Key Points
Question
What are the trends in laryngeal complications reported to the US Food and Drug Administration (FDA) after vagus nerve stimulation (VNS) implantation?
Findings
This cross-sectional study of 12 725 documented iatrogenic vagus nerve issues after VNS implantation, including 187 reports of laryngeal adverse effects associated with VNS devices, found that voice changes were reported to the FDA even though nearly all patients have voice changes after VNS surgery. Problems frequently associated with laryngeal adverse effects were high impedance, incorrect frequency delivery, and battery problems; a minority of the reports documented postoperative paresis/paralysis.
Meaning
Surgeons should improve patient counseling regarding vocal changes prior to VNS surgery to prevent unnecessary patient concern and should consider preoperative laryngeal assessment to differentiate preexisting vocal fold paralysis from that caused by VNS surgery.
This cross-sectional study examines trends in laryngeal complications reported to the US Food and Drug Administration after vagus nerve stimulation implantation.
Abstract
Importance
Vagus nerve stimulation (VNS) devices have gained widespread acceptance for treatment of resistant epilepsy and depression. The increasing number of procedures has resulted in an increasing number of iatrogenic injuries to the vagus nerve, which can have a significant effect on vocalization and quality of life.
Objective
To determine the relative frequency of laryngeal adverse effects reported to the US Food and Drug Administration (FDA) after VNS implantation and to analyze associated VNS device problems.
Design, Setting, and Participants
This retrospective cross-sectional analysis queried the FDA Manufacturer and User Facility Device Experience database of adverse events in the US between 1996 and 2020.
Main Outcomes and Measures
The primary outcome was the percent of adverse events reported to the FDA that included patients who received VNS with laryngeal adverse effects and the associated proportion of device problems after VNS surgery.
Results
A total of 12 725 iatrogenic vagus nerve issues were documented after VNS implantation, with apnea (n = 395; 3.1%) being the most common patient problem. Overall, 187 reports of laryngeal adverse effects associated with VNS devices were identified and represented the eighth most common iatrogenic vagus nerve problem reported to the FDA. Laryngeal adverse effects included 78 reports of voice alteration and 57 reports of paresis/paralysis. The VNS device problems frequently associated with laryngeal adverse effects were high impedance (n = 15, 8.02%), incorrect frequency delivery (n = 10, 5.35%), and battery problems (n = 11, 5.88%). The number of laryngeal adverse effect reports per year peaked in 2012 with 43 cases.
Conclusions and Relevance
This cross-sectional study found that although the literature demonstrates that vocal changes occur with nearly all VNS devices, the FDA receives adverse event reports of voice changes. Our results emphasize a potential need to improve patient counseling prior to VNS surgery to better set patient expectations regarding vocal changes and to prevent unnecessary patient concern. In addition, reports of vocal fold paresis/paralysis potentially suggest that patients may benefit from preoperative laryngeal assessment to differentiate preexisting vocal fold paralysis from that caused by VNS surgery.
Introduction
Vagus nerve stimulation (VNS) using an implantable neuromodulator has been employed in the treatment of refractory epilepsy in the US since 1997.1 The device functions through a lead attached to the vagus nerve that provides a stimulus from an implanted pulse generator. It has been shown that around 50% of epilepsy patients report a decrease in the severity of their seizures with VNS therapy, and the efficacy of VNS therapy is well supported in the literature.2,3,4 When researchers were conducting VNS studies, they incidentally noticed that the therapy improved symptoms of depression in some patients.5 In 2005, VNS therapy was approved by the US Food and Drug Administration (FDA) for treatment of resistant depression. In the US, epilepsy affects 2% to 5% of older adults, and depression has a lifetime prevalence of 21%.6,7,8 Given that both epilepsy and depression have high disease burden, studies aimed at improving outcomes after VNS surgery have the potential to help a considerable number of patients.
Frequent complications of VNS therapy include paresthesia, dyspnea, and infection, but damage to the vagus nerve is one of the most well-known complications of VNS implantation.1,2,9 Iatrogenic vagus nerve damage is a serious complication because the vagus nerve is vital to both somatic and autonomic functions.10 The literature on VNS implantation has shown that iatrogenic injury to the vagus nerve can produce a variety of negative outcomes such as headaches, coughing, and unilateral vocal fold paralysis.3,11 Unilateral vocal fold paralysis is known to have a severe effect on quality of life, including aspiration, shortness of breath, and hoarseness.12 The adverse nature of this complication underscores the importance of better understanding the etiologies (eg, device parameters vs surgical issues) to mitigate the risk of vocal fold paralysis.
In this study, we sought to evaluate reports of laryngeal adverse effects associated with VNS implantation using the nationwide Manufacturer and User Facility Device Experience (MAUDE) database from the FDA. This database allows for determination of the relative frequency of VNS-specific adverse effects and for analysis of VNS-specific problems. There are few large-scale nationwide studies of vagus nerve dysfunction after VNS implantation even though laryngeal alterations are a commonly documented complication.2,10,11,13 Given that vocal adverse effects are commonly reported postoperatively and more than 90 000 patients have been implanted with the device globally, investigating the relationship between VNS therapy and laryngeal changes is an important clinical question.2,14 We hope the findings of this study will provide evidence to improve patient counseling and optimize patient treatment in VNS device implantation.
Methods
The MAUDE database documents adverse event reports from mandatory reporters (eg, manufacturers and importers) as well as voluntary reporters (eg, physicians and patients) and is maintained by the FDA.15 The voluntary nature of physician and patient reporting in the MAUDE database means adverse event reports are likely an underestimate of the actual number of complications. The database was queried from January 1, 1996, to December 31, 2020, for all adverse events related to VNS devices using the product code (“LYJ”) assigned to all VNS devices by the FDA. Vagus nerve problems were defined based on previous studies of complications associated with VNS implantation and the categories were derived from the “Patient Problem” variable.3,11,16 The authors chose this definition to exclude complications secondary to persistent seizures. Therefore, the overall number of reported complications was derived from the Patient Problem variable excluding reports of persistent seizure or seizure-related death. Vocal fold paresis/paralysis was defined by the “Patient Problem” variable in the database and not directly based on laryngoscopic findings. Laryngeal adverse effects included voice changes, hoarseness, dysphonia, or paresis/paralysis in the Patient Problem or Event Text variable. Device problems were defined by the “Device Problem” variable. A single adverse event report could contribute to multiple patient problems and device problems. The year of the adverse event was determined by the “Event Date” variable. Descriptive statistics were performed using Tableau Desktop (version 2021.1; Tableau Software, LLC) and Microsoft Excel (version 16.16, Microsoft) statistical software. This study was deemed exempt from review by the Cleveland Clinic institutional review board due to the public nature of the database.
Results
Table 1 shows the 10 most commonly reported iatrogenic vagus nerve problems (n = 12 725). Laryngeal adverse effects occurred in 187 of these (1.5%) and comprised the eighth most common iatrogenic vagus nerve problem associated with VNS therapy and reported to the FDA. In the 187 cases of laryngeal adverse effects, there were 78 reports of voice alteration and 57 reports of paresis/paralysis. Apnea was the most common vagus nerve complication having 395 reports and accounting of 3.1% of all iatrogenic vagus nerve injuries. This was followed by dyspnea and arrhythmia with 350 (2.8%) and 311 (2.4%) reports, respectively. Less common vagus nerve issues following VNS surgery were syncope (n = 153; 1.2%) and cardiac arrest (n = 121; 1.0%).
Table 1. The 10 Most Common Vagus Nerve Problems Associated With Implantable Vagus Nerve Stimulation.
| Category | No. (%) |
|---|---|
| Total No. | 12 725 |
| Apnea | 395 (3.1) |
| Dyspnea | 350 (2.8) |
| Arrhythmia | 311 (2.4) |
| Dysphagia/odynophagia | 255 (2.0) |
| Sleep dysfunction | 241 (1.9) |
| Undesired nerve stimulation | 201 (1.6) |
| Bradycardia | 197 (1.5) |
| Laryngeal adverse effects | 187 (1.5) |
| Syncope | 153 (1.2) |
| Cardiac arrest | 121 (1.0) |
A VNS device malfunction was documented in 100 (53.48%) reports of laryngeal adverse effects. Table 2 depicts the most frequently associated device malfunctions. The remaining 87 (46.52%) adverse event reports had cases of laryngeal adverse effects without an identified device problem. The most common problem among device malfunctions, with 51 (27.27%) reports, was a device that operated differently than expected. High impedance accounted for 15 (8.02%) instances of laryngeal side effects associated with VNS device malfunction. In addition, there were 11 (5.88%) reports of battery problems, 10 (5.35%) reports of incorrect frequency delivery, and 8 (4.28%) reports of migration or expulsion of device.
Table 2. The VNS Device Malfunctions Associated With Laryngeal Adverse Effectsa.
| Category | No. (%) |
|---|---|
| Total No. | 187 (100) |
| Device operates differently than expected | 51 (27.27) |
| High impedance | 15 (8.02) |
| Battery problems | 11 (5.88) |
| Incorrect frequency delivery | 10 (5.35) |
| Migration or expulsion of device | 8 (4.28) |
| Break/facture | 6 (3.21) |
| Patient-device incompatibility | 4 (2.14) |
| Malposition of device | 3 (1.60) |
| Misfiring | 2 (1.07) |
Abbreviation: VNS, vagus nerve stimulation.
For categories with more than 1 report.
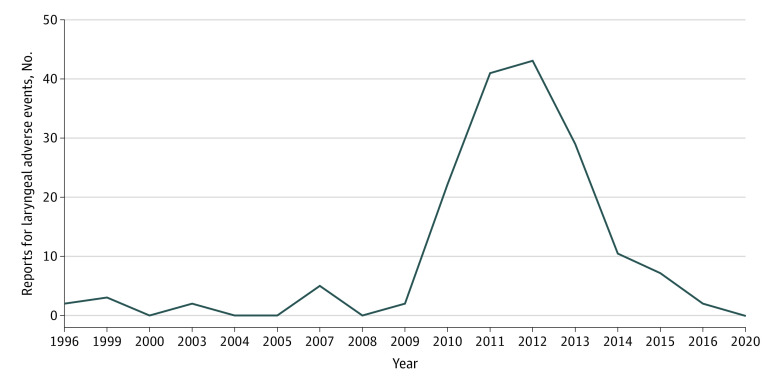
The Figure shows the number of laryngeal adverse effect reports associated with VNS therapy in each year. The range of cases was 43 to 1 with a median of 3 cases per year. The highest number of laryngeal adverse effect events was recorded in 2012.
Figure. Temporal Trends in Laryngeal Adverse Effects Associated With Implantable Vagus Nerve Stimulation.
Discussion
Epilepsy affects between 2% and 5% of older adults in the US, and depression has a lifetime prevalence of 21%.6,7,8 For epilepsy and depression, VNS implantation has become a standard treatment in refractory cases.5 Previous studies have documented the complications associated with VNS therapy including dyspnea, infection, and vocal fold paralysis.1,2,9 In particular, vocal fold paralysis is known to be a severe complication, and vocal fold paralysis can affect more than than 20% of patients receiving VNS postoperatively.2,12 However, the literature on VNS-related laryngeal adverse effects comprises predominately single-institutional studies with small cohorts. This study, to the best of our knowledge, is the largest study investigating the association between VNS device implantation and vagus nerve dysfunction.
We used a national database, the MAUDE database, to evaluate the association between VNS devices and laryngeal adverse effects. We looked at more than 12 000 adverse event reports following VNS implantation, and we found that laryngeal adverse effects were reported to the FDA. Previous work showed a 95% rate of vocal changes following VNS surgery,14 which was not apparent in our study due to the nature of FDA reporting that includes both mandatory and voluntary reporters. It is also important to note that the MAUDE database does not provide the total number of devices and cannot be used to determine the rate of a specific complication. The strength of the MAUDE database is in understanding the specific postoperative complications patients and clinicians are most concerned about and therefore willing to report. In this regard, the reports of voice changes found in this study were surprising given that the literature indicates nearly 100% of patients reporting voice changes after activation of the VNS device.14,17 More specifically, our results are surprising because patients and clinicians would not be taking the time and would not be concerned enough to report such common laryngeal adverse effects if they were properly informed of the standard postoperative voice outcomes associated with VNS implantation. Essentially all patients with VNS implants have their laryngeal function altered to a degree, and essentially all patients have their objective vocal function altered after surgery.17,18 Our results may indicate a need to increase clinician knowledge regarding the adverse effects of VNS implantation. In clinical practice, the literature examining outcomes after VNS surgery indicates that most patients would undergo VNS implantation again even knowing the degree of the laryngeal adverse effects.14 Our results may also point to the importance of improving patient counseling prior to VNS surgery to better set patient expectations regarding standard vocal changes and to avoid unnecessary patient concern.
Properly setting expectations should take into consideration that there are 2 distinct patient groups, those with voice changes secondary to standard VNS device parameters and those with voice changes secondary to vocal fold paralysis. This means surgeons should be educating patients on both the signs of vocal fold paralysis and the standard postoperative voice outcomes. The results of our study underscore these 2 distinct patient groups, which is well established in the literature.10,11 One interpretation of these findings is that otolaryngologists may be valuable in helping determine the cause of the voice changes after VNS implantation through fiberoptic laryngeal assessment to ensure the voice changes are not due to vocal fold paralysis. This is particularly true given that vocal changes are the most commonly reported adverse effect associated with VNS implantation and that vocal fold paralysis presents in approximately 1% of patients, which directly points to the magnitude of the issue.3,11,19 In the time since VNS therapy was approved, more than 90 000 patients have been implanted with the device, which means even a less frequently occurring complication will have an effect on a large number of patients.2 Vocal changes cannot be entirely perceived as normal because a small subset of patients with voice changes after VNS implantation do have issues secondary to vocal fold paralysis. The results of our study emphasize the potential role of postoperative laryngeal assessment by otolaryngologists to facilitate early recognition of vocal fold paralysis.
A number of FDA reports included patients with VNS who were referred after surgery for laryngeal assessment to evaluate whether explantation would benefit their vocal fold issues. However, the value of reoperating was found to be difficult to determine without a preoperative evaluation. Studies have shown that vocal fold paralysis cannot always be determined based on voice symptoms.20,21 Without preoperative laryngeal assessment there is no ability to differentiate preexisting vocal fold paralysis from vocal fold paralysis caused by VNS surgery. Recognizing vocal fold paralysis before VNS surgery would also be valuable for preoperative surgical planning and for discussing the risk of laryngeal complications with patients. This could be particularly important for patients with previous neck surgery as well as those with preexisting vocal fold paralysis to determine the laterality of the implant. Knowing that a patient vocal fold is already paralyzed would influence the placement of the VNS device to avoid damaging the functional vocal fold. Preoperative laryngeal assessment by otolaryngologists may help prevent bilateral vocal fold paralysis and may help reduce the risk of dyspnea, dysphagia and/or odynophagia, and other iatrogenic vagus nerve complications seen in this study. It is worth mentioning that preoperative laryngeal assessments are routinely employed for patients undergoing thyroidectomy, particularly in those considered to be at high risk for vocal fold complications.22 One study23 found that 0.3% of patients with noninvasive thyroid cancer and 70% of patients with invasive thyroid disease had vocal fold paralysis identified by preoperative laryngoscopy. The results of our study point to the need for preoperative laryngeal assessment by otolaryngologists, especially given hoarseness was by far the most commonly reported adverse effect in a randomized clinical trial of VNS implantation.19 We believe that surgeons and patients would benefit from preoperative laryngeal assessment to facilitate early identification of vocal fold paralysis and to optimize postoperative outcomes following VNS device implantation.
Limitations
The primary limitation of this study is the retrospective nature of the MAUDE database and that the database is compiled from mandatory (eg, manufacturers) and voluntary (eg, physicians) reporters. Voluntary reports may contribute to selection bias because the voluntary reporters may not consistently document adverse events, which may influence the accuracy of these findings and may also contribute to underreporting of laryngeal adverse effects. The MAUDE database does not contain demographic information, degree of stimulus, or other factors that could be used to adjust our analysis. In addition, the MAUDE database does not allow for determination of complication rates because the database does not collect information on the total number of devices used per year. Documentation of adverse event reports did not include follow-up information to permit the determination of transient vs permanent vocal fold paralysis, although the impetus to voluntarily report an adverse event is likely related to the degree of the injury. Even though this study has inherent limitations, it also provides valuable insights into the postoperative outcomes following VNS implantation that can be used by surgeons to optimize outcomes.
Conclusions
The findings of this cross-sectional study show that reports of VNS-related laryngeal symptoms are being sent to the FDA even though voice changes are expected after surgery. Surgeons can improve the quality of care for patients with epilepsy through better setting expectations regarding laryngeal adverse effects associated with VNS devices. There were also a number of documented vocal fold paresis and/or paralysis cases. The VNS-specific device problems associated with laryngeal adverse effects included high impedance, incorrect frequency delivery, and battery problems. The findings of this study indicate that there is also opportunity to improve outcomes for patients with VNS through preoperative laryngeal assessment and early recognition of vocal fold paralysis.
References
- 1.Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation in the treatment of refractory epilepsy. Neurotherapeutics. 2009;6(2):228-237. doi: 10.1016/j.nurt.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao H, Chen Y, Ge Q, Ye L, Cheng H. Short- and long-term response of vagus nerve stimulation therapy in drug-resistant epilepsy: a systematic review and meta-analysis. Neuromodulation. 2022;25(3):327-342. doi: 10.1111/ner.13509 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Menachem E, Revesz D, Simon BJ, Silberstein S. Surgically implanted and non-invasive vagus nerve stimulation: a review of efficacy, safety and tolerability. Eur J Neurol. 2015;22(9):1260-1268. doi: 10.1111/ene.12629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher RS, Afra P, Macken M, et al. Automatic vagus nerve stimulation triggered by ictal tachycardia: clinical outcomes and device performance—The U.S. E-37 Trial. Neuromodulation. 2016;19(2):188-195. doi: 10.1111/ner.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austelle CW, O’Leary GH, Thompson S, et al. A comprehensive review of vagus nerve stimulation for depression. Neuromodulation. 2022;25(3):309-315. doi: 10.1111/ner.13528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faught E, Richman J, Martin R, et al. Incidence and prevalence of epilepsy among older U.S. Medicare beneficiaries. Neurology. 2012;78(7):448-453. doi: 10.1212/WNL.0b013e3182477edc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi H, Pack A, Elkind MS, Longstreth WT Jr, Ton TG, Onchiri F. Predictors of incident epilepsy in older adults: The Cardiovascular Health Study. Neurology. 2017;88(9):870-877. doi: 10.1212/WNL.0000000000003662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hasin DS, Sarvet AL, Meyers JL, et al. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry. 2018;75(4):336-346. doi: 10.1001/jamapsychiatry.2017.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parhizgar F, Nugent K, Raj R. Obstructive sleep apnea and respiratory complications associated with vagus nerve stimulators. J Clin Sleep Med. 2011;7(4):401-407. doi: 10.5664/JCSM.1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: a single center longitudinal study of 143 patients. Seizure. 2013;22(10):827-833. doi: 10.1016/j.seizure.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 11.Révész D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18(1):97-104. doi: 10.3171/2016.1.PEDS15534 [DOI] [PubMed] [Google Scholar]
- 12.Spector BC, Netterville JL, Billante C, Clary J, Reinisch L, Smith TL. Quality-of-life assessment in patients with unilateral vocal cord paralysis. Otolaryngol Head Neck Surg. 2001;125(3):176-182. doi: 10.1067/mhn.2001.117714 [DOI] [PubMed] [Google Scholar]
- 13.Granbichler CA, Nashef L, Selway R, Polkey CE. Mortality and SUDEP in epilepsy patients treated with vagus nerve stimulation. Epilepsia. 2015;56(2):291-296. doi: 10.1111/epi.12888 [DOI] [PubMed] [Google Scholar]
- 14.Charous SJ, Kempster G, Manders E, Ristanovic R. The effect of vagal nerve stimulation on voice. Laryngoscope. 2001;111(11 Pt 1):2028-2031. doi: 10.1097/00005537-200111000-00030 [DOI] [PubMed] [Google Scholar]
- 15.MAUDE - Manufacturer and User Facility Device Experience . U.S. Food and Drug Administration. Accessed June 12, 2021. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfmaude/search.cfm
- 16.Nishino T. Dyspnoea: underlying mechanisms and treatment. Br J Anaesth. 2011;106(4):463-474. doi: 10.1093/bja/aer040 [DOI] [PubMed] [Google Scholar]
- 17.Shaffer MJ, Jackson CE, Szabo CA, Simpson CB. Vagal nerve stimulation: clinical and electrophysiological effects on vocal fold function. Ann Otol Rhinol Laryngol. 2005;114(1 Pt 1):7-14. doi: 10.1177/000348940511400103 [DOI] [PubMed] [Google Scholar]
- 18.Van Lierde K, Kryshtopava M, Gadeyne S, et al. Impact of vagal nerve stimulation on objective vocal quality, a pilot study. J Voice. 2015;29(6):777.e9-777.e15. doi: 10.1016/j.jvoice.2015.01.010 [DOI] [PubMed] [Google Scholar]
- 19.The Vagus Nerve Stimulation Study Group . A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology. 1995;45(2):224-230. doi: 10.1212/WNL.45.2.224 [DOI] [PubMed] [Google Scholar]
- 20.Walgama E, Randolph GW, Lewis C, et al. Cost-effectiveness of fiberoptic laryngoscopy prior to total thyroidectomy for low-risk thyroid cancer patients. Head Neck. 2020;42(9):2593-2601. doi: 10.1002/hed.26312 [DOI] [PubMed] [Google Scholar]
- 21.Soares AB, Moares BT, Araújo ANB, de Biase NG, Lucena JA. Laryngeal and vocal characterization of asymptomatic adults with sulcus vocalis. Int Arch Otorhinolaryngol. 2019;23(3):e331-e337. doi: 10.1055/s-0039-1688457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KN, Yip L, Lubitz CC, et al. The American Association of Endocrine Surgeons guidelines for the definitive surgical management of thyroid disease in adults. Ann Surg. 2020;271(3):e21-e93. doi: 10.1097/SLA.0000000000003580 [DOI] [PubMed] [Google Scholar]
- 23.Randolph GW, Kamani D. The importance of preoperative laryngoscopy in patients undergoing thyroidectomy: voice, vocal cord function, and the preoperative detection of invasive thyroid malignancy. Surgery. 2006;139(3):357-362. doi: 10.1016/j.surg.2005.08.009 [DOI] [PubMed] [Google Scholar]