Key Points
Question
Do prostate cancer trials adequately report and include racial and ethnic minority groups and older adults?
Findings
In this meta-analysis of 286 randomized clinical trials including 9552 patients, participation by racial, ethnic, and age subgroups was reported in 69.2%, 26.2%, and 26.2% of trials; clinical outcomes by race and age were reported in 3.1% and 15.0%, respectively, of trials among those with reporting. There was significant underrepresentation of Asian (enrollment incidence ratio [EIR], 0.48), Black (EIR, 0.70), and Hispanic (EIR, 0.62) patients compared with their cancer incidence.
Meaning
The study results suggest that steps should be taken to improve racial and ethnic minority group reporting, representation, and health equity in future prostate cancer trials.
Abstract
Importance
Prostate cancer (PCa) is marked by disparities in clinical outcomes by race, ethnicity, and age. Equitable enrollment in clinical trials is fundamental to promoting health equity.
Objective
To evaluate disparities in the inclusion of racial and ethnic minority groups and older adults across PCa clinical trials.
Data Sources
MEDLINE, Embase, and ClinicalTrials.gov were searched to identify primary trial reports from each database's inception through February 2021. Global incidence in age subgroups and US population-based incidence in racial and ethnic subgroups were acquired from the Global Burden of Disease and Surveillance, Epidemiology, and End Results 21 incidence databases respectively.
Study Selection
All phase 2/3 randomized PCa clinical trials were eligible for age disparity analyses. Trials recruiting exclusively from the US were eligible for primary racial and ethnic disparity analyses.
Data Extraction and Synthesis
This study was reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines. Data were pooled using a random-effects model.
Main Outcomes and Measures
Enrollment incidence ratios (EIRs), trial proportions (TPs) of participants 65 years or older or members of a racial and ethnic subgroup divided by global incidence in the corresponding age group, or US population–based incidence in the corresponding racial and ethnic subgroup, were calculated. Meta-regression was used to explore associations between trial characteristics and EIRs and trends in EIRs during the past 3 decades.
Results
Of 9552 participants among trials reporting race, 954 (10.8%) were African American/Black, 80 (1.5%) were Asian/Pacific Islander, and 8518 (78.5) were White. Of 65 US trials, 45 (69.2%) reported race and only 9 (13.8%) reported data on all 5 US racial categories. Of 286 global trials, 75 (26.2%) reported the enrollment proportion of older adults. Outcomes by race and age were reported in 2 (3.1%) and 41 (15.0%) trials, respectively. Black (EIR, 0.70; 95% CI, 0.59-0.83) and Hispanic (EIR, 0.70; 95% CI, 0.59-0.83) patients were significantly underrepresented in US trials. There was no disparity in older adult representation (TP, 21 143 [71.1%]; EIR, 1.00; 95% CI, 0.95-1.05). The representation of Black patients was lower in larger trials (meta-regression coefficient, −0.06; 95% CI, −0.10 to −0.02; P = .002).
Conclusions and Relevance
The results of this meta-analysis suggest that Black and Hispanic men are underrepresented in trials compared with their share of PCa incidence. The representation of Black patients has consistently remained low during the past 2 decades.
This meta-analysis examines disparities in the inclusion of racial and ethnic minority groups and older adults across prostate cancer clinical trials.
Introduction
Prostate cancer (PCa) is marked by a large disparity in clinical outcomes by race, ethnicity, and age. Black men are twice as likely as White men to receive a diagnosis of PCa,1 significantly more likely to develop cancer recurrence and metastasis, and 2 to 3 times more likely to die of PCa.2 Certain subpopulations of Hispanic/Latino men have also been shown to experience higher rates of PCa-specific mortality compared with non-Hispanic White individuals.3 Moreover, older men of racial and ethnic minority groups are more likely to die prematurely of PCa than younger men,4 indicating an association of age with racial disparities. In general, older men not only harbor an increased risk of PCa, but also are more likely to have an aggressive disease course.5 Mandates have been introduced that emphasize racial and ethnic minority group inclusion in clinical trials (eg, the 1993 National Institutes of Health Revitalization Act6) and the reporting of demographic characteristics on public platforms like ClinicalTrials.gov (eg, the 2007 US Food and Drug Administration [FDA] Amendments Act and Final Rule7). However, the extent to which these mandates are followed is not well known, and assessing their association with racial and ethnic minority group recruitment in modern clinical trials is essential.
Given marked differences in PCa epidemiology across racial, ethnic, and age-related subgroups, representation in clinical trials must be evaluated in the context of incidence in the population. Moreover, regional8 and temporal9 variations in PCa incidence are associated with the recruitment of underrepresented populations to trials. Precise quantification of enrollment disparities is critical because of their implications on policy, clinical decision-making, and future strategies for racial and ethnic minority group recruitment to clinical trials.
The objective of this study was to assess reporting and enrollment disparity by racial, ethnic, and age-related subgroups across PCa clinical trials while accounting for baseline incidence. We also assessed enrollment trends and associations between enrollment disparity and specific trial characteristics across PCa clinical trials.
Methods
Search Strategy and Study Selection
Ovid MEDLINE was searched from the database’s inception through February 2021 to retrieve published reports of oncology clinical trials. The detailed search strategy and eligibility criteria are provided in the eMethods in the Supplement. This study was exempt from institutional review board oversight as it exclusively used published data. This study was exempt from the requirement of informed patient consent as it was based exclusively on published trials and involved no new patients.
Data Extraction and Reporting
Two independent reviewers (W.I. and H.M.) extracted data for trial characteristics, and on reporting and representation of predefined subgroups of race, ethnicity, and age as per the FDA position statement (eMethods in the Supplement). Since the unit of analysis was a study rather than a patient, we followed an adaptation of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines for meta-epidemiologic research.10
Statistical Analysis and Outcome Measures
Selection of Trials
For assessment of racial and ethnic disparity, we included trials that reported race and/or ethnicity and recruited patients exclusively from within the US. For assessment of age-related disparity, we included all trials (region-specific as well as global) that reported the number of patients 65 years or older and younger than 65 years.
Estimation of Trial Proportions
We used trial proportions (TPs) to describe relative representation of different categories within each subgroup of interest (race, ethnicity, and age) in each trial. The TP was calculated as the number of participants in each relevant category of a subgroup divided by the total number of enrolled participants in the trial.
Acquisition of Population-Based Estimates for PCa Incidence
For each racial and ethnic category, we acquired US population–based estimates of PCa incidence from the National Cancer Institute Surveillance, Epidemiology and End Results (SEER) program database (SEER 21: 2000-2018).11 The acquisition of US population–based estimates was tailored and matched to each trial to account for time-specific differences. We used SEER as the reference for PCa incidence estimates in racial and ethnic categories because (1) racial and ethnic disparity in clinical trial enrollment is a US-centric issue, and (2) most US trials define race and ethnicity in accordance with the FDA position statement12 and the definitions reported in SEER.13
For each age category, we acquired global population–based estimates of PCa incidence from the Global Burden of Disease database.14 The acquisition of global estimates was tailored and matched to each trial to account for regional and time-specific differences. The acquired estimates were then used to compute the proportion of incident cases in each subgroup category, calculated as the number of men with a diagnosis of PCa in the subgroup divided by the total number of men with a diagnosis of PCa.
Estimation of Enrollment Incidence Ratios
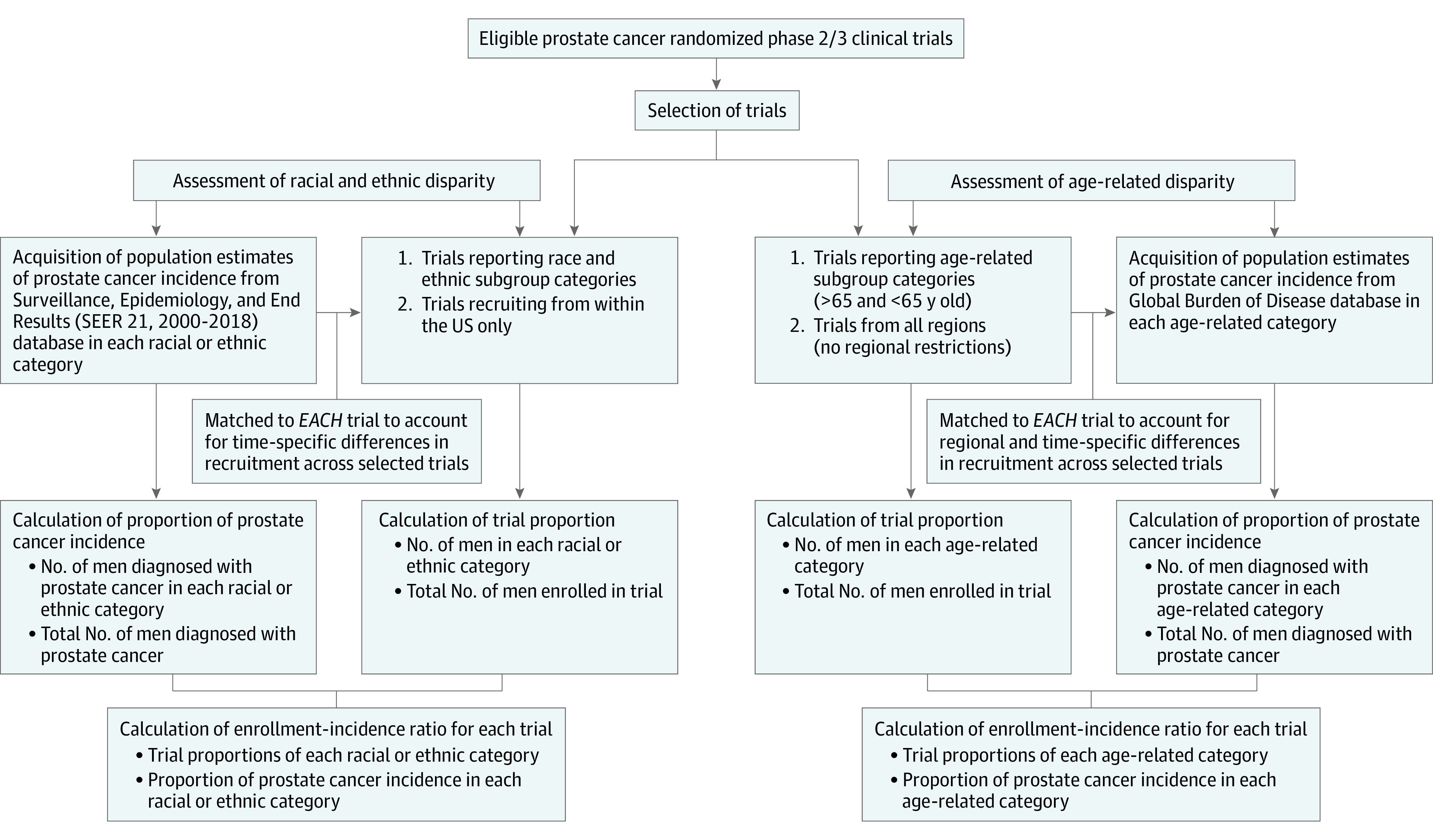
Enrollment incidence ratios (EIRs) were used to assess disparity in the enrollment of each subgroup. For each trial, EIR was calculated as the ratio between the TP and PCa incidence. Asian and Pacific Islander individuals are reported as 1 combined category in SEER, and we followed the same approach in calculating the EIR for maximum comparability between TP and population incidence. The methods used to quantify imprecision in EIR are described in the eMethods in the Supplement. The details of trial selection, acquisition of population-based PCa incidence, and calculation of EIRs are outlined in Figure 1.
Figure 1. Details of Trial Selection, Acquisition of Population-Based Prostate Cancer Incidence, and Calculation of Enrollment Incidence Ratios.
Meta-analysis
Log-transformed EIRs and the associated SE estimated from individual trials were pooled using an inverse variance approach. A random-effects meta-analysis was then conducted to summarize the overall enrollment disparity for racial, ethnic, and age-related subgroups among PCa trials. Heterogeneity was estimated using the I2 statistic.
Meta-regression
To assess the association between enrollment disparity and various trial characteristics, we conducted a random-effects multivariable meta-regression of study-specific EIR (eMethods in the Supplement). We also used meta-regression to show trends in enrollment disparity by assessing the association between logarithmic EIR and publication year from 1990 to 2020.
Sensitivity Analyses
To account for the nature of drug development as an international process, we conducted several post hoc sensitivity analyses to evaluate racial and ethnic disparity in (1) international trials that recruited from the US, (2) all trials that recruited from the US, and (3) trials that led to FDA drug approvals.
All statistical analyses were conducted in R, version 4.0.2 (R Foundation). The meta (version 5.2) and metafor (version 3.0) packages were used to synthesize meta-analysis results and produce forest plots.15
Results
Of 35 876 trials screened, 286 PCa trials were included in this study (eFigure 1 in the Supplement). These trials spanned from 1989 to 2020 and included a total of 104 205 study participants. Of these, 115 (40.2%) were trials that recruited patients exclusively from outside the US, 65 (22.7%) were US-only trials, and 62 (21.7%) were international trials that recruited patients from the US (Table 1).
Table 1. Summary of Trial Characteristics.
| Trial characteristics | No. (%) |
|---|---|
| Total | 286 |
| Trial phase | |
| 2 | 126 (44.1) |
| 3 | 103 (36.0) |
| Unspecified | 57 (19.9) |
| Arms | |
| 2 | 262 (91.6) |
| ≥3 | 24 (8.4) |
| Size of trial, No. of participants | |
| Small (<100) | 106 (37.1) |
| Intermediate (100-500) | 117 (40.9) |
| Large (>500) | 63 (22.0) |
| Clinical setting | |
| Localized | 43 (15.0) |
| Biochemical recurrence | 11 (3.8) |
| Metastatic, castration-sensitive | 61 (21.3) |
| Metastatic, castration-resistant | 161 (56.3) |
| Othera | 10 (3.5) |
| Class of treatment regimenb | |
| Antiandrogen | 141 (49.3) |
| Chemotherapy | 105 (36.7) |
| LHRH agonist/antagonist | 28 (9.8) |
| Liquid radiation | 8 (2.8) |
| Immunotherapy | 6 (2.1) |
| PARPi | 3 (1.0) |
| Primary end point | |
| OS | 58 (20.3) |
| PFS | 48 (16.8) |
| Other | 112 (39.2) |
| Unspecified | 68 (23.8) |
| Positive trial | |
| Yes | 92 (32.2) |
| No | 113 (39.5) |
| Unclear | 81 (28.3) |
| Funding source | |
| Industry | 166 (58.0) |
| Nonindustry | 100 (35.0) |
| Unspecified | 20 (7.0) |
| Center | |
| Single-center | 28 (9.8) |
| Multicenter | 245 (85.7) |
| Unspecified | 13 (4.5) |
| Regional recruitment | |
| US only | 65 (22.7) |
| International with US recruitment | 62 (21.7) |
| Excluding US | 115 (40.2) |
| Region unspecified | 44 (15.4) |
Abbreviations: LHRH, luteinizing hormone-releasing hormone; PARPi, polyadenosine diphosphate ribose polymerase inhibitor; OS, overall survival; PFS, progression-free survival.
Other clinical settings: all stages (n = 7), asymptomatic (n = 1), relapsed (n = 1), unspecified-metastatic (n = 1).
Total percentages exceed 100% due to some trials having multiple classes of treatment in their experimental arm.
Reporting of Participation and Clinical Outcomes
Race and Ethnicity Reporting
Of 65 US trials, 45 (69.2%) reported the race of participants. Nine trials (13.8%) reported the participation of all 5 racial categories across all sources of trial data. For individual races, 10 trials (15.4%) reported the participation of American Indian/Alaska Native patients, 20 trials (30.8%) reported the participation of Asian patients, 38 trials (58.5%) reported the participation of Black patients, 9 trials (13.8%) reported the participation of Pacific Islander individuals, and 45 trials (69.2%) reported the participation of White patients. Likewise, 17 trials (26.2%) reported the ethnicity (Hispanic or non-Hispanic) of participants in addition to race. Only 2 trials (3.1%) reported clinical outcomes by race in a subgroup analysis. No trials reported clinical outcomes by ethnicity.
Age Reporting
A total of 274 trials (95.8%) reported the ages of participants; 260 trials (90.9%) reported the mean/median age of included patients, either with (232 trials [81.1%]) or without (28 trials [9.8%]) a range/standard deviation. Only 75 trials (26.2%) reported patient numbers by prespecified age categories (≥65 and <65 years). Among all trials with age reporting, 41 trials (15.0%) reported clinical outcomes by age in a subgroup analysis, of which the subgroup analyses in 5 trials were exploratory. Trends in the reporting of race, ethnicity, and age are summarized in eFigure 2 in the Supplement.
TPs and Pooled EIRs
Across the 45 US trials reporting race, White participants comprised most of enrolled patients (TP, 8518 [78.5%]), followed by Black (TP. 954 [10.8%]), and Asian/Pacific Islander participants (TP, 80 [1.5%]) (Table 2). A meta-analysis of racial representation in US trials showed no significant disparity in the enrollment of White patients (EIR, 1.00; 95% CI, 0.96-1.04). However, significant underrepresentation was observed for Asian/Pacific Islander patients (EIR, 0.48; 95% CI, 0.34-0.66) and Black patients (EIR, 0.70; 95% CI, 0.59-0.83), as shown in Figure 2A. The EIR for American Indian/Alaska Native individuals was not calculated due to scarcity of US trials reporting American Indian/Alaska Native participation (10 [15.4%]), as well as very few American Indian/Alaska Native individuals included in trials with reporting (3 [4.61%], in total). eFigures 3 to 10 in the Supplement summarize enrollment disparity in different subgroups. Meta-analysis of ethnic representation showed significant underrepresentation of Hispanic patients (TP, 4.4%; EIR, 0.62; 95% CI, 0.42-0.90; Figure 2B) across the 17 US trials that reported ethnicity.
Table 2. Participation of Racial and Ethnic Subgroups in US Prostate Cancer Trials Compared With US Population Estimates.
| Subgroup | Trial participants, No. (%) | Overall proportion in US population, %a | Enrollment incidence ratio | No. of participants/1000 | |
|---|---|---|---|---|---|
| Observed | Expectedb | ||||
| Race | |||||
| African American/Black | 954 (10.8) | 15.1 | 0.7 | 108 | 160 |
| Asian/Pacific Islander | 80 (1.5) | 4.0 | 0.48 | 15 | 39 |
| White | 8518 (78.5) | 78.3 | 1.0 | 785 | 810 |
| Ethnicity | |||||
| Hispanic | 191 (4.4) | 8.6 | 0.62 | 44 | 78 |
| Non-Hispanic | 4174 (95.6) | 91.4 | 1.04 | 956 | 922 |
Proportions calculated from 2000 to 2018 incident cases.
For each individual trial, the expected number of participants of each subgroup category was calculated by multiplying the total observed number of trial participants with the proportion of time-specific prostate cancer incidence in the subgroup.
Figure 2. Enrollment Incidence Ratios (EIRs) for Subgroups of Race, Ethnicity, and Age.
Across the 49 trials that reported the proportion of patients older or younger than 65 years, older adults comprised most of the trial population (TP, 71.1%) compared with younger adults (TP, 27.1%). A meta-analysis of all trials reporting the number of participants older or younger than 65 years showed no significant disparity in the enrollment of older adults (EIR, 1.00; 95% CI, 0.95-1.05) or younger adults (EIR, 0.94; 95% CI, 0.80-1.09), as shown in Figure 2C.
Association With Trial Characteristics
Black patients were significantly more underrepresented in trials with larger sample sizes. Conversely, greater enrollment disparity was observed for older adults in smaller trials. None of the preselected trial characteristics were significantly associated with disparity in the representation of Hispanic patients (eTable 1 in the Supplement).
Trends in Enrollment Disparity
Trial proportions and EIRs for Black patients in PCa trials at each year are outlined in eTable 2 in the Supplement. The representation of Hispanic patients has increased significantly (meta-regression coefficient, 0.76; 95% CI, 0.11-1.42 per decade; P = .02), as shown in Figure 3.
Figure 3. Trends in Enrollment Disparity in Prostate Cancer Clinical Trials Conducted From 1989 to 2020 for Asian/Pacific Islander Patients, Black Patients, Hispanic Patients, and Older Adults.
Sensitivity Analyses
The inclusion of international trials recruiting from the US seemed to worsen enrollment disparity for Black patients but was associated with improved representation of Asian/Pacific Islander individuals. Analysis of FDA trials followed the same trend, showing substantial underrepresentation of Black patients (EIR, 0.26; 95% CI, 0.16-0.43) and overrepresentation of Asian/Pacific Islander individuals (EIR, 2.03; 95% CI, 1.41-2.91). eTable 3 in the Supplement summarizes and compares the results of these analyses.
Discussion
The results of this meta-analysis suggest that there is persistent underrepresentation of racial and ethnic minority groups in PCa clinical trials, even after adjusting for population estimates of PCa incidence. The representation of Black patients has consistently remained low and is significantly worse in larger trials, highlighting the ineffectiveness of efforts to improve health care equity in PCa. This, coupled with the fact that less than 10% of trials report clinical outcomes by race and ethnicity, is an indication of the collective failure to account for differential health outcomes in historically marginalized populations. The finding that the underrepresentation of patients of racial and ethnic minority groups was consistent across US-only trials, international trials with US recruitment, and practice-changing trials that led to FDA approval potentially reinforces the validity of the results.
During the past 3 decades, non-Hispanic White men have constituted most of the trial participants in PCa. The continued prioritization of predominantly White participants parallels the decline of Black participant representation by nearly 5% each year. As a result, the proportion of Black patient representation has declined by more than 65% since 2004.16 The failure of clinical trials to adequately represent the Black population reflects a lack of sufficient health care for these patients. Even with more aggressive disease, Black patients with PCa demonstrate superior responses to therapy17,18,19 and experience a greater survival benefit from participating in trials than counterparts of different racial and ethnic groups.20 However, the statistical power of clinical trials that report such differences is often limited by low accrual of Black patients, and whether their results can be generalized to the wider population is uncertain.21 Perhaps due to this, there is underuse of modern agents in Black and Hispanic populations.22 This problem is likely to persist with newer agents, especially with financial toxic effects widening gaps in care.23
Finally, the representation of different ages in trials may also play a role in the disparity between racial and ethnic subgroups. The mortality gap between Black and White patients with PCa is considerably worse in younger age groups.4 Thus, given that younger adults constitute a much smaller percentage of the PCa trial population than older adults, allocating resources toward their early identification and recruitment may help to decrease racial disparity.
It is critical to acknowledge that racial and ethnic groups are not monolithic and to understand how social determinants of health vary within and across racial groups. Aside from race and ethnicity, several other markers of disparity must be tracked to determine which individuals within racial and ethnic minority groups experience more adversity than others and especially require attention (eg, how immigrant/foreign-born individuals from an underrepresented group may experience greater inequity than US-born individuals of the same race).24 Similarly, most trials are restrictive in their eligibility criteria and are, at times, not representative of a real-world clinical setting. Data-driven artificial intelligence algorithms can be used to evaluate eligibility criteria and predict the association of removing/broadening specific criteria with trial safety and patient response.25
Prior studies have suggested that improved reporting of race and ethnicity may uncover additional, initially unreported enrollment disparities.26 Unfortunately, current underreporting of racial and ethnic minority group participation in clinical trials indicates a lack of data transparency and poor compliance with federal reporting mandates. Journal editors should routinely ask for racial and ethnic demographic data and outcomes data reported by race and ethnicity in the clinical trials that they publish. Precedents set by journals for manuscript publication were previously associated with improved data transparency in industry-sponsored clinical trial publications,27 and a policy for race and ethnicity reporting could have a similar association.
Finally, the role of sponsors must be emphasized. The FDA has recommended that clinical trial sponsors develop a race and ethnicity diversity plan for the inclusion of underrepresented, clinically relevant populations, and compliance with this recommendation during submission of investigational new drug applications is crucial.28 Thus, we propose a practical framework to mandate a level of racial and ethnic minority group accrual to studies necessary to establish clinical validity of the overall trial results for those populations. Because delays in discovery and evidence can be fatal for patients waiting for the next breakthrough, accrual to subpopulations can be designed to continue beyond the accrual required for the primary analysis.
Strengths and Limitations
This study looked at PCa clinical trials during the last 30 years and uses a novel meta-analytic approach to precisely quantify enrollment disparities by pooling individual trial EIRs. The use of an incidence-based measure (EIR) to assess disparities allows interpretation of TPs in the context of PCa epidemiology. This provides a truer measure of disparity than TP alone, as it directly compensates for any epidemiologic differences in incidence between subgroups at the population level. To account for regional and temporal differences in incidence, we analyzed age disparities in all included trials globally using region-specific and time-specific population estimates for incidence at the level of each individual trial and analyzed racial and ethnic disparity in US-only trials using time-specific incidence at the level of each trial. We also conducted several sensitivity analyses to assess the consistency of the results across larger, practice-changing trials and performed several secondary analyses to assess the association of socioeconomic status as well as trial characteristics. Regarding limitations, there is a possibility of missing undocumented cancer cases (which SEER does not record), as well as missing data due to the exclusion of any trials that may have been published as conference abstracts only or not published at all, as well as subgroup data from any trials that may have reported subgroup analyses as separate publications. Finally, while this study focused on the social construct of race, there is also emerging evidence of differential responses to PCa based on genetic ancestry.29
Conclusions
The results of this meta-analysis suggest that Black and Hispanic representation in PCa clinical trials is significantly less than the expected share based on cancer incidence, and Black participation has remained consistently low during the past 2 decades. An actionable framework involving legislation, institutional support, and journal policy for data transparency is required to tackle enrollment inequity in future clinical trials.
eMethods. Detailed search strategy, data extraction and analysis, and selection of variables for meta-regression
eTable 1. Significant predictors of enrollment-incidence ratio after multivariable metaregression across Black, Hispanic, Asian/Pacific Islander, and older patients
eTable 2. Trial proportions and Black patients’ enrollment in prostate cancer clinical trials by years.
eTable 3. Racial/ethnic representation across trials with different levels of U.S. recruitment and across trials that led to FDA drug approvals
eFigure 1. A PRISMA flowchart outlining the studies included, excluded and analyzed
eFigure 2. Trends in the reporting of race, ethnicity, and age in U.S. prostate cancer clinical trials by 5-year intervals
eFigure 3. Observed and expected numbers of under-enrolled minority patients in prostate cancer clinical trials
eFigure 4. Meta-analysis of the enrollment-incidence ratios of Black patients across U.S. prostate cancer trials
eFigure 5. Meta-analysis of the enrollment-incidence ratios of White patients across U.S. prostate cancer trials
eFigure 6. Meta-analysis of the enrollment-incidence ratios of Hispanic patients across U.S. prostate cancer trials
eFigure 7. Meta-analysis of the enrollment-incidence ratios of Hispanic patients across U.S. prostate cancer trials
eFigure 8. Meta-analysis of the enrollment-incidence ratios of non-Hispanic patients across U.S. prostate cancer trials
eFigure 9. Meta-analysis of the enrollment-incidence ratios of older adults across all prostate cancer trials (all regions)
eFigure 10. Meta-analysis of the enrollment-incidence ratios of younger adults across all prostate cancer trials (all regions)
References
- 1.Yamoah K, Lee KM, Awasthi S, et al. Racial and ethnic disparities in prostate cancer outcomes in the Veterans Affairs health care system. JAMA Netw Open. 2022;5(1):e2144027. doi: 10.1001/jamanetworkopen.2021.44027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietro GD, Chornokur G, Kumar NB, Davis C, Park JY. Racial differences in the diagnosis and treatment of prostate cancer. Int Neurourol J. 2016;20(suppl 2):S112-S119. doi: 10.5213/inj.1632722.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinea FM, Patel VN, Kwon D, et al. Ethnic heterogeneity and prostate cancer mortality in Hispanic/Latino men: a population-based study. Oncotarget. 2017;8(41):69709-69721. doi: 10.18632/oncotarget.19068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He T, Mullins CD. Age-related racial disparities in prostate cancer patients: a systematic review. Ethn Health. 2017;22(2):184-195. doi: 10.1080/13557858.2016.1235682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgans AK, Dale W, Briganti A. Screening and treating prostate cancer in the older patient: decision making across the clinical spectrum. Am Soc Clin Oncol Educ Book. 2017;37:370-381. doi: 10.1200/EDBK_175491 [DOI] [PubMed] [Google Scholar]
- 6.United States Congress House Committee on Energy and Commerce, Subcommittee on Health and the Environment . NIH Revitalization Act: hearing before the Subcommittee on Health and the Environment of the Committee on Energy and Commerce, House of Representatives, One Hundred Third Congress, first session, on H.R. 4, a bill to amend the Public Health Service Act. Accessed. https://books.google.com/books/about/NIH_Revitalization_Act.html?id=HWkhAAAAMAAJ
- 7.DeVito NJ, Bacon S, Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. Lancet. 2020;395(10221):361-369. doi: 10.1016/S0140-6736(19)33220-9 [DOI] [PubMed] [Google Scholar]
- 8.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10(2):63-89. doi: 10.14740/wjon1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai Q, Chen Y, Zhang D, et al. Estimates of over-time trends in incidence and mortality of prostate cancer from 1990 to 2030. Transl Androl Urol. 2020;9(2):196-209. doi: 10.21037/tau.2020.02.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22(4):139-142. doi: 10.1136/ebmed-2017-110713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute . End results program, SEER Incidence Database. Accessed July 15, 2021. https://seer.cancer.gov/data/
- 12.US Food and Drug Administration . Collection of race and ethnicity data in clinical trials. Accessed May 11, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/collection-race-and-ethnicity-data-clinical-trials
- 13.National Cancer Institute . End results program, race recode changes. Accessed March 22, 2022. https://seer.cancer.gov/seerstat/variables/seer/race_ethnicity/
- 14.Global Health Data Exchange . GBD results. Accessed March 22, 2022. https://ghdx.healthdata.org/gbd-results-tool
- 15.R Core Team . R: a language and environment for statistical computing. Accessed May 9, 2022. https://www.R-project.org/
- 16.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- 17.Efstathiou E, Deshpande H, George D, et al. Abstract CT313: an exploratory analysis of efficacy and safety of abiraterone acetate (AA) in black patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) without prior chemotherapy (ctx). Cancer Res. 2014;74(19)(suppl):CT313-CT313. doi: 10.1158/1538-7445.AM2014-CT313 [DOI] [Google Scholar]
- 18.Halabi S, Dutta S, Tangen CM, et al. Overall survival of Black and White men with metastatic castration-resistant prostate cancer treated with docetaxel. J Clin Oncol. 2019;37(5):403-410. doi: 10.1200/JCO.18.01279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sartor O, Armstrong AJ, Ahaghotu C, et al. Survival of African-American and Caucasian men after sipuleucel-T immunotherapy: outcomes from the PROCEED registry. Prostate Cancer Prostatic Dis. 2020;23(3):517-526. doi: 10.1038/s41391-020-0213-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spratt DE, Chen YW, Mahal BA, et al. Individual patient data analysis of randomized clinical trials: impact of Black race on castration-resistant prostate cancer outcomes. Eur Urol Focus. 2016;2(5):532-539. doi: 10.1016/j.euf.2016.03.010 [DOI] [PubMed] [Google Scholar]
- 21.Aldrighetti CM, Niemierko A, Van Allen E, Willers H, Kamran SC. Racial and ethnic disparities among participants in precision oncology clinical studies. JAMA Netw Open. 2021;4(11):e2133205. doi: 10.1001/jamanetworkopen.2021.33205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mouzannar A, Atluri VS, Mason M, et al. PD34-03 : racial disparity in the utilization of new therapies for advanced prostate cancer. J Urol. 2021;206(suppl 3):e583-e583. doi: 10.1097/JU.0000000000002038.03 [DOI] [Google Scholar]
- 23.Abrams HR, Durbin S, Huang CX, et al. Financial toxicity in cancer care: origins, impact, and solutions. Transl Behav Med. 2021;11(11):2043-2054. doi: 10.1093/tbm/ibab091 [DOI] [PubMed] [Google Scholar]
- 24.Benson AB III, Carlos RC. Clinical trials as a path toward equity. Cancer. 2021;127(20):3717-3719. doi: 10.1002/cncr.33648 [DOI] [PubMed] [Google Scholar]
- 25.Liu R, Rizzo S, Whipple S, et al. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592(7855):629-633. doi: 10.1038/s41586-021-03430-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hantel A, Luskin MR, Garcia JS, Stock W, DeAngelo DJ, Abel GA. Racial and ethnic enrollment disparities and demographic reporting requirements in acute leukemia clinical trials. Blood Adv. 2021;5(21):4352-4360. doi: 10.1182/bloodadvances.2021005148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeAngelis CD, Fontanarosa PB, Flanagin A. Reporting financial conflicts of interest and relationships between investigators and research sponsors. JAMA. 2001;286(1):89-91. doi: 10.1001/jama.286.1.89 [DOI] [PubMed] [Google Scholar]
- 28.U.S. Food and Drug Administration . Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials. Accessed April 28, 2022. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations
- 29.Awasthi S, Berglund A, Abraham-Miranda J, et al. Comparative genomics reveals distinct immune-oncologic pathways in African American men with prostate cancer. Clin Cancer Res. 2021;27(1):320-329. doi: 10.1158/1078-0432.CCR-20-2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Detailed search strategy, data extraction and analysis, and selection of variables for meta-regression
eTable 1. Significant predictors of enrollment-incidence ratio after multivariable metaregression across Black, Hispanic, Asian/Pacific Islander, and older patients
eTable 2. Trial proportions and Black patients’ enrollment in prostate cancer clinical trials by years.
eTable 3. Racial/ethnic representation across trials with different levels of U.S. recruitment and across trials that led to FDA drug approvals
eFigure 1. A PRISMA flowchart outlining the studies included, excluded and analyzed
eFigure 2. Trends in the reporting of race, ethnicity, and age in U.S. prostate cancer clinical trials by 5-year intervals
eFigure 3. Observed and expected numbers of under-enrolled minority patients in prostate cancer clinical trials
eFigure 4. Meta-analysis of the enrollment-incidence ratios of Black patients across U.S. prostate cancer trials
eFigure 5. Meta-analysis of the enrollment-incidence ratios of White patients across U.S. prostate cancer trials
eFigure 6. Meta-analysis of the enrollment-incidence ratios of Hispanic patients across U.S. prostate cancer trials
eFigure 7. Meta-analysis of the enrollment-incidence ratios of Hispanic patients across U.S. prostate cancer trials
eFigure 8. Meta-analysis of the enrollment-incidence ratios of non-Hispanic patients across U.S. prostate cancer trials
eFigure 9. Meta-analysis of the enrollment-incidence ratios of older adults across all prostate cancer trials (all regions)
eFigure 10. Meta-analysis of the enrollment-incidence ratios of younger adults across all prostate cancer trials (all regions)




