Abstract
The Clostridium botulinum C2 toxin ADP-ribosylates monomeric actin, thereby inducing disassembly of actin filaments, alteration of focal adhesions, and rounding of cells. After treatment with C2 toxin, cells stop to proliferate but remain viable for about 2 days. In view of reported correlations between the structure of the actin cytoskeleton and cell cycle transition, the effects of C2 toxin on the G2/M phase transition of the cell division cycle were studied. Since C2 toxin delayed entry into mitosis in HeLa cells, those enzymes which control entry into mitosis, the cyclin-dependent protein kinase mitosis-promoting factor (MPF) and the phosphatase cdc25-C were examined after treatment of synchronized cells with C2 toxin. MPF is composed of the regulatory cyclin B and the enzymatic p34cdc2 kinase subunits. For its activation at the G2/M border, p34cdc2 needs to be associated with cyclin B and additionally dephosphorylated at Tyr-15 by the specific phosphatase cdc25-C. Treatment of synchronized cells in S or G2 phase with C. botulinum C2 toxin prevented p34cdc2 protein kinase activation by inhibiting its tyrosine dephosphorylation at the G2/M border. Furthermore, the activity of cdc25-C phosphatase was decreased after treatment of cells with C2 toxin. Our results suggest that the prevented activation of the mitotic inducers p34cdc2 kinase and cdc25-C phosphatase represents the final downstream events in the action of C2 toxin resulting in a G2 phase cell cycle delay in synchronized HeLa cells.
The eukaryotic cell division cycle is driven by the precisely coordinated and controlled action of cyclin-dependent kinases (31). Entry into mitosis is under control of the cyclin-dependent kinase mitosis-promoting factor (MPF), which is composed of the enzymatic subunit p34cdc2, harboring serine/threonine kinase activity, and the regulatory subunit cyclin B (3, 13). For activation of p34cdc2 kinase at the G2/M border of the cell cycle, its assembly with cyclin B and subsequent dephosphorylation at Thr-14 and Tyr-15 by the specific phosphatase cdc25-C are essential (17, 23). Activated MPF phosphorylates a variety of substrate proteins which play key roles in the mechanism of cell division. Thus, active MPF is essential for entry into mitosis, and so its activation represents an important endogenous cell cycle control system (19).
Before activation of MPF at the G2/M border, the cell experiences a physiological restriction point in the G2 phase of the cell division cycle. At this cell cycle checkpoint, the necessary prerequisites for subsequent mitosis are controlled (for reviews, see references 8 and 39). At this point the cell can also integrate exogenous growth control signals from its environment—mediated by, for example, growth factors or cell-cell interaction and matrix attachment—with the endogenous key regulator of cell division, i.e., the superimposed activation machinery of MPF (18). Inhibition of the G2/M transition of the eukaryotic cell cycle seems to represent a protective mechanism, allowing the cell to react to various extracellular influences such as ionizing radiation (7, 33) or other DNA-damaging agents (32).
In recent years, correlations between the structure of the actin cytoskeleton and cell cycle transition have been reported. The Escherichia coli toxins cytotoxic necrotizing factor 1 (CNF-1) and cytolethal distending toxin both lead to a stabilization of actin filaments and, in parallel, inhibit the G2/M transition in HeLa cells (12, 16). In contrast, the F-actin-destroying drug dihydrocytochalasin B inhibits cell division by blocking cleavage into interphase but has no influence on mitotic processes (34).
In this study, we investigated the effects of the actin-ADP-ribosylating Clostridium botulinum C2 toxin on the G2/M transition of eukaryotic cells. The binary C2 toxin consists of the enzymatic component C2I (49 kDa) and the binding component C2II (activated form about 60 kDa [40]). The two components represent separate proteins. When exhibiting its cytotoxic effects, C2II binds to the cell surface, thereby creating a binding site for C2I. Subsequently, the proteins enter the cell via receptor-mediated endocytosis and C2I is released into the cytosol (46), where it ADP-ribosylates monomeric actin at arginine-177 (1, 49). This ADP-ribosylation of G-actin leads to a complete disassembly of the actin filaments and thereby to a total breakdown of the actin cytoskeleton (51). Consequently, cells round up and focal adhesions are altered. After C2 toxin treatment, a significant decrease of cell division was observed. The destruction of actin filaments could be the underlying mechanism for inhibition of cytokinesis, as in the case of cytochalasin treatment (34). Using synchronized HeLa cells, we show that destruction of the actin cytoskeleton induced by C. botulinum C2 toxin is accompanied by a transiently delayed entry of cells into mitosis. The activating tyrosine dephosphorylation of the p34cdc2 protein kinase at the G2/M border was prevented after C2 toxin incubation, and the kinase remained inactive. Furthermore, the cdc25-C phosphatase activity was decreased after treatment of synchronized cells with C2 toxin.
MATERIALS AND METHODS
Materials.
Cell culture medium and trypan blue were obtained from Biochrom (Berlin, Germany), fetal calf serum was obtained from PAN Systems (Aidenbach, Germany), and cell culture materials were obtained from Falcon (Heidelberg, Germany). Amethopterin and thymidine were from Calbiochem (Frankfurt, Germany). Paraformaldehyde was from Merck (Darmstadt, Germany). The C2II binding component from C. botulinum C2 toxin was purified and activated with trypsin as described previously (40, 42). The C2I enzyme component was purified as a recombinant glutathione S-transferase fusion protein as described (6). The pGEX2T vector (included in the glutathione S-transferase gene fusion system) and glutathione-Sepharose 4B were purchased from Pharmacia Biotech (Uppsala, Sweden). The low-molecular-mass protein marker was from Bio-Rad (Hercules, Calif.), and the nitrocellulose blotting membrane was from Schleicher & Schuell (Dassel, Germany). Protein A/G PLUS-agarose beads and anti-cyclin B- and antiphosphotyrosine antibodies were from Santa Cruz (Heidelberg, Germany). Anti-p34cdc2 antibody was from Gibco (Karlsruhe, Germany). Anti-mouse antibody coupled to peroxidase was from Dianova (Hamburg, Germany), and donkey anti-rabbit antibody coupled to peroxidase and the enhanced chemiluminescence detection kit were obtained from Amersham (Braunschweig, Germany). Thrombin and phalloidin-rhodamine were purchased from Sigma (Deisenhofen, Germany), [32P]ATP (specific activity, 3 Ci/mmol) was from Amersham Buchler (Braunschweig, Germany), and [32P]NAD (30 Ci/mmol) was from DuPont NEN (Bad Homburg, Germany). Histone H1 was obtained from Boehringer (Mannheim, Germany). Aquasafe 500 scintillation cocktail was from Zinsser Analytic (Frankfurt, Germany). The basic fuchsin for Feulgen staining was from Janssen-Pharma (Geel, Belgium).
Cell culture, synchronization, and cell cycle analysis.
HeLa cells were cultivated in tissue culture flasks as monolayers at 37°C and 5% CO2 in Eagle’s minimal essential medium with Earl’s salts containing 10% fetal calf serum, 2 mM l-glutamate, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cells were routinely trypsinized and reseeded twice a week. For experiments, subconfluent growing monolayer cells (about 105 cells/cm2) in 3-cm-diameter plastic dishes were synchronized as described by Mueller and Kajiwara by blockage with 10−6 M amethopterin for 16 h in complete medium and subsequent release by thymidine (10 μg/106 cells) (37). Because the C. botulinum C2 toxin exhibits its full effects on the actin cytoskeleton of HeLa cells after about 2 to 3 h, at 4 or 6 h after release, i.e., when most of the cells were in the S phase, the C2 toxin was added to the synchronized cells (200 ng of activated C2II and 100 ng of C2I per ml) and cells were incubated at 37°C. The degree of cell synchrony was analyzed by flow cytometric measurements of DNA distribution (26) and by counting of mitotic figures, i.e., rounded cells (25). Viability of the cells was tested with a 30-min incubation at 37°C with trypan blue. The cell number was determined with a Neubauer chamber. For a detailed cell cycle analysis, a combined morphological and flow cytometric determination was carried out. For the former, the monolayer cells were removed from the dishes with 0.05% trypsin and heat fixed on glass slides. The chromatin was stained with the Feulgen reagent (43). This procedure allows analysis of mitotic cells among the cell fraction in which rounding was induced by the action of C2 toxin.
Fluorescence staining of F-actin.
For fluorescence staining of F-actin, HeLa cells treated with or without C2 toxin were fixed for 30 min at 25°C in phosphate-buffered saline (PBS) containing 4% paraformaldehyde and 0.1% Triton X-100. Thereafter, cells were briefly washed and incubated for 30 min with phalloidin-rhodamine (600 ng/ml) at room temperature in the dark (6).
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method of Laemmli (29). For kinase assays, gels were stained with 0.1% Coomassie brilliant blue R-250 in methanol-acetic acid-water (40:10:50), destained in this solution without dye, and dried for autoradiography. For immunoblot analysis, the proteins (100 μg per lane; determined by the method of Bradford [9]) were transferred from the gel onto a nitrocellulose membrane by using a semidry transfer cell (Bio-Rad, Munich, Germany). The membrane was blocked for 30 min with 5% non fat dry milk in PBS containing 0.05% Tween 20 (PBS-T), and then the proteins were probed with either anti-p34cdc2 antibody (rabbit; 1:2,000 in PBS-T), anti-cyclin B antibody (rabbit; 1:2,000), or antiphosphotyrosine (anti-P-Tyr) antibody (mouse, 1:2,000). After washing with PBS-T, the blots were incubated for 1 h with donkey anti-rabbit antibody coupled to horseradish peroxidase (1:2,000 in PBS-T) or with anti-mouse antibody coupled to peroxidase (1:2,000), respectively. The membrane was washed again, and proteins were visualized by enhanced chemiluminescence according to the manufacturer’s instructions.
ADP-ribosylation assay.
To test the C2 toxin effect on cells, in vitro analysis of the ADP-ribosylation state of cellular actin was done as described previously (1). Cells were washed with cold PBS, scraped into 500 μl of lysis buffer (50 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 1 mM dithiothreitol [DTT]), and sonicated, and 100 μg of protein (determined by the method of Bradford [9]) was incubated with 500 nM [adenylate-32P]NAD (about 25 nCi) and 50 ng of C2I toxin for 15 min at 37°C. The reaction was stopped by addition of Laemmli buffer, aliquots (50 μg protein of the reaction mixture) were subjected to SDS-PAGE in a 12.5% gel, and [32P]ADP-ribosylated proteins were detected by autoradiography with a PhosphorImager from Molecular Dynamics (Krefeld, Germany).
Immunoprecipitation of p34cdc2 and histone H1 kinase assay.
Immunoprecipitation was performed as described previously (4). Cells were washed with cold PBS, scraped into 1 ml of cold lysis buffer (50 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 1 mM DTT, 0.1 mM sodium orthovanadate, 50 mM NaF, 50 μg of phenylmethylsulfonyl fluoride per ml), and gently sonicated on ice. After protein determination, p34cdc2 was immunoprecipitated from 100 μg of cell lysate protein in 1 ml of lysis buffer with 2 μl of anti-p34cdc2 antibody (1 mg/ml) and 50 μl of a 1:1 slurry of protein A/G-agarose beads for 2 h at 4°C. The immunoprecipitates were pelleted (2,000 rpm in an Eppendorf centrifuge) and washed three times with cold lysis buffer. The immunoprecipitates were used for histone H1 kinase assay or immunoblot analysis. Histone kinase assays were carried out by addition of 10 μl of a buffer containing 50 mM Tris-HCl (pH 7.4), 10 mM MgCl2, 1 mM DTT, 3.3 μM ATP, 16 μg of histone H1, and 5 μCi of [γ-32P]ATP (specific activity, 3 Ci/mmol) to the immunoprecipitated p34cdc2. The reaction mixture was incubated for 10 min at 37°C, and the reaction was stopped by addition of 10 μl of 2× Laemmli sample buffer and heating for 2 min at 95°C. The agarose beads were pelleted at 420 × g (Eppendorf centrifuge model 5417R) for 3 min, and the proteins were separated by SDS-PAGE on a 12.5% gel. The 32P-labeled histone H1 proteins were excised and incubated overnight with 2 ml of a scintillation cocktail at 25°C, and radioactivity was determined by scintillation counting (4).
Immunoprecipitation and phosphatase assay of cdc25-C.
Immunoprecipitation of cdc25-C and the subsequent phosphatase assay were performed as described earlier (23). In brief, cells were lysed in the buffer described above (without sodium orthovanadate) and sonicated, and 3 mg of lysate protein was incubated for 2 h at 4°C with cdc25-C antiserum (IH37) and for additional 2 h at 4°C with 50 μl of a 1:1 slurry of protein A/G-agarose beads. The collected immunoprecipitates were washed three times and used for cdc25-C phosphatase assay with inactive p34cdc2 as the substrate. Therefore, inactive p34cdc2 was immunoprecipitated from 500 μg of S-phase HeLa lysate protein (without sodium orthovanadate) as described above. Immunoprecipitated p34cdc2 kinase and cdc25-C phosphatase were mixed and incubated together for 15 min at 30°C. The reaction was stopped on ice, and the samples were washed three times with buffer (50 mM Tris-HCl [pH 7.4], 10 mM MgCl2, 1 mM DTT, 0.1 mM sodium orthovanadate). Subsequently, the samples were assayed for p34cdc2 kinase activity at 37°C for 10 min as described above.
All experiments were carried out at least two times. Data from representative experiments are presented.
RESULTS
C. botulinum C2 toxin delays the G2/M phase transition in synchronized HeLa cells.
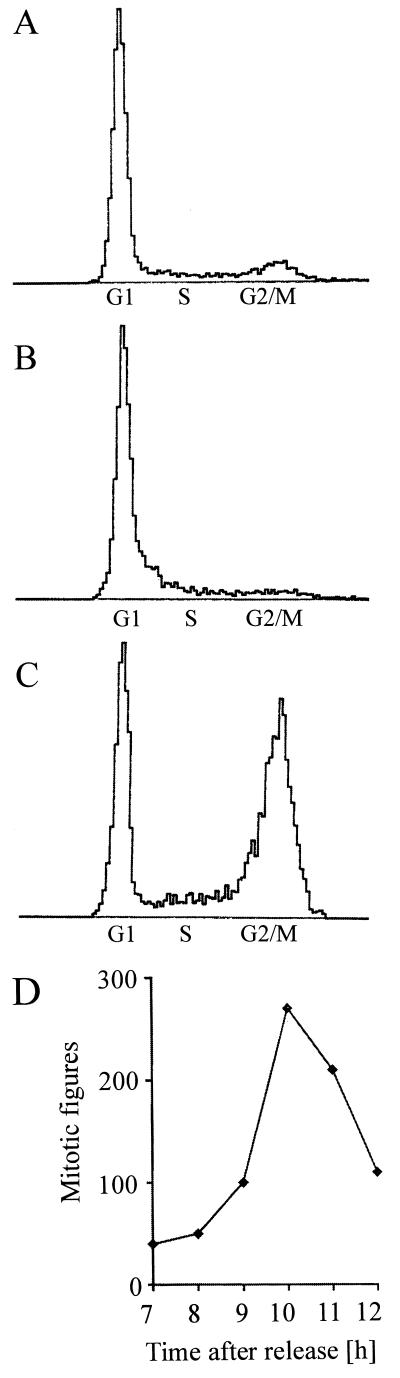
C. botulinum C2 toxin ADP-ribosylates monomeric actin in eukaryotic cells, thereby leading to disassembly of actin filaments and breakdown of the actin cytoskeleton (51). As a consequence, C2 toxin-treated cells round up and their substrate attachment is altered. We observed that C2 toxin-treated logarithmically growing HeLa cells stopped proliferating. Up to 72 h after C2 toxin addition, no significant increase in cell number was detectable (compared with control cells), while the majority of cells appeared to be viable as indicated by trypan blue exclusion even after a 48-h C2 toxin treatment (Table 1). Cells treated with C2 toxin did not recover and did not start to proliferate again. Most of the cells exposed to C2 toxin for longer than 3 days became detached from the substrate and were no more able to exclude trypan blue. Based on these findings, we tested the influence of C2 toxin on the division of synchronized HeLa cells. Cells were blocked in the S phase of the cell cycle with amethopterin and subsequently released from this block with thymidine (37). The degree of cell cycle synchrony achieved by this method is demonstrated in Fig. 1 by DNA histograms obtained by flow cytometry (26). Compared with an asynchronously growing culture (Fig. 1A), a high proportion (about 98%) of cells treated for 16 h with amethopterin accumulated in S phase (inclusive of cells at the G1/S border) (Fig. 1B). At 9 h after release from the block with thymidine, the majority (50 to 70%) of cells were in the G2/M phase of the cell cycle (Fig. 1C). Because cell cycle analysis by fluorescence-activated cell sorting does not allow one to distinguish between cells in G2 phase and cells in mitosis, we determined the amount of cells in mitosis by microscopic counting of mitotic figures, i.e., rounded cells with the typical condensed chromosomes (25, 47). Figure 1D shows the time course of mitotic figures from 7 to 12 h after release of the cells from amethopterin blockage. The majority of cells entered mitosis between 9 and 10 h after release from the S-phase block. The experiment is representative of more than 20 similar control experiments in which the number of mitotic figures per field determined in viable cultures increased between 7 and 10 h after release at least seven times.
TABLE 1.
Cell number and viability of HeLa cells treated with C. botulinum C2 toxina
| Cells | No. of cells/dish (dead cells as % of total cell no.) after incubation for:
|
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Control | 1.7 × 105 (<10) | 3.2 × 105 (<10) | 6.0 × 105 (<10) |
| C2 treated | 1.0 × 105 (<10) | 1.0 × 105 (<10) | 1.2 × 105 (30) |
HeLa cells were grown in complete medium at 37°C in the absence (control) or in the presence of C2 toxin (200 ng of C2II and 100 ng of C2I per ml). After 24, 48, and 72 h, the cells were incubated for 30 min with 0.36% trypan blue in complete medium, and the total cell number and the number of cells unable to exclude trypan blue were determined with a Neubauer chamber.
FIG. 1.
Cell cycle phase distribution of synchronized HeLa cells. HeLa cells were synchronized with amethopterin and thymidine as described in the text. At the indicated times, cells were fixed and analyzed by flow cytometry. DNA histograms represent asynchronous cells (A), amethopterin-blocked cells (16 h; B) and cells 9 h after release from the amethopterin block (C). Abscissa, relative fluorescence; ordinate, relative cell number. (D) Time course of mitotic figures of amethopterin-thymidine-synchronized HeLa cells starting 7 h after release from the block.
To demonstrate that the C2 toxin exerted its cytotoxic effects even on cells synchronized with the described procedure, C2 toxin was added to the medium 6 h after release from the amethopterin block, and cells were further incubated at 37°C. Every 30 min, a culture was lysed and subjected to an in vitro ADP-ribosylation assay with C2I. The autoradiogram in Fig. 2A shows a significantly decreased signal of [32P]ADP-ribosylated G-actin in the lysates after a 2 to 3 h incubation of cells with C2 toxin. This indicates that after about 3 h, the majority of actin was ADP-ribosylated by the C2 toxin in intact cells and no longer constituted a substrate for subsequent in vitro ADP-ribosylation by C2I. This observation is confirmed by F-actin staining of synchronized cells treated with C2 toxin for 2 h. C2 toxin was added at 6 h after release from the amethopterin block to the cells (for control without toxin), cells were fixed and the F-actin was stained with phalloidin-rhodamine. Figure 2B shows the C2 toxin caused disassembly of the actin filaments.
FIG. 2.
Cytotoxic effect of C. botulinum C2 toxin on synchronous HeLa cells. (A) Time course of C2 toxin-induced actin ADP-ribosylation. At 6 h after release from the amethopterin block, C2 toxin (200 ng of C2II and 100 ng of C2I per ml) was added to synchronized HeLa cells. Cells were incubated at 37°C; immediately and every 30 min after toxin addition, cells were lysed and lysate proteins (100 μg) were subjected to an in vitro ADP-ribosylation assay with C2I. The autoradiogram of [32P]ADP-ribosylated actin is shown. Lane 1, control (without C2 toxin); lanes 2 to 8, incubation for 30 min with C2 toxin, 60 min with C2, 90 min with C2, 120 min with C2, 150 min with C2, 180 min with C2, and 210 min with C2, respectively. (B) C2 toxin-induced morphological changes and F-actin redistribution. Synchronized control cells (8 h after release from the amethopterin block) as well as synchronized cells treated with C2 toxin for 2 h (6 to 8 h after release from the block; 200 ng of C2II and 100 ng of C2I per ml) were fixed, and F-actin was stained with phalloidin-rhodamine.
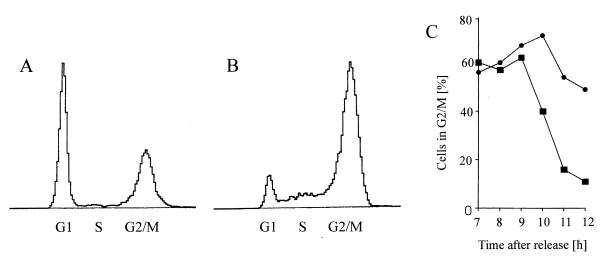
To test whether the C2 toxin inhibits the division of HeLa cells, C2 toxin was added to synchronized cells 4 h after release from the block. The toxin needs 2 to 3 h to exhibit its full effects. During this time, most of the cells were in late S phase. The cells were further incubated in the presence of the toxin (200 ng of C2II and 100 ng of C2I per ml) in complete medium at 37°C. To determine their cell cycle progression, control cells and C2 toxin-treated cells were analyzed at 1-h intervals by flow cytometry starting at 7 h after release from the block. The passage of cells through S phase was apparently not altered by the toxin; however, that through G2 phase was affected. Figure 3 shows the DNA histograms of control cells (Fig. 3A) and C2-treated cells (Fig. 3B) at 11.5 h after release from the amethopterin block, i.e., 7.5 h after addition of C2 toxin. While in the control culture only 16% of the cells were in G2/M but 74% were in G1, after C2 treatment 54% of the cells were in G2/M and only 25% were in the G1. This result indicates that C2 toxin treatment delayed the cell division and thereby entry into the G1 phase of the cell cycle. These findings were confirmed by the time course of cells in the G2/M phase, determined by flow cytometry (Fig. 3C). While the control cells started to leave the G2/M phase at 9 h after release from the S-phase block, cells treated at 4 h after release with C2 toxin remained in G2/M. Because C2 toxin-treated cells rounded up after about 2 to 3 h (Fig. 2B), it was not possible to determine the amount of mitotic figures in these cultures through morphological criteria by microscopic counting. From the results obtained by flow cytometry described above, it was not clear whether the C2 toxin-treated cells were blocked in mitosis, or whether C2 toxin treatment of cells in late S or early G2 phase, respectively, prevented their subsequent entry into mitosis and delayed the cells at the G2/M border. For a more detailed characterization of that topic, cells were analyzed with respect to their cell cycle phase and especially their mitotic phase. Cells were treated at 4 h after release from the amethopterin block with C2 toxin. At 9.75 and 11.25 h after release (i.e., at 5.75 and 7.25 h after C2 toxin addition), C2 toxin-treated and control cells were collected from the dish and fixed on glass slides, and their DNA was stained with Feulgen reagent. The cell cycle phases of these cells are given in Table 2. The data revealed that C2 toxin treatment provoked a significant but transient delay of entry into mitosis. Subsequently cytokinesis might be blocked because of the destruction of the actin cytoskeleton. In the following experiments, we focused on mechanistic aspects of this C2 toxin-induced delay of the G2/M transition.
FIG. 3.
G2 delay of synchronous HeLa cells induced by the C. botulinum C2 toxin. Four hours after release from the amethopterin block, the cells were treated with C2 toxin (200 ng of C2II and 100 ng of C2I per ml). Starting at 3 h after addition of the C2 toxin (i.e., 7 h after release from the block), cells were fixed and analyzed by flow cytometry. DNA histograms represent control cells (A) and C2 toxin-treated cells (B) at 11.5 h after release from the block (i.e., after 7.5 h of C2 toxin treatment). (C) Time course of the percentage of control (■) and C2-treated (●) cells in G2/M phase, determined by flow cytometry.
TABLE 2.
Cell cycle distribution of synchronized HeLa cells treated with C. botulinum C2 toxina
| Cells | % of cells (mean ± SD [n = 6])
|
|||||
|---|---|---|---|---|---|---|
| 9.75 h after release
|
11.25 h after release
|
|||||
| G2 | M | G1/S | G2 | M | G1/S | |
| Control | 62 ± 8 | 19 ± 5 | 19 ± 6 | 7 ± 5 | 10 ± 4 | 83 ± 8 |
| C2 treated | 85 ± 4 | 9 ± 3 | 6 ± 3 | 54 ± 4 | 38 ± 5 | 8 ± 3 |
HeLa cells were synchronized with amethopterin and thymidine as described in the text, and at 4 h after release from the amethopterin block were treated with C2 toxin (200 ng of C2II and 100 ng of C2I per ml). At 9.75 and 11.25 h after release, toxin-treated and untreated control cells were collected from the dish by trypsin treatment and subsequent centrifugation. Part of the cells were fixed on glass slides, and their DNA was stained with the Feulgen reagent. Morphological analysis was carried out on enlarged photographic prints. The other part of the cells was analyzed by flow cytometry.
C2 toxin treatment prevents the activation of p34cdc2 kinase at the G2/M border.
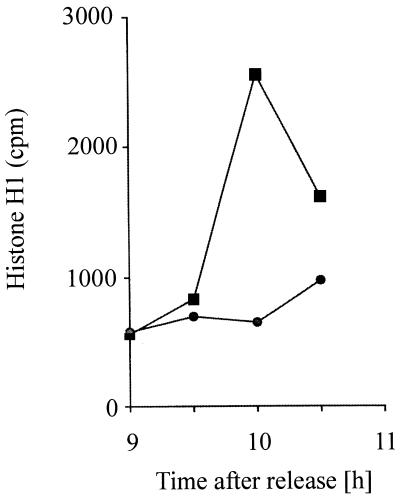
Since C2 toxin treatment of cells delayed their entry into mitosis prior to G2/M transition, i.e., at a physiological restriction point of the cell cycle, the p34cdc2 kinase activity from C2 toxin-treated cells was analyzed. This enzyme normally becomes activated at the G2/M border and catalyzes entry into mitosis (13). The p34cdc2 kinase activity was measured by immunoprecipitation of p34cdc2 and subsequent in vitro phosphorylation assay with histone H1 as the substrate (3, 36). To test whether p34cdc2 kinase activation of C2 toxin-treated cells is prevented, a time course of p34cdc2 protein kinase activity of synchronized HeLa cells treated with or without C2 toxin was performed. For this purpose, C2 toxin was added at 6 h after release from the amethopterin block to the cells (200 ng of C2II and 100 ng of C2I per ml), which were further incubated at 37°C. Starting at 9 h after release from the block, i.e., when the toxin had been present in the medium for 3 h, every 30 min control cells or C2 toxin-treated cells were lysed. Thereafter, p34cdc2 was immunoprecipitated from 100 μg of cell lysate protein and analyzed for histone kinase activity. As shown in Fig. 4, p34cdc2 kinase activity from control cells dramatically increased between 9.5 and 10 h after release from the S-phase block, reflecting the time course of mitotic figures (Fig. 1D). The kinase activity rapidly decreased after the mitotic peak due to the rapid inactivation of this enzyme after mitotic metaphase. In contrast, p34cdc2 kinase activity from C2 toxin-treated cells showed no significant increase. This means that C2 toxin treatment of synchronized HeLa cells in the late S or early G2 phase of the cell cycle inhibits the subsequent activation of the mitotic inducer p34cdc2 kinase.
FIG. 4.
Time course of p34cdc2 protein kinase activity of synchronized HeLa cells untreated (control) or treated with C. botulinum C2 toxin. At 6 h after release from the amethopterin block, C2 toxin (200 ng of C2II and 100 ng of C2I per ml) was added to the medium, and the cells were further incubated at 37°C. Starting at 9 h after release from the block, every 30 min control cells (■) and cells treated with C2 toxin (●) were lysed, and p34cdc2 was immunoprecipitated and analyzed for histone H1 kinase activity.
C2 toxin prevents the activating tyrosine dephosphorylation of p34cdc2.
Based on the finding that C2 toxin delays cells at the G2/M border by preventing activation of p34cdc2 kinase, we attempted to analyze the underlying mechanism. MPF, which is composed of the enzymatic Ser/Thr kinase p34cdc2 and the regulatory protein cyclin B, is activated after assembly of these two subunits at the G2/M border by dephosphorylation of tyrosine-15 (and threonine-14) of the p34cdc2 subunit (23). It is feasible that the prevention of MPF activation by C2 toxin is caused by (i) effects on the cellular amounts of p34cdc2 and/or cyclin B, (ii) alteration of complex assembly, or (iii) prevention of tyrosine dephosphorylation of p34cdc2.
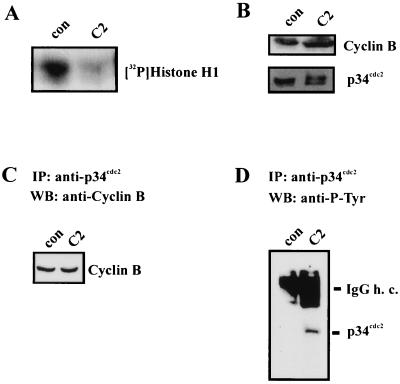
To elucidate the effects of C2 toxin on activation of the MPF complex of synchronized HeLa cells, C2 toxin (200 ng of C2II and 100 ng of C2I per ml) was added to the cells at 6 h after release from the amethopterin block. After incubation at 37°C for a further 4 h, p34cdc2 was immunoprecipitated for histone kinase assay. The autoradiogram of the phosphorylated histone H1 is shown in Fig. 5A. To estimate the amounts of the p34cdc2 and cyclin B subunits, lysate proteins were subjected to immunoblot analysis with anti-p34cdc2 and anti-cyclin B antibodies, respectively. As shown in Fig. 5B, C2 toxin treatment did not affect the amounts of the MPF subunits. Furthermore, C2 did not disturb the assembly of p34cdc2 and cyclin B because the complete complex was immunoprecipitated by anti-p34cdc2 antibody and protein A/G-agarose beads (Fig. 5C). After toxin treatment, the higher-migrating, i.e., tyrosine-phosphorylated inactive, form of p34cdc2 was still detectable, while in control cells the complete p34cdc2 was dephosphorylated, i.e., activated (Fig. 5C). To test any effect of C2 toxin treatment on tyrosine phosphorylation of p34cdc2, a blot of p34cdc2 immunoprecipitates was probed with anti-P-Tyr antibody. Figure 5D shows that after C2 toxin treatment, p34cdc2 was tyrosine phosphorylated to a significantly higher degree than p34cdc2 from control cells. These results indicate that C. botulinum C2 toxin prevents tyrosine dephosphorylation and activation of the catalytic p34cdc2 subunit of MPF. The lack of MPF activation seems to represent the reason for the C2 toxin-induced G2 delay of synchronized HeLa cells.
FIG. 5.
Effect of C. botulinum C2 toxin on the MPF complex of synchronized HeLa cells. At 6 h after release from the amethopterin block, C2 toxin (200 ng of C2II and 100 ng of C2I per ml) was added to the cells. After 4 h at 37°C, cells were lysed and p34cdc2 was immunoprecipitated for determination of histone kinase (A). Lysate proteins (100 μg) were subjected to immunoblot analysis with anti-p34cdc2 antibody and anti-cyclin B antibody (B). Anti-p34cdc2 immunoprecipitates (IP) from the same lysates were probed by Western blotting (WB) with anti-cyclin B antibody (C). The influence of C2 toxin on the tyrosine phosphorylation of p34cdc2 was analyzed by immunoblot analysis of p34cdc2 immunoprecipitates with anti-P-Tyr (D). con, control; IgG h. c., immunoglobulin G heavy chain.
C2 toxin treatment prevents the activation of cdc25-C phosphatase at the G2/M border.
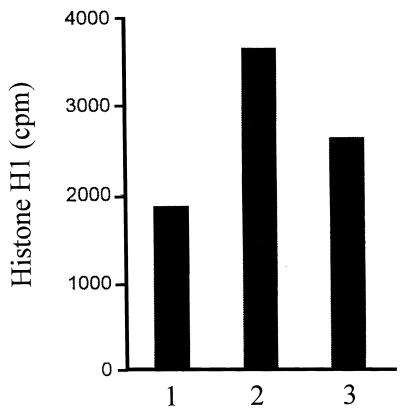
The finding that C2 toxin treatment of cells prevented the activating tyrosine dephosphorylation of p34cdc2 kinase led us to investigate the influence of the toxin on the activity of cdc25-C phosphatase. Synchronized cells were treated at 6 h after release from the amethopterin block with or without C2 toxin and incubated for further 4 h, i.e., until 10 h after release. Then the cells were lysed, and cdc25-C was immunoprecipitated for phosphatase assay. The cdc25-C immunoprecipitates were incubated for 15 min at 30°C with immunoprecipitated inactive p34cdc2 kinase as the substrate. The inactive kinase was immunoprecipitated from S-phase HeLa cells. Finally, the activity of the activated p34cdc2 kinase was measured by histone H1 phosphorylation assay. The data shown in Fig. 6 demonstrate that the activity of the inactive p34cdc2 kinase (bar 1) was significantly increased by treatment of the kinase with cdc25-C phosphatase from control cells (bar 2). In contrast, a weaker activation was measured on incubation with cdc25-C isolated from C2 toxin-treated cells. This result indicates that C2 toxin treatment of cells prevents activation of cdc25-C phosphatase at the G2/M border.
FIG. 6.
Effect of C. botulinum C2 toxin on cdc25-C phosphatase of synchronized HeLa cells. At 6 h after release from the amethopterin block, C2 toxin (200 ng of C2II and 100 ng of C2I per ml) was given to the cells (for control without toxin). After further 4 h at 37°C, i.e., at 10 h after release, cells were lysed and cdc25-C was immunoprecipitated for determination of phosphatase activity. The cdc25-C immunoprecipitates were incubated for 15 min at 30°C with inactive p34cdc2 immunoprecipitated from 500 μg of S-phase HeLa lysate protein. Activity of p34cdc2 kinase was measured by histone H1 phosphorylation assay. Histone H1 bands were measured by scintillation counting. Bar 1, S-phase p34cdc2 kinase (without cdc25-C); bar 2, S-phase p34cdc2 kinase plus cdc25-C from control cells; bar 3, S-phase p34cdc2 kinase plus cdc25-C from C2 toxin-treated cells.
DISCUSSION
A variety of extracellular signals can delay proliferating cells either reversibly or irreversibly at one of two major physiological restriction points of the cell division cycle (24). One of these checkpoints is in G1 phase; the other is in G2 phase prior to mitosis. At these restriction points, the cell can integrate exogenous growth-controlling signals from its environment via various signal cascades with the endogenous control systems of cell division (18, 20). The endogenous cell cycle control system is represented by the enzyme family of cyclin-dependent protein kinases, which drive the cell through the individual phases of the division cycle. While much progress has been made in detailed elucidation of the G1-phase cell cycle checkpoint, the mechanisms leading to a G2 arrest are less understood.
In this study, we addressed the question of whether the actin ADP-ribosylating C. botulinum C2 toxin has any influence on the cell cycle transition of eukaryotic cells since cells treated with C2 toxin seem to stop their proliferation, even when the toxin is removed from the medium. C2 toxin is known to disassemble the cellular actin filaments within a few hours of cell treatment, leading to a breakdown of the actin cytoskeleton and alterations in adhesion of the cell to the substrate. Cytokinesis itself might be influenced because of the disrupted actin cytoskeleton, as observed after cytochalasin treatment (34). Synchronized HeLa cells were treated with C2 toxin during S phase, and analysis of their passage through G2/M by flow cytometry showed a significant but transient delay in G2. After the G2 delay, the C2 toxin-treated cells entered mitotic prophase.
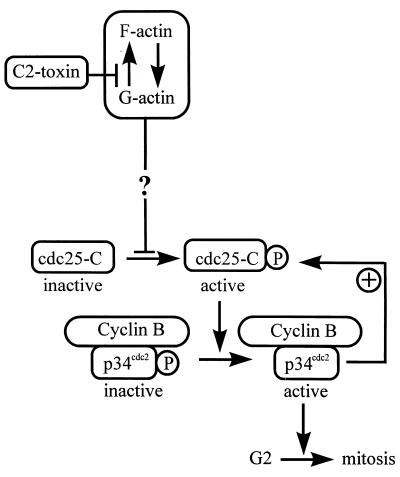
With respect to the mechanism of the G2 delay caused by C2 toxin, we analyzed its influence on the cyclin-dependent protein kinase mitosis-promoting factor, MPF. This enzyme controls entry into mitosis by phosphorylating specific proteins involved in cell division, such as histone H1 or the lamins. MPF consists of the catalytic subunit p34cdc2, which associates with the regulatory protein cyclin B to form the inactive pre-MPF complex during G2 phase (17). Pre-MPF is activated at the G2/M border by dephosphorylation of p34cdc2 at Thr-14 and Tyr-15, catalyzed by the specific phosphatase cdc25-C. Cells treated with C2 toxin failed to activate p34cdc2 kinase by dephosphorylation, indicating that the C2 toxin-induced inhibition of cell division is due to a block prior to mitosis, i.e., in late G2 phase of the cell cycle. Furthermore, C2 toxin treatment prevented the activation of cdc25-C phosphatase at the G2/M border. Since p34cdc2 kinase and cdc25-C phosphatase are thought to activate each other via an autocatalytic loop (23), one cannot distinguish which of the two enzymes may act as a primary target for toxin-induced signals leading to the G2-phase delay. As shown in Fig. 7, C2 toxin may induce intracellular signals acting upstream of MPF or cdc25-C.
FIG. 7.
Model for the G2 phase cell cycle arrest induced by C. botulinum C2 toxin. Cyclin B/p34cdc2 is activated at the G2/M border by dephosphorylation of p34cdc2 by the phosphatase cdc25-C. Active p34cdc2 kinase drives the cell into mitosis. Furthermore, active p34cdc2 kinase activates cdc25-C phosphatase via an autocatalytic loop. Treatment of HeLa cells with C2 toxin delays the G2/M transition by preventing tyrosine dephosphorylation and thereby activation of the p34cdc2 protein kinase. C2 toxin treatment also prevents activation of cdc25-C phosphatase, which indicates that C2 toxin may affect intracellular signal cascades upstream of the mitotic inducers p34cdc2 and cdc25-C.
The phosphatase cdc25-C itself is active in the phosphorylated form and becomes inactivated by dephosphorylation through protein phosphatase 2A (PP2A) (11, 23). The lack of active (i.e., phosphorylated) cdc25-C caused by C2 toxin might be due to an activated PP2A. It has been reported that active PP2A (called INH) may inhibit cells in G2 phase (30), while inhibition of PP2A leads to a mitosis-like state of cells (21). Since on the one hand PP2A can be inactivated by tyrosine phosphorylation of the catalytic subunit (10) and on the other hand C2 toxin treatment of cells was shown to lead to an alteration in tyrosine phosphorylation of various proteins, probably due to an activation of protein tyrosine phosphatases (45), the superimposed machinery of MPF activation, including PP2A, could represent a target for the C2 toxin-induced effects. At present, however, the pathway connecting the destruction of the actin cytoskeleton to the enzymatic machinery responsible for mitotic control is not clear. A possible link between the actin cytoskeleton and PP2A may be indicated by the observation that actin can be specifically coprecipitated by the use of monoclonal antibodies directed toward the amino-terminal domain of PR65, the conserved regulatory subunit of PP2A (28). The in vivo relevance of this observation, however, is not yet known.
An involvement of cytoskeletal structures and cell adhesion molecules such as integrins in cell cycle control has been discussed (44, 48, 50, 52). In the case of destruction of the actin cytoskeleton by dihydrocytochalasin B, a G2 delay was not observed but there was an inhibition of the division of synchronized HeLa cells. These cells are arrested in mitosis because their cleavage furrow is blocked. While the machinery for cell cleavage was disturbed, no influence on the mitotic processes could be observed (34).
Recently other bacterial toxins which act on the actin cytoskeleton of the cell have been analyzed for their influence on the cell cycle transition. Cytolethal distending toxin, produced by E. coli and Campylobacter jejuni (2, 12), and CNF-1, from E. coli (15, 16), both stabilize the actin filaments and, in parallel, induce a G2 arrest in eukaryotic cells. Cytolethal distending toxin thereby prevents p34cdc2 protein kinase dephosphorylation and activation (2). That means that the actin-disrupting C2 toxin shows similar effects with respect to the G2/M transition as the actin-stabilizing bacterial toxins CNF-1 and cytolethal distending toxin and acts via a different mechanism than dihydrocytochalasin D, which disassembles the actin cytoskeleton as C2 toxin does. In no case so far has a mechanistic link between changes of the cytoskeleton and the cell cycle regulatory enzymes been established. The results point, however, to the same molecular targets for C2 toxin and cytolethal distending toxin from E. coli. Genetic evidence for a connection of the actin cytoskeleton and the G2/M cell cycle control comes from studies in yeast. Saccharomyces cerevisiae lacking the TOR2-unique function which mediates the cell cycle-dependent organization of the actin cytoskeleton may arrest in G2/M of the cell cycle (22).
A delay of cells at the G2/M border can be caused by exposure to exogenous influences inducing DNA damage, as reported in detail for ionizing radiation, where signal transduction leads to failure of MPF activation at the G2/M border (7, 14, 32, 36, 38). Since in the present study HeLa cells were treated with C2 toxin during S phase, a DNA-damaging effect cannot be excluded. A delay in the traverse through S phase, however, could not be observed. On the other hand, damage to DNA seems not to be a prerequisite for inducing a block in G2 phase. Synchronized HeLa cells are reversibly delayed at the G2/M boundary when they are treated with epidermal growth factor (27). Here too, a failure to activate MPF, due to a lack of tyrosine dephosphorylation of p34cdc2, was observed (5).
Thus, our results leave unresolved whether the C2 toxin-induced G2 arrest and the prevented activation of p34cdc2 kinase and cdc25-C phosphatase are directly caused by destruction of the actin cytoskeleton and decrease of focal adhesions or, on the other hand, whether the toxin triggers intracellular signal cascades, such as activation or inactivation of protein kinases and phosphatases, or even DNA damage independent from its effects on actin filaments (Fig. 7).
In conclusion, we have found that (i) C. botulinum C2 toxin treatment causes a transient delay of the G2/M transition of synchronized HeLa cells; (ii) the action of the toxin involves a prevented activation of p34cdc2 protein kinase; (iii) the prevented activation of the p34cdc2 kinase is based on the lack of an activating tyrosine dephosphorylation of that protein; and (iv) C2 toxin treatment lowers the activation of cdc25-C phosphatase, which is responsible for the activation of the p34cdc2 protein kinase. The underlying direct mechanism remains to be elucidated.
ACKNOWLEDGMENTS
We thank Ulrike Müller and James Richards for expert technical assistance, Michael Stöhr for flow cytometric analysis, Ingrid Hoffmann for anti-cdc25-C antiserum (IH37), and Jennifer Reed for critical reading of the manuscript.
This study was financially supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 388 and Ki 173/14-2).
REFERENCES
- 1.Aktories K, Bärmann M, Ohishi I, Tsuyama S, Jakobs K H, Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986;322:390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- 2.Aragon V, Chao K, Dreyfus L A. Effect of cytolethal distending toxin on F-actin assembly and cell division in Chinese hamster ovary cells. Infect Immun. 1997;65:3774–3780. doi: 10.1128/iai.65.9.3774-3780.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arion D, Meijer L, Brizuela L, Beach D. Cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988;55:371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- 4.Barth H, Kinzel V. Phorbol ester TPA rapidly prevents activation of p34cdc2 histone H1 kinase and concommitantly the transition from G2 phase to mitosis in synchronized Hela cells. Exp Cell Res. 1994;212:383–388. doi: 10.1006/excr.1994.1158. [DOI] [PubMed] [Google Scholar]
- 5.Barth H, Kinzel V. Epidermal growth factor rapidly impairs activation of p34cdc2 protein kinase in HeLa cells at the G2-M boundary. J Cell Physiol. 1995;162:44–51. doi: 10.1002/jcp.1041620107. [DOI] [PubMed] [Google Scholar]
- 6.Barth H, Hofmann F, Olenik C, Just I, Aktories K. The N-terminal part of the enzyme component (C2I) of the binary Clostridium botulinum C2 toxin interacts with the binding component C2II and functions as a carrier system for a Rho ADP-ribosylating C3-like fusion toxin. Infect Immun. 1998;66:1364–1369. doi: 10.1128/iai.66.4.1364-1369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barth H, Hoffmann I, Kinzel V. Radiation with 1 Gy prevents the activation of the mitotic inducers mitosis-promoting factor (MPF) and cdc25-C in HeLa cells. Cancer Res. 1996;56:2268–2272. [PubMed] [Google Scholar]
- 8.Beach D, Kirschner M W. The cell cycle coming under control. Science. 1989;245:252–255. doi: 10.1126/science.2526371. [DOI] [PubMed] [Google Scholar]
- 9.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Martin B L, Brautigan D L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 11.Clarke P R, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Mol Biol Cell. 1993;4:397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comayras C, Tasca C, Pérès S Y, Ducommun B, Oswald E, De Rycke J. Escherichia coli cytolethal distending toxin blocks the HeLa cell cycle at the G2/M transition by preventing cdc2 protein kinase dephosphorylation and activation. Infect Immun. 1997;65:5088–5095. doi: 10.1128/iai.65.12.5088-5095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cyert M S, Kirschner M W. Regulation of MPF activity in vitro. Cell. 1988;53:185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- 14.Datta R, Hass R, Gunji H, Weichselbaum R, Kufe D. Downregulation of cell cycle control genes by ionizing radiation. Cell Growth Differ. 1992;3:637–644. [PubMed] [Google Scholar]
- 15.De Rycke J, Nougayrede J P, Oswald E, Mazars P. Interaction of Escherichia coli producing cytotoxic necrotizing factor with HeLa epithelial cells. Adv Exp Med Biol. 1997;412:363–366. doi: 10.1007/978-1-4899-1828-4_58. [DOI] [PubMed] [Google Scholar]
- 16.De Rycke J, Mazars P, Nougayrede J P, Tasca C, Boury M, Herault F, Valette A, Oswald E. Mitotic block and delayed lethality in HeLa epithelial cells exposed to Escherichia coli BM2-1 producing cytotoxic necrotizing factor type 1. Infect Immun. 1996;64:1694–1705. doi: 10.1128/iai.64.5.1694-1705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988;54:17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 18.Dunphy W G. The decision to enter mitosis. Trends Cell Biol. 1994;4:202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 19.Dunphy W G, Newport J W. Unraveling of mitotic control mechanisms. Cell. 1988;55:925–928. doi: 10.1016/0092-8674(88)90234-6. [DOI] [PubMed] [Google Scholar]
- 20.Elledge S J. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 21.Félix A, Cohen P, Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990;9:675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helliwell S B, Howald I, Barbet N, Hall M N. TOR2 is part of two related signaling pathways coordinating cell growth in Saccharomyces cerevisiae. Genetics. 1998;148:99–112. doi: 10.1093/genetics/148.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann I, Clarke P R, Marcote M J, Karsenti E. Phosphorylation and activation of human cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt T. Under arrest in the cell cycle. Nature. 1989;342:483–484. doi: 10.1038/342483a0. [DOI] [PubMed] [Google Scholar]
- 25.Kinzel V, Bonheim G, Richards J. Phorbol ester-induced G2 delay in HeLa cells analyzed by time lapse photography. Cancer Res. 1988;48:1759–1762. [PubMed] [Google Scholar]
- 26.Kinzel V, Richards J, Stöhr M. Responses of synchronized HeLa cells to the tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate as evaluated by flow cytometry. Cancer Res. 1981;41:306–309. [PubMed] [Google Scholar]
- 27.Kinzel V, Kaszkin M, Blume A, Richards J. Epidermal growth factor inhibits transiently the progression from G2 phase to mitosis: a receptor-mediated phenomenon in various cells. Cancer Res. 1990;50:7932–7936. [PubMed] [Google Scholar]
- 28.Kremmer E, Ohst K, Kiefer J, Brewis N, Walter G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol Cell Biol. 1997;217:1692–1701. doi: 10.1128/mcb.17.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee T H, Solomon M J, Mumby M C, Kirschner M W. INH, a negative regulator of MPF, is a form of protein phosphatase 2A. Cell. 1991;64:415–423. doi: 10.1016/0092-8674(91)90649-j. [DOI] [PubMed] [Google Scholar]
- 31.Lewin B. Driving the cell cycle: M phase kinase, its partners and substrates. Cell. 1990;61:743–752. doi: 10.1016/0092-8674(90)90181-d. [DOI] [PubMed] [Google Scholar]
- 32.Lock R B, Killing P K. Responses of HeLa and Chinese hamster ovary cdc2/cyclin-B kinase in relation to cell cycle perturbations induced by etoposide. Int J Oncol. 1993;3:33–42. doi: 10.3892/ijo.3.1.33. [DOI] [PubMed] [Google Scholar]
- 33.Lock R B, Ross W E. Inhibition of p34cdc2 kinase activity by etoposide or irradiation as a mechanism of G2 arrest in Chinese hamster ovary cells. Cancer Res. 1990;50:3761–3766. [PubMed] [Google Scholar]
- 34.Martineau S N, Andreassen P R, Margolis R L. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meijer L, Pondaven P. Cyclic activation of histone H1 kinase during sea urchin mitotic divisions. Exp Cell Res. 1988;174:116–129. doi: 10.1016/0014-4827(88)90147-4. [DOI] [PubMed] [Google Scholar]
- 36.Metting N F, Little J B. Transient failure to dephosphorylate the cdc2-cyclin B1 complex accompanies radiation-induced G2-phase arrest in HeLa cells. Radiat Res. 1995;143:286–292. [PubMed] [Google Scholar]
- 37.Mueller G C, Kajiwara K. Synchronization of cells for DNA synthesis. In: Habel K, Salzman N P, editors. Fundamental techniques in virology. New York, N.Y: Academic Press, Inc.; 1969. pp. 21–27. [Google Scholar]
- 38.Muschel R J, Zhang H B, Iliakis G, McKenna W G. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res. 1991;51:5113–5117. [PubMed] [Google Scholar]
- 39.Norbury C, Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- 40.Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987;55:1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohishi I, Yanagitomo A. Visualizations of binding and internalization of two nonlinked protein components of botulinum C2 toxin in tissue culture cells. Infect Immun. 1992;60:4648–4655. doi: 10.1128/iai.60.11.4648-4655.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohishi I, Iwasaki M, Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980;30:668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paul J. Cell and tissue culture. 4th ed. Edinburgh, Scotland: E. & S. Livingstone Ltd.; 1970. [Google Scholar]
- 44.Podesta F, Roth T, Ferrara F, Cagliero E, Lorenzi M. Cytoskeletal changes induced by excess extracellular matrix impair endothelial cell replication. Diabetologia. 1997;40:879–886. doi: 10.1007/s001250050763. [DOI] [PubMed] [Google Scholar]
- 45.Prepens U, Barth H, Wilting J, Aktories K. Influence of Clostridium botulinum C2 toxin on FceRI-mediated secretion and tyrosine phosphorylation in RBL cells. Naunyn-Schmiedeberg’s Arch Pharmacol. 1998;357:323–330. doi: 10.1007/pl00005174. [DOI] [PubMed] [Google Scholar]
- 46.Simpson L L. The binary toxin produced by Clostridium botulinum enters cells by receptor-mediated endocytosis to exert its pharmacologic effects. J Pharmacol Exp Ther. 1989;251:1223–1228. [PubMed] [Google Scholar]
- 47.Stoehr M, Gebhardt U, Goerttler K. Computer assistance in multiparameter flow cytometry of mammalian cells. Biotechnol Bioeng. 1976;18:1057–1074. doi: 10.1002/bit.260180804. [DOI] [PubMed] [Google Scholar]
- 48.Udagawa T, McIntyre B W. ADP-ribosylation of the G-protein Rho inhibits integrin regulation of tumor cell growth. J Biol Chem. 1996;271:12542–12548. doi: 10.1074/jbc.271.21.12542. [DOI] [PubMed] [Google Scholar]
- 49.Vandekerckhove J, Schering B, Bärmann M, Aktories K. Botulinum C2 toxin ADP-ribosylates cytoplasmic beta/gamma-actin in arginine 177. J Biol Chem. 1988;263:696–700. [PubMed] [Google Scholar]
- 50.Wary K K, Mariotti A, Zurzolo C, Giancotti F G. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 51.Wegner A, Aktories K. ADP-ribosylated actin caps the barbed ends of actin filaments. J Biol Chem. 1988;263:13739–13742. [PubMed] [Google Scholar]
- 52.Whitehouse C A, Balbo P B, Pesci E C, Cottle D L, Mirabito P M, Pickett C L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect Immun. 1998;66:1934–1940. doi: 10.1128/iai.66.5.1934-1940.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]