Key Points
Question
Does adjuvant therapy (AT) improve survival outcomes for patients with node-negative (N0) disease after neoadjuvant therapy (NAT) and surgical resection for pancreatic cancer?
Findings
In this multi-institutional study of 430 patients with N0 disease after NAT for localized pancreatic cancer, AT was associated with significantly improved progression-free and overall survival (4.1 vs 2.1 and 5.3 vs 3.5 years, respectively). The magnitude of this benefit on overall survival was reduced in patients who received neoadjuvant radiation and amplified in patients with perineural invasion.
Meaning
These findings suggest a survival benefit for AT in patients with N0 disease after NAT, particularly for those with perineural invasion.
This cohort study evaluates survival outcomes with adjuvant chemotherapy for patients with node-negative disease after neoadjuvant therapy and surgical resection for pancreatic cancer.
Abstract
Importance
Neoadjuvant therapy (NAT) is rarely associated with a complete histopathologic response in patients with pancreatic ductal adenocarcinoma (PDAC) but results in downstaging of regional nodal disease. Such nodal downstaging after NAT may have implications for the use of additional adjuvant therapy (AT).
Objectives
To examine the prognostic implications of AT in patients with node-negative (N0) disease after NAT and to identify factors associated with progression-free (PFS) and overall survival (OS).
Design, Setting, and Participants
A retrospective review was conducted using data from 2 high-volume, tertiary care academic centers (University of Pittsburgh Medical Center and the Medical College of Wisconsin). Prospectively maintained pancreatic cancer databases at both institutes were searched to identify patients with localized PDAC treated with preoperative therapy and subsequent surgical resection between 2010 and 2019, with N0 disease on final histopathology.
Exposures
Patients received NAT consisting of chemotherapy with or without concomitant neoadjuvant radiation (NART). For patients who received NART, chemotherapy regimens were gemcitabine or 5-fluoururacil based and included stereotactic body radiotherapy (SBRT) or intensity-modulated radiation therapy (IMRT) after all intended chemotherapy and approximately 4 to 5 weeks before anticipated surgery. Adjuvant therapy consisted of gemcitabine-based therapy or FOLFIRINOX; when used, adjuvant radiation was commonly administered as either SBRT or IMRT.
Main Outcomes and Measures
The association of AT with PFS and OS was evaluated in the overall cohort and in different subgroups. The interaction between AT and other clinicopathologic variables was examined on Cox proportional hazards regression analysis.
Results
In this cohort study, 430 consecutive patients were treated between 2010 and 2019. Patients had a mean (SD) age of 65.2 (9.4) years, and 220 (51.2%) were women. The predominant NAT was gemcitabine based (196 patients [45.6%]), with a median duration of 2.7 cycles (IQR, 1.5-3.4). Neoadjuvant radiation was administered to 279 patients (64.9%). Pancreatoduodenectomy was performed in 310 patients (72.1%), and 160 (37.2%) required concomitant vascular resection. The median lymph node yield was 26 (IQR, 19-34); perineural invasion (PNI), lymphovascular invasion (LVI), and residual positive margins (R1) were found in 254 (59.3%), 92 (22.0%), and 87 (21.1%) patients, respectively. The restricted mean OS was 5.2 years (95% CI, 4.8-5.7). On adjusted analysis, PNI, LVI, and poorly differentiated tumors were independently associated with worse PFS and OS in N0 disease after NAT, with hazard ratios (95% CIs) of 2.04 (1.43-2.92; P < .001) and 1.68 (1.14-2.48; P = .009), 1.47 (1.08-1.98; P = .01) and 1.54 (1.10-2.14; P = .01), and 1.90 (1.18-3.07; P = .008) and 1.98 (1.20-3.26; P = .008), respectively. Although AT was associated with prolonged survival in the overall cohort, the effect was reduced in patients who received NART and strengthened in patients with PNI (AT × PNI interaction: hazard ratio, 0.55 [95% CI, 0.32-0.97]; P = .04).
Conclusions and Relevance
The findings of this cohort study suggest a survival benefit for AT in patients with N0 disease after NAT and surgical resection. This survival benefit may be most pronounced in patients with PNI.
Introduction
Patients with localized pancreatic ductal adenocarcinoma (PDAC) are most often treated with multimodal therapy. The optimal treatment sequencing, based on disease stage, remains somewhat controversial.1,2,3 Until recently, a surgery-first approach followed by adjuvant therapy (AT) was favored for patients with resectable disease, with several trials demonstrating a benefit with single and multiagent regimens over observation alone.4,5,6,7 Currently, most contemporary clinical trials for resectable and borderline resectable PDAC are exploring neoadjuvant therapy (NAT) delivered either as perioperative or total NAT.6,8,9,10 Moreover, nearly half of patients who undergo upfront resection do not receive AT because of postoperative complications or decreased performance status; thus, NAT has emerged as a valuable strategy to treat the systemic nature of PDAC up front and ensure access to chemotherapy.11,12,13,14,15
Neoadjuvant therapy has rarely been associated with a complete or near complete pathologic response in the primary tumor. In contrast, NAT has been linked to an increased frequency of node-negative (N0) resections compared with a surgery-first approach.10,16,17 In the setting of N0 disease after NAT, the incremental benefit derived from AT remains to be evaluated. Furthermore, the effects of other high-risk pathologic features such as margin status, tumor grade, lymphovascular invasion (LVI), and perineural invasion (PNI) have not yet been evaluated in N0 specimens after NAT.18,19 Conceptually, the detection of tumor-specific features, or the lack thereof, in patients with N0 disease after NAT may allow investigators to identify a cohort of patients for whom AT may be indicated or omitted.
This study aimed to examine the implications of AT in a cohort of patients with N0 PDAC who received NAT followed by curative-intent resection at 2 large academic centers in the US.
Methods
Study Design, Patient Population, and Definitions
This cohort study was approved by the institutional review boards (IRBs) of the University of Pittsburgh Medical Center (UPMC) and the Medical College of Wisconsin (MCW). Both IRBs waived informed consent because deidentified data were used. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
A data use agreement was executed between UPMC and MCW, and prospectively maintained pancreatic cancer databases at both institutes were queried for this study. We identified all patients with a pathologic diagnosis of PDAC who received NAT followed by curative-intent surgical resection and had N0 disease on final pathology between 2010 and 2019. Patients with node-positive or metastatic disease were excluded from the analysis.
Neoadjuvant and Adjuvant Treatment
Multidisciplinary management of PDAC was considerably similar at both study sites. All patients received NAT consisting of chemotherapy with or without concomitant radiation therapy (NART). Given the retrospective nature of the study, different chemotherapy regimens were used, all of which were gemcitabine or 5-fluoururacil based. In this study, 1 cycle of gemcitabine-based therapy was defined as a doublet (2-weekly infusions followed by a 1- to 2-week break) or triplet (3-weekly infusions followed by a 1-week break), whereas 1 cycle of FOLFIRINOX (combined fluorouracil, leucovorin, irinotecan, and oxaliplatin) consisted of 2 treatments administered 2 weeks apart. When used, NART was administered commonly as either stereotactic body radiotherapy (SBRT) or intensity-modulated radiation therapy (IMRT) at a dose of 30 to 36 Gy over 3 to 5 fractions (≥6.6 Gy/fraction) or 50 to 54 Gy over 25 to 28 fractions (1.8-2 Gy/fraction), respectively, after all intended chemotherapy and approximately 4 to 5 weeks before anticipated surgery. For patients who received NAT, cancer was typically restaged at 2- to 3-month intervals with imaging and laboratory investigations. Based on physician assessment that included a combination of factors such as radiographic response, carbohydrate antigen 19-9 (CA19-9) response, and performance status, these patients were continued on the same regimen, switched to an alternative neoadjuvant regimen, or taken to surgery for resection.
After surgical resection, AT was administered based on physician discretion or multidisciplinary tumor board discussions, taking into consideration CA19-9 response, pathologic response to NAT, disease stage, and ability to tolerate further chemotherapy. Similar to NAT, AT consisted of gemcitabine-based therapy or FOLFIRINOX, whereas adjuvant radiation was commonly administered as either SBRT or IMRT at a dose of 30 to 36 Gy over 3 to 5 fractions (≥6.6 Gy/fraction) or 50 to 54 Gy over 25 to 28 fractions (1.8-2 Gy/fraction), respectively, before or after AT.
Data Collection and Definitions
Data on baseline demographics, pre- and postsurgical treatments, and pathologic features were collected at each institute and shared after a deidentification process as specified in the data use agreement between both centers. Resectability status was classified according to National Comprehensive Cancer Network (NCCN) criteria.20 Pathology information was abstracted from pathology reports; the pathology slides were not rereviewed for this study. The pathologic assessment of pancreatectomy specimens was performed in a systematic fashion, as described previously.21 Each pancreatectomy specimen was evaluated for tumor size, tumor location, involvement of resection margins, LVI, PNI, and regional lymph node metastases. Lymphovascular invasion was defined by the presence of neoplastic cells within lymphatic spaces and muscular vessels; for a subset of patients, confirmatory immunohistochemical stains (eg, D2-40, CD34, etc) were used judiciously. Similarly, neoplastic cells identified along a nerve or within a nerve (intraneural) were considered to be positive for PNI. Pathologic staging (TNM) was classified according to the American Joint Committee on Cancer Staging Manual (8th edition), and a tumor at or within 1 mm of the resection margin was considered microscopically positive (R1 margin).22 Progression-free survival (PFS) was defined as the time from diagnosis to the time of recurrence or date of last follow-up; overall survival (OS) was defined as the time from diagnosis to the time of death or date of last follow-up. Patients who died within 90 days of their operation were excluded from the survival analyses.
Statistical Analysis
Median (IQR) or mean (SD) values are reported for quantitative variables, whereas counts with percentages are reported for categorical variables. Kaplan-Meier estimates were used to examine estimated restricted mean PFS and OS and were compared using the log-rank test.
All potentially relevant variables were used to fit Cox proportional hazards regression models with each variable tested individually (eTables 1 and 2 in the Supplement). Any variable showing a significant association with survival (P < .2) was then used in a multivariable model to reach a best-fit model. Of further interest was whether AT had differing associations with survival for various subgroups; therefore, interactions between AT and other variables were tested in a similar fashion. In the multivariable model, variables were then removed based on statistical insignificance and/or guidance from clinical experts. This process was completed to fit a multivariable Cox proportional hazards regression model for OS and another for PFS as outcomes. α was set to .05, and all analyses were performed using Stata version 16 (StataCorp).
Results
Patient Demographics and Treatment Characteristics
Of 976 patients treated with NAT followed by complete surgical resection, 430 (44.1%) had negative regional lymph nodes (N0) on final histopathology and represent the study cohort (Table 1). The mean (SD) patient age was 65.2 (9.4) years; 220 patients were women (51.2%) and 210 were men (48.8%). By NCCN criteria as applied to computed tomography images at the time of diagnosis, 166 patients (38.7%) had resectable disease, 188 (43.8%) had borderline resectable disease, and 75 (17.5%) had locally advanced disease. Gemcitabine-based NAT (mono- or, more commonly, multiagent therapy) was administered to 196 patients (45.6%), whereas 115 (26.7%) received 5-fluorouracil–based therapy (multiagent) and 119 (27.7%) received a combination. The median number of NAT cycles was 2.7 (IQR, 1.5-3.4) and 279 patients (64.9%) received NART. Of the 282 patients with an elevated pre-NAT CA19-9 response, 129 (45.7%) experienced a decrease in CA19-9 to normal levels (<37) after all intended NAT; the response remained elevated for 153 patients (54.3%).
Table 1. Demographic, Clinicopathologic, and Outcome Variables .
| Variable | Value (N = 430) |
|---|---|
| Age, y, mean (SD) | 65.2 (9.4) |
| Sex, No. (%) | |
| Women | 220 (51.2) |
| Men | 210 (48.8) |
| Body mass index, mean (SD)a | 27.1 (5.1) |
| Age-adjusted Charlson Comorbidity Index score, median (IQR) | 5 (4-6) |
| Resectability, No. (%) | |
| Resectable | 166 (38.7) |
| Borderline resectable | 188 (43.8) |
| Locally advanced | 75 (17.5) |
| Neoadjuvant therapy regimen, No. (%) | |
| Gemcitabine based | 196 (45.6) |
| 5-Fluorouracil based | 115 (26.7) |
| Combination therapy | 119 (27.7) |
| No. of neoadjuvant therapy cycles, median (IQR) | 2.7 (1.5-3.4) |
| Neoadjuvant radiation, No. (%) | 279 (64.9) |
| CA19-9 level after neoadjuvant therapy, No. (%) | |
| Nonsecretors | 90 (20.9) |
| Normalized | 129 (30.0) |
| Elevated | 153 (35.6) |
| Unknown | 58 (13.5) |
| Surgical approach, No. (%) | |
| Open | 330 (76.7) |
| Robotic | 95 (22.1) |
| Laparoscopic | 5 (1.2) |
| Operation performed, No. (%) | |
| Whipple | 310 (72.1) |
| Distal | 68 (15.8) |
| DP-CAR | 27 (6.3) |
| Total | 25 (5.8) |
| Vascular resection (yes) , No. (%) | 160 (37.2) |
| Tumor size, cm, mean (SD) | 2.4 (1.6) |
| T stage, No. (%) | |
| T0 | 27 (6.4) |
| T1 | 159 (37.7) |
| T2 | 192 (45.5) |
| T3 | 44 (10.4) |
| Grade, No. (%) | |
| Well differentiated | 50 (11.6) |
| Moderately differentiated | 262 (60.9) |
| Poorly differentiated | 61 (14.2) |
| Undifferentiated | 20 (4.7) |
| Complete response | 27 (6.4) |
| Unknown | 10 (2.3) |
| No. of harvested lymph nodes, median (IQR) | 26 (19-34) |
| Lymphovascular invasion, No. (%) | 92 (22.0) |
| Perineural invasion, No. (%) | 254 (59.3) |
| R1 margins, No. (%) | 87 (21.1) |
| 90-d Mortality, No. (%) | 7 (1.6) |
| Adjuvant chemotherapy, No. (%) | 217 (51.8) |
| Adjuvant chemotherapy regimen, No. (%) | |
| Gemcitabine based | 123 (57.2) |
| 5-Fluorouracil based | 52 (24.0) |
| Combination | 40 (18.4) |
| Unknown | 2 (0.9) |
| No. of adjuvant cycles, median (IQR) | 3 (2-5) |
| Adjuvant radiation, No. (%) | 37 (8.9) |
Abbreviations: CA19-9, carbohydrate antigen 19-9; DP-CAR, distal pancreatectomy with celiac axis resection.
Calculated as weight in kilograms divided by height in meters squared.
Pancreaticoduodenectomy was performed in 310 patients (72.1%), distal pancreatectomy in 68 (15.8%), distal pancreatectomy with celiac artery resection in 27 (6.3%), and total pancreatectomy in 25 (5.8%). An open approach was used in 330 patients (76.7%), whereas 95 (22.1%) underwent robotic surgery and 5 (1.2%) underwent laparoscopic resection. Vascular resection was performed in 160 patients (37.2%). After 11 patients (2.6%) with 90-day mortality or unknown 90-day mortality status were excluded, 217 (51.8%) received AT. Of these 217 patients, 123 (57.2%) received gemcitabine-based treatment for a median of 3 cycles (IQR, 2-5). Adjuvant radiation was administered to 37 patients (8.9%).
On pathologic examination, 27 patients (6.4%) had a complete pathologic response and microscopic positive margins (R1) were present in 87 (21.1%). Perineural invasion was present in 254 patients (59.3%), whereas LVI was seen in 92 (22.0%). Notably, collinearity existed between PNI and other locoregional and/or tumoral prognosticators commonly seen in PDAC. For example, 81 of the 91 patients (89.0%) with LVI had PNI. Similarly, 75 of the 87 patients (86.2%) with R1 margins had PNI, and 57 of 81 patients (70.4%) with poorly differentiated or undifferentiated tumors had PNI.
Unadjusted Survival Outcomes for AT in N0 PDAC After NAT
When survival outcomes were examined, 11 patients (2.6%) with 90-day mortality or unknown 90-day mortality status were excluded. For investigations related to PFS, an additional 6 patients were excluded for missing recurrence status or PFS time. For the remaining 413 patients, the restricted mean values (95% CIs) for OS and PFS were 5.2 (4.8-5.7) and 4.0 (3.6-4.4) years, respectively. The 217 patients who received AT displayed improved PFS and OS (restricted mean [95% CI] 4.4 [3.8-4.9] vs 3.4 [2.8-3.8 ] years and 5.4 [4.9-5.9] vs 4.7 [4.1-5.2] years; log-rank test, P < .001 vs P = .009, respectively; Figure 1).
Figure 1. Kaplan-Meier Estimates of Restricted Mean Progression-Free and Overall Survival, Stratified by Receipt of Adjuvant Therapy.

A, Restricted mean progression-free survival of 413 patients was 4.4 (95% CI, 3.8-4.9) vs 3.4 (95% CI, 2.8-3.8) years (P < .001) with vs without adjuvant therapy, respectively. B, Restricted mean overall survival of 419 patients was 5.4 (95% CI, 4.9-5.9) vs 4.7 (95% CI, 4.1-5.2) years (P = .009) with vs without adjuvant therapy, respectively.
Next, we explored the effect of AT on various subgroups to identify subsets of patients with N0 disease who may benefit most from AT. Notably, AT was associated with improved PFS (restricted mean [95% CI] 4.1 [3.4-4.7] vs 2.1 [1.7-2.5] years; P < .001) and OS (5.3 [4.6-5.9] vs 3.5 [2.9-4.0]; P < .001) in patients with PNI (Figure 2), but not in those without PNI (4.9 [4.0-5.7] vs 4.8 [3.9-4.7] and 5.7 [4.9-6.5] vs 6.0 [5.0-6.8] years; P = .65 vs P = .84, respectively). Adjuvant therapy did not significantly improve OS in patients without PNI regardless of receipt of NART (Figure 3). For patients with PNI, the survival benefit of AT was most pronounced in the group who did not receive NART (5.1 [95% CI, 4.3-5.9] vs 2.0 [95% CI, 1.6-2.4] years; P < .001; Figure 3C) and was diminished (albeit still significant) in those who received NART (5.1 [95% CI, 4.2-6.0] vs 3.8 [95% CI, 3.1-4.3] years; P = .02; Figure 3D). These findings suggested the need to include interaction terms (AT × PNI and AT × NART) in multivariate modeling.
Figure 2. Kaplan-Meier Curves Evaluating Effects of Adjuvant Therapy on Progression-Free and Overall Survival in Patients With or Without Perineural Invasion.

A, Progression-free survival of 168 patients without perineural invasion (PNI), with or without adjuvant therapy (AT). B, Progression-free survival of 243 patients with PNI, with or without AT. C, Overall survival of 170 patients without PNI, with or without AT. D, Overall survival of 247 patients with PNI, with or without AT.
Figure 3. Kaplan-Meier Estimates Examining Differences in Effects of Adjuvant Therapy on Overall Survival of Patients With or Without Perineural Invasion and Receipt of Neoadjuvant Radiation.
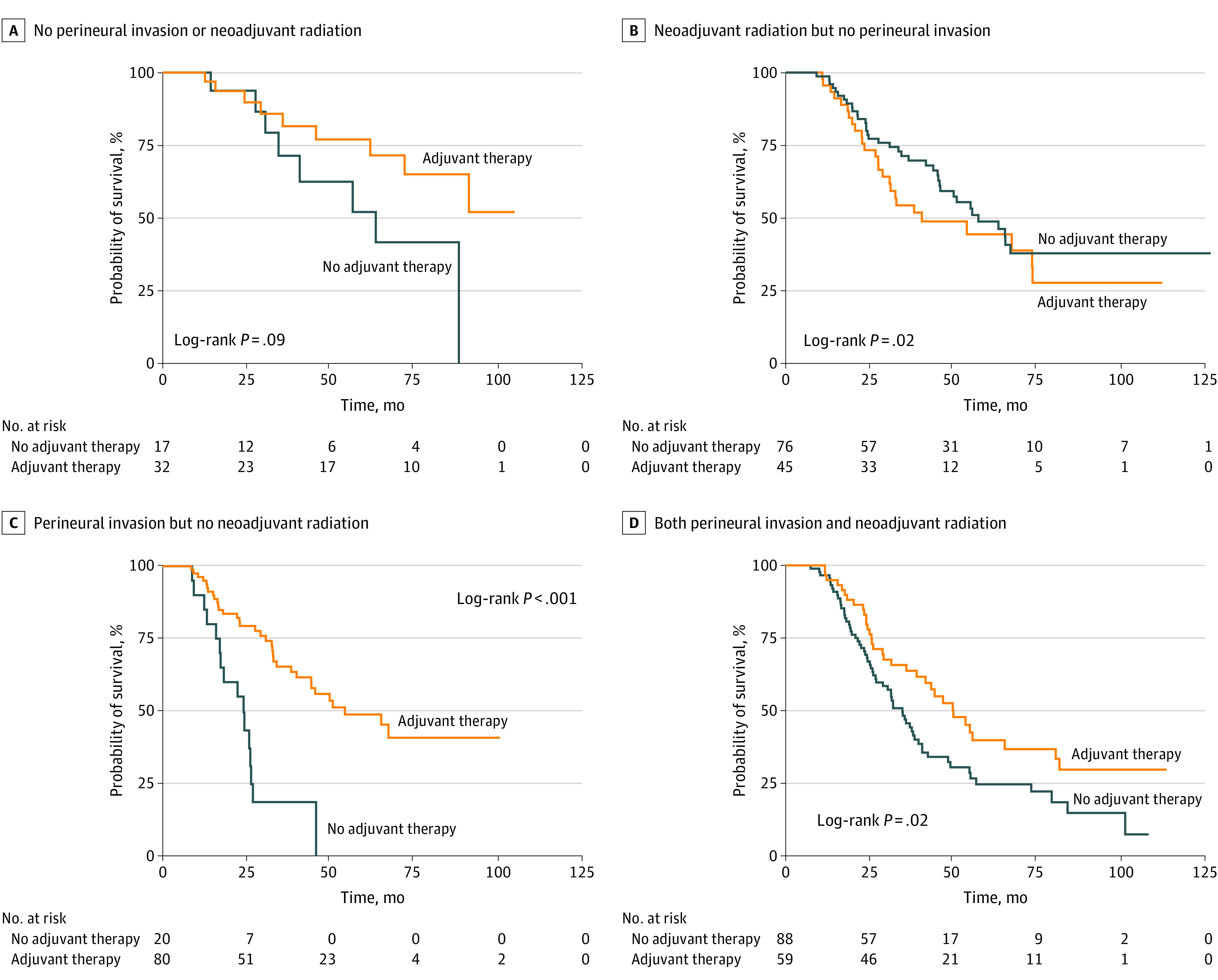
A, Restricted mean overall survival (OS) of 49 patients without perineural invasion (PNI) who did not receive neoadjuvant radiation (NART), with or without adjuvant therapy (AT) (6.8 [95% CI, 6.3-7.8] vs 5.1 [95% CI, 3.9-6.3] years; P = .09). B, Restricted mean OS of 121 patients without PNI who received NART, with or without AT (4.8 [95% CI, 3.8-5.9] vs 6.0 [95% CI, 5.1-7.0] years; P = .23). C, Restricted mean OS of 100 patients with PNI who did not receive NART, with or without AT (5.1 [95% CI, 4.3-5.9] vs 2.0 [95% CI, 1.6-2.4] years; P < .001). D, Restricted mean OS of 147 patients with PNI who received NART, with or without AT (5.1 [95% CI, 4.2-6.0] vs 3.8 [95% CI, 3.1-4.3] years; P = .02).
Risk Adjustment for Estimators of Survival in Patients With N0 Disease After NAT
In constructing a Cox proportional hazards regression model examining estimators of PFS and OS (Table 2) for patients with N0 disease, significant interactions were noted between AT and several treatment or pathologic variables, including NART, PNI, LVI, and margin status (particularly for AT × PNI and AT × NART). The associations of these variables with each other and with survival were carefully examined and accounted for when fitting the multivariable Cox proportional hazards regression model.
Table 2. Multivariate Cox Proportional Hazards Regression Analysis Examining Factors Associated With Progression-Free and Overall Survival.
| Variablea | PFS | OS | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Gradeb | ||||
| Well differentiated | 1 [Reference] | NA | 1 [Reference] | NA |
| Moderately differentiated | 1.19 (0.79-1.79) | .42 | 1.04 (0.68-1.59) | .85 |
| Poorly differentiated | 1.90 (1.18-3.07) | .008 | 1.98 (1.20-3.26) | .008 |
| Undifferentiated | 0.92 (0.46-1.83) | .82 | 0.93 (0.45-1.90) | .84 |
| Complete response | 0.50 (0.23-1.05) | .07 | 0.33 (0.13-0.86) | .02 |
| LVIb | 1.47 (1.08-1.98) | .01 | 1.54 (1.10-2.14) | .01 |
| PNIb | 2.04 (1.43-2.92) | <.001 | 1.68 (1.14-2.48) | .009 |
| NART | 0.72 (0.46-1.11) | .14 | 0.70 (0.44-1.11) | .13 |
| Total lymph nodes harvested | 0.99 (0.98-1.00) | .02 | NS | NS |
| Vascular resection | NS | NS | 1.40 (1.06-1.83) | .02 |
| AT | 0.56 (0.32-0.99) | .04 | 0.51 (0.27-0.94) | .03 |
| AT × PNI | 0.51 (0.31-0.84) | .009 | 0.55 (0.32-0.97) | .04 |
| AT × NART | 1.95 (1.13-3.39) | .02 | 2.36 (1.29-4.30) | .005 |
Abbreviations: AT, adjuvant therapy; HR, hazard ratio; LVI, lymphovascular invasion; NA, not applicable; NART, neoadjuvant radiation; NS, not significant; OS, overall survival; PFS, progression-free survival; PNI, perineural invasion.
All of these variables were coded as indicator variables (0/1) and missing values were all imputed at the mean. The inclusion of the imputed values did not have a significant effect on HRs and SEs. Six patients were excluded for having either an unknown recurrence status or an unknown recurrence date.
Grade was missing 10 values, LVI was missing 11 values, and PNI was missing 2 values.
Accordingly, on multivariate analysis, poorly differentiated tumors, LVI, and PNI were all associated with worse PFS and OS (Table 2). The need for vascular resection was also associated with worse OS, whereas higher lymph node yield was associated with improved PFS. The use of NART favored a benefit in PFS (hazard ratio [HR], 0.72 [95% CI, 0.46-1.11]; P = .14) and OS (HR, 0.70 [95% CI, 0.44-1.11]; P = .13) but did not reach statistical significance.
In the unadjusted comparison of survival discussed previously, AT was not associated with improved survival for patients who did not receive NART or had PNI on final pathology. However, on multivariate analysis after adjusting for other factors in a Cox proportional hazards regression model, AT was significantly associated with improved PFS for patients who did not receive NART or had PNI on final pathology (HR, 0.56 [95% CI, 0.32-0.99]; P = .045). Interaction terms on multivariate analysis revealed that receipt of NART would weaken the benefit of AT (AT × NART; HR, 1.95 [95% CI, 1.13-3.39]; P = .02), whereas the presence of PNI would enhance the benefit (AT × PNI; HR, 0.51 [95% CI, 0.31-0.84]; P = .009; Table 2). Similarly, AT was associated with improved OS for patients who did not receive NART or had PNI on final pathology (HR, 0.51 [95% CI, 0.27-0.94]; P = .03). However, NART decreased its benefit (AT × NART; HR, 2.36 [95% CI, 1.29-4.30]; P = .005), whereas the presence of PNI increased it (AT × PNI; HR, 0.55 [95% CI, 0.32-0.97]; P = .04; Table 2).
Discussion
The results of this cohort study suggest that AT may be beneficial for patients with resected PDAC who have N0 disease after NAT and surgical resection. This survival benefit was most pronounced in patients with PNI on final pathology. Our findings must be interpreted with the understanding that most of the patients reported herein received perioperative treatment sequencing that included less than 4 months of systemic NAT. In addition, physician judgment was used in the recommendation for AT; therefore, AT receipt was likely a surrogate marker for improved performance status. Considering these limitations, PNI tumor grade (high), LVI, and the need for vascular resection seem to be high-risk features associated with shorter OS in this cohort of patents with N0 disease. Our data also suggest that the maximal benefit of AT was observed in patients with PNI and may be less pronounced in patients with N0 disease who received NART.
Neoadjuvant therapy, especially when combined with NART, is successful in sterilizing regional lymph node metastases and has been shown to improve long-term survival compared with a surgery-first approach.23,24,25,26 As NART is incorporated into NAT regimens more frequently, N0 disease may be more commonly encountered. The decision to administer AT to patients who have received NAT is difficult because cumulative toxicities make AT delivery challenging for many patients. Physicians and patients are informed that N0 status still carries a high risk for recurrence; therefore, being able to dichotomize this population into higher and lower risk is clinically meaningful. The results reported herein suggest a benefit for AT in the subset of patients with N0 disease who had PNI identified on final histopathology. Although historical data report a PNI rate of up to 92.0% for patients treated with a surgery-first approach, NART has been associated with reduced rates of PNI, LVI, and margin-positive resection and improved pathologic tumor response.27 However, such local antitumor effects have not translated into improved median survival when NART is compared with chemotherapy alone.27,28,29 Recently reported results of the PREOPANC trial show a benefit in long-term (5-year) survival associated with NAT (including NART), which is likely due to improved local disease control.26
This study further identifies several “high-risk” features in N0 PDAC after NAT (including higher tumor grade, PNI, and LVI) and confirms their association with OS. These factors are known to be negative prognostic indicators in most gastrointestinal cancers, including colon and gastric cancer, cholangiocarcinoma, and hepatoma.30,31,32 In this study, tumors that required vascular resection were another high-risk feature associated with decreased survival. PDAC that requires vascular resection is often associated with larger tumor size, higher PNI incidence, and worse survival.33,34,35 In colon cancer, such locoregional pathologic features inform the need for AT in stage II disease, an entity that would not otherwise require AT. In this study, PNI was the most prevalent adverse pathologic feature, and its presence was suggestive of a benefit for AT receipt. However, PNI coexisted with other features, including positive microscopic margins, LVI, and higher tumor grade. Given the substantial overlap of PNI and these other high-risk pathologic features, it was not possible to isolate the benefit of AT for each high-risk feature without including PNI. Larger studies are needed to discern whether AT is beneficial in these specific subsets after NAT.
Limitations
This study is not without limitations, including its retrospective nature and the potential for selection bias. As mentioned earlier, the use of AT may have been a surrogate marker for improved patient performance status. In addition, NAT duration and NART use were not standardized in this study. Although both UPMC and MCW represent high-volume pancreatic cancer referral centers with experienced pathologists, the assessment of pathologic features such as margin status, PNI, and LVI is subject to variation because all data were abstracted from pathology reports; the pathology specimens were not rereviewed. Finally, although both centers used a multidisciplinary approach for the multimodal treatment of PDAC, the treatment algorithms were different, particularly regarding NAT duration, AT use, and the utility of minimally invasive techniques. Nonetheless, to our knowledge, this study is the first of its kind to evaluate a large cohort of patents with N0 PDAC after NAT among whom we attempted to delineate high-risk features that warrant consideration of AT.
Conclusions
The results of this cohort study of patients with N0 disease after NAT suggest a survival benefit for AT, particularly for patients with PNI. High-risk features in N0 PDAC after NAT included poorly differentiated tumors, LVI, PNI, and the need for vascular resection. These findings may apply largely to patients who received less than 4 months of systemic therapy before surgery and may not apply to patients treated with a total neoadjuvant approach.
eTable 1. Univariate Analysis for Variables Included in the Multivariate Model for Progression-Free Survival
eTable 2. Univariate Analysis for Variables Included in the Multivariate Model for Overall Survival
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913-2921. doi: 10.1158/0008-5472.CAN-14-0155 [DOI] [PubMed] [Google Scholar]
- 3.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10-27. doi: 10.14740/wjon1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Dunn JA, Stocken DD, et al. ; European Study Group for Pancreatic Cancer . Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576-1585. doi: 10.1016/S0140-6736(01)06651-X [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, et al. ; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup . FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825. doi: 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- 6.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310(14):1473-1481. doi: 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 7.Rangarajan K, Pucher PH, Armstrong T, Bateman A, Hamady Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: a systematic review and meta-analysis. Ann R Coll Surg Engl. 2019;101(7):453-462. doi: 10.1308/rcsann.2019.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Palmer DH, Ghaneh P, et al. ; European Study Group for Pancreatic Cancer . Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389(10073):1011-1024. doi: 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 9.Ahmad SA, Duong M, Sohal DPS, et al. Surgical outcome results from SWOG S1505: a randomized clinical trial of mFOLFIRINOX versus gemcitabine/nab-paclitaxel for perioperative treatment of resectable pancreatic ductal adenocarcinoma. Ann Surg. 2020;272(3):481-486. doi: 10.1097/SLA.0000000000004155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Versteijne E, Vogel JA, Besselink MG, et al. ; Dutch Pancreatic Cancer Group . Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br J Surg. 2018;105(8):946-958. doi: 10.1002/bjs.10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed H, Hnoosh D, Huang B, et al. Defining the optimal timing of adjuvant therapy for resected pancreatic adenocarcinoma: a statewide cancer registry analysis. J Surg Oncol. 2016;114(4):451-455. doi: 10.1002/jso.24314 [DOI] [PubMed] [Google Scholar]
- 12.Bakens MJ, van der Geest LG, van Putten M, et al. ; Dutch Pancreatic Cancer Group . The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: a nationwide population-based analysis. Cancer Med. 2016;5(10):2825-2831. doi: 10.1002/cam4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim RY, Christians KK, Aldakkak M, et al. Total neoadjuvant therapy for operable pancreatic cancer. Ann Surg Oncol. 2021;28(4):2246-2256. doi: 10.1245/s10434-020-09149-3 [DOI] [PubMed] [Google Scholar]
- 14.Truty MJ, Kendrick ML, Nagorney DM, et al. Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 2021;273(2):341-349. doi: 10.1097/SLA.0000000000003284 [DOI] [PubMed] [Google Scholar]
- 15.Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma: a phase 2 clinical trial. JAMA Oncol. 2018;4(7):963-969. doi: 10.1001/jamaoncol.2018.0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schorn S, Demir IE, Haller B, et al. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—a systematic review and meta-analysis. Surg Oncol. 2017;26(1):105-115. doi: 10.1016/j.suronc.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 17.van Roessel S, van Veldhuisen E, Klompmaker S, et al. ; European-African Hepato-Pancreato-Biliary Association . Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol. 2020;6(11):1733-1740. doi: 10.1001/jamaoncol.2020.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olecki EJ, Stahl KA, Torres MB, et al. Adjuvant chemotherapy after neoadjuvant chemotherapy for pancreatic cancer is associated with improved survival for patients with low-risk pathology. Ann Surg Oncol. 2021;28(6):3111-3122. doi: 10.1245/s10434-020-09546-8 [DOI] [PubMed] [Google Scholar]
- 19.Barnes CA, Chavez MI, Tsai S, et al. Survival of patients with borderline resectable pancreatic cancer who received neoadjuvant therapy and surgery. Surgery. 2019;166(3):277-285. doi: 10.1016/j.surg.2019.05.010 [DOI] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Pancreatic Adenocarcinoma 2021, version 2.2021. February 25, 2021. Accessed July 15, 2021. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- 21.Das R, McGrath K, Seiser N, et al. Tumor size differences between preoperative endoscopic ultrasound and postoperative pathology for neoadjuvant-treated pancreatic ductal adenocarcinoma predict patient outcome. Clin Gastroenterol Hepatol. 2022;20(4):886-897. doi: 10.1016/j.cgh.2020.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell F, Smith RA, Whelan P, et al. Classification of R1 resections for pancreatic cancer: the prognostic relevance of tumour involvement within 1 mm of a resection margin. Histopathology. 2009;55(3):277-283. doi: 10.1111/j.1365-2559.2009.03376.x [DOI] [PubMed] [Google Scholar]
- 23.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26(21):3496-3502. doi: 10.1200/JCO.2007.15.8634 [DOI] [PubMed] [Google Scholar]
- 24.Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol. 2002;20(10):2537-2544. doi: 10.1200/JCO.2002.11.064 [DOI] [PubMed] [Google Scholar]
- 25.Pisters PW, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998;16(12):3843-3850. doi: 10.1200/JCO.1998.16.12.3843 [DOI] [PubMed] [Google Scholar]
- 26.Versteijne E, van Dam JL, Suker M, et al. ; Dutch Pancreatic Cancer Group . Neoadjuvant chemoradiotherapy versus upfront surgery for resectable and borderline resectable pancreatic cancer: long-term results of the Dutch randomized PREOPANC trial. J Clin Oncol. 2022;40(11):1220-1230. doi: 10.1200/JCO.21.02233 [DOI] [PubMed] [Google Scholar]
- 27.Chopra A, Hodges JC, Olson A, et al. Outcomes of neoadjuvant chemotherapy versus chemoradiation in localized pancreatic cancer: a case-control matched analysis. Ann Surg Oncol. 2021;28(7):3779-3788. doi: 10.1245/s10434-020-09391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz MHG, Shi Q, Meyers JP, et al. Alliance A021501: preoperative mFOLFIRINOX or mFOLFIRINOX plus hypofractionated radiation therapy (RT) for borderline resectable (BR) adenocarcinoma of the pancreas. J Clin Oncol. 2021;39(3 suppl):377. doi: 10.1200/JCO.2021.39.3_suppl.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Versteijne E, Suker M, Groothuis K, et al. ; Dutch Pancreatic Cancer Group . Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch randomized phase III PREOPANC trial. J Clin Oncol. 2020;38(16):1763-1773. doi: 10.1200/JCO.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan H, Dong Q, Zheng B, Hu X, Xu JB, Tu S. Lymphovascular invasion is a high risk factor for stage I/II colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(28):46565-46579. doi: 10.18632/oncotarget.15425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Huang X, Sun J, et al. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointest Surg. 2015;19(6):1113-1122. doi: 10.1007/s11605-015-2761-z [DOI] [PubMed] [Google Scholar]
- 32.Quah HM, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51(5):503-507. doi: 10.1007/s10350-008-9246-z [DOI] [PubMed] [Google Scholar]
- 33.Peng C, Zhou D, Meng L, et al. The value of combined vein resection in pancreaticoduodenectomy for pancreatic head carcinoma: a meta-analysis. BMC Surg. 2019;19(1):84. doi: 10.1186/s12893-019-0540-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delpero JR, Boher JM, Sauvanet A, et al. Pancreatic adenocarcinoma with venous involvement: is up-front synchronous portal-superior mesenteric vein resection still justified? A survey of the Association Française de Chirurgie. Ann Surg Oncol. 2015;22(6):1874-1883. doi: 10.1245/s10434-014-4304-3 [DOI] [PubMed] [Google Scholar]
- 35.Giovinazzo F, Turri G, Katz MH, Heaton N, Ahmed I. Meta-analysis of benefits of portal-superior mesenteric vein resection in pancreatic resection for ductal adenocarcinoma. Br J Surg. 2016;103(3):179-191. doi: 10.1002/bjs.9969 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Univariate Analysis for Variables Included in the Multivariate Model for Progression-Free Survival
eTable 2. Univariate Analysis for Variables Included in the Multivariate Model for Overall Survival


