Abstract
ATP and UTP have been proposed for use as therapeutic treatment of the abnormal ion transport in the airway epithelium in cystic fibrosis (CF), the most characteristic feature of which is permanent infection by Pseudomonas aeruginosa. As for diverse gram-negative bacteria, this pathogenic bacterium accumulates diffusible N-acylhomoserine lactone (AHL) signal molecules, and when a threshold concentration is reached, virulence factor genes are activated. Human submucosal tracheal gland serous (HTGS) cells are believed to play a major role in the physiopathology of CF. Since ATP and UTP stimulate CF epithelial cells through P2Y receptors, we sought to determine whether CF HTGS cells are capable of responding to the AHLs N-butanoyl-l-homoserine lactone (BHL), N-hexanoyl-l-homoserine lactone (HHL), N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL), and N-(3-oxohexanoyl)-l-homoserine lactone (OHHL), with special reference to P2Y receptors. All AHLs inhibited ATP- and UTP-induced secretion by CF HTGS cells. The 50% inhibitory concentrations were as high as 10 and 5 μM for BHL and HHL, respectively, but were only 0.3 and 0.4 pM for OdDHL and OHHL, respectively. Furthermore, all AHLs down-regulated the expression of the P2Y2 and P2Y4 receptors. Ibuprofen and nordihydroguaiaretic acid were able to prevent AHL inhibition of the responses to nucleotides, but neither dexamethasone nor indomethacin was able to do this. These data indicate that AHLs may alter responsiveness to ATP and UTP by CF HTGS cells and suggest that, in addition to ATP and/or UTP analogues, ibuprofen may be of use for a combinational pharmacological therapy for CF.
Cystic fibrosis (CF) is a genetic disease characterized by hypersecretion of mucus and especially by persistent severe bacterial infection and inflammation in the airways. The defect lies in mutations of a membrane protein called CF transmembrane conductance Regulator (CFTR), which possesses a cyclic AMP-dependent chloride channel activity (34) that is defective in CF. However, since calcium-dependent chloride channels remain functional in CF airway epithelial cells (46), the use of agents inducing rises in intracellular calcium was proposed for pharmacological therapy in order to bypass the basic defect in CF. Among the possible secretagogues acting on the intracellular calcium pathway, nucleotides such as ATP or UTP analogues are thought to be of most interest, as they were demonstrated to act on receptors located on the apical side of the airway epithelium (21). ATP or UTP analogues were shown to induce chloride secretion by CF epithelial cells (21) and also to induce bronchial relaxation (1). When tested clinically, nucleotides or nucleotide analogues were described as having positive and efficient effects on mucociliary clearance (3, 29).
The most characteristic feature of CF is the persistent infection of the airways by the gram-negative bacterium Pseudomonas aeruginosa. This bacterium is a major pathogen in CF which persists continuously (17) in the airways of CF patients despite intense antibiotic therapy. A large number of P. aeruginosa virulence factors are regulated by two unique quorum-sensing systems, LasRI and RhlRI (23, 32). These two systems form a hierarchical quorum-sensing cascade (23), and there is considerable overlap within this dual-level control system in the regulation of many virulence factors. Quorum-sensing systems generally consist of two components, a small diffusible signal molecule (N-acylhomoserine lactone [AHL]) and a transcriptional activator protein (41). When present in sufficient amounts, the free diffusible signal molecule binds to the regulator, which activates a set of specific target genes. The threshold concentration of autoinducer necessary for the induction of the genes is reached when cultures achieve a sufficiently high bacterial density (for a review, see reference 15). Two major AHLs [N-(3-oxododecanoyl)-l-homoserine lactone (OdDHL) and N-butanoyl-l-homoserine lactone (BHL)] and two minor AHLs [N-(3-oxohexanoyl)-l-homoserine lactone (OHHL) and N-hexanoyl-l-homoserine lactone (HHL)] have been identified in P. aeruginosa (47).
Since AHLs appear to readily diffuse across cell membranes (16), it is possible that these bacterial signal molecules may influence the physiology of pulmonary epithelial cells and therefore modulate the possible outcome of infection. This important aspect, that is, the capacity of bacterial AHLs to be perceived by, and to act upon, not only other bacteria but the eucaryotic host itself, has been largely ignored. Recent findings have shown that the signal molecule OdDHL could stimulate interleukin-8 (IL-8) production by respiratory epithelial cells (12) and could also influence the Th1-Th2 balance in the infected host (43). Despite the fact that these effects have been observed at supramaximal concentrations (10 to 30 μM), higher than those normally encountered in laboratory media (33), these data provided us with preliminary evidence for a contribution of AHLs to the infection process.
Engelhardt et al. (14) have demonstrated that CFTR is almost undetectable in the surface epithelial cells of the human bronchus, while the serous component of the bronchotracheal glands expresses CFTR at a very high level. Human tracheal gland serous (HTGS) cells are responsible for the secretion of antibacterial and antiproteolytic proteins like lactoferrin, lysozyme, and the secretory leukocyte proteinase inhibitor (SLPI) (2). Furthermore, tracheal gland cells are able to respond to nucleotides by stimulation of SLPI secretion (26) and by an increase in chloride transport (48). The corresponding nucleotide receptors were identified as P2Y2 and P2Y4 (27). We recently developed a stable cell line of CF HTGS cells, the CF-KM4 cell line (20). In culture, these cells were shown to have retained most of their original epithelial and secretory characteristics, such as constitutive secretion of SLPI. They have also kept their CF-specific defects, such as an absence of cyclic AMP-dependent CFTR-associated chloride channel activity and an inability to respond to adrenergic and cholinergic agonists. However, like the original cells, CF-KM4 cells still respond to nucleotide stimulation. Furthermore, all of these CF-specific defects were shown to be corrected by adenovirus-mediated CFTR gene transfer.
The present work was aimed at determining the possible effects of AHLs on HTGS cells, with special reference to P2 receptors. We demonstrate here that the quorum-sensing oxo-AHLs (at up to picomolar concentrations) dramatically repressed the stimulatory effects of SLPI secretion by nucleotides, probably due to the repression of P2Y2 and P2Y4 receptor expression, in CF but not normal HTGS cells. Ibuprofen (but not glucocorticoids or indomethacin) was able to prevent this deleterious effect of OdDHL on CF cells. It is therefore possible that efficiency of pharmacological CF therapy with nucleotide analogues may be hindered by minimal secretion of the quorum-sensing molecule OdDHL by P. aeruginosa. However, we suggest that, in addition to use of ATP or UTP analogues for CF therapy, ibuprofen may be of potential use for a combinational pharmacological therapy of CF.
MATERIALS AND METHODS
Chemicals and solutions.
ATP, UTP, epinephrine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), indomethacin, nordihydrogaiaretic acid (NDGA), dexamethasone, cortisone, prednisone, ibuprofen, and Dulbecco’s modified Eagle’s medium-Ham’s F12 medium were obtained from Sigma (St. Louis, Mo.). Ultroser G was from Biosepra (Villeneuve la Garenne, France). All other chemicals were of cell culture grade.
Cell culture.
The normal HTGS cell line MM39 (24) and the CF-KM4 cell line (20) were grown in a Dulbecco’s modified Eagles medium-Ham’s F12 medium mixture supplemented with 1% Ultroser G, 0.22 g of sodium pyruvate per liter, and 8 g of glucose per liter. Epinephrine (2.5 μM, from a 2.5 mM stock solution made in 0.001 N HCl and stored at −80°C) was routinely added to the cell culture medium in order to provide optimal growth and differentiation. Cells were passaged by using 0.025% trypsin (GIBCO) and 0.02% EDTA. Type 1 collagen-coated Falcon disposable tissue culture flasks were used. Adenovirus-mediated CFTR gene transfer in CF-KM4 cells was carried out as previously described (20).
AHL synthesis.
The general method described by Chhabra et al. (8) was used to synthesize BHL and HHL, while OdDHL and OHHL were prepared as described by Dekhane et al. (11). Each compound was purified by liquid chromatography to homogeneity as determined by high-pressure liquid chromatography. The structures were confirmed by infrared as well as 1H and 13C nuclear magnetic resonance spectroscopy, and their optical purities were controlled by measurement of their optical rotations. The biological activity of each compound was tested as described previously (23).
Cytotoxicity.
The cytotoxicities of the AHLs were assessed by measuring cellular dehydrogenase activity. Stock solutions of AHLs (100 μM in dimethyl sulfoxide) were diluted in complete culture medium. For the cytotoxicity assay, cells were grown until confluency in 24-well tissue culture plates and exposed to the different AHLs for the durations and at the concentrations indicated in Results. After incubation, dehydrogenase activity was measured biochemically by using the water-soluble tetrazolium salt MTT, which is converted to purple formazan. Results are expressed as changes in optical density compared to that of a 100% lysis control.
Pharmacological stimulation of SLPI secretion.
Confluent cultures of CF-KM4 cells grown on 24-well plates were rinsed four times for 1 h each with serum-free culture medium and then exposed for 30 min to 100 μM nucleotides. Forty microliters of the culture medium was harvested, and the SLPI was directly measured by enzyme-linked immunosorbent assay (44). The ratio of the SLPI secreted in the assays to that secreted in the assays to that secreted in control experiments was calculated, and agonist-induced stimulations are expressed as the percentage of SLPI secretion above the control value. Drugs or vehicle solutions were added at the same times to the respective wells. Vehicle additions were shown to have no effect on SLPI secretion by CF-KM4 cells. In each experiment the mean SLPI secretion rate from quadruplicate assays was determined.
Reverse transcription-PCR amplification.
Total RNA was purified from cellular pellets of CF-KM4 cells (approximately 107 cells) by the technique of Chomczynski and Sacchi (9). Total RNA was quantified by absorbance at 260 nm (1 optical density unit = 40 mg/liter), and the quality of the preparations was monitored by agarose gel electrophoresis in the presence of formaldehyde. For detection of the expression of P2Y2 (31) and P2Y4 (10) mRNA transcripts, PCR amplification of mRNA (after conversion to cDNA) was performed with a Gene AMP RNA PCR kit (Perking-Elmer Cetus). Specific amplifications were performed with the primers 5′-CTTCAACGAGGACTTCAAGTACGTGC-3′ (nucleotides 323 to 348 of the P2Y2 gene) and 5′-CATGTTGATGGCGTTGAGGGTGTGG-3′ (nucleotides 1079 to 1103 of the P2Y2 gene) for P2Y2 and 5′-ATCCTGCCACCCTCACTTCTCC-3′ (nucleotides 137 to 159 of the P2Y4 gene) and 5′-AGGCGAGAAGACGACTGTGC-3′ (nucleotides 882 to 902 of the P2Y4 gene) for P2Y4. Thirty-five cycles of amplification were used, with cycle times as follows: (i) denaturation for 60 s at 94°C, (ii) primer annealing for 60 s at 56°C (P2Y2) or 55°C (P2Y4), and (iii) extension for 150 s at 72°C. An aliquot of the final amplification solution was analyzed after ethidium bromide staining of a 2% agarose gel to assess the sizes of the amplified fragments. Sequences of the PCR products were checked by the dideoxy chain termination method with a Sequenase sequencing kit (U.S. Biochemical Corp., Cleveland, Ohio).
Statistics.
All results are expressed as means ± standard deviations (SDs). The significance of differences between the effects of the concentrations of agents or between the effects of the agonists was determined by analysis of variance. The difference between agents or between concentrations of agents was isolated by the Scheffé multiple-comparison test.
RESULTS
Cytotoxicity of AHLs to CF-KM4 cells.
We first checked for a possible cytotoxic effect of AHLs by using measurements of cellular dehydrogenase activity through MTT metabolization, which appeared to be a good method, since HTGS cells were shown to have a high metabolic activity (25). When added to the cells at up to 100 μM for 24 h, neither BHL, HHL, OHHL, nor OdDHL showed cytotoxicity to normal HTGS (MM39) or CF HTGS (CF-KM4) cells (data not shown).
Effects of AHLs on constitutive secretion by CF-KM4 cells.
We next determined whether AHLs may alter the physiology of HTGS cells. Since these cells are glandular, we looked for protein secretion by using SLPI as a representative specific secretory marker. None of the studied AHLs were found to induce any significant change in the rate of constitutive secretion of SLPI by MM39 or CF-KM4 cells (data not shown).
Effects of AHLs on regulated secretion by CF-KM4 cells.
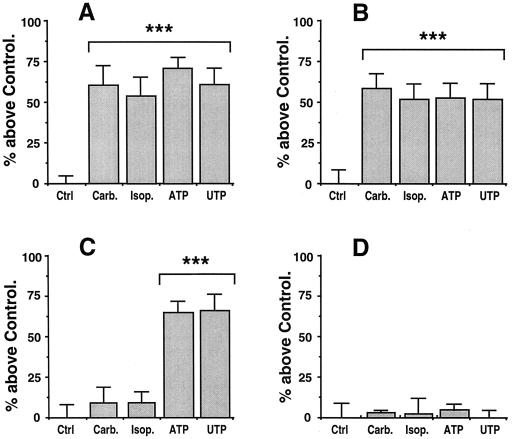
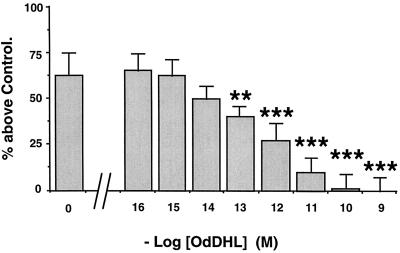
We next looked for effects of AHLs on the ability of cells to be stimulated by secretagogues for the secretion of SLPI. MM39 cell secretion can be stimulated by either cholinergic (carbachol), adrenergic (isoproterenol), or purinergic (ATP and UTP) agonists (24, 27). A 100 μM concentration of each secretagogue gives maximal stimulation of secretion with the following respective values above the control level: 60% ± 12% (P < 0.001), 52% ± 12% (P < 0.001), 65% ± 6% (P < 0.001), and 60% ± 10% (P < 0.001) (Fig. 1A). CF-KM4 cells do not respond to carbachol or isoproterenol but are still responsive to ATP and UTP, with stimulation of 62% ± 6% (P < 0.001) and 65% ± 10% (P < 0.001) above the control value, respectively (Fig. 1C). Pretreatment of the cells for 24 h with 10 μM OdDHL had no significant effects on MM39 cells when they were stimulated by any of the secretagogues tested (Fig. 1B). A similar absence of effects was also observed with 10 μM OHHL, HHL, or BHL (data not shown). However, 1 nM OdDHL led to a total loss of cell responsiveness to ATP and UTP by CF-KM4 cells (Fig. 1D). Similar results were obtained with the AHL analogue OHHL (1 nM), and with the analogues BHL and HHL but at much higher concentrations (10 μM) (data not shown). To rule out the possibility that the differences seen between normal (MM39) and CF (CF-KM4) cells were not due to CFTR differences but to other genetic differences, we repeated the experiments with the CF-KM4 cells corrected by adenovirus-mediated gene transfer of CFTR. Under these conditions, the transfected cells showed the same phenotype as MM39 cells in response to AHLs (data not shown). This inhibition of the ability of CF-KM4 cells to be stimulated by ATP was shown to be dependent on the concentration of AHLs. Dose-response data for the action of OdDHL are presented in Fig. 2. Inhibition was shown to be total up to an OdDHL concentration of 10−10 M. The concentration giving half of the maximal inhibition (50% inhibitory concentration [IC50]) was 0.3 pM. The calculated IC50s were 0.4 pM for OHHL and 10 and 5 μM for BHL and HHL, respectively.
FIG. 1.
Effects of OdDHL on the ability of MM39 cells (A and B) and CF-KM4 cells (C and D) to be stimulated by secretagogues. (A and C) No pretreatment; (B and D) pretreatment with OdDHL. After cells were cultured to confluency, they were washed and exposed for 30 min to the secretagogues. For each experiment, secretion of SLPI in response to 100 μM carbachol (Carb.), isoproterenol (Isop.), ATP, and UTP are presented and expressed as the percentage of SLPI secretion above the control value (Ctrl). Each bar represents the mean and SD for quadruplicate determinations from three different experiments (n = 12). The statistical significance of the difference between responses to agents and the control are indicated as follows: ∗∗, P < 0.01; ∗∗∗, P < 0.001. (A) MM39 cells respond to each of the secretagogues with a significant stimulation of SLPI secretion. (B) Pretreatment of the cells with 10 μM OdDHL for 24 h does not significantly change the ability of the MM39 cells to be stimulated by any of the agonists. (C) CF-KM4 cells do not respond to carbachol and isoproterenol but still respond to ATP and UTP. (D) Pretreatment of CF-KM4 cells with 1 nM OdDHL for 24 h totally abolishes responsiveness to ATP and UTP.
FIG. 2.
Dose dependency of the inhibition by OdDHL of stimulation of SLPI secretion by ATP. After CF-KM4 cells were cultured to confluency, increasing concentrations of OdDHL were added and left for 24 h with the cells. The cells were then washed and exposed for 30 min to 100 μM ATP. SLPI secretion was determined as described for Fig. 1. Each bar represents the mean and SD for four experiments (n = 12). Statistical significance is indicated as described for Fig. 1.
Effects of AHLs on P2Y2 and P2Y4 receptor expression by CF-KM4 cells.
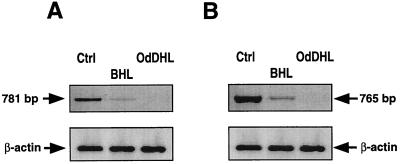
Since the stimulatory effects of UTP and ATP were inhibited by AHLs, and especially by oxo-AHLs, at concentrations not compatible with those expected for receptor antagonists, we checked for the presence of both the P2Y2 and P2Y4 receptors by PCR amplification before and after incubation of CF-KM4 cells with AHLs. We chose primers from the sequences of the cloned genes in zones with less than 40% sequence identity between the two receptors (10, 31). Electrophoresis of the PCR products revealed that CF-KM4 cells express both the P2Y2 and the P2Y4 receptor mRNAs (Fig. 3). Incubation of CF-KM4 cells with BHL (10 μM) or OdDHL (1 nM) dramatically reduced (for BHL) or completely inhibited (for OdDHL) the expression of both the P2Y2 and P2Y4 receptors. This inhibition was not due to a general reduction of cell transcription, since neither of the two AHLs changed the β-actin transcript levels in CF-KM4 cells (Fig. 3).
FIG. 3.
Effects of OdDHL and BHL on P2Y2 (A) and P2Y4 (B) mRNA expression in CF-KM4 cells. Cells were cultured to confluency as described in the text. Cells were then exposed to 1 nM OdDHL or 10 μM BHL for 24 h. After total RNA extraction and reverse transcription, cDNAs were amplified by using primers specific for P2Y2, P2Y4, and β-actin. The amplification products (781 bp for P2Y2 and 765 bp for P2Y4) were separated by electrophoresis on a 2% agarose gel and stained with ethidium bromide. Ctrl, cells without treatments.
Effects of anti-inflammatory agents.
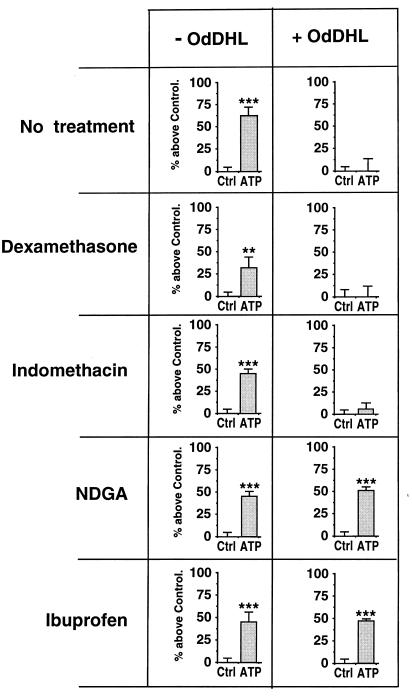
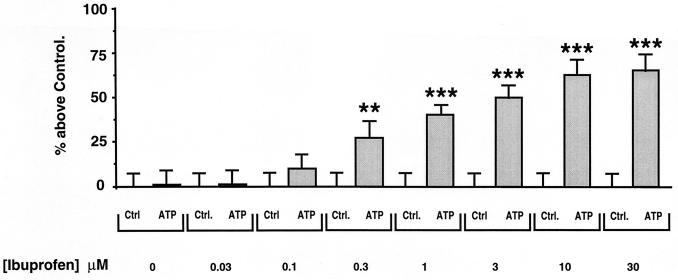
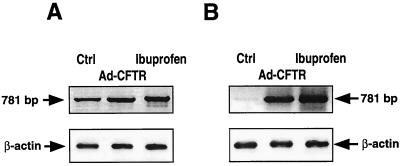
Since AHLs were shown to have immunomodulatory effects (43), we investigated whether anti-inflammatory agents might be able to modify the inhibitory effects of OdDHL (1 nM). We analyzed the effects of dexamethasone (10−7 M), ibuprofen (10 μM), the cyclo-oxygenase inhibitor indomethacin (10 μM), and the lipo-oxygenase inhibitor NDGA (10 μM). These agents were added to the cells at the same time as OdDHL and left to incubate for 24 h. Figure 4 shows that neither dexamethasone nor indomethacin changed the inhibitory effects of OdDHL. Other glucocorticoids (cortisone and prednisone) gave similar results (data not shown). However, both NDGA and ibuprofen reversed the inhibitory effects of OdDHL. We observed a concentration dependence for ibuprofen prevention of the inhibitory effects of OdDHL (Fig. 5). The IC50 of ibuprofen was 0.8 μM. We also assessed the effects of ibuprofen on the P2Y2 receptor mRNA by PCR amplification before and after CF-KM4 cell incubation with OdDHL (Fig. 6). Electrophoresis of the PCR products revealed that ibuprofen (10 μM) prevented OdDHL-induced inhibition of P2Y2 receptor expression in CF-KM4 cells. In addition, as for MM39 cells, CF-KM4 cells with the defect corrected by adenovirus-mediated CFTR gene transfer expressed the P2Y2 receptor mRNA in presence of 1 nM OdDHL (Fig. 6).
FIG. 4.
Effects of immunosuppressors on OdDHL-induced inhibition of secretory responses to ATP by CF-KM4 cells. Confluent CF-KM4 cells were exposed for 24 h to 1 nM OdDHL with or without 0.1 μM dexamethasone or 10 μM indomethacin, NDGA, or ibuprofen. The cells were then washed and exposed for 30 min to 100 μM ATP. The SLPI secretion was determined as described for Fig. 1. Values are given as means ± SDs for three experiments (n = 9). Where no agent was added, only the vehicle was added to the cells. Statistical significance is indicated as described for Fig. 1. Note that only NDGA and ibuprofen prevented the inhibition of stimulation of SLPI secretion by ATP in CF-KM4 cells.
FIG. 5.
Dose dependency of the prevention by ibuprofen of OdDHL inhibition of ATP-induced stimulation of SLPI secretion by CF-KM4 cells. Cells grown to confluency were exposed to 1 nM OdDHL and to increasing concentrations of ibuprofen for 24 h. The cells were then washed and exposed for 30 min to 100 μM ATP. For each concentration of ibuprofen tested, secretion of SLPI in response to 100 μM ATP is presented and compared to the control value (Ctrl). Means and SDs are shown (n = 12). Statistical significance is indicated as described for Fig. 1.
FIG. 6.
Effects of OdDHL on P2Y2 mRNA expression in CF-KM4 cells with the defect corrected by adenovirus-mediated CFTR gene transfer (Ad-CFTR) or treated with ibuprofen. Cells were cultured to confluency as described in the text. Cells in absence of OdDHL (A) were compared to cells exposed to 1 nM OdDHL for 24 h (B). When used, all products (OdDHL, ibuprofen, or adenovirus) were added to the cells at the same time. After total RNA extraction and reverse transcription, cDNAs were amplified by using primers specific for P2Y2 and β-actin. The amplification products were separated by electrophoresis as described for Fig. 3.
DISCUSSION
The aim of the present study was to determine the effects of the quorum-sensing signal molecules AHLs secreted by P. aeruginosa on normal and CF HTGS cells. Normal cells respond to cholinergic, adrenergic, and purinergic agonists by an increase in protein secretion, whereas CF cells are not responsive to adrenergic and cholinergic agonists but still remain responsive to nucleotides. In the present paper we show that CF HTGS cells, but not the normal cells, are no longer able to respond to nucleotides by an increase in the secretion of SLPI in presence of minute amounts of oxo-AHLs (IC50 of about 0.3 pM). This absence of responsiveness is related to the repression of both the P2Y2 and P2Y4 receptor mRNAs. It is likely that this excessive sensitivity is related to the CF defect, since adenovirus-mediated CFTR gene transfer confered resistance to oxo-AHLs that was similar to that of the normal cells. Another finding of this study was that ibuprofen was able to prevent the oxo-AHL-induced repression of P2Y receptors.
In CF, virulence of P. aeruginosa has been demonstrated to be linked to quorum-sensing density with subsequent relatively high concentrations of the autoinducers BHL and OdDHL (38). In CF lungs, P. aeruginosa grows to a high density, (concentrations of 107 to 108 CFU per g are common in CF sputa), at which virulence factors are expressed. This observation suggests that P. aeruginosa has properties which make the CF lung a good environment for bacterial development and that quorum-sensing molecules may play a role in chronic infections associated with CF. P. aeruginosa grows in microcolonies or in biofilms. These densities of bacteria should produce concentrations of autoinducers that would trigger the expression of specific target genes. The role of P. aeruginosa quorum-sensing systems in infectious processes has not been extensively studied. It has recently been shown that P. aeruginosa biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro (37). It has also been found that a lasR mutant is significantly less virulent in a neonatal mouse model of infection (42) and that the lasR gene is expressed in the lungs of CF patients (38). Taken together, these results strongly suggest that quorum-sensing systems may play a role in infection process by regulating synthesis of several virulence factors. Nevertheless, probably because of technical difficulties, concentrations of AHLs in sputa of CF patients have never been reported. As the concentration of OdDHL normally produced in laboratory media is approximately 5 μM (33), it is reasonable to postulate that high local concentrations may be attained in sputa of CF patients, because bacteria grow as microcolonies. Since AHLs appear to readily diffuse across cell membranes, it is conceivable that these bacterial signal molecules per se may influence the outcome of the infection by modulating the host cell response. One possibility is that the quorum-sensing AHLs produced by P. aeruginosa could be able to modify the behavior of the CF epithelial cells which are thought to be most implicated in the pulmonary physiopathology of the disease, i.e., the CF HTGS cells (14). This was the hypothesis that we wanted to address in this study.
There are relatively few data in the literature regarding the effects of AHLs on airway cells. The signal molecule OdDHL has been reported by some authors (12) but not by others (30) to stimulate IL-8 secretion by respiratory epithelial cells. However, this effect was observed at very high concentrations of OdDHL (over 10 μM), which are over the quorum-sensing level, and in a physiological situation where toxins and virulence factors should be secreted by the bacteria. IL-8 is a potent chemoattractant for neutrophils, whose role, when activated, is to attack, digest, and destroy bacteria at the site of infection. However, since in these reports no difference in OdDHL-induced IL-8 secretion between normal and CF cells was observed, it is unlikely (i) that this phenomenon is pathological rather than a positive reaction against infection, and (ii) that this phenomenon could be involved in the pathophysiology of CF. The reasons for this specific affinity of P. aeruginosa for the CF airway are still unclear. Several authors have suggested that P. aeruginosa may bind to some membrane receptors that are up-regulated in CF epithelial cells (7). However, these findings were not observed in vivo (45) and hardly explain the failure of the nonspecific immune system. Other authors have suggested that the up-regulation of IL-8 and down-regulation of IL-10 found in CF would generate an inflammatory state prone to P. aeruginosa development (5). However, this imbalance in inflammatory cytokines would, rather, protect the lung tissue and cannot explain the specificity of P. aeruginosa.
In contrast to the above-mentioned data, our results indicate that the quorum-sensing signal molecules OdDHL and OHHL can inhibit expression of nucleotide receptors by CF HTGS cells but not by their homologous normal, non-CF cells. This phenomenon was also observed with BHL and HHL but at very high concentrations (>10 μM), which are reached only in vitro (33). The IC50s of OdDHL and OHHL are very low (0.3 and 0.4 pM, respectively), and since the secretion of oxo-AHLs by bacteria is proportional to bacterial density, these low values imply that the presence of only a small amount of bacteria is needed to lead to dramatic effects on expression of the P2Y2 and P2Y4 receptors. Very large quantities of P. aeruginosa in CF airways (≥107 CFU/g of sputum) are found during infection, and the quantities are still significant (about 106 CFU/g) when infection is overcome (18). Therefore, it is possible that, due to this persistence of bacteria, the expression of P2 receptors might be continuously repressed in the airways of CF patients.
The fact that CF gland cells are normally able to respond to nucleotides by mobilization of intracellular calcium (25a), by an increase in chloride transport (48), and by stimulation of protein secretion (27) strengthens the case for the eventual use of nucleotide analogues for therapeutic purposes. ATP and UTP analogues have already been tested on healthy volunteers and on CF patients with mild CF injuries. Data about these human trials are scarce; the trials were first performed with CF patients with mild lung disease, and the data concerned chloride transport in the nose (22). Furthermore, these results were from experiments performed on epithelial tissue devoid of bacterial contamination, and no data are available for infected tissue. Currently, clinical trials are being carried out in the United States and in the United Kingdom (35). It would be very interesting to know whether and how much the benefit of the clinical treatment is impaired by infection and/or by the residual presence of bacteria.
The fact that OdDHL may induce abolition of expression of P2Y2 and P2Y4 receptors by CF-KM4 cells suggests that the action of OdDHL may lead directly or indirectly to the mobilization of inhibitory transcription factors involved in the expression of the P2Y genes. One possibility is that leukotrienes may be involved in that process, since inhibitors of leukotriene synthesis, but not inhibitors of the cyclo-oxygenase pathway, inhibited the action of oxo-AHLs. Furthermore, the high sensitivity of CF HTGS cells to oxo-AHLs, in comparison to the normal HTGS cells, suggests a CF-specific defect linked with arachidonate metabolism. It has long been known that there is an alteration of arachidonate metabolism in CF (39). However, although many investigations have detailed some cellular mechanisms involved in phospholipase A2 overactivation in CF (4, 28), neither the reasons for the overactivation (including the link with the CFTR defect) nor its physiological consequences have been determined so far. On the other hand, in contrast to ibuprofen and NDGA, glucocorticoids were shown to be inefficient in preventing OdDHL negative effects. Glucocorticoids are known to activate transcription of lipocortin-1, which is a physiological intracellular inhibitor of phospholipase A2. Glucocorticoids are thus indirectly able to inhibit generation of eicosanoides. Both NDGA, by inhibiting lipo-oxygenase, and ibuprofen, by inhibiting both cyclo-oxygenase and lipo-oxygenase, also inhibit generation of eicosanoides. Thus, the difference in the effects of (i) glucocorticoids and (ii) ibuprofen or NDGA is intriguing. A possibility is that glucocorticoids, in contrast to lipo-oxygenase inhibitors, do not function normally in CF cells. Defective inhibition of leukotriene production by leukocytes in patients with CF was reported (36), resistance of CF B lymphocytes to dexamethasone was observed (13), and inefficiency of dexamethasone in inhibiting lipopolysaccharide-induced cytokine production by CF HTGS cells was shown (19). Another possibility is that ibuprofen and NDGA may act through mechanisms other than inhibition of leukotriene production. Ibuprofen was shown to stabilize the NF-κB–IκB complex in the cytoplasm (40). NF-κB is a transcription factor which is located in the cytoplasm in an inactive form, where it is bound to a protein called inhibitor-κBα (IκBα). Upon stimulation, IκBα is phosphorylated and degraded, resulting in the release and translocation of NF-κB into the nucleus, where it activates the expression of numerous genes. Ibuprofen was shown to be able to block the degradation of IκBα and thereby to inhibit activation and translocation of NF-κB into the nucleus (40). NDGA was also shown to inhibit IκB degradation (6). Further understanding of the molecular mechanism of ibuprofen action should provide insights into the process of oxo-AHL action and, more generally, into the emergence of infection and inflammation in CF patients. Here we have not been able to assess how oxo-AHLs have this particular action on CF-KM4 cells, but the consequences of this observation might be relevant. Ibuprofen has been used with success in decreasing inflammation associated with CF overinfection. That the negative effect of oxo-AHLs on P2Y expression was abolished by ibuprofen suggests a possible beneficial effect of ATP and/or UTP analogues in association with ibuprofen for CF therapy.
In conclusion, the results presented here show that oxo-AHLs from P. aeruginosa induce the repression of P2Y2 and P2Y4 receptors by CF-KM4 cells. It is our understanding that this work represents the first demonstration of an effect of AHLs on a secretory mechanism involved in the lung defense system in CF HTGS cells but not in normal, non-CF HTGS cells. The ability of ibuprofen to prevent this deleterious effect suggests that a possible use of ibuprofen in combination with ATP and/or UTP analogues would be of considerable interest for pharmacological therapy of CF.
ACKNOWLEDGMENTS
This work was supported by grants from the Association Française de Lutte contre la Mucoviscidose (AFLM). A. Saleh is the recipient of a fellowship from AFLM.
We thank M. Foglino for fruitful discussions and Anne Shildrake for her help with the English text.
REFERENCES
- 1.Aksoy M O, Kelsen S G. Relaxation of rabbit tracheal smooth muscle by adenine nucleotides: mediation by P2-purinoceptors. Am J Respir Cell Mol Biol. 1994;10:230–236. doi: 10.1165/ajrcmb.10.2.8110478. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum C B, Jany B, Finkbeiner W E. The serous cell. Annu Rev Physiol. 1990;52:97–113. doi: 10.1146/annurev.ph.52.030190.000525. [DOI] [PubMed] [Google Scholar]
- 3.Bennett W D, Olivier K N, Zeman K L, Hohneker K W, Boucher R C, Knowles M R. Effect of uridine 5′-triphosphate plus amiloride on mucociliary clearance in adult cystic fibrosis. Am J Respir Crit Care Med. 1996;153:1796–1801. doi: 10.1164/ajrccm.153.6.8665037. [DOI] [PubMed] [Google Scholar]
- 4.Berguerand M, Klapisz E, Thomas G, Humbert L, Jouniaux A M, Olivier J L, Bereziat G, Masliah J. Differential stimulation of cytosolic phospholipase A2 by bradykinin in human cystic fibrosis cell lines. Am J Respir Cell Mol Biol. 1997;17:481–490. doi: 10.1165/ajrcmb.17.4.2734. [DOI] [PubMed] [Google Scholar]
- 5.Bonfield T L, Panuska J R, Konstan M W, Hilliard K A, Hilliard J B, Ghnaim H, Berger M. Binding of Pseudomonas aeruginosa to respiratory epithelial cells from patients with various mutations in the cystic fibrosis transmembrane regulator. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 6.Brennan P, O’Neill L A. Inhibition of nuclear factor kappaB by direct modification in whole cells—mechanism of action of nordihydroguaiaritic acid, curcumin and thiol modifiers. Biochem Pharmacol. 1998;55:965–973. doi: 10.1016/s0006-2952(97)00535-2. [DOI] [PubMed] [Google Scholar]
- 7.Bryan R, Kube D, Perez A, Davis P, Prince A. Overproduction of the CFTR R domain leads to increased levels of asialoGM1 and increased Pseudomonas aeruginosa binding by epithelial cells. Am J Respir Cell Mol Biol. 1998;19:269–277. doi: 10.1165/ajrcmb.19.2.2889. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra S R, Stead P, Bainton N J, Salmond G P, Stewart G S, Williams P, Bycroft B W. Autoregulation of carbapenem biosynthesis in Erwinia carotovora by analogues of N-(3-oxohexanoyl)-l-homoserine lactone. Antibiotics (Tokyo) 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 9.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 10.Communi D, Pirotton S, Parmentier M, Boeynaems J M. Cloning and functional expression of a human uridine nucleotide receptor. J Biol Chem. 1995;270:30849–30852. doi: 10.1074/jbc.270.52.30849. [DOI] [PubMed] [Google Scholar]
- 11.Dekhane M, Douglas K T, Gilbert P. A novel convenient route to the naturally occuring 3-oxoacyl-l-homoserinelactones and related bacterial autoinducers. Tetrahedron Lett. 1996;37:1883–1884. [Google Scholar]
- 12.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emilie D, Crevon M C, Chicheportiche R, Auffredou M T, Barot-Ciorbaru R, Lenoir G, Dayer J M, Galanaud P. Cystic fibrosis patients’ B lymphocyte response is resistant to the in vitro enhancing effect of corticosteroids. Eur J Clin Invest. 1990;20:620–626. doi: 10.1111/j.1365-2362.1990.tb01910.x. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt J F, Yankaskas J R, Ernst S A, Yang Y P, Marino C R, Boucher R C, Cohn J A, Wilson J M. Submucosal glands are the predominant site of CFTR expression in human bronchus. Nat Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 15.Fuqua C, Winans S C, Greenberg E P. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu Rev Microbiol. 1996;50:727–751. doi: 10.1146/annurev.micro.50.1.727. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua W C, Winans S C, Greenberg E P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata J S, Fick R B., Jr Pseudomonas aeruginosa and the airway disease of cystic fibrosis. Clin Chest Med. 1988;9:679–689. [PubMed] [Google Scholar]
- 18.Jaffar-Bandjee M C, Lazdunski A, Bally M, Carrere J, Chazalette J P, Galabert C. Production of elastase, exotoxin A, and alkaline protease in sputa during pulmonary exacerbation of cystic fibrosis in patients chronically infected by Pseudomonas aeruginosa. J Clin Microbiol. 1995;33:924–929. doi: 10.1128/jcm.33.4.924-929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kammouni W, Figarella C, Marchand S, Merten M D. Altered cytokine production by cystic fibrosis tracheal gland serous cells. Infect Immun. 1997;65:5176–5183. doi: 10.1128/iai.65.12.5176-5183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kammouni W, Moreau B, Becq F, Saleh A, Pavirani A, Figarella C, Merten M D. A cystic fibrosis tracheal gland cell line, CF-KM4. Correction by adenovirus-mediated CFTR gene transfer. Am J Respir Cell Mol Biol. 1999;20:684–691. doi: 10.1165/ajrcmb.20.4.3341. [DOI] [PubMed] [Google Scholar]
- 21.Knowles M R, Clarke L L, Boucher R C. Activation by extracellular nucleotides of chloride secretion in the airway epithelia of patients with cystic fibrosis. N Engl J Med. 1991;325:533–538. doi: 10.1056/NEJM199108223250802. [DOI] [PubMed] [Google Scholar]
- 22.Knowles M R, Clarke L L, Boucher R C. Extracellular ATP and UTP induce chloride secretion in nasal epithelia of cystic fibrosis patients and normal subjects in vivo. Chest. 1992;101(Suppl. 3):60S–63S. doi: 10.1378/chest.101.3_supplement.60s. [DOI] [PubMed] [Google Scholar]
- 23.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 24.Merten M, Kammouni W, Renaud W, Birg F, Mattei M G, Figarella C. A transformed human tracheal gland serous cell line, MM39, that retains serous secretory functions. Am J Respir Cell Mol Biol. 1996;15:520–528. doi: 10.1165/ajrcmb.15.4.8879186. [DOI] [PubMed] [Google Scholar]
- 25.Merten M. Human tracheal gland cells in primary culture. In: Jones G E, editor. Methods in molecular medicine: cell culture protocols. Totowa, N.J: Humana Press; 1996. pp. 201–216. [DOI] [PubMed] [Google Scholar]
- 25a.Merten, M. Unpublished data.
- 26.Merten M D, Breittmayer J P, Figarella C, Frelin C. ATP and UTP increase secretion of bronchial inhibitor by human tracheal gland cells in culture. Am J Physiol. 1993;265:L479–L484. doi: 10.1152/ajplung.1993.265.5.L479. [DOI] [PubMed] [Google Scholar]
- 27.Merten M D, Saleh A, Kammouni W, Marchand S, Figarella C. Characterization of two distinct P2Y receptors in human tracheal gland cells. Eur J Biochem. 1998;251:19–24. doi: 10.1046/j.1432-1327.1998.2510019.x. [DOI] [PubMed] [Google Scholar]
- 28.Miele L, Cordella-Miele E, Xing M, Frizzell R, Mukherjee A B. Cystic fibrosis gene mutation (deltaF508) is associated with an intrinsic abnormality in Ca2+-induced arachidonic acid release by epithelial cells. DNA Cell Biol. 1997;16:749–759. doi: 10.1089/dna.1997.16.749. [DOI] [PubMed] [Google Scholar]
- 29.Olivier K N, Bennett W D, Hohneker K W, Zeman K L, Edwards L J, Boucher R C, Knowles M R. Acute safety and effects on mucociliary clearance of aerosolized uridine 5′-triphosphate ± amiloride in normal human adults. Am J Respir Crit Care Med. 1996;154:217–223. doi: 10.1164/ajrccm.154.1.8680683. [DOI] [PubMed] [Google Scholar]
- 30.Palfreyman R W, Watson M L, Eden C, Smith A W. Induction of biologically active interleukin-8 from lung epithelial cells by Burkholderia (Pseudomonas) cepacia products. Infect Immun. 1997;65:617–622. doi: 10.1128/iai.65.2.617-622.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parr C E, Sullivan D M, Paradiso A M, Lazarowski E R, Burch L H, Ohlsen J C, Erb L, Weisman G A, Boucher R C, Turner J T. Cloning and expression of a human P2u nucleotide receptor, a target for cystic fibrosis. Proc Natl Acad Sci USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Passador L, Cook J M, Gambello M J, Rust L, Iglewski B H. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science. 1993;260:1127–1130. doi: 10.1126/science.8493556. [DOI] [PubMed] [Google Scholar]
- 33.Pearson J P, Passador L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riordan J R, Rommens J M, Kerem B S, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou J L, Drumm M L, Iannuzzi M C, Collins F S, Tsui L C. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 35.Shaffer C, Jacobus K, Foy C, Pue C, Donohue J, Bennet W, Ye H, Graham C, Noone P, Drutz D. Controlled clinical studies indicate that INS316 (uridine 5′-triphosphate), a P2Y2 receptor agonist, stimulates mucociliary clearance and enhances sputum expectoration. Am J Respir Crit Care Med. 1998;157:A796. [Google Scholar]
- 36.Shimizu T, Hannson G C, Strandvik B. Defective inhibition by dexamethesone of leukotriene B4 and C4 production by leukocytes in patients with cystic fibrosis. Prostaglandins Leukoc Essent Fatty Acids. 1994;51:407–410. doi: 10.1016/0952-3278(94)90056-6. [DOI] [PubMed] [Google Scholar]
- 37.Stickler D J, Morris N S, McLean R J, Fuqua C. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl Environ Microbiol. 1998;64:3486–3490. doi: 10.1128/aem.64.9.3486-3490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Storey D G, Ujack A E, Rabin H R, Mitchell I. Pseudomonas aeruginosa lasR transcription correlates with the transcription of lasA, lasB, and toxA in chronic lung infections associated with cystic fibrosis. Infect Immun. 1998;66:2521–2528. doi: 10.1128/iai.66.6.2521-2528.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Strandvik B, Bronnegard M, Gilljam H, Carlstedt-Duke J. Relation between defective regulation of arachidonic acid release and symptoms in cystic fibrosis. Scand J Gastroenterol. 1988;143(Suppl):1–4. doi: 10.3109/00365528809090205. [DOI] [PubMed] [Google Scholar]
- 40.Stuhlmeier K M, Li H, Kao J J. Ibuprofen: new explanation for an old phenomenon. Biochem Pharmacol. 1999;57:313–320. doi: 10.1016/s0006-2952(98)00301-3. [DOI] [PubMed] [Google Scholar]
- 41.Swift S, Throup J P, Salmond G P C, Williams P, Stewart G S A B. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 42.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Telford G, Wheeler D, Williams P, Tomkins P T, Appleby P, Sewell H, Stewart G S, Bycroft B W, Pritchard D I. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect Immun. 1998;66:36–42. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tournier J M, Jacquot J, Sadoul P, Bieth J G. Noncompetitive enzyme immunoassay for the measurement of bronchial inhibitor in biological fluids. Anal Biochem. 1983;131:345–350. doi: 10.1016/0003-2697(83)90181-1. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich M, Herbert S, Berger J, Bellon G, Louis D, Munker G, Doring G. Localization of Staphylococcus aureus in infected airways of patients with cystic fibrosis and in a cell culture model of S. aureus adherence. Am J Respir Cell Mol Biol. 1998;19:83–91. doi: 10.1165/ajrcmb.19.1.3137. [DOI] [PubMed] [Google Scholar]
- 46.Wagner J A, McDonald T V, Nghiem P T, Lowe A W, Schulman H, Gruenert D C, Stryer L, Gardner P. Antisense oligodeoxynucleotides to the cystic fibrosis transmembrane conductance regulator inhibit cAMP-activated but not calcium-activated chloride currents. Proc Natl Acad Sci USA. 1992;89:6785–6789. doi: 10.1073/pnas.89.15.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Winson M K, Camara M, Latifi A, Foglino M, Chhabra S R, Daykin M, Bally M, Chapon V, Salmond G P, Bycroft B W, Lazdunski A, Stewart G S A B, Williams P. Multiple N-acyl-l-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:9427–9431. doi: 10.1073/pnas.92.20.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaya M, Sekizawa K, Kakuta Y, Ohrui T, Sawai T, Sasaki H. P2u-purinoceptor regulation of chloride secretion in cultured human tracheal submucosal glands. Am J Physiol. 1996;270:L979–L984. doi: 10.1152/ajplung.1996.270.6.L979. [DOI] [PubMed] [Google Scholar]