Key Points
Question
Is the robotic approach a safe strategy for hepatocellular carcinoma (HCC) resection with good long-term outcomes?
Findings
In this cohort study of 398 patients and comparing, after propensity score matching, 106 patients who underwent robotic with 106 who underwent open liver resection for HCC, overall survival and cumulative incidence of death related to tumor recurrence were comparable. There was a significant reduction of perioperative morbidity after the robotic approach.
Meaning
These findings suggest that robotic liver resection is a safe treatment strategy for patients with HCC and those with compromised liver function while achieving oncologic efficacy.
This international, multicenter cohort study assesses the safety, reproducibility, and oncologic efficacy of robotic vs open liver resection for hepatocellular carcinoma.
Abstract
Importance
Long-term oncologic outcomes of robotic surgery remain a hotly debated topic in surgical oncology, but sparse data have been published thus far.
Objective
To analyze short- and long-term outcomes of robotic liver resection (RLR) for hepatocellular carcinoma (HCC) from Western high-volume centers to assess the safety, reproducibility, and oncologic efficacy of this technique.
Design, Setting, and Participants
This cohort study evaluated the outcomes of patients receiving RLR vs open liver resection (OLR) for HCC between 2010 and 2020 in 5 high-volume centers. After 1:1 propensity score matching, a group of patients who underwent RLR was compared with a validation cohort of OLR patients from a high-volume center that did not perform RLR.
Main Outcomes and Measures
A retrospective analysis was performed of prospectively maintained databases at 2 European and 2 US institutions of patients who underwent RLR for HCC between January 1, 2010, and September 30, 2020. The main outcomes were safety and feasibility of RLR for HCC and its oncologic outcomes compared with a European OLR validation cohort. A 2-sided P < .05 was considered significant.
Results
The study included 398 patients (RLR group: 125 men, 33 women, median [IQR] age, 66 [58-71] years; OLR group: 315 men, 83 women; median [IQR] age, 70 [64-74] years), and 106 RLR patients were compared with 106 OLR patients after propensity score matching. The RLR patients had a significantly longer operative time (median [IQR], 295 [190-370] minutes vs 200 [165-255] minutes, including docking; P < .001) but a significantly shorter hospital length of stay (median [IQR], 4 [3-6] days vs 10 [7-13] days; P < .001) and a lower number of admissions to the intensive care unit (7 [6.6%] vs 21 [19.8%]; P = .002). Incidence of posthepatectomy liver failure was significantly lower in the RLR group (8 [7.5%] vs 30 [28.3%]; P = .001), with no cases of grade C failure. The 90-day overall survival rate was comparable between the 2 groups (RLR, 99.1% [95% CI, 93.5%-99.9%]; OLR, 97.1% [95% CI, 91.3%-99.1%]), as was the cumulative incidence of death related to tumor recurrence (RLR, 8.8% [95% CI, 3.1%-18.3%]; OLR, 10.2% [95% CI, 4.9%-17.7%]).
Conclusions and Relevance
This study represents the largest Western experience to date of full RLR for HCC. Compared with OLR, RLR performed in tertiary centers represents a safe treatment strategy for patients with HCC and those with compromised liver function while achieving oncologic efficacy.
Introduction
Minimally invasive liver surgery is a safe and effective procedure for resecting both primary and metastatic liver tumors.1,2 However, complex laparoscopic liver resections, particularly of posterior segments or in the treatment of cirrhosis, require a longer learning curve than standard resections to be performed proficiently and safely.3 Therefore, open hepatectomies may still be preferred in certain cases as an oncologically adequate procedure. Another minimally invasive approach to hepatectomy is robotic liver resection (RLR), which may also reduce the risk of conversion to open liver resection (OLR) during complex hepatectomies.4 The innate characteristics of the robotic platform, including increased stability, tremor filtration, magnified 3-dimensional vision, and instrument flexibility, allow for gentle manipulation of the liver, precise dissection, and increased dexterity in narrow spaces.
Despite these technical advantages, long-term oncologic outcomes of robotic surgery remain a hotly debated topic in surgical oncology because sparse data have been published thus far.5,6 We analyzed the short- and long-term outcomes of RLR for hepatocellular carcinoma (HCC) from Western high-volume centers7 to assess the safety, reproducibility, and oncologic efficacy of this technique.
Methods
Study Groups
This cohort study was a retrospective analysis of prospectively maintained databases from 2 European institutions (University of Modena and Reggio Emilia and University Hospital Zurich) and 2 US institutions (New York-Presbyterian/Columbia University Irving Medical Center and Weill Cornell Medical College) of patients who underwent RLR for HCC (RLR group) between January 1, 2010, and September 30, 2020. This cohort was compared with a control group of patients who underwent OLR for HCC (OLR group) in the same time interval from an international referral center for HCC surgical management with experience in nonrobotic, minimally invasive surgery (Istituto Nazionale Tumori) to reduce the potential selection bias from the robotic centers.
Data Collection
After institutional review board and ethics committee approval for data collection (protocol No. 215/2013/OSS/AOUMO), all institutions obtained their respective approvals according to their local center requirements. All patients signed an informed consent before surgery to authorize anonymized data collection and audiovisual registration of the surgical procedure. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.8
A diagnosis of HCC is achieved on the basis of internationally approved radiologic criteria with computed tomography or magnetic resonance imaging.9,10 Baseline characteristics of the 2 study groups were age, sex, body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), Child-Pugh-Turcotte (CPT) status, Charlson Comorbidity Index (CCI),11 and platelet count. Data were extracted from the latest laboratory examination before liver resection. In addition, difficulty of each surgical procedure was evaluated according to the IWATE score12,13,14,15 for a fair comparison of the OLR and RLR cases. This score stratifies liver resection complexity into low, intermediate, high, and expert difficulty according to tumor characteristics (including size, location, and proximity to major vessels); type of resection; and liver function. Finally, the preoperative Early Recurrence After Surgery for Liver tumor model (ERASL-pre) was calculated for patients in both groups.16 This model is used to estimate the risk of HCC recurrence in an individual patient on the basis of sex, albumin-bilirubin score,17 serum α-fetoprotein concentration, tumor size in centimeters, and tumor number. Perioperative outcomes included estimated blood loss (EBL); need for packed red blood cell (pRBC) transfusion; operative time, including docking time in the RLR group; length of stay in the intensive care unit (ICU); and postoperative hospital length of stay. Evaluation of EBL differs between RLR and OLR because of the closed system of the minimally invasive approach that allows exact calculation of blood loss compared with the open abdomen approach. Therefore, cases of EBL of less than 100 mL were categorized as 100 mL for a fair comparison between the 2 groups. Incidence rates of post–hepatectomy liver failure (PHLF) and biliary fistula were evaluated according to International Study Group for Liver Surgery definitions.18,19 After discharge from the hospital, all patients were followed up at their outpatient clinic according to each institution’s local protocol and evaluated for potential liver transplant.
Open Liver Resection
For the OLR group, the surgical technique followed the same principles already reported from the Istituto Nazionale Tumori group.20 Laparotomy was performed through a right-side subcostal incision extended to the midline for tumors located in the right lobe and through a midline incision for those located in the left lobe. Parenchymal transection was performed using an ultrasonic dissector (Cavitron Ultrasonic Surgical Aspirator [CUSA]; Valleylab) and irrigated monopolar/bipolar forceps or irrigated bipolar coagulator (Aquamantys; Salient Surgical Technologies). Portal triad clamping (Pringle maneuver) was used occasionally in the event of major bleeding.
Robotic Liver Resection
Robotic procedures were performed using the da Vinci Si or Xi platform (Intuitive Surgical). Parenchymal transection was performed using a Kelly clamp crush technique combined with either ultrasonic (Harmonic ACE; Intuitive Surgical) or radiofrequency (Vessel Sealer; Intuitive Surgical) advanced hemostasis energy devices. Indocyanine green (ICG) fluorescence for tumor mapping required a dose of 0.25 mg per kilogram of body weight 12 hours before surgery or, alternatively, 1 mg ICG at induction, which is effective for HCC visualization in patients without cirrhosis (Figure 1).
Figure 1. Hepatocellular Carcinoma (HCC) Identification With Integrated Firefly.
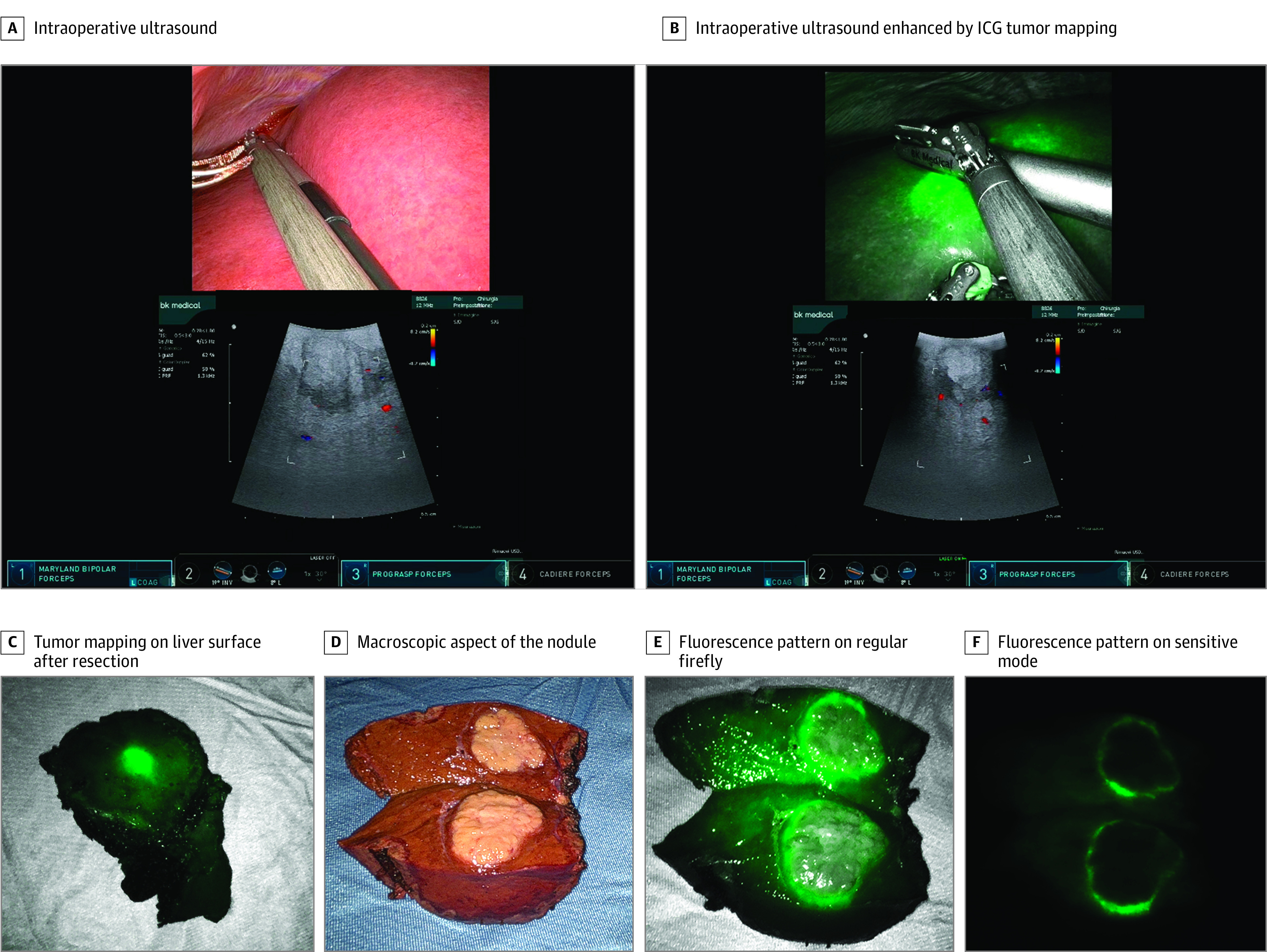
Tumor cells can be either hypofluorescent or hyperfluorescent after indocyanine green (ICG) injection according to histologic features. Well-differentiated HCC tumors usually show a homogenous fluorescence pattern, whereas poorly differentiated HCC tumors show an inhomogeneous one or even a rim-type fluorescence pattern.21
Statistical Analysis
Continuous data are reported as median (IQR). Categorical data are reported as counts and percentages. Comparisons between the OLR and RLR groups were performed using the Student t test for continuous variables, χ2 test or Fisher exact test for categorical variables, and Cochran-Armitage test for trend for ordinal variables. Balance in covariates between treatment groups was also evaluated by the standardized mean difference (SMD). An SMD of less than 0.1 was deemed to be the ideal balance.
To adjust for the nonrandom assignment of the procedure, a 1:1 propensity score matching (PSM) algorithm without replacement was used, with caliper of 0.1. The propensity score was estimated using a multivariable logistic regression, with type of surgery as the dependent variable and age, BMI, CCI, platelet count, IWATE score, ERASL-pre score, and CPT status as covariates.
The overall survival (OS) function was estimated using the Kaplan-Meier method. The cumulative incidence function (CIF) of death related to tumor recurrence was estimated according to the method described by Kalbfleisch and Prentice,22 considering death as a result of other causes as a competing event. For both OS and CIF, patients who underwent a liver transplant were censored at the date of transplant. To evaluate the association between surgery and both OS and CIF of death related to tumor recurrence, hazard ratios (HRs) were estimated from Cox proportional hazards regression and Fine and Gray models, respectively, and reported with their 95% CIs. To account for covariate imbalances, HRs were adjusted for covariates with an SMD greater than 0.1 in the PSM cohort.23 A 2-sided P < .05 was considered significant in all the analyses. All statistical analyses were performed using SAS, version 9.4 software (SAS Institute Inc).
Results
Demographic Characteristics
A total of 398 patients were included in the analysis, with 158 in the RLR group (125 men, 33 women) and 240 in the OLR group (190 men, 50 women). Table 1 shows demographic and clinical characteristics of the study population. Patients in the RLR group were significantly younger than those in the OLR group (median [IQR], 66 [58-71] years vs 70 [64-74] years; P < .001) and had a higher CCI score (median [IQR], 6 [5-8] vs 6 [5-7]; P = .004). Moreover, the IWATE score differed significantly between the RLR and OLR groups before PSM (median [IQR], 6 [4-8] vs 7 [5-9]; P < .001). Patients in the OLR group also had a significantly higher ERASL-pre score than the RLR group (median [IQR], 1.9 [1.5-2.3] vs 1.8 [1.4-2.1]; P = .01). The intraoperative conversion rate from RLR to OLR was 3.2%. eTable 1 in Supplement 1 shows the distribution of patients in the 2 groups according to the extension of the resection; nonanatomic liver resections were performed in 60 patients (25.0%) in the OLR group and 37 patients (23.4%) in the RLR group before PSM.
Table 1. Demographic and Clinical Characteristics of the Study Population Before and After Propensity Score Matching (PSM).
| Variable | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| OLR (n = 240) | RLR (n = 158) | P valuea | SMD | OLR (n = 106) | RLR (n = 106) | P valuea | SMD | |
| Age, median (IQR), y | 70 (64-74) | 66 (58-71) | <.001 | 0.425 | 69 (63-72) | 67 (59-72) | .71 | 0.052 |
| BMI, median (IQR) | 27.55 (24.00-30.95) | 25.97 (23.67-28.70) | .02 | 0.239 | 26.85 (22.20-29.60) | 25.77 (23.67-29.01) | .90 | 0.017 |
| CCI, median (IQR) | 6 (5-7) | 6 (5-8) | .004 | 0.291 | 6 (5-7) | 6 (5-7) | .52 | 0.088 |
| Platelet count, median (IQR), 103/μL | 173 (127-225) | 155 (127-214) | .38 | 0.089 | 156 (117-216) | 155 (127-201) | .63 | 0.066 |
| IWATE difficulty score, median (IQR) | 7 (5-9) | 6 (4-8) | <.001 | 0.424 | 6 (4-8) | 7 (4-8) | .72 | 0.049 |
| ERASL-pre score, median (IQR)b | 1.9 (1.5-2.3) | 1.8 (1.4-2.1) | .01 | 0.265 | 1.8 (1.5-2.1) | 1.8 (1.5-2.1) | .63 | 0.067 |
| Sex, No. (%) | ||||||||
| Female | 50 (20.8) | 33 (20.9) | .99 | 0.001 | 18 (17.0) | 21 (19.8) | .59 | 0.073 |
| Male | 190 (79.2) | 125 (79.1) | 88 (83.0) | 85 (80.2) | ||||
| CPT status, No. (%) | ||||||||
| A | 232 (96.7) | 147 (93.0) | .10 | 0.165 | 100 (94.3) | 101 (95.3) | .76 | 0.043 |
| B | 8 (3.3) | 11 (7.0) | 6 (5.7) | 5 (4.7) | ||||
Abbreviations: BMI, body mass index (weight in kilograms divided by height in meters squared); CCI, Charlson Comorbidity Index; CPT, Child-Pugh-Turcotte; ERASL-pre, preoperative Early Recurrence After Surgery for Liver Tumor; OLR, open liver resection; RLR, robotic liver resection; SMD, standardized mean difference.
To convert platelent count to ×109/L, multiply by 1.
t Test for continuous variables, χ2 test for categorical variables.
Eight missing among patients before PSM: 5 in the OLR group and 3 in the RLR group.
PSM Results
After applying PSM according to the items listed in the Methods, 106 patients from the RLR group were compared with 106 control patients in the OLR group (Table 2). Operative time was significantly longer in the RLR group compared with the OLR group (median [IQR], 295 [190-370] minutes vs 200 [165-255] minutes, including docking; P < .001). Furthermore, the intraoperative EBL was higher in the RLR group (median [IQR], 200 [100-500] mL vs 100 [100-150] mL; P < .001), despite a higher Pringle maneuver rate (13.2% vs 1%; P < .001). Interestingly, no significant differences were found in the number of patients requiring blood transfusions in the 2 groups (both groups, 9 [8.5%]; P > .99) and in the number of pRBC units transfused (both groups, 9 [8.5%]; P = .76). Nevertheless, patients in the RLR group had a significantly shorter hospital length of stay compared with the OLR group (median [IQR], 4 [3-6] vs 10 [7-13] days; P < .001) and lower number of admissions to the ICU (7 [6.6%] vs 21 [19.8%]; P = .002). Despite comparable rates of postoperative complications between the 2 groups, patients receiving an OLR had a significantly higher risk of developing severe complications according to Clavien-Dindo grade (3-4 vs 0-1-2, 12 [11.3%] vs 3 [2.8%]; P = .029). Similarly, the incidence of PHLF was significantly lower in the RLR group than in the OLR group (8 [7.5%] vs 30 [28.3%]; P < .001), and no cases of grade C PHLF occurred among patients who underwent RLR. The incidence of grade B bile leak was seen only in the RLR group (4 [3.8%]; P = .52). Resection margins were similar between the OLR and RLR groups (median [IQR], 9 [5-10] vs 8 [3-10] mm; P = .56), without significant differences in the number of patients with an R0 rate between groups.
Table 2. Outcome Variables by Type of Surgery Before and After Propensity Score Matching (PSM).
| Variable | Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|---|
| No. (%) | P valuea | No. (%) | P valuea | ||||
| OLR (n = 240) | RLR (n = 158) | OLR (n = 106) | RLR (n = 106) | ||||
| EBL, median (IQR), mLb,c | 100 (100-100) | 200 (100-500) | <.001 | 100 (100-150) | 200 (100-500) | <.001 | |
| Operative time, median (IQR), mind | 210 (174-259) | 290 (190-380) | <.001 | 200 (165-255) | 295 (190-370) | <.001 | |
| Transfusion | |||||||
| No | 219 (91.3) | 144 (91.1) | >.99 | 97 (91.5) | 97 (91.5) | >.99 | |
| Yes | 21 (8.8) | 14 (8.9) | 9 (8.5) | 9 (8.5) | |||
| No. of pRBC units | |||||||
| 0 | 219 (91.3) | 144 (91.1) | .73 | 97 (91.5) | 97 (91.5) | .76 | |
| 1 | 11 (4.6) | 5 (3.2) | 5 (4.7) | 3 (2.8) | |||
| >1 | 10 (4.2) | 9 (5.7) | 4 (3.8) | 6 (5.7) | |||
| Hospital length of stay, median (IQR), d | 10 (7-13) | 4 (3-6) | <.001 | 10 (7-13) | 4 (3-6) | <.001 | |
| Margin size, median (IQR), mme | 10 (5-10) | 8 (3-12) | .60 | 9 (5-10) | 8 (3-10) | .56 | |
| ICU length of stay, df | |||||||
| 0 | 195 (81.2) | 145 (92.4) | .003 | 85 (80.2) | 99 (93.4) | .002 | |
| 1 | 32 (13.3) | 9 (5.7) | 15 (14.2) | 7 (6.6) | |||
| >1 | 13 (5.4) | 3 (1.9) | 6 (5.7) | 0 | |||
| PHLFg | |||||||
| No | 176 (73.3) | 136 (91.9) | <.001 | 76 (71.7) | 98 (92.5) | <.001 | |
| A | 28 (11.7) | 10 (6.8) | 13 (12.3) | 8 (7.5) | |||
| B | 27 (11.2) | 2 (1.4) | 13 (12.3) | 0 | |||
| C | 9 (3.8) | 0 | 4 (3.8) | 0 | |||
| Bile leak | |||||||
| No | 230 (95.8) | 150 (94.9) | .21 | 100 (94.3) | 101 (95.3) | .52 | |
| A | 10 (4.2) | 4 (2.5) | 6 (5.7) | 1 (0.9) | |||
| B | 0 | 4 (2.5) | 0 (0.0) | 4 (3.8) | |||
| Clavien-Dindo grade | |||||||
| 0 | 140 (58.3) | 73 (46.2) | .36 | 59 (55.7) | 49 (46.2) | .91 | |
| 1-2 | 72 (30.0) | 76 (48.1) | 35 (33.0) | 54 (50.9) | |||
| >2 | 28 (11.7) | 9 (5.7) | 12 (11.3) | 3 (2.8) | |||
| Resection margine | |||||||
| R0 | 214 (100) | 157 (99.4) | .42 | 106 (100) | 105 (99.1) | >.99 | |
| R1 | 0 | 1 (0.6) | 0 | 1 (0.9) | |||
| 90-d OS (95% CI), % | 96.2 (92.9-98.0) | 98.7 (95.0-99.7) | .16 | 97.1 (91.3-99.1) | 99.1 (93.5-99.9) | .33 | |
Abbreviations: EBL, estimated blood loss; ICU, intensive care unit; OLR, open liver resection; OS, overall survival; PHLF, posthepatectomy liver failure; pRBC, packed red blood cell; RLR, robotic liver resection.
t Test for continuous variables, Fisher exact test or Cochran-Armitage test for trend for categorical variables, and log-rank test for 90-day OS.
EBL values of 100 mL or less were considered equal to 100 mL.
Data for 14 patients missing before PSM, including 7 in the OLR group and 7 in the RLR group.
Data for 2 patients missing before PSM in the OLR group.
Data for 26 patients missing before PSM in the OLR group.
Data for 1 patient missing before PSM in the RLR group.
Data for 10 patients missing before PSM in the RLR group.
With respect to the oncologic features of the resected specimen (Table 3), tumor size was comparable between the OLR and RLR groups (median [IQR], 30 [21-45] vs 35 [23-50] mm; P = .76), with 18 (17.0%) experiencing satellitosis in the RLR group and 22 (20.8%) in the OLR group (P = .48). In the OLR group, 83 patients (78.3%) had unifocal disease vs 95 (89.6%) in the RLR group (P = .03). α-Fetoprotein levels were comparable between the OLR and RLR groups (median [IQR], 5.9 [2.8-25.0] ng/mL vs 5.3 [3.3-25.6] ng/mL; P = .66). Robotically resected tumors were more likely to have a grade 3 or higher differentiation according to Edmonson criteria (48 [45.3%] vs 18 [17.0%]; P = .002) but showed less incidence of microvascular invasion (34 [32.1%] vs 60 [56.6%]; P < .001).
Table 3. Oncologic Features After Propensity Score Matching by Type of Surgery.
| Variable | No. (%) | P valuea | SMD | |
|---|---|---|---|---|
| OLR (n = 106) | RLR (n = 106) | |||
| AFP, median (IQR), ng/mL | 5.9 (2.8-25.0) | 5.3 (3.3-25.6) | .66b | 0.061b |
| Maximum size, median (IQR), mm | 30 (21-45) | 35 (23-50) | .76 | 0.042 |
| No. of nodules | ||||
| 1 | 83 (78.3) | 95 (89.6) | .03 | 0.312 |
| 2-3-4 | 23 (21.7) | 11 (10.4) | ||
| Grade | ||||
| 1 | 17 (16.0) | 18 (17.0) | .002 | 0.697 |
| 2 | 71 (67.0) | 40 (37.7) | ||
| 3 | 18 (17.0) | 46 (43.4) | ||
| 4 | 0 (0.0) | 2 (1.9) | ||
| Satellitosis | ||||
| No | 84 (79.2) | 88 (83.0) | .48 | 0.097 |
| Yes | 22 (20.8) | 18 (17.0) | ||
| Microvascular invasion | ||||
| No | 46 (43.4) | 72 (67.9) | <.001 | 0.510 |
| Yes | 60 (56.6) | 34 (32.1) | ||
Abbreviations: AFP, α-fetoprotein; OLR, open liver resection; RLR, robotic liver resection; SMD, standardized mean difference.
t Test for continuous variables or χ2 test or Cochran-Armitage test for trend for categorical variables.
Evaluated on log-transformed values.
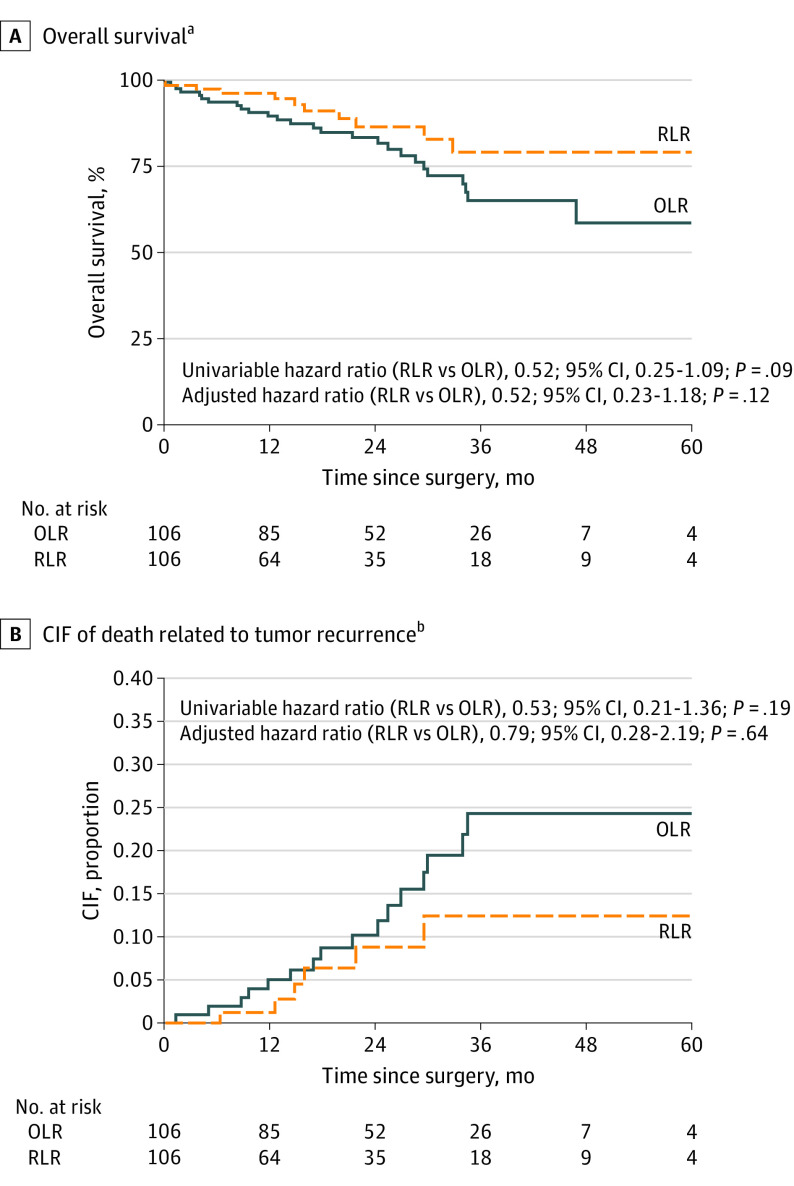
Survival
Overall survival and CIF of death related to tumor recurrence curves are shown in Figure 2. Seventeen patients who received a liver transplant were censored at the date of transplant (2 in the OLR group without recurrence and 15 in the RLR group [9 without recurrence and 6 undergoing liver transplant after recurrence]). After PSM, the 2 groups showed similar 90-day OS rates after surgery for HCC (OLR, 97.1% [95% CI, 91.3%-99.1%]; RLR, 99.1% [93.5%-99.9%]; P = .33) (Table 2).
Figure 2. Overall Survival and Cumulative Incidence Function (CIF) of Death Related to Tumor Recurrence After Propensity Score Matching by Type of Surgery.
Seventeen patients received a liver transplant and were censored on the date of transplant (2 in the open liver resection [OLR] group without recurrence, and 15 in the robotic liver resection [RLR] group [9 without recurrence and 6 with liver transplant after recurrence]). Deaths related to tumor recurrence were considered as events; deaths as a result of other causes were considered as competing events.
aMedian (IQR) follow-up, 19 (10-33) months.
bHazard ratio of surgery adjusted for number of nodules, grading, and microvascular invasion.
Before PSM, 8 perioperative deaths (6 in the OLR group, 2 in the RLR group) were observed in the whole cohort (P = .49 by univariable analysis). All perioperative deaths occurred in patients with CPT A status. However, because of the small sample of patients with CPT B status (n = 19), the difference between perioperative mortality risk in CPT A vs B was not significant. After propensity score analysis, only 1 event each was observed in the OLR and RLR groups.
The estimated 24-month OS rate was 83.8% (95% CI, 74.4%-90.0%) in the OLR group and 86.9% (95% CI, 74.7%-93.5%) in the RLR group. The HR for OS adjusted for number of nodules, grading, and microvascular invasion was 0.52 (95% CI, 0.23-1.18; P = .12). As a sensitivity analysis, a multivariable Cox proportional hazards regression for OS was performed that included all relevant prognostic factors as covariates. The HR for OS of RLR vs OLR was 0.51 (95% CI, 0.26-1.01), which was similar to that estimated after PSM (HR, 0.52; 95% CI, 0.23-1.16) (eTable 2 in Supplement 1).
The 24-month CIF of death related to tumor recurrence for the OLR group was 10.2% (95% CI, 4.9%-17.7%) vs 8.8% (95% CI, 3.1%-18.3%) for the RLR group. The HR of CIF of death related to tumor recurrence adjusted for number of nodules, grading, and microvascular invasion was 0.79 (95% CI, 0.28-2.19; P = .64). A subgroup analysis of patients within Milan criteria did not show superiority of the RLR approach, as shown in the eFigure in Supplement 1.
Discussion
This cohort study is, to our knowledge, the largest Western series of consecutive patients treated with the full RLR approach for HCC. The results show that RLR is associated with better perioperative tolerability than OLR in patients with HCC after a PSM analysis based on clinical, oncologic, and technical criteria. After PSM, the 2 populations became homogeneous on the basis of not only baseline characteristics but also surgical difficulty and preoperative risk of tumor recurrence, representing an important refinement in this kind of analysis. Patients in the RLR group experienced shorter hospital lengths of stay and were less likely to spend more than 1 day in the ICU in the PSM analysis. Consistently, incidence of PHLF and a Clavien-Dindo grade of more than 2 complications were significantly less frequent after RLR vs OLR, which is of relevance when treating patients with cirrhosis who are at higher risk of liver decompensation after surgery. Of note, these results were achieved despite shorter operative times and lower blood loss in the OLR group. The incidence of grade B bile leak was higher but not significantly different in the RLR group. A learning curve effect may explain these outcomes because 30 cases are required to obtain a plateau of operative time in expert hepatobiliary centers and up to 40 cases to standardize major hepatectomy techniques.24,25 Therefore, the 3 included robotic centers are currently at different stages of proficiency compared with the well-established open procedure. However, the small difference in median EBL was not clinically associated with either procedure because no differences were found in terms of patients requiring transfusions and pRBC units transfused.
Most importantly, reduced ICU and hospital lengths of stay as well as a lower incidence of severe complications may ultimately result in a reduction of overall costs and compensate for the higher technology-related expenses. It is well known that one of the major criticisms to the use of robotic technology in surgery is cost. Unfortunately, we could not run a cost comparison and cost-effectiveness analysis because of the different reimbursement systems and uneven costs of each tool across countries. However, intuitively, a median difference of 6 days of hospitalization represents a major saving for any hospital, as well as a 93.4% vs 80.2% rate of RLR and OLR patients, respectively, not requiring ICU admission.
Hepatocellular carcinoma is a complex disease with a mosaic of possible therapeutic strategies that vary according to patient age, tumor characteristics, and biology.26 Even so, surgical resection and liver transplant can be curative for patients with HCC, meaning achievement of a life expectancy comparable to that of patients with cirrhosis without HCC.27,28 Recently, the classical Barcelona Clinic Liver Cancer staging system was updated to focus on pathophysiologic characteristics of patients with HCC.29 In particular, great emphasis has been given to the need for tailored approaches and to the pivotal role of portal pressure to determine the most appropriate therapeutic approach. Nevertheless, minimally invasive resections of the liver still deserve a better definition inside HCC therapeutic algorithms for both radical approaches and downstaging strategies.30,31,32 Minimally invasive liver resections have been shown to be safe and effective in the treatment of HCC, even in the context of advanced cirrhosis and decompensation, with more favorable outcomes compared with open surgery.33,34 In particular, Sposito et al20 reported in 2016 that laparoscopy was the only independent factor to reduce the risks of complications after surgery in a PSM study of laparoscopic vs OLR for HCC. In this context, the RLR approach may confer additional improvements compared with standard laparoscopy, thus increasing the safety and reproducibility of the procedure, especially when performing highly demanding procedures.4 These improvements are possible because of the special features of the robotic platform, namely tremor filtration, motion scaling, and magnified 3-dimensional vision, that increase the precision of the surgical moves, especially when operating in narrow spaces. Moreover, instrument articulation outperforms in vascular control and parenchymal dissection for posterior segments. The integrated 3-dimensional firefly detection with 2 levels of sensitivity may improve tumor identification and radical excision in the near future.35 Although not routinely used in this study (31% of all RLRs), the use of ICG fluorescence is increasing for tumor identification and surface mapping because of its greater safety and effectiveness.21,35,36
Instrumentation is another hotly debated topic, with a particular focus on the lack of CUSA among robotic tools. Hybrid techniques with the use of laparoscopic CUSA by the tableside surgeon have been described, claiming increasing safety and reduced morbidity.37 The use of CUSA from the tableside results in 2 major issues. First, it shifts the role of the robot from operating to exposing, which becomes detrimental from the cost-effectiveness point of view. Second, during robotic surgery, the tableside surgeon may become physically uncomfortable due to the narrow robotic trocar lines and encumbrance of robotic arms. The presence of a robotic CUSA would be helpful to further increase the performance of RLR, particularly in the setting of anatomic resection and Glissonian approach. Even so, ultrasonic instruments cannot fulfill the robotic concept because they lack a flexible design, like the Harmonic ACE. Therefore, bipolar-mediated parenchymal dissection represents the highest expression of robotic technology because of the agility of its movements and the 7 degrees of freedom. Nevertheless, the ratio between anatomic and nonanatomic liver resection was comparable between the OLR and RLR groups. Thus, there are still margins of improvement with regard to RLR outcomes that will depend on future innovations in the armamentarium of robotic instruments.
In 2017, Chen et al38 reported the first comparison of oncologic results between OLR and RLR for HCC. After PSM, they compared 81 patients with HCC treated with RLR with 81 OLR control patients, showing similar pathologic results, longer operative time in the RLR group, similar EBL, and shorter hospital length of stay with less requirement for analgesics after RLR. Moreover, disease-free survival and OS were comparable between the 2 groups. In our study, we confirmed through a Western population the same effectiveness of the RLR approach, with a specific advantage on reduced risk of liver failure and comparable survival rates after careful matching of the oncologic and technical risk factors. A positive oncologic effect of the minimally invasive approach might be foreseen, particularly in patients at an earlier stage of disease. However, a subgroup analysis of patients within Milan criteria did not show superiority of the RLR approach. Additional prospective studies on this issue are warranted. Surprisingly, the incidence of microvascular invasion in this study reached a significant difference, but this result probably depends on the absence of a centralized review of the histologic examination.
Limitations
Limitations of this study include the retrospective design and potential biases of a multicenter, nonrandomized protocol.
Conclusions
The findings of this cohort study suggest that RLR confers significant advantages over OLR in patients with HCC in expert centers, with reasonable safety also at the beginning of the learning curve. In particular, RLR may reduce morbidity, expanding the potential number of patients able to receive treatment from which they are currently excluded because of the risk of liver decompensation. This study represents the largest Western experience to date of full RLR for HCC vs OLR. Robotic liver resection performed in tertiary centers represents a safe treatment strategy for patients with HCC and those with compromised liver function and is associated with the prevention of incident PHLF and achievement of oncologic efficacy.
eTable 1. Resection Type Before and After Propensity Score Matching
eTable 2. Multivariable Cox Proportional Hazards Regression for Overall Survival (OS)
eFigure. Overall Survival and Cumulative Incidence of Death Related to Tumor Recurrence After Propensity Score Matching by Type of Surgery Among Patients Within Milan Criteria
Nonauthor Collaborators
References
- 1.Abu Hilal M, Aldrighetti L, Dagher I, et al. The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg. 2018;268(1):11-18. doi: 10.1097/SLA.0000000000002524 [DOI] [PubMed] [Google Scholar]
- 2.Chin KM, Linn YL, Cheong CK, et al. Minimally invasive vs open major hepatectomies for liver malignancies: a propensity score-matched analysis. J Gastrointest Surg. 2022;26(5):1041-1053. doi: 10.1007/s11605-021-05226-4 [DOI] [PubMed] [Google Scholar]
- 3.Chong CC, Fuks D, Lee KF, et al. ; International Robotic and Laparoscopic Liver Resection Study Group Investigators . Propensity score–matched analysis comparing robotic and laparoscopic right and extended right hepatectomy. JAMA Surg. 2022;157(5):436-444. doi: 10.1001/jamasurg.2022.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cipriani F, Fiorentini G, Magistri P, et al. Pure laparoscopic versus robotic liver resections: multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci. Published online July 22, 2021. doi: 10.1002/jhbp.1022 [DOI] [PubMed] [Google Scholar]
- 5.Magistri P, Tarantino G, Assirati G, et al. Robotic liver resection for hepatocellular carcinoma: a systematic review. Int J Med Robot. 2019;15(4):e2004. doi: 10.1002/rcs.2004 [DOI] [PubMed] [Google Scholar]
- 6.Di Benedetto F, Petrowsky H, Magistri P, Halazun KJ. Robotic liver resection: hurdles and beyond. Int J Surg. 2020;82S:155-162. doi: 10.1016/j.ijsu.2020.05.070 [DOI] [PubMed] [Google Scholar]
- 7.Torzilli G, Viganò L, Giuliante F, Pinna AD. Liver surgery in Italy: criteria to identify the hospital units and the tertiary referral centers entitled to perform it. Updates Surg. 2016;68(2):135-142. doi: 10.1007/s13304-016-0373-0 [DOI] [PubMed] [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver . EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 10.Elsayes KM, Hooker JC, Agrons MM, et al. 2017 version of LI-RADS for CT and MR imaging: an update. Radiographics. 2017;37(7):1994-2017. doi: 10.1148/rg.2017170098 [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Ban D, Tanabe M, Ito H, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21(10):745-753. doi: 10.1002/jhbp.166 [DOI] [PubMed] [Google Scholar]
- 13.Tanaka S, Kubo S, Kanazawa A, et al. Validation of a difficulty scoring system for laparoscopic liver resection: a multicenter analysis by the endoscopic liver surgery study group in Japan. J Am Coll Surg. 2017;225(2):249-258.e1. doi: 10.1016/j.jamcollsurg.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 14.Labadie KP, Droullard DJ, Lois AW, et al. IWATE criteria are associated with perioperative outcomes in robotic hepatectomy: a retrospective review of 225 resections. Surg Endosc. 2022;36(2):889-895. doi: 10.1007/s00464-021-08345-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luberice K, Sucandy I, Modasi A, et al. Applying IWATE criteria to robotic hepatectomy: is there a “robotic effect”? HPB (Oxford). 2021;23(6):899-906. doi: 10.1016/j.hpb.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 16.Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol. 2018;69(6):1284-1293. doi: 10.1016/j.jhep.2018.08.027 [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach—the ALBI grade. J Clin Oncol. 2015;33(6):550-558. doi: 10.1200/JCO.2014.57.9151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680-688. doi: 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 19.Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-724. doi: 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 20.Sposito C, Battiston C, Facciorusso A, et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103(7):871-880. doi: 10.1002/bjs.10137 [DOI] [PubMed] [Google Scholar]
- 21.Franz M, Arend J, Wolff S, et al. Tumor visualization and fluorescence angiography with indocyanine green (ICG) in laparoscopic and robotic hepatobiliary surgery—valuation of early adopters from Germany. Innov Surg Sci. 2021;6(2):59-66. doi: 10.1515/iss-2020-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. John Wiley & Sons, 1980. [Google Scholar]
- 23.Nguyen TL, Collins GS, Spence J, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. BMC Med Res Methodol. 2017;17(1):78. doi: 10.1186/s12874-017-0338-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magistri P, Guerrini GP, Ballarin R, Assirati G, Tarantino G, Di Benedetto F. Improving outcomes defending patient safety: the learning journey in robotic liver resections. Biomed Res Int. 2019;2019:1835085. doi: 10.1155/2019/1835085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen PD, Wu CY, Hu RH, et al. Robotic major hepatectomy: is there a learning curve? Surgery. 2017;161(3):642-649. doi: 10.1016/j.surg.2016.09.025 [DOI] [PubMed] [Google Scholar]
- 26.Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien PA. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17(12):755-772. doi: 10.1038/s41575-020-0314-8 [DOI] [PubMed] [Google Scholar]
- 27.Pinna AD, Yang T, Mazzaferro V, et al. Liver transplantation and hepatic resection can achieve cure for hepatocellular carcinoma. Ann Surg. 2018;268(5):868-875. doi: 10.1097/SLA.0000000000002889 [DOI] [PubMed] [Google Scholar]
- 28.Cucchetti A, Zhong J, Berhane S, et al. The chances of hepatic resection curing hepatocellular carcinoma. J Hepatol. 2020;72(4):711-717. doi: 10.1016/j.jhep.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 29.Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol. 2022;76(3):681-693. doi: 10.1016/j.jhep.2021.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magistri P, Tarantino G, Guidetti C, et al. Laparoscopic versus robotic surgery for hepatocellular carcinoma: the first 46 consecutive cases. J Surg Res. 2017;217:92-99. doi: 10.1016/j.jss.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Magistri P, Olivieri T, Assirati G, et al. Robotic liver resection expands the opportunities of bridging before liver transplantation. Liver Transpl. 2019;25(7):1110-1112. doi: 10.1002/lt.25477 [DOI] [PubMed] [Google Scholar]
- 32.Magistri P, Catellani B, Frassoni S, et al. Robotic liver resection versus percutaneous ablation for early HCC: short- and long-term results. Cancers (Basel). 2020;12(12):E3578. doi: 10.3390/cancers12123578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon YI, Kim KH, Kang SH, et al. Pure laparoscopic versus open right hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score matched analysis. Ann Surg. 2017;265(5):856-863. doi: 10.1097/SLA.0000000000002072 [DOI] [PubMed] [Google Scholar]
- 34.Troisi RI, Berardi G, Morise Z, et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child-Pugh B cirrhosis: multicentre propensity score-matched study. Br J Surg. 2021;108(2):196-204. doi: 10.1093/bjs/znaa041 [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi T, Cacciaguerra AB, Abe Y, et al. Indocyanine green fluorescence navigation in liver surgery: a systematic review on dose and timing of administration. Ann Surg. 2022;275(6):1025-1034. doi: 10.1097/SLA.0000000000005406 [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez-Ciccarelli LF, Quadri P, Daskalaki D, Milone L, Gangemi A, Giulianotti PC. Robotic approach to hepatobiliary surgery. Chirurg. 2017;88(suppl 1):19-28. doi: 10.1007/s00104-016-0223-0 [DOI] [PubMed] [Google Scholar]
- 37.Hawksworth J, Radkani P, Nguyen B, et al. Improving safety of robotic major hepatectomy with extrahepatic inflow control and laparoscopic CUSA parenchymal transection: technical description and initial experience. Surg Endosc. 2022;36(5):3270-3276. doi: 10.1007/s00464-021-08639-z [DOI] [PubMed] [Google Scholar]
- 38.Chen PD, Wu CY, Hu RH, et al. Robotic versus open hepatectomy for hepatocellular carcinoma: a matched comparison. Ann Surg Oncol. 2017;24(4):1021-1028. doi: 10.1245/s10434-016-5638-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Resection Type Before and After Propensity Score Matching
eTable 2. Multivariable Cox Proportional Hazards Regression for Overall Survival (OS)
eFigure. Overall Survival and Cumulative Incidence of Death Related to Tumor Recurrence After Propensity Score Matching by Type of Surgery Among Patients Within Milan Criteria
Nonauthor Collaborators