. Information on hospital admission in relation to COVID-19 was retrieved from electronic patient records.
Lung Function Tests
All participants performed 2 lung function tests. A pre–SARS-CoV-2 single-breath maneuver spirometry was performed as previously described [17]. Forced expiratory volume in 1 second (FEV1), FVC, and FEV1/FVC were measured. At post–SARS-CoV-2 examinations, dynamic lung volumes, diffusing capacity for carbon monoxide (DLCO), and estimated total lung capacity (TLC) were measured by a single-breath device (EasyOne Pro, ndd Medical Technologies). Lung function data were collected as raw data and as percent predicted values for FEV1, FVC, DLCO, and TLC. Percent predicted was based on previously described and published reference equations [20]. The lower limit of normal (LLN) of FEV1/FVC was calculated based on the same reference equations.
Chest CT Scans
All participants underwent an unenhanced chest CT scan post–SARS-CoV-2 using an Aquillon One Vision Edition scanner (Toshiba Medical Systems). A subset of included participants had an identical chest CT scan performed prior to infection using the same scanner and an identical protocol, except that pre–SARS-CoV-2 CT scans were acquired using only a deep inspiratory breath-hold without expiration. Post–SARS-CoV-2 infection CT scans were acquired during both inspiratory and expiratory breath-hold. Settings were 120 kVp and automated exposure control (SD15 and SD55). Images were reconstructed using filter back projection and a soft tissue kernel (1 mm slice thickness). Details on CT scanner and protocol have previously been described [21].
Chest Image Interpretation
All CT images were reviewed unblinded in random order by 2 board-certified radiologists (T. S. K. and J. J.). Images were reviewed independently and images were reviewed if any disagreements. Abnormalities were assessed using the standard terms defined in the Fleischner Society nomenclature and peer-reviewed literature for the presence of the following interstitial and noninterstitial features: GGO, reticulation, thickening of the adjacent pleura, pleural effusion, honey combing, interlobar traction, emphysema, bronchiectasis, peribronchial thickening, and presence of nodules and masses [22, 23]. For each CT scan, the radiologists were asked to assess the predominant pattern if any interstitial abnormalities were present; GGO, reticular pattern, or a combination if no pattern was predominant. Our main outcome was degree of interstitial disease in each of the 5 lung lobes, which was also graded by a semiquantitative score: 0, no involvement; 1, equivocal (1%–5%); 2, mild (6%–25%); 3, moderate (26%–50%); and 4, severe (51%–100%). A total score was calculated by summarizing the individual score from the 5 lobes with a total possible score ranging from 0 to 20. Emphysema was further graded according to a semiquantitative score of 0–5, where 0 indicates no emphysema (0%); 1, trace emphysema (1%–10%); 2, mild emphysema (11%–25%); 3, moderate emphysema (26%–50%); 4, severe emphysema (51%–75%); and 5, very severe (>75%) visual emphysema. Bronchiectasis was further graded according to the bronchiectasis radiologically indexed CT score: 0, absent; 1, mild (lumen just greater than the diameter of the adjacent vessel); 2, moderate (lumen 2–3 times greater than the diameter of the adjacent vessel); and 3, severe (lumen >3 times the diameter of the adjacent vessel).
Statistical Analysis
Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR) and group comparisons were made using paired t test. Categorical variables were expressed as absolute and relative frequencies (%) and groups were compared with McNemar-Bowker test of symmetry or χ2 test, as appropriate. Spearman correlation and scatterplots were used to evaluate the relationship between measures from lung functions tests and CT scores. A P value < .05 was considered statistically significant. Analyses were conducted using R statistical software version 4.1.0 and Stata/SE 15.1 for Windows (StataCorp, College Station, Texas).
RESULTS
Demographics and Clinical Characteristics
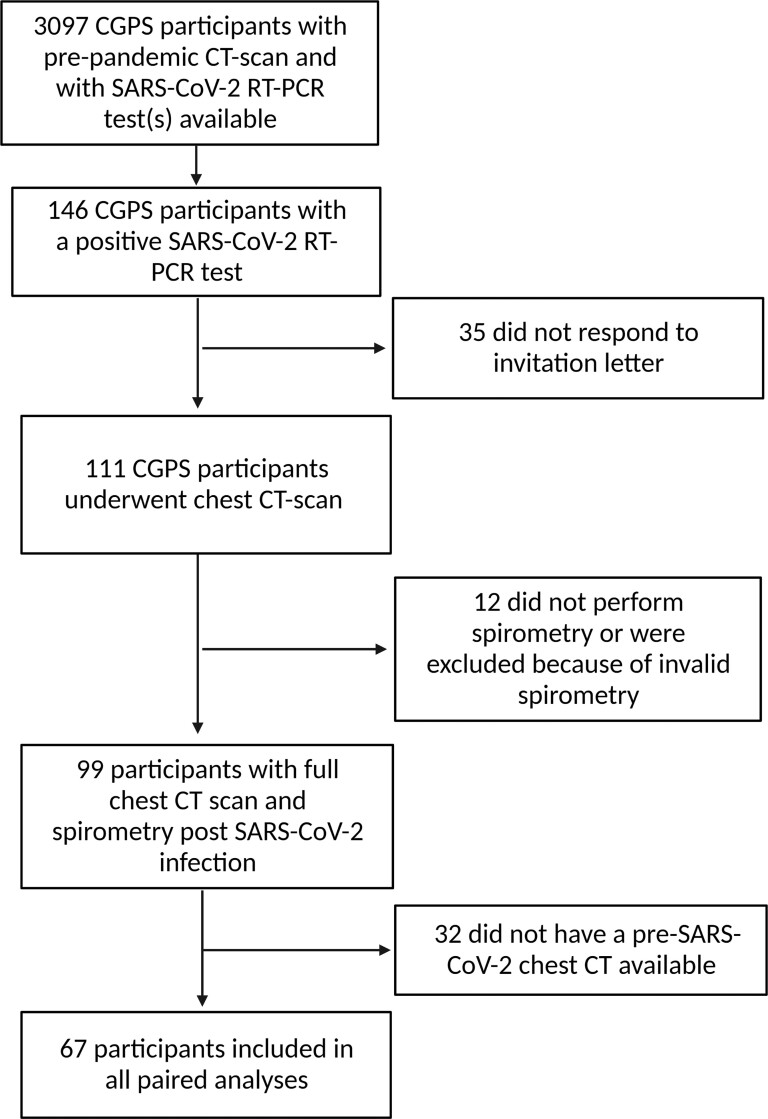
A total of 111 participants were included in the study and 99 participants performed a satisfactory lung function test (Figure 1). At pre–SARS-CoV-2 examination, the average age was 56.9 (SD, 8.7) years, 56 (50.5%) were female, and all were White. Fifty-two (46.8%) were former smokers with a median of 17.8 pack-years (IQR, 8.7–27.6) for current or former smokers. Pre–SARS-CoV-2, the mean FEV1 was 3.1 L (SD, 0.8), corresponding to 95.4% (SD,14.9) of predicted, and 11 (10.6%) participants had an FEV1/FVC ratio below the LLN (Table 1). Most study participants (86.5%) experienced symptoms in relation to SARS-CoV-2 infection and mean symptom duration was 13.8 (SD, 8.3) days. 12 (11.2%) were hospitalized with a median length of stay of 7.0 (IQR, 3.7–9.3) days. One (0.9%) participant required mechanical ventilation (Table 1).
Figure 1.
Flowchart of study participants. Sixty-seven participants had paired chest computed tomography scans (see Methods for further details). Abbreviations: CGPS, Copenhagen General Population Study; CT, computed tomography; rt-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2 (created with BioRender.com).
Table 1.
Baseline Characteristics of Participants
| Characteristic | Pre–SARS-CoV-2 Assessment (n = 111) | Pre–SARS-CoV-2 Assessment in Participants With Paired CT Scans (n = 67) |
|---|---|---|
| Age, y, mean (SD) | 56.9 (8.7) | 59.3 (8.7) |
| Sex, female | 56 (53.8) | 34 (50.1) |
| Ethnicity, White | 104 (100.0) | 67 (100.0) |
| BMI, kg/m2, mean (SD) | 26.5 (3.8) | 26.9 (4.3) |
| Smoking status | ||
| Never-smoker | 44 (42.3) | 23 (37.3) |
| Former smoker | 52 (50.0) | 29 (43.3) |
| Current smoker | 8 (7.7) | 5 (7.5) |
| Cumulative smoking, pack-yearsa, median (IQR) | 17.1 (8.7–27.6) | 18.0 (9.9–26.0) |
| Date of pre–SARS-CoV-2 assessment, median | 7 Jul 2015 | 24 Aug 2016 |
| Dynamic lung volumes | ||
| FEV1, L, mean (SD) | 3.1 (0.8) | 2.96 (0.7) |
| FEV1% predicted, mean (SD) | 95.4 (14.9) | 94.5 (14.1) |
| FVC, L, mean (SD) | 4.1 (0.9) | 4.02 (0.8) |
| FVC % predicted, mean (SD) | 100.6 (13.3) | 100.5 (12.8) |
| FEV1/FVC, mean (SD) | 0.7 (0.1) | 0.7 (0.1) |
| FEV1/FVC <LLN | 11 (10.6) | 9 (13.4) |
| COVID-19 characteristics | ||
| Any SARS-CoV-2–related symptoms | 90 (86.5) | 55 (82.1) |
| Duration of symptoms, d (SD) | 13.8 (8.3) | 12.7 (10.1) |
| Fever | 74 (66.7) | 43 (64.1) |
| Dyspneab | 43 (38.7) | 22 (32.8) |
| Fatigue | 92 (82.8) | 50 (74.6) |
| Muscle or body aches | 63 (56.7) | 35 (52.2) |
| Sore throat | 28 (25.2) | 15 (22.3) |
| Loss of smell or taste | 53 (47.7) | 31 (46.2) |
| Hospital admission | 12 (11.2) | 8 (11.9) |
| Days, median (IQR) | 7 (3.7–9.3) | 7 (4.5–9.3) |
| Oxygen therapy | 2 (1.9) | 1 (1.5) |
| CPAP | 3 (2.8) | 3 (4.5) |
| Mechanical ventilation | 1 (0.9) | 0 (0.0) |
| Outcome assessments | ||
| Pre–SARS-CoV-2 chest CT available | 67 (60.3) | … |
| Time between LFT and chest CT scan, d, median (IQR) | 103 (78.4–127.6) | … |
| Time between positive SARS-CoV-2 rt-PCR and post–SARS-CoV-2 examinations, d, median (IQR) | 154.0 (122.8–235.0) | … |
| Time between pre–SARS-CoV-2 and post–SARS-CoV-2 chest CT scan, y, median (IQR) | NA | 3.48 (2.59–4.63) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; CPAP, continuous positive airway pressure; CT, computed tomography; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; IQR, interquartile range; LFT, lung function test; LLN, lower limit of normal; NA, not available; rt-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Only for current or former smokers.
Individuals were asked: “Did you experience difficulties in breathing in relation to SARS-CoV-2 infection”?
CT Assessment and Semiquantitative CT Score
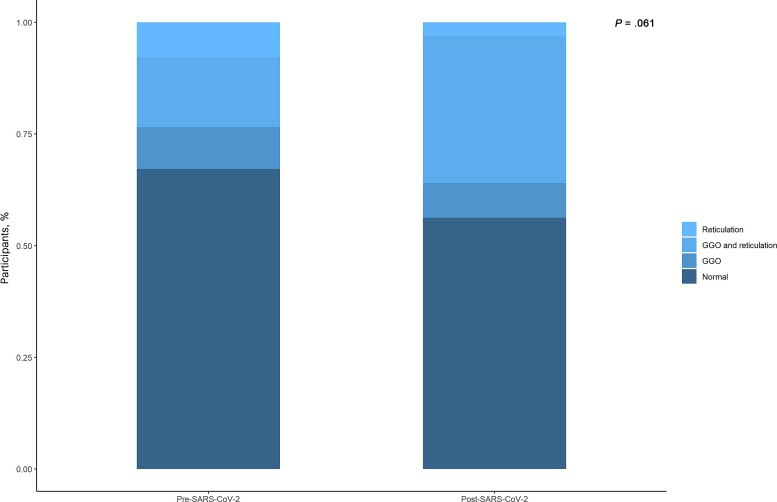
Sixty-seven (60.3%) patients had a full chest CT scan prior to SARS-CoV-2 infection, and the remaining 44 (39.7%) had a cardiac chest CT performed pre–SARS-CoV-2 infection; thus, assessment of the lungs pre–SARS-CoV-2 infection was not possible. Median time between pre– and post–SARS-CoV-2 CT scans was 3.48 (IQR, 2.59–4.63) years. Characteristics of the 67 individuals with paired CT scans were comparable with the entire cohort (Table 1). The chest CT features and distribution across the 67 participants with a full pre–SARS-CoV-2 CT scan are shown in Table 2 and for the entire cohort in Supplementary Table 1. Twenty-three (34.4%) had 1 or more interstitial abnormality prior to SARS-CoV-2 infection and 31 (46.3%) post–SARS-CoV-2 infection (P = .061). The predominant pattern of interstitial abnormalities was a combination of GGO and reticulation at both pre– and post–SARS-CoV-2 CT scan (Figure 2). A similar pattern was observed when hospitalized individuals were excluded (Supplementary Table 4). One participant had signs of severe interstitial abnormalities (honey combing) post–SARS-CoV-2 infection; however, this individual had radiologically signs interstitial lung disease prior to infection as well. Noninterstitial lung abnormalities, including bronchiectasis and nodules, occurred at a similar prevalence pre– and post–SARS-CoV-2 infection (Supplementary Tables 2 and 3).
Table 2.
Interstitial Lung Abnormalities Before and After Severe Acute Respiratory Syndrome Coronavirus 2 Infection
| Visual CT Abnormalities | Pre–SARS-CoV-2 Chest CT Scan (n = 67) | Post–SARS-CoV-2 Chest CT Scan (n = 67) | P Value |
|---|---|---|---|
| Any interstitial abnormality (yes) | 23 (34.3) | 31 (46.3) | .061 |
| Pattern of interstitial abnormality | |||
| GGO exclusively | 8 (11.9) | 7 (11.1) | |
| Reticulation exclusively | 5 (7.5) | 2 (3.2) | |
| GGO and reticulation | 10 (14.9) | 22 (34.9) | |
| Honey combing | 0 (0.0) | 1 (1.5) | 1 |
| Parenchymal bands | 20 (29.9) | 27 (42.9) | .15 |
| Interlobar pleural traction | 6 (9.0) | 10 (15.9) | .13 |
| Thickening of the adjacent pleura | 5 (7.5) | 6 (9.5) | 1 |
| Visual semiquantitative scores and location of lesions | |||
| Total CT score, mean (SD) | 1.3 (3.1) | 3.0 (4.7) | .011 |
| Total CT score, mean (SD) | |||
| <6 mo post–SARS-CoV-2 infection (n = 34) | … | 3.03 (5.20) | |
| >6 mo post–SARS-CoV-2 infection (n = 33) | … | 2.78 (4.2) | .97 |
Data are presented as No. (%) unless otherwise indicated. Significant p-values (<0.05) are in bold.
Abbreviations: CT, computed tomography; GGO, ground glass opacity; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.
Figure 2.
Distribution of pattern of interstitial lung abnormalities before and after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Distribution of pattern of interstitial abnormalities pre– and post–SARS-CoV-2 infection is depicted. P values compare the prevalence of any interstitial abnormality pre– and post–SARS-CoV-2 infection. Abbreviations: GGO, ground glass opacity; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
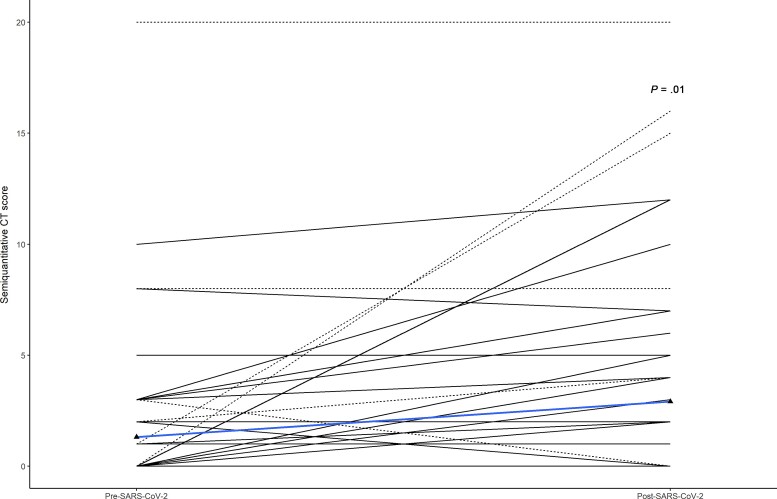
Mean pre–SARS-CoV-2 total CT score was 1.3 (SD, 3.1) and increased to 3.0 (SD, 4.7) in post–SARS-CoV-2 scans (P = .011). After admitted participants were excluded from analysis, an increase from 0.9 (SD, 1.9) to 2.0 (SD, 2.0) (P = .002) was observed. Individual changes in semiquantitative CT-score pre– and post–SARS-CoV-2 infection are shown in Figure 3. No significant change in mean CT score was observed if individuals were divided into groups of follow-up more or less than 6 months after SARS-CoV-2 infection.
Figure 3.
Computed tomography (CT) scores before and after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Shown is the individual dynamic in CT score at pre– and post–SARS-CoV-2 examination. Admitted individuals are represented as dotted lines. The blue line represents median score before and after SARS-CoV-2 infection.
CT Abnormalities and Lung Function
Post–SARS-CoV-2 examinations showed an inverse association between CT score and % predicted DLCO (r = −0.35, P < .01) and between % predicted TLC and CT score (r = −0.33, P < .01) (Supplementary Figure 1). Similar associations remained after limiting analysis to nonhospitalized individuals. The percentage of predicted DLCO in participants with interstitial abnormalities was lower compared to participants without abnormalities (82.98% vs 93.20%, P < .01). TLC was significantly lower in subjects with interstitial abnormalities compared to subjects without interstitial abnormalities, whereas there was no difference in FEV1, FVC, or FEV1/FVC between groups (Table 3).
Table 3.
Associations Between Respiratory Symptoms, Lung Function Test, and Post–Severe Acute Respiratory Syndrome Coronavirus 2 Interstitial Lung Abnormalities
| Characteristic | Entire Cohort (N = 99) | Any Interstitial Abnormality Post–SARS-CoV-2 (n = 48) | No Interstitial Abnormality Post–SARS-CoV-2 Detected (n = 51) | P Value |
|---|---|---|---|---|
| Respiratory symptoms, No. (%) | ||||
| Sputum | 12 (12.1) | 5 (10.4) | 7 (13.7) | .84 |
| Wheezing | 6 (6.1) | 3 (6.2) | 3 (5.9) | 1 |
| Dyspnea (MRC scale >2) | 12 (12.1) | 8 (16.7) | 4 (7.8) | .28 |
| Lung function measures | ||||
| FEV1, L, mean (SD) | 2.78 (0.71) | 2.74 (0.78) | 2.83 (0.64) | .55 |
| FVC, L, mean (SD) | 3.65 (0.85) | 3.58 (0.90) | 3.73 (0.82) | .28 |
| FEV1/FVC, mean (SD) | 0.76 (0.08) | 0.77 (0.09) | 0.76 (0.07) | .56 |
| Mean decline in FEV1, mL, mean (SD) | 38.97 (41.67) | 39.47 (45.50) | 38.92 (38.61) | .95 |
| Mean decline in FVC, mL, mean (SD) | 63.58 (56.51) | 66.05 (68.36) | 62.59 (57.65) | .79 |
| DLCO, mmol/min/kPa, mean (SD) | 7.73 (2.03) | 7.18 (2.16) | 8.24 (1.76) | .01 |
| DLCO predicted, %, mean (SD) | 87.75 (16.56) | 82.98 (18.02) | 92.98 (13.20) | <.01 |
| TLC, L, mean (SD) | 5.52 (1.07) | 5.41 (1.09) | 5.62 (1.06) | .34 |
| TLC predicted, %, mean (SD) | 90.17 (10.72) | 87.02 (10.16) | 93.20 (10.47) | <.01 |
Abbreviations: DLCO, diffusing capacity of the lungs; FEV1, forced expiratory volume in first second; FVC, forced vital capacity; MRC, Medical Research Council; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; TLC, total lung capacity. Significant p-values (<0.05) are in bold.
DISCUSSION
To our knowledge, this is the first longitudinal study with access to chest CT prior to SARS-CoV-2 infection to describe changes in relation to the infection. We showed that individuals with predominantly mild disease, of which most were not hospitalized, experienced a small but significant increase in interstitial lung abnormalities by chest imaging. Furthermore, presence of interstitial lung abnormalities was associated with lower DLCO and TLC. SARS-CoV-2 infection was not associated with development of noninterstitial abnormalities.
Multiple studies have investigated the prevalence of radiologic abnormalities at mid- to long-term follow-up of individuals hospitalized with COVID-19. A systematic review found that 49% of patients had inflammatory abnormalities defined as GGO or consolidation and 34% had fibrotic changes defined as either reticulation, lung architectural distortion, interlobar septal thickening, traction bronchiectasis, or honey combing at 3–6 months after discharge [23]. To what extent these abnormalities were present prior to the infection is unknown. In our cohort, in which most individuals were not hospitalized, we found a comparable prevalence (43.2%) of GGO at a median of 5.4 months of follow-up. However, similar findings were present in 18 (26.8%) individuals prior to infection, indicating a relatively high prevalence of fibrotic-like changes not related to SARS-CoV-2 infection. A combination of GGO and reticulation was the most prevalent finding post–SARS-CoV-2 infection, which may suggest a combination of residual inflammation and fibrotic changes. Although the degree of abnormalities was small, with a mean total CT score of 3.0, it is comparable to studies of individuals with moderate to severe disease [8, 11, 15]. A study using a similar scoring approach of 209 individuals with moderate to severe disease observed a mean CT score of 2.7 at 3 months’ follow-up [12]. Overall, we and others have shown that interstitial and overall lung abnormalities are present months after infection regardless of disease severity at the time of infection. Whether the changes will persist and transform to more recognizable signs of fibrosis over time is unknown. It is encouraging that 1 study with longer follow-up showed that most lung abnormalities resolve within a year for most individuals, particularly for individuals with mild disease [15]. Nevertheless, the study found that 40% of participants had persistent abnormalities at 1 year if they had any abnormality at 6 months after infection.
We did not observe any increase in the presence of noninterstitial abnormalities, and limited data on this topic exist. In particular, the association between viral infections, including COVID-19, and development of bronchiectasis may be of interest but is not well understood. A study by Han et al found presence of bronchiectasis in 24% of participants hospitalized with COVID-19 at 3–6 months of follow-up but only in 7% during acute disease, indicating a late onset in relation to COVID-19 [14]. We did not find any increase in the presence of bronchiectasis after infection in this study. It seems likely that only individuals with more severe disease may develop bronchiectasis.
Our findings of an inverse relationship between the extent of interstitial abnormalities and gas diffusing impairment has been observed in other follow-up studies [10, 25–28]. A study of patients who had been hospitalized with mild to moderate disease found that the presence of any lung abnormality was associated with a 3-fold odds of impaired DLCO at 6 and 12 months of follow-up after adjustment for differences in age, sex, cigarette smoking, comorbidity, and disease severity [15]. Our findings suggest that even nonhospitalized individuals with mild disease may experience diffuse parenchymal changes leading to visible interstitial abnormalities by CT and impairment of DLCO. In terms of improvement of initial impairment of DLCO due to SARS-CoV-2 infection, a study with 86 individuals with mild to severe disease showed that a low diffusing capacity at discharge improved by 3 and 6 months of follow-up but remained below normal, suggesting that changes are either permanent or regress slowly [26]. More long-term follow-up studies are needed to fully establish the impact of SARS-CoV-2 infection on lung function.
Our study has limitations. First, not all study participants had a pre–SARS-CoV-2 chest CT scan available, which limited our sample size for the paired analysis and thus did not allow us to analyze all clinically relevant subgroups. Data on DLCO and TLC were only collected after SARS-CoV-2 infection. Therefore, the full impact of SARS-CoV-2 on DLCO and its relation to CT scores over time cannot be established. Although median time between CT reexaminations was reasonable, in terms of what we can expect to be associated with SARS-CoV-2 infection, we only have 2 data points and are unable to provide a full trajectory of the interstitial lung abnormalities.
In conclusion, we found that asymptomatic to mild SARS-CoV-2 infection was associated with an increase in interstitial lung abnormalities including mostly GGO and reticulation. Furthermore, presence of these abnormalities was associated with a decrease in DLCO and TLC. Long-term follow-up with serial assessments in individuals with mild disease will be relevant to understand the impact of SARS-CoV-2 infection on the lungs. The relationship between interstitial abnormalities, lung function, and pulmonary symptoms also needs to be further investigated.
Supplementary Material
Notes
Author contributions. A. R., S. A., T. B., and K. F. K. conceived the idea and planning of the study. K. K. I., T. S. K., J. J., and A. K. collected the data. K. K. I. performed statistical analysis. All authors revised the manuscript for important intellectual content. T. B., B. G. N., and K. F. K. obtained funding and provided administrative, technical, and material support. K. K. I., A. R., and T. B. wrote the draft manuscript. All authors contributed to subsequent writing and revisions and approved the final version of the manuscript.
Acknowledgments. We thank all COVID-19 patients for their participation. We also thank the staff at Department of Radiology, Rigshospitalet, for their dedicated participation. The support of the Cardiac CT-Copenhagen General Population Substudy, Rigshospitalet, is highly appreciated.
Patient consent. The patients provided written consent (available from the corresponding author) for the use of their data in the study.
Ethics approval. The study was approved by the Regional Ethics Committee of Copenhagen (H-KF-01-144/0; H-20072296).
Financial support. This work was supported by the Research Council of Rigshospitalet, AP Møller og Hustru Chastine McKinney Møllers Fond, the Danish Heart Foundation, and the Brodie Foundation.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Katrine K Iversen, Department of Infectious Diseases 144, Copenhagen University Hospital–Amager Hvidovre, Hvidovre, Denmark.
Andreas Ronit, Department of Infectious Diseases 144, Copenhagen University Hospital–Amager Hvidovre, Hvidovre, Denmark.
Thomas S Kristensen, Department of Radiology and Cardiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Shoaib Afzal, Department of Clinical Biochemistry, Copenhagen University Hospital–Herlev and Gentofte, Herlev, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; The Copenhagen General Population Study, Copenhagen University Hospital–Herlev and Gentofte, Herlev, Denmark.
Jelena Jankovic, Department of Radiology and Cardiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Anna Kalhauge, Department of Radiology and Cardiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Magnus G Ahlström, Department of Clinical Microbiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Børge G Nordestgaard, Department of Clinical Biochemistry, Copenhagen University Hospital–Herlev and Gentofte, Herlev, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark; The Copenhagen General Population Study, Copenhagen University Hospital–Herlev and Gentofte, Herlev, Denmark.
Klaus F Kofoed, Department of Radiology and Cardiology, Copenhagen University Hospital–Rigshospitalet, Copenhagen, Denmark.
Thomas Benfield, Department of Infectious Diseases 144, Copenhagen University Hospital–Amager Hvidovre, Hvidovre, Denmark.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Hu B, Guo H, Zhou P, Shi Z-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021; 19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li P, Zhang J-F, Xia X-D, et al. Serial evaluation of high-resolution CT findings in patients with pneumonia in novel swine-origin influenza A (H1N1) virus infection. Br J Radiol 2012; 85:729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ooi GC, Khong PL, Müller NL, et al. Severe acute respiratory syndrome: temporal lung changes at thin-section CT in 30 patients. Radiology 2004; 230:836–44. [DOI] [PubMed] [Google Scholar]
- 4. Poitevineau T, Chassagnon G, Bouam S, et al. Computed tomography after severe COVID-19 pneumonia: findings at 6 months and beyond. ERJ Open Res 2021; 7:00488-02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu N, He G, Yang X, et al. Dynamic changes of chest CT follow-up in coronavirus disease-19 (COVID-19) pneumonia: relationship to clinical typing. BMC Med Imaging 2020; 20:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blomberg B, Mohn KG-I, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med 2021; 27:1607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020; 6:00542-02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frija-Masson J, Debray M-P, Boussouar S, et al. Residual ground glass opacities three months after Covid-19 pneumonia correlate to alteration of respiratory function: the post Covid M3 study. Respir Med 2021; 184:106435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lewis KL, Helgeson SA, Tatari MM, Mallea JM, Baig HZ, Patel NM. COVID-19 and the effects on pulmonary function following infection: a retrospective analysis. EClinicalMedicine 2021; 39:101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Wang D, Mei N, et al. Longitudinal radiological findings in patients with COVID-19 with different severities: from onset to long-term follow-up after discharge. Front Med (Lausanne) 2021; 8:711435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu D, Zhang W, Pan F, et al. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res 2020; 21:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Y, Ding C, Yu L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med 2021; 19:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qureshi S, Nasir N, Rashid N, Ahmed N, Haq Z, Qamar F. Long term impact on lung function of patients with moderate and severe COVID-19. A prospective cohort study. Front Public Health 2021; 9:663076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology 2021; 299:E177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021; 398:747–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020; 323:1239–42. [DOI] [PubMed] [Google Scholar]
- 17. Iversen KK, Afzal S, Ahlström MG, et al. Lung function decline in relation to COVID-19 in the general population: a matched cohort study with pre-pandemic assessment of lung function. J Infect Dis 2022; 225:1308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Çolak Y, Afzal S, Nordestgaard BG, Marott JL, Lange P. Combined value of exhaled nitric oxide and blood eosinophils in chronic airway disease: the Copenhagen General Population Study. Eur Respir J 2018; 52:1800616. [DOI] [PubMed] [Google Scholar]
- 19. Çolak Y, Afzal S, Nordestgaard BG, Lange P, Vestbo J. Importance of early COPD in young adults for development of clinical COPD: findings from the Copenhagen general population study. Am J Respir Crit Care Med 2021; 203:1245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40:1324–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ronit A, Kristensen T, Hoseth VS, et al. Computed tomography quantification of emphysema in people living with HIV and uninfected controls. Eur Respir J 2018; 52:1800296. [DOI] [PubMed] [Google Scholar]
- 22. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 23. Balbi M, Conti C, Imeri G, et al. Post-discharge chest CT findings and pulmonary function tests in severe COVID-19 patients. Eur J Radiol 2021; 138:109676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fabbri L. Post-viral parenchymal lung disease following COVID-19 and viral pneumonitis hospitalisation: a systematic review and meta-analysis [manuscript published online ahead of print 25 March 2022]. Thorax 2022. doi:10.1136/thoraxjnl-2021-218275 [Google Scholar]
- 25. Zhou M, Xu J, Liao T, et al. Comparison of residual pulmonary abnormalities 3 months after discharge in patients who recovered from COVID-19 of different severity. Front Med (Lausanne) 2021; 8:682087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen M, Liu J, Peng P, et al. Dynamic changes of pulmonary diffusion capacity in survivors of non-critical COVID-19 during the first six months. EClinicalMedicine 2022; 43:101255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao Y, Yang C, An X, et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis 2021; 112:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Writing Committee for the COMEBAC Study Group; Morin L, Savale L, Pham T, et al. Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA 2021; 325:1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.