Key Points
Question
What is the rate of structural recurrent disease in patients with low-risk papillary thyroid microcarcinoma after hemithyroidectomy?
Findings
In this nationwide cohort study that included 1636 patients with papillary thyroid microcarcinoma, recurrent disease after hemithyroidectomy was rare (<2%).
Meaning
It seems reasonable to deescalate follow-up of patients with low-risk papillary thyroid microcarcinoma after hemithyroidectomy.
This national cohort study assesses the occurrence and risk factors of recurrent disease in all Dutch patients with papillary thyroid microcarcinoma who received surgical treatment according to the Dutch guideline between January 2000 and December 2020.
Abstract
Importance
Structural recurrent disease (RD) after surgical treatment of papillary thyroid microcarcinoma (mPTC) is rare. We hypothesized that the RD rate after hemithyroidectomy in low-risk patients with mPTC is low.
Objective
To assess the occurrence of RD in Dutch patients with mPTC who received surgical treatment according to the Dutch guidelines.
Design, Setting, and Participants
This nationwide retrospective cohort study included all patients who had undergone surgery with a diagnosis of cN0/cNx mPTC in the Netherlands between January 2000 and December 2020 were identified from the Netherlands Cancer Registry database. Patients with preoperative lymph node metastases were excluded. Two groups were defined: group 1 (incidental), mPTC in pathology report after thyroid surgery for another indication; and group 2 (nonincidental), patients with a preoperative highly suspect thyroid nodule (Bethesda 5) or proven mPTC (Bethesda 6). Dutch guidelines state that a hemithyroidectomy is sufficient in patients with unifocal, intrathyroidal mPTC.
Main Outcomes and Measures
The occurrence of RD in patients with low-risk mPTC after hemithyroidectomy.
Results
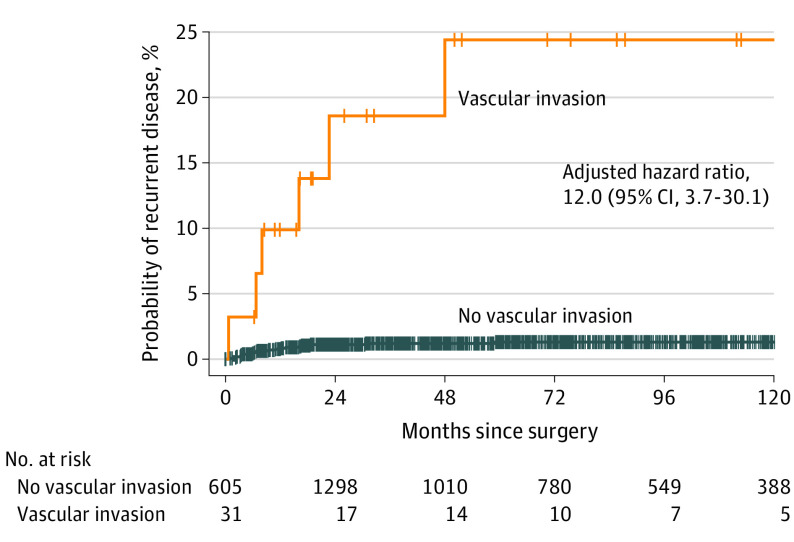
In total, 1636 patients with mPTC were included. Patients had a median (IQR) follow-up time of 71 (32-118) months. Median (IQR) age at time of diagnosis was 51 (41-61) years and 1292 (79.0%) were women. Overall, RD after initial treatment was seen in 25 patients (1.5%). The median (IQR) time to RD was 8.2 (3.6-16.5) months and 22 of the 25 (88%) patients developed RD within 2 years. Recurrent disease was not significantly different between both groups (group 1, n = 15 [1.3%]; group 2, n = 10 [2.1%]; difference, 0.8%; 95% CI, –0.5% to 2.5%). Of the 484 patients with nonincidental mPTC (group 2), 246 (50.8%) patients were treated with a hemithyroidectomy and follow-up in accordance with Dutch guidelines. Lymph node metastases were found in 1 of 246 (0.4%) patients after hemithyreoidectomy, and new mPTC in the contralateral thyroid was detected in 3 of 246 (1.2%) patients. Median (IQR) follow-up of this patient group was 37 (18-71) months. The 10-year probability of RD was 1.3% for patients without vascular invasion and 24.4% for patients with vascular invasion.
Conclusions and Relevance
This nationwide cohort study found that overall, RD after hemithyroidectomy for patients with low-risk mPTC was rare (<2%). Based on these results, it seems reasonable to deescalate follow-up of patients with low-risk mPTC without vascular invasion after hemithyroidectomy. From a health care perspective, deescalation of follow-up may contribute to increased sustainability and accessibility to health care, both large challenges for the future.
Introduction
The worldwide incidence of small papillary thyroid carcinoma (≤10 mm), also known as papillary microcarcinoma (mPTC), has increased over the past decades.1,2,3 The rising incidence of mPTC alongside a stable mortality rate1,4,5 is highly suggestive of overtreatment of patients with mPTC.6,7,8 Currently, restrictive diagnostic workup strategies for thyroid nodules smaller than 1 cm are advocated,9,10,11 which resulted in a decrease in mPTC diagnosis since 2014.12 In addition, several international guidelines have shifted from immediate surgery to a less aggressive treatment approach by advocating the use of active surveillance with serial ultrasonographic examinations of the neck.10 However, surgery will likely remain important in countries with restrictive diagnostic workup strategies due to selection of patients with biologically more aggressive disease or for patients anxious to undergo active surveillance.13
Dutch guidelines state that a hemithyroidectomy is sufficient in patients with unifocal, intrathyroidal mPTC without lymph node metastases, and a total thyroidectomy is indicated in cases of multifocal disease, extrathyroidal extension or pathology in the contralateral lobe.14 The 2015 ATA guideline suggests that patients with low-risk mPTC should receive follow-up after hemithyroidectomy including clinical examinations, Tg measurements, and periodic neck ultrasonography.10 However, structural recurrent disease (RD) rates after surgical treatment of N0 mPTC have been shown to be low.15,16 We hypothesized that the RD rate after hemithyroidectomy in patients with low-risk mPTC in the Netherlands is low. The objective of this nationwide study was therefore to assess the occurrence and risk factors of RD in all Dutch patients with mPTC who received surgical treatment according to the Dutch guideline between January 2000 and December 2020.
Methods
Patients and Data Collection
As previously described,13 all adult patients treated for mPTC in the Netherlands from January 2000 to December 2020 were included in this study. Data were obtained from the Dutch Registry of Histo- and Cytopathology Reports (PALGA) and the Netherlands Cancer Registry managed by the Netherlands Comprehensive Cancer Organisation (IKNL). All reports that were identified by IKNL as T1a and T1 were reviewed. Patients were identified using the American Joint Committee on Cancer (AJCC) Staging Manual, Eighth Edition, primary tumor stage classification (TNM),17 in which the tumor had to be 1.0 cm or smaller in the largest dimension on the pathology reports. Exclusion criteria were PTC larger than 1 cm, preoperative pathologic findings confirmed lymph node metastases, follicular or medullar microcarcinoma, and unknown tumor diameter of the PTC. Patients with unknown tumor diameter were also included if the report explicitly stated that a microcarcinoma was found in the specimen. After inclusion, patients were divided into 2 groups: group 1 (incidental), mPTC in pathology report after thyroid surgery for another indication; and group 2 (nonincidental), patients with a preoperative highly suspect thyroid nodule (Bethesda 5) or proven mPTC (Bethesda 6).
Baseline characteristics (age, sex, and follow-up time), findings from the pathology reports (type of mPTC, tumor diameter, vascular invasion, [extra]capsular invasion of the thyroid, multifocality, bilaterality, number of localizations, coexistence of thyroiditis, BRAF mutation, encapsulating tumor, invasion through tumor capsule, lymph node involvement, and radical [R0] or less than radical resection [R1 or R2]), RD, and mortality data were collected. Follow-up of patients at low risk, according to the Dutch guidelines, consists of an annual determination of Tg during thyroxine replacement in combination with neck palpation for 5 years. Recurrent disease was detected and proven by using ultrasonography and fine-needle aspiration cytology, and was defined as structural, pathology proven local RD, the occurrence of lymph node metastases, or distant metastases after initial treatment. Recurrent disease was treated with surgical excision and/or radioactive iodine treatment (RAI). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines was used.18 The medical ethics committee of the Erasmus Medical Center approved this study (MEC-2018-1195), and informed consent was waived.
Statistical Analysis
Descriptive statistics were used to express continuous variables with normal distribution as mean with standard deviation (SD) or abnormal distribution as median with interquartile range (IQR). Distribution was assessed using the Shapiro-Wilk normality test. Categorical variables are described as count (n) and percentage (%). To describe the magnitude of the difference and precision of the estimate when comparing both groups, the risk difference with 95% CI for dichotomous variables and the standardized mean difference (Cohen d) with 95% CIs for continuous variables was used.19
A multivariable Cox proportional hazards regression model was created to analyze risk of RD. Variables for the Cox proportional hazards model were included in the analysis based on their association with cancer RD in literature.20,21,22 Covariates within the Cox proportional hazards assumption were constant over time, meeting the proportional hazards assumption. Cox regression results are presented as hazard ratios with 95% CIs. Time to RD was estimated using the Kaplan-Meier estimator.23 All statistical tests were performed using the R Project for Statistical Computing (version 4.1.2, R Foundation; https://www.r-project.org/). All test hypotheses were 2-sided and P < .05 was considered significant.
Outcome Measures
The primary outcome was the occurrence of RD in patients with low-risk mPTC after hemithyroidectomy. Secondary outcomes were risk factors for RD and time to RD of patients with mPTC.
Results
Patient Characteristics
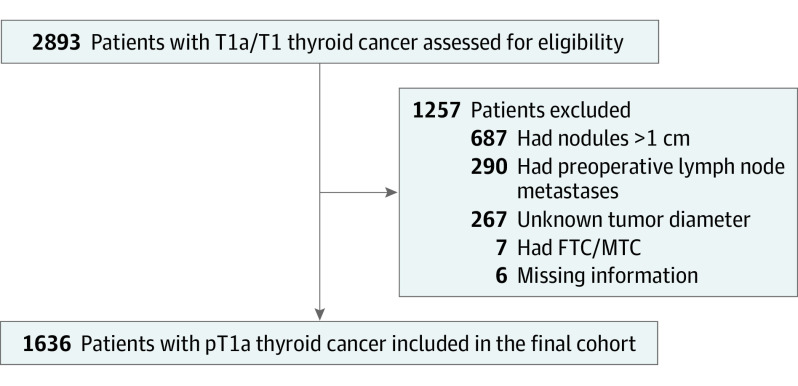
In total, 2893 patients were identified with pT1a or pT1 thyroid cancer from 2000 until 2020 in the Netherlands, of which 1636 mPTC patients were included in this study (Figure 1). Reasons for exclusion were unknown tumor diameter (n = 267), tumor larger than 1 cm (n = 687), preoperative lymph node metastases (n = 290), and other missing information (n = 13). Of these 1636 patients with mPTC, 1152 patients were incidentally diagnosed postoperatively (group 1) and 484 patients were diagnosed preoperatively (group 2). Patients had a median (IQR) follow-up time of 71 (32-118) months. The median age at time of diagnosis was 51 (41-61) years and 1292 (79.0%) were female. The median (IQR) tumor diameter in the pathologic specimen was 6.0 (3.0-8.0) mm. In total 372 (22.7%) patients underwent a total thyroidectomy, 367 (22.4%) underwent a completion thyroidectomy, and 874 (53.4%) patients underwent a hemithyroidectomy. Cervical lymph node dissection was performed in 76 (4.6%) patients, and 402 (24.6%) patients received RAI treatment after total or completion thyroidectomy.
Figure 1. Flowchart of Patient Enrollment.
FTC indicates follicular thyroid carcinoma; MTC, medullary thyroid carcinoma.
Patients with nonincidental mPTC were more likely to be men compared with patients with incidental mPTC (123 [25.4%] vs 221 [19.2%]; difference 6.2%; 95% CI, 1.8%-10.8%). Tumor diameter was larger in the group with nonincidental mPTC than in the group with incidental mPTC (6.0 mm vs 5.0 mm). Vascular invasion, bilaterality, and (extra)capsular invasion of thyroid were more frequent in patients with nonincidental mPTC. Patients with nonincidental mPTC underwent more total or completion thyroidectomies (232 [47.9%]) and RAI treatments (173 [35.7%]) than patients with incidental mPTC (507 [44.0%] and 229 [19.9%] respectively). Of all 376 patients who underwent initial total thyroidectomy, bilaterality of the mPTC was found in 24 of 228 (10.5%) patients with incidental mPTC and in 33 of 144 (22.9%) patients with nonincidental mPTC. Refer to Table 1 for additional characteristics.
Table 1. Clinical and Histopathologic Characteristics of Patients With mPTC in the Netherlandsa.
| Characteristic | No. (%) | Estimate with percentage difference (95% CI) | ||
|---|---|---|---|---|
| Total | Incidental mPTC | Nonincidental mPTC | ||
| No. of patients | 1636 (100) | 1152 (70.3) | 484 (29.7) | |
| Age, median (IQR), y | 51 (41-61) | 51 (42-61) | 52 (41-60) | 0.00 (–0.11 to 0.10) |
| Male sex | 344 (21.0) | 221 (19.2) | 123 (25.4) | 6.2 (1.7 to 10.7) |
| Follow-up, median (IQR), mo | 71 (32-118) | 78 (39-127) | 47 (21-93) | –0.43 (–0.54 to –0.32) |
| Surgical treatment | ||||
| Hemithyroidectomy | 874 (53.4) | 632 (54.9) | 242 (50.0) | –4.9 (–10.2 to 0.4) |
| Total thyroidectomy | 372 (22.7) | 228 (19.8) | 144 (29.8) | 10.0 (5.3 to 14.6) |
| Completion thyroidectomy | 367 (22.4) | 279 (24.2) | 88 (18.2) | –6.0 (–10.2 to –1.8) |
| Isthmus resection | 13 (0.8) | 4 (0.3) | 9 (1.9) | 1.6 (0.3 to 2.9) |
| Thyroglossal duct cyst resection | 9 (0.6) | 8 (0.7) | 1 (0.2) | –0.5 (–1.1 to 0.1) |
| Lateral cyst | 1 (0.1) | 1 (0.1) | 0 | –0.1 (–0.3 to 0.1) |
| CLNDb | 76 (4.6) | 35 (3.0) | 41 (8.5) | 5.5 (2.8 to 8.2) |
| CCLND | 58 | 25 | 31 | NA |
| LCLND | 25 | 18 | 7 | |
| Location unknown | 14 | 5 | 9 | |
| Nonsurgical treatment | ||||
| Radioactive iodine treatment | 402 (24.6) | 229 (19.9) | 173 (35.7) | 15.8 (10.9 to 20.6) |
| Pathology report | ||||
| Diameter, mm (n = 40)c | 6.0 (3.0-8.0) | 5.0 (2.0-7.0) | 8.0 (6.0-9.0) | 1.05 (0.94 to 1.17) |
| Diameter, ≥5.0 mm (n = 40) | 995 (62.3) | 559 (50.1) | 436 (90.6) | 40.5 (36.6 to 44.4) |
| Multifocality | 343 (21.0) | 230 (20.0) | 113 (23.3) | 3.3 (–1.1 to 7.7) |
| Bilaterality (n = 10) | 139 (8.5) | 83 (7.2) | 56 (11.7) | 4.5 (1.3 to 7.7) |
| Less than radical resection | 78 (4.8) | 46 (4.0) | 32 (6.6) | 2.6 (0.1 to 5.1) |
| (Extra)capsular invasion of thyroid | 54 (3.3) | 25 (2.2) | 29 (6.0) | 4.4 (2.5 to 6.3) |
| Vascular invasion | 31 (1.9) | 9 (0.8) | 22 (4.5) | 3.7 (1.8 to 5.6) |
| BRAF mutation (n = 1501) | 98 (72.6) | 68 (82.9) | 30 (56.6) | –26.3 (–41.9 to –10.7) |
| Thyroiditis | 252 (15.4) | 196 (17.0) | 56 (11.6) | –5.4 (–9.0 to –1.8) |
| Type of mPTC | ||||
| Classic mPTC | 1329 (81.2) | 906 (78.6) | 423 (87.4) | 8.8 (5.0 to 12.6) |
| FVmPTC | 293 (17.9) | 237 (20.6) | 56 (11.6) | –9.0 (–12.7 to –5.3) |
| Other | 14 (0.9) | 9 (0.8) | 5 (1.0) | 0.2 (–0.8 to 1.2) |
| Metastases | ||||
| N-stage | ||||
| NX/N0 | 1552 (94.9) | 1119 (97.1) | 433 (89.5) | –7.6 (–10.5 to –4.7) |
| N1a | 61 (3.7) | 21 (1.8) | 40 (8.3) | 6.5 (3.9 to 9.1) |
| N1b | 23 (1.4) | 13 (1.1) | 11 (2.3) | 1.2 (–0.3 to 2.7) |
| M-stage (M1) | 1 (0.1) | 1 (0.1) | 0 | –0.1 (–0.3 to 0.1) |
| Recurrence after initial treatment | ||||
| Overall recurrence | 25 (1.5) | 15 (1.3) | 10 (2.1) | 0.8 (–0.5 to 2.5) |
| Lymph node | 15 (0.9) | 8 (0.7) | 7 (1.4) | 0.7 (–0.5 to 1.9) |
| Distant | 0 | 0 | 0 | 0 |
| Local | 10 (0.6) | 7 (0.6) | 3 (0.6) | 0 (–0.8 to 0.8) |
Abbreviations: FVmPTC, follicular variant of mPTC; mPTC, papillary thyroid microcarcinoma; NA, not applicable.
Data are expressed as numbers (percentage) or as median [IQR]; Estimate is expressed as risk difference for dichotomous variables and as standardized mean difference for continuous variables; Missing data are presented in parentheses behind variables.
Concomitant cervical lymph node dissection (CLND); CCLND, Central CLND (level VI); LCLND, Lateral CLND (level II-IV) (total number of dissections).
Certain mPTC but precise diameter unknown.
Follow-up of All 1636 Patients With mPTC
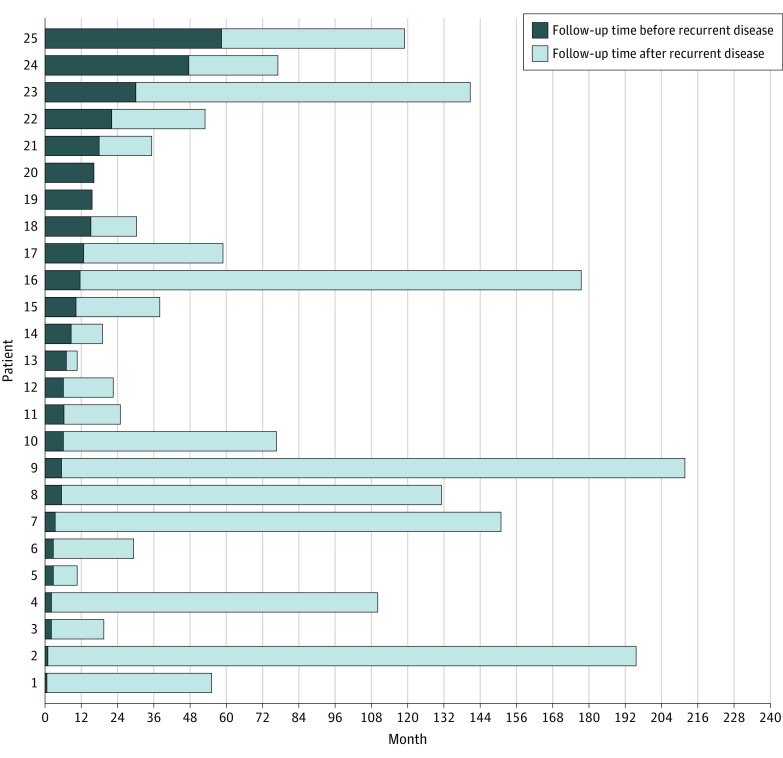
Lymph node metastases were found in 85 (5.2%) of the total cohort. Of these 85 patients, 69 had lymph node involvement during surgery that was not seen preoperatively and were not defined as RD. The percentage of distant metastases was 0.1% (n = 1). Recurrent disease after initial treatment was seen in 25 patients (1.5%), of which there were 15 patients with lymph node metastases, 8 patients with new mPTC in the contralateral lobe, and 2 patients with local RD after total thyroidectomy. The median (IQR) time to RD was 8.2 (3.6-16.5) months. Figure 2 displays the time distribution of all patients with mPTC with RD in the Netherlands. In total, 16 of 25 (64%) patients with RD were found in the first year after follow-up and 22 of 25 (90%) within the first 2 years of follow-up.
Figure 2. Duration of Follow-up and Time of Recurrence of All 25 Patients With Recurrent Disease.
Subgroup analysis showed that patients with nonincidental mPTC had a higher rate of lymph node metastases than patients with incidental mPTC (n = 51 [10.5%] vs n = 34 [3.0%]; difference, 7.6%; 95% CI, 4.9%-10.8%). However, RD was not significantly different between both groups (incidental mPTC, n = 15 [1.3%]; nonincidental mPTC, n = 10 [2.1%]; difference, 0.8%; 95% CI, −0.5% to 2.5%). Of the 484 patients with nonincidental mPTC (group 2), 246 (50.8%) patients were treated with a hemithyroidectomy and follow-up in accordance with Dutch guidelines. Median (IQR) follow-up of this patient group was 37 (18-71) months. Lymph node metastases were found in 1 of 246 (0.4%) patients after hemithyreoidectomy, and a new mPTC in the contralateral thyroid was detected in 3 of 246 (1.2%) patients. All 4 patients underwent completion thyroidectomy after diagnosis of RD. Of the 484 patients with nonincidental mPTC (group 2), 228 (47.1%) patients received total or completion thyroidectomy in accordance with Dutch guidelines. The median (IQR) follow-up of this patient group was 67 (27-111) months. Lymph node metastases were found in 6 of 228 (2.6%). Of these 6 patients, 4 patients received RAI after surgery.
Risk Factors for RD
Univariate Cox proportional hazards model analysis showed that a larger tumor diameter, multifocality, vascular invasion, and (extra)capsular invasion were associated with RD (Table 2). Multivariable Cox proportional hazard model analysis showed that vascular invasion (HR, 12.0; 95% CI, 3.7-30.1) and (extra)capsular invasion (HR, 4.0; 95% CI, 1.3-12.2) were risk factors for RD after initial treatment. The estimated cumulative probability of RD at 1, 3, 5, and 10 years were 0.9%, 1.5%, 1.7%, and 1.7%, respectively. The 10-year estimated cumulative probability of RD was 1.3% for patients without vascular invasion and 24.4% for patients with vascular invasion of the tumor (Figure 3). The total number of patients with RD in the group with vascular invasion was 6, whereas this number was 25 in the group without vascular invasion. Of all 15 patients with lymph node RD, 9 (60%) patients had multifocal disease, 6 (40%) patients had vascular invasion, and 3 (15%) patients had capsular invasion. In total, 4 patients (27%) lacked all of these risk factors described. Overall, 10 of 15 patients with lymph node RD received RAI treatment after initial surgery.
Table 2. Multivariable Cox Proportional Hazards Analysis for Recurrence-Free Survivala.
| Independent variable | Univariate hazard ratio (95% CI) | Independent variable | Multivariable hazard ratio (95% CI) |
|---|---|---|---|
| Age, y | 1.00 (0.97-1.03) | NA | NA |
| Male sex | 1.23 (0.49-3.08) | ||
| Diameter, mm | 1.16 (1.01-1.34) | ||
| Multifocality | 2.52 (1.13-5.62) | ||
| Vascular invasion | 18.51 (7.38-46.41) | Vascular invasion | 12.02 (3.71-30.06) |
| Capsular invasion | 8.39 (3.14-22.41) | Capsular invasion | 4.01 (1.32-12.21) |
| Irradical resection | 2.82 (0.84-9.41) | NA | NA |
| Type of mPTC | |||
| Classic mPTC | 1.70 (0.51-5.68) | ||
| FVmPTC | 1 [Reference] |
Abbreviations: FVmPTC, follicular variant of mPTC; mPTC, papillary thyroid microcarcinoma; NA, not applicable.
Age is expressed in years as a continuous variable; Diameter is expressed in millimeters as a continuous variable. All other independent variables are dichotomous.
Figure 3. Vascular Invasion and Recurrent Disease in All 1636 Patients.
Kaplan-Meier curve of patients with and without vascular invasion. The displayed hazard ratios and 95% CIs are the adjusted estimates from the multivariable Cox proportional hazards analysis.
Discussion
In this study, we described the occurrence and risk factors of RD after surgery in 1636 patients with cN0/cNx mPTC during a 20-year time period in the Netherlands. Overall, RD rates after initial surgical surgery were low (1.5%) and, if RD was detected, a 90% RD rate was found within 2 years of follow-up. The low RD rate in patients without preoperative lymph node metastases demonstrated in this study substantiates findings in other studies. Mehenna et al15 described 2 different entities of mPTC; 1 group with incidental mPTC and 1 group with nonincidental mPTC. In that meta-analysis, nonincidental mPTCs had a significant higher rate of RD than incidentally found mPTCs (7.9% vs 0.5%, respectively). However, in the current study, there was no difference in RD between the groups after initial treatment. Subgroup analysis of patients with nonincidental mPTC receiving a hemithyroidectomy and follow-up showed that the RD rate was 1.6%, similar to the group with incidental mPTC. This is best explained by the fact that patients with preoperative lymph node metastases were excluded in the current study. Another study from Buffet et al16 also showed a low structural recurrence rate (4%) after surgical intervention in 1667 patients with mPTC without preoperative lymph node metastases.
As a result of the low rate of RD shown in the current study, the additional value of follow-up for patients with unifocal intrathyroidal mPTC seems to be low and has to be weighed against the chance of patient anxiousness as a result of false-positive thyroid nodules on ultrasonographic findings during follow-up. In addition, to our knowledge, early detection of RD in patients with mPTC after surgery has never been shown to positively affect prognosis or quality of life. This substantiates the recommendation of the 2014 British Thyroid Association Guideline, which states that patients with low-risk mPTC after hemithyroidectomy require no further follow-up for cancer care and can be discharged to the care of their general practitioner for thyroid function evaluation.24
From a health care perspective, deescalation of follow-up after surgery would contribute to an increased accessibility to health care, which is considered one of the main challenges for the future.25 In addition, deescalating follow-up contributes to lower health care costs due to a decrease in laboratory tests and neck ultrasonography. When compared why active surveillance, Lin et al26 have already suggested that surgery is less costly and more effective than active surveillance for patients with mPTC after 16.2 years. In that study, patients who underwent surgery received 5 years of clinical follow-up with neck ultrasonographic examination. If patients with low-risk mPTC do not remain in follow-up for 5 years, costs would decrease even more. Deescalation of follow-up also comes with more sustainability due to patients travelling less.
Although vascular invasion was not common in patients with mPTC (2%), this determinant showed to be a significant risk factor for the occurrence of RD in the current study. The risk of RD at 5 years after surgery increased to 25% for patients with vascular invasion of the mPTC in the pathology report. This corresponds to findings from several studies in the literature describing vascular invasion as an adverse prognostic factor for RD and survival in patients with papillary thyroid carcinoma.27,28,29 This also substantiates that patients with vascular invasion after hemithyroidectomy could benefit from additional completion thyroidectomy and radioactive iodine and require more extensive follow-up.
Limitations
The results of this study should be addressed with respect to its limitations. First, it is a retrospective registry study and some degree of information bias cannot be ruled out. Second, we did not have access to individual ultrasonography reports. We could not determine if patients with RD in the contralateral lobe after hemithyroidectomy all had negative preoperative ultrasonography results of that contralateral lobe. In addition, we could not determine if patients who had RD in the second year had a negative results on ultrasonography in the first year after surgery. Third, due to a limited number of patients with RD and thus a lack of power, the multivariable cox proportional hazards model did not include all factors significantly related to the outcome. Lastly, disease-specific mortality was not available in the Netherlands Cancer Registry and could not be extracted from the data. Efforts were made to assess these data by combining other national registries.
The current study found that RD after hemithyroidectomy for low-risk mPTC occurred in less than 2% of patients and the 90% of RD occurrences are discovered within the first 2 years. These results suggest that patients could safely refrain from further follow-up after hemithyroidectomy for low-risk mPTC without vascular invasion. However, a one-time follow-up moment with neck ultrasonography after 2 years also seems reasonable. Individual patient engagement through a shared decision-making approach should be conducted to choose the most appropriate follow-up option. Patients with pathologically confirmed vascular invasion should receive more intensive follow-up due to the high chance of RD (25%).
Conclusions
This nationwide retrospective cohort study found that recurrent disease after hemithyroidectomy for patients with unifocal, intrathyroidal mPTC was rare (<2%). Based on the low RD rates in this study, it seems reasonable to deescalate follow-up of patients with low-risk mPTC without vascular invasion after hemithyroidectomy. From a health care perspective, deescalation of follow-up contributes to increased sustainability and accessibility to health care, both large challenges for the future.
References
- 1.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164-2167. doi: 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 2.Leenhardt L, Grosclaude P, Chérié-Challine L; Thyroid Cancer Committee . Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14(12):1056-1060. doi: 10.1089/thy.2004.14.1056 [DOI] [PubMed] [Google Scholar]
- 3.Londero SC, Krogdahl A, Bastholt L, et al. ; Danish Thyroid Cancer Group . Papillary thyroid microcarcinoma in Denmark 1996-2008: a national study of epidemiology and clinical significance. Thyroid. 2013;23(9):1159-1164. doi: 10.1089/thy.2012.0595 [DOI] [PubMed] [Google Scholar]
- 4.Husson O, Haak HR, van Steenbergen LN, et al. Rising incidence, no change in survival and decreasing mortality from thyroid cancer in The Netherlands since 1989. Endocr Relat Cancer. 2013;20(2):263-271. doi: 10.1530/ERC-12-0336 [DOI] [PubMed] [Google Scholar]
- 5.La Vecchia C, Bosetti C, Lucchini F, et al. Cancer mortality in Europe, 2000-2004, and an overview of trends since 1975. Ann Oncol. 2010;21(6):1323-1360. doi: 10.1093/annonc/mdp530 [DOI] [PubMed] [Google Scholar]
- 6.Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L. Worldwide thyroid-cancer epidemic? the increasing impact of overdiagnosis. N Engl J Med. 2016;375(7):614-617. doi: 10.1056/NEJMp1604412 [DOI] [PubMed] [Google Scholar]
- 7.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322. doi: 10.1001/jamaoto.2014.1 [DOI] [PubMed] [Google Scholar]
- 8.Furuya-Kanamori L, Bell KJL, Clark J, Glasziou P, Doi SAR. Prevalence of differentiated thyroid cancer in autopsy studies over six decades: a meta-analysis. J Clin Oncol. 2016;34(30):3672-3679. doi: 10.1200/JCO.2016.67.7419 [DOI] [PubMed] [Google Scholar]
- 9.Kim BW, Yousman W, Wong WX, Cheng C, McAninch EA. Less is more: comparing the 2015 and 2009 American Thyroid Association Guidelines for thyroid nodules and cancer. Thyroid. 2016;26(6):759-764. doi: 10.1089/thy.2016.0068 [DOI] [PubMed] [Google Scholar]
- 10.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(7):709-711. doi: 10.1001/jamaoto.2016.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Megwalu UC, Moon PK. Thyroid cancer incidence and mortality trends in the United States: 2000-2018. Thyroid. 2022;32(5):560-570. doi: 10.1089/thy.2021.0662 [DOI] [PubMed] [Google Scholar]
- 13.Lončar I, van Dijk SPJ, Metman MJH, et al. Active surveillance for papillary thyroid microcarcinoma in a population with restrictive diagnostic work-up strategies. Thyroid. 2021;31(8):1219-1225. doi: 10.1089/thy.2020.0845 [DOI] [PubMed] [Google Scholar]
- 14.Links T. Richtlijn voor de diagnostiek, behandeling en follow-up van patiënten met gedifferentieerd (niet-medullair) schildkliercarcinoom [2015]. Updated February 16, 2015. www.nhg.org/sites/default/files/content/nhg_org/uploads/schildkliercarcinoom.pdf
- 15.Mehanna H, Al-Maqbili T, Carter B, et al. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014;99(8):2834-2843. doi: 10.1210/jc.2013-2118 [DOI] [PubMed] [Google Scholar]
- 16.Buffet C, Golmard JL, Hoang C, et al. Scoring system for predicting recurrences in patients with papillary thyroid microcarcinoma. Eur J Endocrinol. 2012;167(2):267-275. doi: 10.1530/EJE-12-0105 [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene FL. AJCC cancer staging manual. Springer; New York; 2010. [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. doi: 10.1136/bmj.39335.541782.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellis PD. Contents. In: Ellis PD, ed. The Essential Guide to Effect Sizes: Statistical Power, Meta-Analysis, and the Interpretation of Research Results. Cambridge University Press; 2010:vii-viii. [Google Scholar]
- 20.Ryu YJ, Cho JS, Yoon JH, Park MH. Identifying risk factors for recurrence of papillary thyroid cancer in patients who underwent modified radical neck dissection. World J Surg Oncol. 2018;16(1):205. doi: 10.1186/s12957-018-1496-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y, Roh J-L, Gong G, et al. Risk factors for lateral neck recurrence of N0/N1a papillary thyroid cancer. Ann Surg Oncol. 2017;24(12):3609-3616. doi: 10.1245/s10434-017-6057-2 [DOI] [PubMed] [Google Scholar]
- 22.Wreesmann VB, Nixon IJ, Rivera M, Katabi N, Palmer F, Ganly I, et al. Prognostic value of vascular invasion in well-differentiated papillary thyroid carcinoma. Thyroid. 2015;25(5):503-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel MK, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. Int J Ayurveda Res. 2010;1(4):274-278. doi: 10.4103/0974-7788.76794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perros P, Boelaert K, Colley S, et al. ; British Thyroid Association . Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(s1)(suppl 1):1-122. doi: 10.1111/cen.12515 [DOI] [PubMed] [Google Scholar]
- 25.Heath S. 2022. https://patientengagementhit.com/news/top-challenges-impacting-patient-access-to-healthcare
- 26.Lin JF, Jonker PKC, Cunich M, et al. Surgery alone for papillary thyroid microcarcinoma is less costly and more effective than long term active surveillance. Surgery. 2020;167(1):110-116. doi: 10.1016/j.surg.2019.05.078 [DOI] [PubMed] [Google Scholar]
- 27.Gardner RE, Tuttle RM, Burman KD, et al. Prognostic importance of vascular invasion in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2000;126(3):309-312. doi: 10.1001/archotol.126.3.309 [DOI] [PubMed] [Google Scholar]
- 28.Vuong HG, Kondo T, Duong UNP, et al. Prognostic impact of vascular invasion in differentiated thyroid carcinoma: a systematic review and meta-analysis. Eur J Endocrinol. 2017;177(2):207-216. doi: 10.1530/EJE-17-0260 [DOI] [PubMed] [Google Scholar]
- 29.Pontius LN, Youngwirth LM, Thomas SM, Scheri RP, Roman SA, Sosa JA. Lymphovascular invasion is associated with survival for papillary thyroid cancer. Endocr Relat Cancer. 2016;23(7):555-562. doi: 10.1530/ERC-16-0123 [DOI] [PubMed] [Google Scholar]