Key Points
Question
Is the Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) tool valid for guiding skin cancer screening in solid organ transplant recipients (SOTRs)?
Findings
This prognostic study found good prognostic discrimination in a European cohort of 3421 SOTRs. The observed skin cancer incidences were similar to those predicted from the US SOTR population for each risk group.
Meaning
These findings suggest that the SUNTRAC tool can be transported to different populations to stratify SOTRs into distinct skin cancer risk groups and identify those at a very high risk, opening the door to efficient and effective preventive measures.
This prognostic study assesses the Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) tool to validate it in different populations of solid organ transplant recipients.
Abstract
Importance
The Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) tool has been developed in the US to facilitate the identification of solid organ transplant recipients (SOTRs) at a higher risk of developing skin cancer. However, it has not yet been validated in populations other than the one used for its creation.
Objective
To provide an external validation of the SUNTRAC tool in different SOTR populations.
Design, Setting, and Participants
This retrospective external validation prognostic study used data from a prospectively collected cohort of European SOTRs from transplant centers at teaching hospitals in the Netherlands (1995-2016) and Spain (2011-2021). Participants were screened and followed up at dermatology departments. Data were analyzed from September to October 2021.
Main Outcomes and Measures
The discrimination ability of the SUNTRAC tool was assessed via a competing risk survival analysis, cumulative incidence plots, and Wolbers concordance index. Calibration of the SUNTRAC tool was assessed through comparison of projected skin cancer incidences. Skin cancer diagnoses included squamous cell carcinoma, basal cell carcinoma, melanoma, and Merkel cell carcinoma.
Results
A total of 3421 SOTRs (median age at transplant, 53 [quartile 1: 42; quartile 3: 62] years; 2132 [62.3%] men) were assessed, including 72 Asian patients (2.1%), 137 Black patients (4.0%), 275 Latinx patients (8.0%), 109 Middle Eastern and North African patients (3.2%), and 2828 White patients (82.7%). With a total of 23 213 years of follow-up time, 603 patients developed skin cancer. The SUNTRAC tool classified patients into 4 groups with significantly different risks of developing skin cancer during follow-up. Overall, the relative rate for developing skin cancer estimated using subdistribution hazard ratios (SHRs) and using the low-risk group as the reference group, increased according to the proposed risk group (medium-risk group: SHR, 6.8 [95% CI, 3.8-12.1]; P < .001; high-risk group: SHR, 15.9 [95% CI, 8.9-28.4]; P < .001; very-high–risk group: SHR, 54.8 [95% CI, 29.1-102.9]; P < .001), with a concordance index of 0.72. Actual skin cancer incidences were similar to those predicted by the SUNTRAC tool (5-year skin cancer cumulative incidence for medium-risk group: predicted, 6.2%; observed, 7.0%).
Conclusions and Relevance
The findings of this external validation prognostic study support the use of the SUNTRAC tool in European populations for stratifying SOTRs based on their skin cancer risk and also detecting patients at a high risk of developing skin cancer. This can be helpful in prioritizing and providing better screening and surveillance for these patients.
Introduction
Between 14% and 37.5% of solid organ transplant recipients (SOTRs) will develop skin cancer within 10 years of transplantation.1 Among skin malignant neoplasms, cutaneous squamous cell carcinoma (cSCC), basal cell carcinoma (BCC), melanoma, and Merkel cell carcinoma (MCC) are the most frequently observed. Due to its higher incidence and aggressive behavior in the SOTR population, cSCC is often the most concerning skin malignant neoplasm. In particular, cSCC can grow rapidly and metastasize, leading to immunosuppression regimen changes that may compromise graft survival, mutilating surgical treatments, or even death.
Prevention and early detection are key factors to improve outcomes associated with these tumors.2,3 A 2019 expert consensus guideline recommended that dermatological screening should be carried out at different times depending on the estimated skin cancer risk of the target population and that risk assessment should be performed with the aid of an evidence-based risk stratification tool.3
Several skin cancer risk stratification instruments have been developed for the SOTR population.4,5,6 These scores have common variables, such as age, race, or skin phototype, but none was widely used or had large population-based studies backing their validity or usability.4
In 2019, Jambusaria-Pahlajani et al7 proposed the Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) as an easy-to-use screening tool to stratify SOTRs according to their skin cancer risk. This tool considers 5 variables: sex, race, age at transplantation, pretransplant history of skin cancer, and type of transplant, which had been identified as risk factors in a large US-based multicenter study.8 The SUNTRAC tool uses an additive scoring system that classifies patients into 4 risk groups, achieving good prognostic discrimination in the Transplant Skin Cancer Network (TSCN) population study. Jambusaria-Pahlajani et al7 recommend, based on results from a Delphi consensus guideline,3 optimal screening times for each risk group. However, external validation in an independent population is often considered an essential prerequisite for a screening tool before entering clinical practice.9,10 To our knowledge, there are no other published studies evaluating the validity of the SUNTRAC screening tool in an independent sample. We present the results of an external validation study evaluating the performance of the SUNTRAC tool in a large European SOTR cohort.
Methods
The use of the clinical databases for this prognostic external validation study was approved by the hospital ethics committees of each participating institution. Participants provided written informed consent to be included in these prospective clinical databases. This study is reported following the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.
Study Populations
We assessed the transportability of the SUNTRAC tool in a diverse European population; the Netherlands and Spain, 2 countries with significantly different latitudes and well-known differences in skin cancer incidences, were selected to create our European validation cohort.11 We included retrospective data from 3654 patients from 2 ongoing cohorts of SOTRs from the Netherlands and Spain. The Dutch cohort is comprised of 2599 patients who received a kidney or pancreatic transplant between 1995 and 2016, while the Spanish cohort is comprised of 1055 patients who received different types of solid organ grafts (ie, lung, kidney, liver, heart, or pancreas) between 2011 and 2021. Data were derived from university hospitals carrying out dermatological screening visits and follow-ups of SOTRs in Leiden, the Netherlands (52°10′N 4°29′E), and Barcelona, Spain (41°23′N 2°11′E). Information was registered prospectively at the time of transplantation and during follow-up visits. Race and ethnicity of the patients were coded by C.F.P. in Spain and J.B.B. in the Netherlands after asking for the patient’s country of origin and ethnic origin during clinical interview and after clinical examination for their skin phototype and phenotypic traits. Race and ethnicity were categorized as Asian, Black, Latinx, Middle Eastern and North African, and White. Multiracial patients were classified under groups other than White race. First skin cancer event after transplant diagnoses were based on histopathological diagnosis of cSCC, BCC, MCC or melanoma by board-certified pathologists. As the SUNTRAC tool was intended for the adult SOTR population, pediatric patients were omitted, as well as patients with missing information to compute their SUNTRAC score or missing date of skin cancer diagnosis.
The reference or derivation cohort (ie, the population used to generate the SUNTRAC tool) included 6340 patients from the TSCN multicenter study across 26 centers in the US, with a median latitude of 40°N. These patients received a solid organ transplant either in 2003 or 2008. Most of them were men (63.8%), were White race (69.4%), had a median age at transplant of 53 years, and had received a kidney transplant (52.3%). The skin cancer outcome for this cohort included the diagnosis of the first cSCC (91.3%), first melanoma (8.5%), or first MCC (0.2%) but did not consider BCC as a skin cancer outcome. Further details on the derivation cohort have been published elsewhere.7,8,12
Statistical Analysis
Descriptive statistics were computed as customary.13 If variables were nonparametric, nonparametric tests were used. We calculated bivariate comparisons by different clinical and demographic characteristics and by SUNTRAC group. The Kruskal-Wallis rank sum test was used to assess statistically significant differences for quantitative variables. Fisher exact test was used to ascertain differences in proportions.
SUNTRAC scores were computed at the time of transplant, and risk groups were assigned as specified by Jambusaria-Pahlajani et al.7 SUNTRAC scores were calculated using the original scoring (White race = 9 points; pretransplant history of skin cancer = 6 points; age ≥50 years = 4 points; male sex = 2 points; thoracic organ transplant (heart or lung) = 1 point). We then assigned the patients to their corresponding risk group depending on their total SUNTRAC score at the time of transplant (low risk: 0-6 points; medium risk: 7-13 points; high risk: 14-17 points; very high risk:18-22 points). Patients were considered to have a history of skin cancer if they had a registered pretransplant diagnosis of cSCC, BCC, melanoma, or MCC.
We carried out a competing risk survival analysis where the event of interest was the first skin cancer occurrence after transplantation, while death was considered the competing event. A competing event prevents the development or observation of the event of interest in a study population. Due to the higher mortality experienced by transplant recipients, accounting for competing events is usually recommended to provide accurate estimations on the probability of the event of interest.14 To evaluate the power of discrimination of the tool, cumulative incidence functions were plotted to assess incidence of skin cancer by SUNTRAC group. Following the methods used to create the SUNTRAC tool, we computed unadjusted subdistribution hazard ratios (SHRs) and their 95% CIs via a Fine-Gray subdistribution hazard model for every SUNTRAC group and the risk factors included in the tool.15 Predictive performance was evaluated by computing Wolbers concordance index (C-index) and by calculating time-dependent areas under the receiving operator characteristic curves (t-AUROCs) over time and truncated at 5 years after transplant.14,16,17 The C-index indicates the overall discrimination ability of the model by ranking the expected survival times based on the risk attributed to each individual; a C-index of 0.5 indicates a random prediction, while a C-index of 1 would indicate perfect discrimination power.14
In our study, we focused on assessing the discrimination power of the tool, as models can always be recalibrated to provide accurate expected probabilities. Nonetheless, we assessed calibration by visual comparison of cumulative incidence functions and by comparing 5-year cumulative incidences of skin cancer in our cohort with those reported in the TSCN cohort.
We did not carry out formal sample size estimations, as these methods are not well established for validating prognostic scores. However, we included more than half the participants included in the TSCN cohort with a higher event rate than in the derivation cohort (17.6% vs 13.6%). We also performed sensitivity analyses in which BCC was not considered as a skin cancer outcome without resulting in major changes to our estimates or overall conclusions. All tests were 2-tailed, and the level of significance was set at P < .05. All statistical analyses were conducted with R statistical software version 3.6.1 (R Project for Statistical Computing) with the following main additional packages: “survival,” “cmprsk,” “prodlim,” “pec,” “maxstat,” and “rpart.” Data were analyzed from September to October 2021.
Results
Cohort Characteristics
We included 3421 patients in our validation cohort (2132 [62.3%] men; median age at transplant, 53 [quartile 1: 42; quartile 3: 62] years), of whom 603 (17.6%) developed skin cancer within a median follow-up of 5.7 (quartile 1: 2.7; quartile 3: 9.4) years after transplantation, with 23 213 patient-years of contributed follow-up (Table 1). There were 72 Asian patients (2.1%), 137 Black patients (4.0%), 275 Latinx patients (8.0%), 109 Middle Eastern and North African patients (3.2%), and 2828 White patients (82.7%). Detailed information on the clinical and demographic characteristics of the validation cohort stratified by country are presented in Table 1. Significant differences regarding median age at transplant, race and ethnicity distribution, type of transplant, and posttransplant skin cancer rates were found by country, while pretransplant skin cancer history percentages were almost equal. Median SUNTRAC scores differed by country, with a significantly higher median score in patients from Spain, but a larger spread of scores was observed in the Dutch cohort (Table 1; eFigure 1 in the Supplement). Accordingly, the distribution of patients among the SUNTRAC risk groups was quite different, except for the very-high–risk group who displayed almost equal percentages (Table 1; eFigure 1 in the Supplement). Age, sex, and other SUNTRAC risk factors were distributed unevenly across SUNTRAC groups (eTable 1 and eFigure 1 in the Supplement).
Table 1. Clinical and Demographic Characteristics of the Validation Cohort by Country and Overall.
| Characteristic | Patients, No. (%) | P valuea | ||
|---|---|---|---|---|
| Spain (n = 1046) | The Netherlands (n = 2375) | Overall (N = 3421) | ||
| Sex | ||||
| Women | 371 (35.5) | 918 (38.7) | 1289 (37.7) | .08b |
| Men | 675 (64.5) | 1457 (61.3) | 2132 (62.3) | |
| Age at transplant, y | ||||
| Median (Q1-Q3) | 57.0 (48.0-63.0) | 51.0 (40.5-61.0) | 53.0 (42.0-62.0) | <.001c |
| <50 | 296 (28.3) | 1117 (47.0) | 1413 (41.3) | <.001b |
| ≥50 | 750 (71.7) | 1258 (53.0) | 2008 (58.7) | |
| Race and ethnicity | ||||
| Asian | 7 (0.7) | 65 (2.7) | 72 (2.1) | <.001b |
| Black | 15 (1.4) | 122 (5.1) | 137 (4.0) | |
| Latinx | 51 (4.9) | 224 (9.4) | 275 (8.0) | |
| Middle Eastern and North African | 31 (3.0) | 78 (3.3) | 109 (3.2) | |
| White | 942 (90.1) | 1886 (79.4) | 2828 (82.7) | |
| Type of transplant | ||||
| Kidney | 443 (42.4) | 1976 (83.2) | 2419 (70.7) | <.001b |
| Kidney and liver | 9 (0.9) | 14 (0.6) | 23 (0.7) | |
| Kidney and pancreas | 0 | 352 (14.8) | 352 (10.3) | |
| Liver | 143 (13.7) | 0 | 143 (4.2) | |
| Pancreas | 0 | 33 (1.4) | 33 (1.0) | |
| Single-lung | 156 (14.9) | 0 | 156 (4.6) | |
| Double-lung | 293 (28.0) | 0 | 293 (8.6) | |
| Heart and lung | 1 (0.1) | 0 | 1 (<0.1) | |
| Heart | 1 (0.1) | 0 | 1 (<0.1) | |
| Abdominal or thoracic transplant | ||||
| Abdominal | 596 (57.0) | 2375 (100) | 2971 (86.8) | <.001b |
| Thoracic | 450 (43.0) | 0 | 450 (13.2) | |
| Pretransplant history of skin cancer | ||||
| No | 1008 (96.4) | 2288 (96.3) | 3296 (96.3) | 1.00b |
| Yes | 38 (3.6) | 87 (3.7) | 125 (3.7) | |
| SUNTRAC score, median (Q1-Q3), points | 14.0 (11.0-15.0) | 11.0 (9.0-15.0) | 13.0 (9.0-15.0) | <.001d |
| SUNTRAC risk group | ||||
| Low | 93 (8.9) | 487 (20.5) | 580 (17.0) | <.001d |
| Medium | 352 (33.7) | 1221 (51.4) | 1573 (46.0) | |
| High | 566 (54.1) | 587 (24.7) | 1153 (33.7) | |
| Very high | 35 (3.3) | 80 (3.4) | 115 (3.4) | |
| Follow-up time, median (Q1-Q3), y | 3.8 (2.1-6.7) | 6.7 (3.4-11.3) | 5.7 (2.7-9.4) | <.00c |
| Skin cancer after transplant | ||||
| No | 891 (85.2) | 1927 (81.1) | 2818 (82.4) | .004b |
| Yes | 155 (14.8) | 448 (18.9) | 603 (17.6) | |
| Type of skin cancer (first event)e | ||||
| Basal cell carcinoma | 59 (38.1) | 252 (56.2) | 311 (51.6) | <.001b |
| Squamous cell carcinoma, cutaneous | 90 (58.1) | 183 (40.8) | 273 (45.3) | |
| Melanoma, cutaneous | 6 (3.9) | 13 (2.9) | 19 (3.2) | |
| Merkel cell carcinoma | 0 | 0 | 0 | |
Abbreviations: Q, quartile; SUNTRAC, Skin and UV Neoplasia Transplant Risk Assessment Calculator.
P values from tests comparing Spain vs the Netherlands.
Fisher exact test for count data.
Kruskal-Wallis rank sum test.
Trend test for ordinal variables.
Percentages calculated for those who developed a skin cancer event.
Cumulative skin cancer incidences were 2.1% (95% CI, 1.7%-2.7%) at 1 year and 12.1% (95% CI, 11.0%-13.4%) at 5 years. Patients who developed malignant neoplasms after transplant were more commonly men, aged 50 years or older, were White, had history of pretransplant skin cancer, had higher SUNTRAC total scores, and belonged to higher SUNTRAC risk groups (Table 2).
Table 2. Clinical and Demographic Characteristics by Skin Cancer Outcome After Transplant, by Country, and Overall.
| Characteristic | Spain (n = 1046) | The Netherlands (n = 2375) | Overall (N = 3421) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients, No. (%) | P value | Patients, No. (%) | P value | Patients, No. (%) | P value | ||||
| No skin cancer (n = 891) | Skin cancer (n = 155) | No skin cancer (n = 1927) | Skin cancer (n = 786) | No skin cancer (n = 2818) | Skin cancer (n = 603) | ||||
| Sex | |||||||||
| Women | 331 (37.1) | 40 (25.8) | .006a | 780 (40.5) | 138 (30.8) | <.001a | 1111 (39.4) | 178 (29.5) | <.001a |
| Men | 560 (62.9) | 115 (74.2) | 1147 (59.5) | 310 (69.2) | 1707 (60.6) | 425 (70.5) | |||
| Age at transplant, y | |||||||||
| Median (Q1-Q3) | 56.0 (46.0-63.0) | 60.0 (55.0-67.0) | <.001b | 49.0 (39.0-59.5) | 58.0 (48.0-66.0) | <.001b | 51.0 (41.0-61.0) | 58.0 (50.0-66.0) | <.001b |
| <50 | 278 (31.2) | 18 (11.6) | <.001a | 996 (51.7) | 121 (27.0) | <.001a | 1274 (45.2) | 139 (23.1) | <.001a |
| ≥50 | 613 (68.8) | 137 (88.4) | 931 (48.3) | 327 (73.0) | 1544 (54.8) | 464 (76.9) | |||
| Race and ethnicity | |||||||||
| Asian | 7 (0.8) | 0 | <.001a | 62 (3.2) | 3 (0.7) | <.001a | 69 (2.4) | 3 (0.5) | <.001a |
| Black | 15 (1.7) | 0 | 119 (6.2) | 3 (0.7) | 134 (4.8) | 3 (0.5) | |||
| Latinx | 50 (5.6) | 1 (0.6) | 219 (11.4) | 5 (1.1) | 269 (9.5) | 6 (1.0) | |||
| Middle Eastern and North African | 31 (3.5) | 0 | 76 (3.9) | 2 (0.4) | 107 (3.8) | 2 (0.3) | |||
| White | 788 (88.4) | 154 (99.4) | 1451 (75.3) | 435 (97.1) | 2239 (79.5) | 589 (97.7) | |||
| Type of transplant | |||||||||
| Abdominal | 491 (55.1) | 105 (67.7) | .004a | 1927 (100) | 448 (100) | NA | 2418 (85.8) | 553 (91.7) | <.001a |
| Thoracic | 400 (44.9) | 50 (32.3) | 0 | 0 | 400 (14.2) | 50 (8.3) | |||
| Pretransplant history of skin cancer | |||||||||
| No | 866 (97.2) | 142 (91.6) | .002a | 1898 (98.5) | 390 (87.1) | <.001a | 2764 (98.1) | 532 (88.2) | <.001a |
| Yes | 25 (2.8) | 13 (8.4) | 29 (1.5) | 58 (12.9) | 54 (1.9) | 71 (11.8) | |||
| SUNTRAC score, median (Q1-Q3), points | 14.0 (11.0-15.0) | 15.0 (14.0-15.0) | <.001b | 11.0 (9.0-13.0) | 15.0 (11.0-15.0) | <.001b | 11.0 (9.0-15.0) | 15.0 (13.0-15.0) | <.001b |
| SUNTRAC group | |||||||||
| Low risk | 92 (10.3) | 1 (0.6) | <.001c | 476 (24.7) | 11 (2.5) | <.001c | 568 (20.2) | 12 (2.0) | <.001c |
| Medium risk | 321 (36.0) | 31 (20.0) | 1026 (53.2) | 195 (43.5) | 1347 (47.8) | 226 (37.5) | |||
| High risk | 456 (51.2) | 110 (71.0) | 398 (20.7) | 189 (42.2) | 854 (30.3) | 299 (49.6) | |||
| Very high risk | 22 (2.5) | 13 (8.4) | 27 (1.4) | 53 (11.8) | 49 (1.7) | 66 (10.9) | |||
Abbreviations: NA, not applicable; Q, quartile; SUNTRAC, Skin and UV Neoplasia Transplant Risk Assessment Calculator.
Fisher exact test for count data.
Kruskal-Wallis rank sum test.
Trend test for ordinal variables.
All SUNTRAC variables, except for type of transplant, were statistically significant risk factors associated with developing skin cancer (Table 3). White race and previous history of skin cancer were the most relevant risk factors, and most of the variables displayed very similar SHRs to those reported in the TSCN study and were used to determine the points for each variable (Table 3). On the other hand, thoracic transplant was not associated with an increased risk of skin cancer. We also tested the validity of the dichotomization of the age variable at 50 years and found that in our cohort, the most discriminative cut point would be at age 53 years from a survival tree model or at age 52 years via a maximally selected rank statistic method.18 The country of origin was not an independent risk factor associated with developing skin cancer after adjusting for the variables in the SUNTRAC tool.
Table 3. Risk of Skin Cancer for the SUNTRAC Items by Cohort.
| SUNTRAC Item | SHR (95% CI)a | SUNTRAC points, No.b | |||
|---|---|---|---|---|---|
| Spain (n = 1046) | The Netherlands (n = 2375) | Validation Cohort (n = 3421) | TSCN cohort (n = 6340) | ||
| White race | 11.48 (1.63-80.94) | 8.07 (4.66-14.00) | 8.38 (4.95-14.21) | 8.78 (6.05-12.76) | 9 |
| Pretransplant skin cancer | 2.66 (1.46-4.85) | 4.67 (3.29-6.64) | 4.02 (3-5.38) | 4.59 (3.45-6.1) | 6 |
| Age ≥50 y at transplant | 2.59 (1.58-4.25) | 2.63 (2.13-3.24) | 2.68 (2.21-3.23) | 2.46 (2.03-2.98) | 4 |
| Male sex | 1.46 (1.02-2.09) | 1.49 (1.21-1.83) | 1.47 (1.23-1.75) | 1.53 (1.29-1.82) | 2 |
| Thoracic transplant | 0.62 (0.44-0.87) | NA | 0.60 (0.45-0.82) | 1.28 (1.08-1.53) | 1 |
Abbreviations: NA, not applicable; SHR, subdistribution hazard ratio; SUNTRAC, Skin and UV Neoplasia Risk Assessment Calculator; TSCN, Transplant Skin Cancer Network.
SHRs and their 95% CIs are calculated from a multivariate competing risk regression by country and overall and those reported in the TSCN cohort for the SUNTRAC items.
Corresponding points for risk factors included in the SUNTRAC tool.
Discrimination Ability
We verified that SUNTRAC scores were associated with an increased risk of skin cancer and found that a 1-point increase in the SUNTRAC score was associated with a 25% increase in the rate of skin cancer (SHR, 1.25 [95% CI, 1.22-1.28]; P < .001). By replicating the methods used to determine the cut points in the total score defining the original SUNTRAC risk groups, we found that a 4-tier classification system also yielded the best prognostic discrimination in our cohort. The optimal cut points in our cohort aligned almost perfectly with those from the original SUNTRAC tool, with a 1-point offset for the first 3 groups and the exact same cut point for the very-high–risk group (eTable 2 in the Supplement).
We found statistically significant differences in the percentage of skin cancer across SUNTRAC groups, with higher percentages in the higher-risk groups but no differences in the distribution of the different types of skin cancer between SUNTRAC risk groups (eTable 1 in the Supplement).
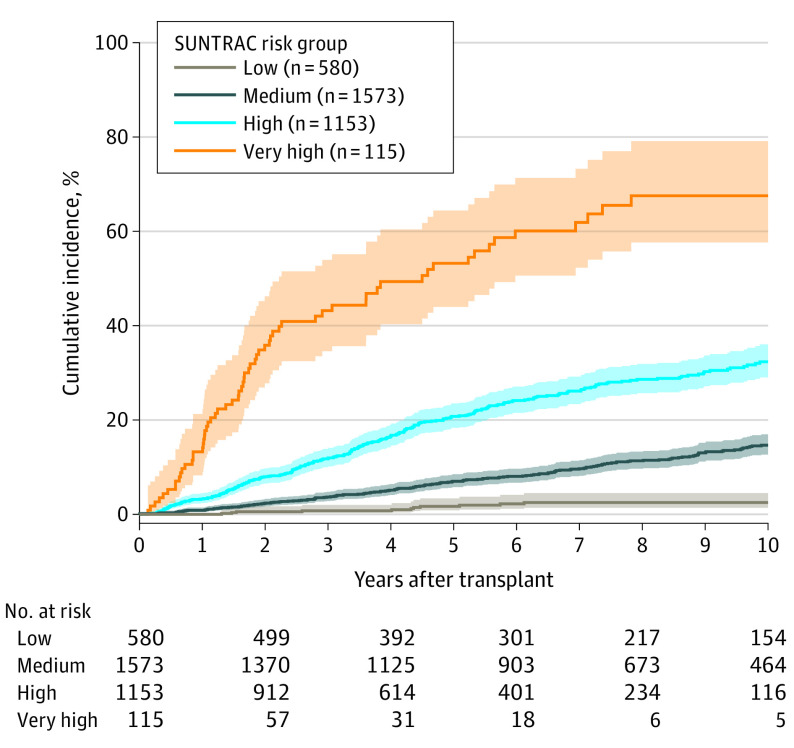
Cumulative incidences of skin cancer by SUNTRAC group are displayed in Figure 1. Higher-risk groups displayed higher skin cancer incidences at all times, with wide separation between curves. By country, the SUNTRAC tool achieved greater discrimination in the Dutch cohort than in the Spanish cohort (eFigure 2 in the Supplement).
Figure 1. Cumulative Incidence of Skin Cancer After Solid Organ Transplantation by Skin and UV Neoplasia Transplant Risk Assessment Calculator (SUNTRAC) Risk Group in Validation Cohort.
To further assess the discrimination ability, a Fine-Gray subdistribution hazard model was fitted with the SUNTRAC group as single covariate. Significantly higher skin cancer rates were found for each increase in SUNTRAC group compared with the low-risk group (medium-risk group: SHR, 6.8 [95% CI, 3.8-12.1]; P < .001; high-risk group: SHR, 15.9 [95% CI, 8.9-28.4]; P < .001; very-high–risk group: SHR, 54.8 [95% CI, 29.1-102.9]; P < .001). Wolbers C-index at 5 years was 0.72 in our validation cohort, whereas the reported C-index in the TSCN cohort was 0.74 (eFigure 3 in the Supplement). Greater power of discrimination was found at 5 years after transplant for the Dutch cohort (t-AUROC, 0.75 [95% CI, 0.72-0.79]) than for the Spanish cohort (t-AUROC, 0.64 [95% CI, 0.59-0.68]). We also computed t-AUROCs of the tool at different time points eliciting quite stable performance over time (eFigure 3 in the Supplement).
Calibration Assessment
To evaluate the concordance in expected skin cancer incidences, we compared cumulative incidence curves and 5-year skin cancer cumulative incidences between the validation cohort and the TSCN cohort (Figure 2). We found quite similar predicted skin cancer incidences, with lower percentages in the TSCN cohort at 5 years after transplant (Figure 2B).
Figure 2. Calibration Assessment.
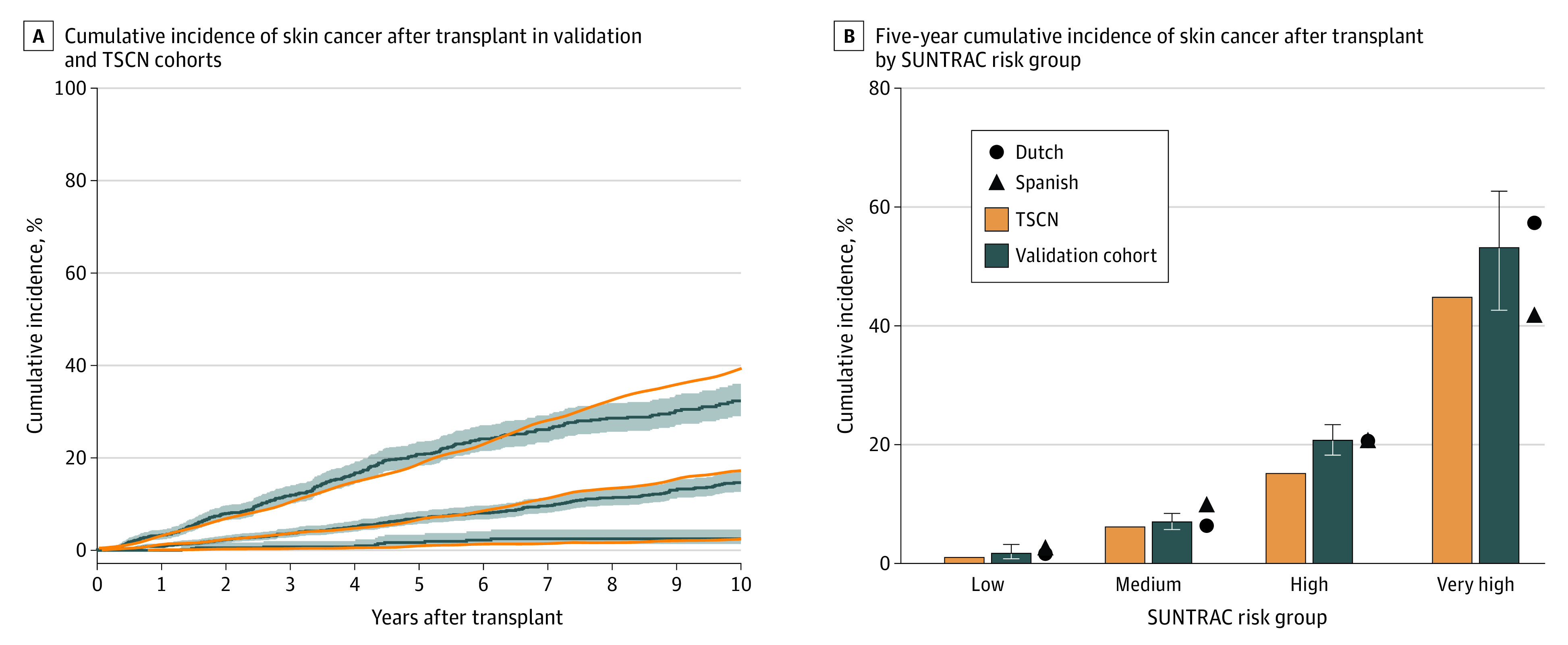
A, shaded areas indicate 95% CI. SUNTRAC indicates Skin and UV Neoplasia Transplant Risk Assessment Calculator; TSCN, Transplant Skin Cancer Network.
Dermatological screening times have been proposed for each SUNTRAC group.7 We assessed whether those times (6 months and 1, 2, and 10 years) were adequate in the European SOTR cohort and found that patients reached the 2% cumulative incidence threshold at similar time points (3 months, 7 months, 2 and 6 years) (Figure 1).
Discussion
This prognostic external validation study focused on validating the use of the SUNTRAC tool in predicting skin cancer in a large European cohort comprised of SOTRs from 2 countries with known differences in skin cancer risk.11 Due to the recent publication of the SUNTRAC tool, our study was retrospective but based on prospectively gathered data.
Skin cancer screening in the SOTR population is a practice recommended by several clinical practice guidelines and the American Society of Transplantation.19,20,21 Most guidelines recommend annual screening for skin malignant neoplasms, and some recommend skin cancer risk stratification.3,19 The SUNTRAC tool has emerged as a risk prediction instrument that could guide this screening while being quick to implement and having the ability to be administered by office staff.7
Although cancer incidences varied between countries, the SUNTRAC tool was able to identify patients at a high risk of developing posttransplant skin cancer. The SUNTRAC tool assigned most patients to the medium-risk category in the Dutch cohort, while most recipients in the Spanish cohort were considered high risk. These findings suggests that the SUNTRAC tool may prove valuable for detecting patients with high risk and referring them to a dermatologist within 6 months of transplant or even assessing them before transplantation.
Compared with the TSCN cohort, there were fewer patients in the low-risk group, as White race is the main risk factor adding 9 points and the score range for the low-risk group is from 0 to 6 points. This uneven distribution among groups will be found in other countries depending on their racial and ethnic mix. Some experts advise caution on considering transplant recipients who are not White (particularly Black individuals of sub-Saharan African descent) as being at low risk for skin cancer, as they might have a higher risk of developing Kaposi sarcoma, a skin cancer end point not considered in the development of the SUNTRAC tool nor in our external validation.22
Even though the Dutch cohort was solely comprised of patients who had received kidney transplants, we found the best overall discrimination in this cohort. This finding could be related to differences in race and age distribution between cohorts, suggesting a higher discriminative ability in countries with more racially diverse populations and with almost equal percentage of people younger than 50 years and those aged 50 years and older. Another explanation for these differences might be residual confounding due to the categorization of the patients into just 4 risk groups.
The points assigned to the variables in the SUNTRAC tool were very similar to those that would be optimal for our cohort, suggesting that the selected variables are indeed relevant risk factors with similar relative contribution to the development of skin cancer in European populations. In our study, having a thoracic transplant was not associated with an increased risk of developing skin cancer. This may be due to residual confounding (since all thoracic transplant recipients were from the Spanish cohort), due to selection bias, or possibly due to differences in immunosuppressive regimens compared with the TSCN cohort. Nonetheless, we found a fairly good prognostic discrimination just below that attained in the derivation cohort.
Regarding calibration, we observed very similar skin cancer incidences to those predicted by the SUNTRAC tool. This finding seems to support the ability of the SUNTRAC tool to recommend fixed time intervals for a first dermatological screening. However, this tool does not explicitly provide orientation on the follow-up intervals after the first dermatological screening. Such a tool ideally would integrate information on posttransplant skin cancer events and immunosuppressive or other photosensitizing medications, among other time-varying clinical variables.4,5,6 While a study by Urwin et al5 developed a skin cancer risk prediction tool for patients who had received kidney transplants and offered follow-up intervals, the SUNTRAC tool provides the clinician with an intuitive skin cancer risk measure to adjust future follow-up visits based on basal skin cancer risk, regardless of the type of organ transplant and with an easy implementation. Recent attempts at incorporating genetic information to skin cancer risk prediction in SOTRs have yielded marginal benefits over just using clinical information suggesting that tools based on clinical variables are still current.23,24 Prospective randomized clinical trials would be desirable to fully assess the effect of the SUNTRAC tool in transplant centers.
Limitations
This study has some limitations, including bias arising from its retrospective design, differences in study time periods and immunosuppressive regimens, incompleteness of cancer registration, and from selecting only 2 European countries. In spite of these limitations, our study had several strengths, such as a generous sample size and the inclusion of BCC diagnoses, a tumor that was not considered in the original TSCN cohort.
Conclusions
The findings of this prognostic external validation study suggest that the SUNTRAC tool was a useful instrument to stratify SOTRs into skin cancer risk groups and provides fairly accurate cumulative skin cancer incidences in populations different from the TSCN study. Having a tool that can quickly and correctly stratify SOTRs according to their relative skin cancer risk is a great aid for the clinician and a fundamental step in defining guidelines to ensure adequate screening and dermatological follow-up of these patients.
eTable 1. Clinical and Demographic Characteristics of the Validation Cohort by Skin and Ultraviolet Neoplasia Risk Assessment Calculator (SUNTRAC) Risk Group
eTable 2. Subdistribution Hazard Ratios (SHR) and Their 95% CIs From a Univariate Competing Risk Regression Analysis Including SUNTRAC Group as Predictor in Validation Cohort and in the TSCN Study
eFigure 1. Density Plots Displaying SUNTRAC Score Distribution in Validation Cohort
eFigure 2. Cumulative Incidence of Skin Cancer After Transplant by SUNTRAC Risk Group and Country
eFigure 3. Time-Dependent Area Under the Receiving Operating Characteristic Curve (t-AUROC) After Transplant and ROC Curves at 5 Years After Transplant by Country
References
- 1.Colegio OR, Billingsley EM. Skin cancer in transplant recipients, out of the woods: scientific retreat of the ITSCC and SCOPE. Am J Transplant. 2011;11(8):1584-1591. doi: 10.1111/j.1600-6143.2011.03645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gjersvik P. How to take the skin cancer risk of your transplant patient seriously. Transpl Int. 2019;32(12):1244-1246. doi: 10.1111/tri.13541 [DOI] [PubMed] [Google Scholar]
- 3.Crow LD, Jambusaria-Pahlajani A, Chung CL, et al. Initial skin cancer screening for solid organ transplant recipients in the United States: Delphi method development of expert consensus guidelines. Transpl Int. 2019;32(12):1268-1276. doi: 10.1111/tri.13520 [DOI] [PubMed] [Google Scholar]
- 4.Lowenstein SE, Garrett G, Toland AE, et al. ; National Cancer Institute Keratinocyte Carcinoma Consortium . Risk prediction tools for keratinocyte carcinoma after solid organ transplantation: a review of the literature. Br J Dermatol. 2017;177(5):1202-1207. doi: 10.1111/bjd.15889 [DOI] [PubMed] [Google Scholar]
- 5.Urwin HR, Jones PW, Harden PN, et al. Predicting risk of nonmelanoma skin cancer and premalignant skin lesions in renal transplant recipients. Transplantation. 2009;87(11):1667-1671. doi: 10.1097/TP.0b013e3181a5ce2e [DOI] [PubMed] [Google Scholar]
- 6.Carroll RP, Ramsay HM, Fryer AA, Hawley CM, Nicol DL, Harden PN. Incidence and prediction of nonmelanoma skin cancer post-renal transplantation: a prospective study in Queensland, Australia. Am J Kidney Dis. 2003;41(3):676-683. doi: 10.1053/ajkd.2003.50130 [DOI] [PubMed] [Google Scholar]
- 7.Jambusaria-Pahlajani A, Crow LD, Lowenstein S, et al. Predicting skin cancer in organ transplant recipients: development of the SUNTRAC screening tool using data from a multicenter cohort study. Transpl Int. 2019;32(12):1259-1267. doi: 10.1111/tri.13493 [DOI] [PubMed] [Google Scholar]
- 8.Garrett GL, Blanc PD, Boscardin J, et al. Incidence of and risk factors for skin cancer in organ transplant recipients in the United States. JAMA Dermatol. 2017;153(3):296-303. doi: 10.1001/jamadermatol.2016.4920 [DOI] [PubMed] [Google Scholar]
- 9.Royston P, Altman DG. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. doi: 10.1186/1471-2288-13-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mallett S, Royston P, Waters R, Dutton S, Altman DG. Reporting performance of prognostic models in cancer: a review. BMC Med. 2010;8:21. doi: 10.1186/1741-7015-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrándiz C, José Fuente M. Skin cancer in patients submitted to organ transplantation: a growing problem. Med Clin (Barc). 2001;116(6):217-219. doi: 10.1016/S0025-7753(01)71775-7 [DOI] [PubMed] [Google Scholar]
- 12.Garrett GL, Yuan JT, Shin TM, Arron ST; Transplant Skin Cancer Network (TSCN) . Validity of skin cancer malignancy reporting to the Organ Procurement Transplant Network: a cohort study. J Am Acad Dermatol. 2018;78(2):264-269. doi: 10.1016/j.jaad.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 13.Daniel WW. Biostatistics: A Foundation for Analysis in the Health Sciences. 9th ed. John Wiley & Sons; 2008. [Google Scholar]
- 14.Wolbers M, Blanche P, Koller MT, Witteman JCM, Gerds TA. Concordance for prognostic models with competing risks. Biostatistics. 2014;15(3):526-539. doi: 10.1093/biostatistics/kxt059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 16.Blanche P, Kattan MW, Gerds TA. The C-index is not proper for the evaluation of $t$-year predicted risks. Biostatistics. 2019;20(2):347-357. doi: 10.1093/biostatistics/kxy006 [DOI] [PubMed] [Google Scholar]
- 17.Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381-5397. doi: 10.1002/sim.5958 [DOI] [PubMed] [Google Scholar]
- 18.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics: Science Direct working paper No S1574-0358(04)70152-5. Accessed June 9, 2021. https://papers.ssrn.com/abstract=3133711
- 19.Acuna SA, Huang JW, Scott AL, et al. Cancer screening recommendations for solid organ transplant recipients: a systematic review of clinical practice guidelines. Am J Transplant. 2017;17(1):103-114. doi: 10.1111/ajt.13978 [DOI] [PubMed] [Google Scholar]
- 20.Kasiske BL, Vazquez MA, Harmon WE, et al. ; American Society of Transplantation . Recommendations for the outpatient surveillance of renal transplant recipients. J Am Soc Nephrol. 2000;11(suppl 15):S1-S86. doi: 10.1681/ASN.V11suppl_1s1 [DOI] [PubMed] [Google Scholar]
- 21.Stasko T, Brown MD, Carucci JA, et al. ; International Transplant-Skin Cancer Collaborative; European Skin Care in Organ Transplant Patients Network . Guidelines for the management of squamous cell carcinoma in organ transplant recipients. Dermatol Surg. 2004;30(4 Pt 2):642-650. doi: 10.1111/j.1524-4725.2004.30150.x [DOI] [PubMed] [Google Scholar]
- 22.Kentley J, Allawh R, Rao S, et al. The burden of cutaneous disease in solid organ transplant recipients of color. Am J Transplant. 2021;21(3):1215-1226. doi: 10.1111/ajt.16210 [DOI] [PubMed] [Google Scholar]
- 23.Stapleton CP, Birdwell KA, McKnight AJ, et al. Polygenic risk score as a determinant of risk of non-melanoma skin cancer in a European-descent renal transplant cohort. Am J Transplant. 2019;19(3):801-810. doi: 10.1111/ajt.15057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stapleton CP, Chang BL, Keating BJ, Conlon PJ, Cavalleri GL. Polygenic risk score of non-melanoma skin cancer predicts post-transplant skin cancer across multiple organ types. Clin Transplant. 2020;34(8):e13904. doi: 10.1111/ctr.13904 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Clinical and Demographic Characteristics of the Validation Cohort by Skin and Ultraviolet Neoplasia Risk Assessment Calculator (SUNTRAC) Risk Group
eTable 2. Subdistribution Hazard Ratios (SHR) and Their 95% CIs From a Univariate Competing Risk Regression Analysis Including SUNTRAC Group as Predictor in Validation Cohort and in the TSCN Study
eFigure 1. Density Plots Displaying SUNTRAC Score Distribution in Validation Cohort
eFigure 2. Cumulative Incidence of Skin Cancer After Transplant by SUNTRAC Risk Group and Country
eFigure 3. Time-Dependent Area Under the Receiving Operating Characteristic Curve (t-AUROC) After Transplant and ROC Curves at 5 Years After Transplant by Country