Abstract
Milk is related to many gastrointestinal disorders from the cradle to the grave due to the many milk ingredients that can trigger gastrointestinal discomfort and disorders. Cow’s milk protein allergy (CMPA) is the most common food allergy, especially in infancy and childhood, which may persist into adulthood. There are three main types of CMPA; immunoglobulin E (IgE)-mediated CMPA, non-IgE-mediated CMPA, and mixed type. CMPA appears before the first birthday in almost all cases. Symptoms may start even during the neonatal period and can be severe enough to simulate neonatal sepsis. CMPA (often non-IgE mediated) can present with symptoms of gastroesophageal reflux, eosinophilic esophagitis, hemorrhagic gastritis, food protein-induced protein-losing enteropathy, and food protein-induced enterocolitis syndrome. Most CMPAs are benign and outgrown during childhood. CMPA is not as common in adults as in children, but when present, it is usually severe with a protracted course. Lactose intolerance is a prevalent condition characterized by the development of many symptoms related to the consumption of foods containing lactose. Lactose intolerance has four typical types: Developmental, congenital, primary, and secondary. Lactose intolerance and CMPA may be the underlying pathophysiologic mechanisms for many functional gastrointestinal disorders in children and adults. They are also common in inflammatory bowel diseases. Milk consumption may have preventive or promoter effects on cancer development. Milk may also become a source of microbial infection in humans, causing a wide array of diseases, and may help increase the prevalence of antimicrobial resistance. This editorial summarizes the common milk-related disorders and their symptoms from childhood to adulthood.
Keywords: Cow’s milk; Adults; Children; Functional gastrointestinal disorders; Cow’s milk protein allergy; Lactose intolerance, Inflammatory bowel disease; Zoonosis
Core Tip: Milk has been a basic human food for hundreds of centuries with a high nutritional value. However, milk can cause various gastrointestinal disorders from early childhood to late adulthood, as many milk ingredients, such as lactose and cow’s milk proteins, can trigger gastrointestinal discomfort and disorders. Cow’s milk protein allergy and lactose intolerance are the most common milk-related disorders. However, milk consumption is related to many functional gastrointestinal disorders, inflammatory bowel disease, milk-related cancer, and milk-born zoonotic infections. Awareness of these disorders is crucial for physicians and patients to avoid unnecessary nutritional mismanagement.
INTRODUCTION
Milk is a comprehensive dietary liquid containing adequate amounts of highly bioavailable nutrients humans need. Humans have consumed animal milk for about 10000 years and as baby food for about 8000 years, as evidenced by the dental remains of Neolithic humans, ancient clay pottery vessels, and ancient baby bottles[1]. Many gastrointestinal disorders humans suffer are related to dietary components, and diet modification could be an essential step in disease management. As milk is a critical component in the human diet, milk is related to many gastrointestinal disorders from the cradle to the grave[2]. Many milk ingredients, such as lactose and cow’s milk proteins, can trigger gastrointestinal discomfort and disorders. Milk decreases gut bacterial diversity. Dairy and dairy products, such as yogurt and kefir, can modulate and alter the gut microbiota[3]. In addition to its effects on gut microbiota, cow’s milk may make humans prone to many food-borne infectious diseases. In this editorial, we discuss the various cow’s milk-induced gastrointestinal disorders from infancy to adulthood that will be highlighted in the topics of this special issue. Table 1 summarizes the various gastrointestinal effects of cow’s milk on humans.
Table 1.
The various gastrointestinal effects of cow’s milk in humans
|
Items
|
Details
|
| Cow’s milk protein allergy | Pediatric onset: (1) IgE-mediated; (2) Non-IgE-mediated; and (3) Mixed type |
| Adult onset: (1) Mostly IgE-mediated; and (2) Pregnancy-induced CMPA | |
| Pediatric onset with adulthood persistence | |
| Lactose intolerance | Developmental |
| Congenital (inherited) | |
| Primary (aging-induced) | |
| Secondary | |
| Cow’s milk-related functional gastrointestinal disorders | Functional dyspepsia |
| Persistent regurgitation and gastroesophageal reflux | |
| Infant colic | |
| Functional constipation | |
| Irritable bowel syndrome | |
| Cow’s milk and inflammatory bowel diseases | Crohn’s disease |
| Cow’s milk-related gastrointestinal cancer | Anti-colorectal cancer |
| Milk-born gastrointestinal infections | Mycobacterium avium, Mycobacterium bovis, Salmonella species, brucellosis, streptococcal infections, “summer diarrhea”, Yersinia enterocolitica, diphtheria, Escherichia coli (E. coli), Campylobacter jejuni, Citrobacter species, Shiga toxin-producing E. coli, Pseudomonas aeruginosa, Proteus mirabilis, and Klebsiella species |
E. coli: Escherichia coli.
METHODS AND RESULTS
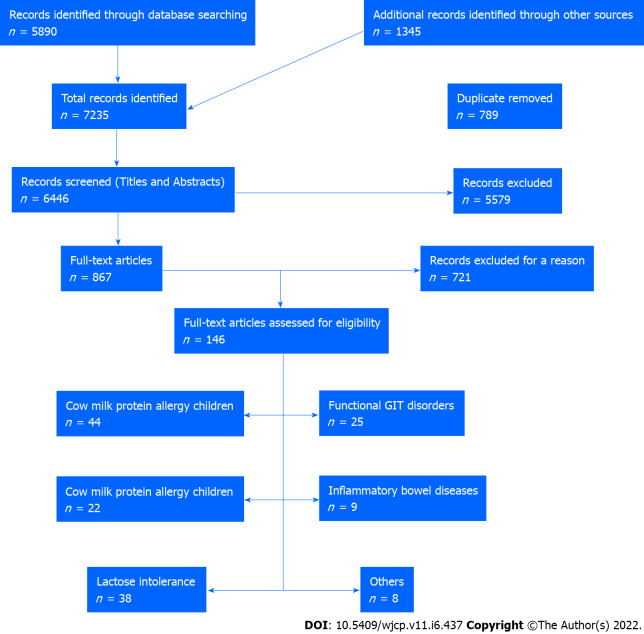
In this editorial, we conducted a comprehensive literature review by searching electronic databases such as PubMed, Embase, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature, Web of Science, Scopus, Library and Information Science Abstracts, and the National Library of Medicine catalog up to July 31, 2022, related to cow milk effects on the gastrointestinal tract in children and adults. Reference lists were inspected, and citation searches were performed on the included studies. We included open-access papers on English-language studies. Figure 1 shows the flow chart of the reviewed articles. We included 146 articles concerned with the various effects of cow’s milk on humans, from birth to the elderly. We also cited high-quality articles in Reference Citation Analysis (https://www.referencecitationanalysis.com).
Figure 1.
The flow chart of the included studies. GIT: Gastrointestinal tract.
COW’S MILK PROTEIN ALLERGY
Cow’s milk protein allergy (CMPA) is the most frequent food allergy, especially in infancy and childhood, but it can persist into adulthood. It is due to an abnormal immune response to CMP. It should be distinguished from the other adverse effects of cow’s milk, such as lactose intolerance and infection-related disorders[4]. Both casein and whey (α-lactalbumin and β-lactoglobulin) proteins can cause allergic reactions. α-lactalbumin and casein are the most common cow’s milk allergenic proteins, while β-lactoglobulin is associated with severe anaphylaxis[5]. In addition, β-lactoglobulin has a relative resistance to enzymatic degradation. Therefore, β-lactoglobulin could be implicated in non-immunoglobulin E (IgE)-immune-mediated CMPA with delayed gastrointestinal symptoms[6]. Besides cow’s milk and dairy products, CMPs can be detected in some probiotics, oral polio vaccines, and lactulose. Some dry powder inhalers containing lactulose (such as Fluticasone/Salmeterol or Lanimavir) could be contaminated with CMPs. Some parenteral vaccines, such as the diphtheria-tetanus-pertussis vaccine, can be contaminated with CMP[7].
It should also be noted that cow’s milk allergy is not only against cow’s milk proteins but can also be triggered by other additives that could be added to modify cow’s milk, such as artificial flavors or preservatives. Cross-reactivities with other mammals’ milk (e.g., goats and sheep) and raw beef are prevalent due to the composition of homologies of amino acids[8,9]. About 13%-20% of children with CMPA have a beef meat allergy. In addition, patients with beef meat allergies mostly have CMPA. Camel’s milk proteins are unlikely to cross-react with cow’s milk proteins due to phylogenetic differences, and consequently, camel’s proteins cannot be recognized by circulating IgEs and monoclonal antibodies[10]. Even though soy milk is being used as a possible substitute for cow’s milk in the case of CMPA, cross-reactivity between both occasionally exists due to cross-reactivity with bovine caseins and soybean protein p34[11].
There are three main types of CMPA: IgE-mediated CMPA, non-IgE-mediated CMPA, and mixed allergic reactions. The role of other kinds of immune-mediated reactions to CMP, especially those associated with IgG and IgA antibody isotypes, is presently controversial[12]. The rate of IgE-mediated CMPA decreases while other non-IgE-mediated CMPA increases with increasing age. High cow’s milk-specific IgE levels are rare in adults[13]. CMPA may manifest as an isolated gut reaction or be associated with other systemic manifestations such as skin, respiratory, or cardiovascular manifestations. CMPA can affect any part of the gastrointestinal tract, from the mouth to the anus, and at any age, from newborn to the elderly[14].
CMPA in infancy and childhood
CMPA commonly occurs early in life, and almost all cases appear before the first birthday. The average age for CMPA to appear during childhood is 3.5 mo (ten days-to-ten months). The symptoms usually appear in the first week after the introduction of CMP (95% of cases). About 60% of patients developed symptoms with the first formula feeding[15].
Risk factors for CMPA include immature gastric acid production, defective intestinal and pancreatic enzymes, low vitamin D levels, and a deficit of regulatory T cells[16,17]. Other risk factors include male sex, parental atopy, maternal allergy, maternal smoking during pregnancy, amnionitis, maternal vaginitis, febrile infection during pregnancy, gestational diabetes, hypertension, stress, decreasing maternal age, and difficult delivery (early or threatened labor, malpresentation of the fetus, cesarean section, breech and instrumental delivery, low APGAR score). Post-natal risk factors include neonatal jaundice, Erythema toxicum neonatorum, antibiotic use in the first week of life, and indoor air contaminants such as mold and smoking[18,19]. Another important risk factor for CMPA during infancy is gut microbial dysbiosis. Gut microbiotas play a vital role in modifying intestinal regulatory T (Treg) cell responses to develop an oral tolerance that could protect against IgE-mediated CMPA and other types of food allergies[20]. On the other hand, non-IgE-mediated CMPA can induce gut microbial dysbiosis, affecting intestinal immune homeostasis and tolerance[21]. Tan et al[22] showed that Lactobacillus rhamnosus (L. rhamnosus) could help promote oral tolerance in children suffering from CMPA and assist with intestinal symptom recovery.
Sensitization can occur before and after birth, resulting in non-IgE mediated CMPA as indicated by the presence of CMP-specific tumor necrosis factor-α in the cord blood and the appearance of the symptoms shortly after birth[23]. The onset of symptoms in infancy and childhood usually develops within a week of cow’s milk exposure, although symptoms may take many weeks (up to 24 and 36 wk). Most cases appear after cow’s milk exposure (raw or formula milk), but some may appear after cow’s milk-based foods. However, some exclusively breastfed infants may develop CMPA after exposure to CMP excreted in breast milk[24,25]. Most CMPAs are benign and outgrown during childhood. Non-IgE-mediated CMPA usually resolves more quickly than IgE-mediated allergy. Signs of persistence of CMPA include the presence of acute manifestations, multiple food allergies, especially to eggs, concomitant bronchial asthma, allergic rhinitis, and reactivity to CMP in baked milk on exposure or first challenge[26,27].
Gastrointestinal symptoms of CMPA in infancy and children
Acute manifestations of IgE-mediated CMPA include nausea, vomiting, diarrhea, bloody stools, gastro-esophageal reflux (GER), and colicky abdominal pain. The symptoms usually appear rapidly within minutes of exposure. Anaphylaxis may also manifest in young infants with angioedema of the lips, tongue, and palate, oral pruritus, pallor, and floppiness[28]. Symptoms may occur even during the neonatal period and can be so severe as to be misdiagnosed as neonatal sepsis[29]. Non-IgE-mediated CMPA is common (50% of pediatric CMPA) and is presented with faltering growth, frequent “spitting up”, feeding problems, food refusal or aversion, pallor and tiredness, abdominal colic, upper digestive bleeding, gastroesophageal reflux with or without disease (GERD), abnormal stool habitus such as chronic diarrhea or constipation, blood and/or mucus in the stools, perianal redness, and skin manifestations such as atopic eczema[30,31].
CMPA (often non-IgE mediated) can present with symptoms of GERD, such as poor appetite, crying, fussiness, regurgitation, vomiting, and sleep disturbances. Oral milk elimination and rechallenge tests, esophageal pH impedance, and gastrointestinal endoscopy are advised for a correct clinical diagnosis, but they are not always achievable in all patients[32]. Eosinophilic esophagitis is an immune-mediated disease by Th2 interleukins affecting the esophagus with age-dependent symptoms such as GER, abdominal pain, and food impaction. Various allergens, including CMP can trigger it. Endoscopy shows characteristic features of mucosal eosinophilic infiltration of more than 15 eosinophils/high power field[33]. It can be treated with proton-pump inhibitors, but dietary treatment is the cornerstone of therapy after confirming the diagnosis with endoscopy and a 4- to 12-wk elimination test. The dietary approaches involve amino acid-based formula, allergy testing-based directed diet, or empirical six-food elimination diet (milk, soy, wheat, eggs, fish/seafood, and peanut/nuts)[34,35].
Hemorrhagic gastritis: Hemorrhagic gastritis is not common in infants with CMPA but is recorded in some cases. A possible cause is in utero CMP sensitization. It may be present with persistent vomiting and with subacute or chronic hematemesis. Occasionally, it may be present with subclinical hemorrhage. Endoscopy is usually needed to confirm the diagnosis. Nevertheless, etiological diagnosis is made according to clinical guidelines to diagnose CMPA with an elimination diet and rechallenge test. The outcome is usually favorable, with complete spontaneous resolution within one week after a period of bowel rest using either parenteral nutrition or amino acid formula[36,37]. Acute pancreatitis was recorded in one case with eosinophilic gastroenteritis due to CMPA[38].
Food protein-induced protein-losing enteropathy: Food protein-induced protein-losing enteropathy in infancy is a mixed IgE and non-IgE immune-mediated food allergy characterized by villous atrophy that leads to enteral loss of proteins, causing hypoproteinemia/hypoalbuminemia, diarrhea, edema, malabsorption, and poor weight gain. Laboratory work-up showed anemia, eosinophilia, hypoalbuminemia, raised fecal α1-antitrypsin (α1AT), raised specific-IgE, and positive allergy skin prick test (SPT) for milk proteins[39]. Hypoalbuminemia results from protein loss in the stool. α1AT is a part of the plasma protein not digested in the intestines and is not present in animal or plant food. The increased fecal α1AT is a good marker of protein loss through the digestive system[40]. A diagnosis relies mainly on the response to an elimination diet, with clinical improvement usually occurring within 3-4 d, but it may take weeks to resolve fully[41].
Food protein-induced enterocolitis syndrome: Food protein-induced enterocolitis syndrome (FPIES) is mainly a non-IgE-mediated disease caused mainly by CMP. It is caused by IgE-mediated mechanisms in 25% of cases, especially in patients with more protracted and persistent courses. Those with multiple allergies present with copious, repetitive vomiting, abdominal pain, and frequent diarrhea, causing acute dehydration with lethargy. Weight loss and failure to thrive occur in chronic conditions. FPIES is frequently misdiagnosed as acute viral gastroenteritis, sepsis, or surgical conditions, which delays the diagnosis for many months. FPIES occasionally results in symptoms similar to protein-induced protein-losing enteropathy and protein-induced proctocolitis[42,43]. The diagnosis is mainly clinical. However, open food challenges, milk-specific IgE, and SPTs can help diagnose FPIES. Milk-specific IgE is positive in about 25% of cases. Ondansetron may help in acute conditions. FPIES usually resolves by 3-5 years[44]. However, we still need future investigations and treatment guidelines to improve patient care for those with FPIES.
Food protein-induced allergic proctocolitis: Food protein-induced allergic proctocolitis (FPIAP) is a benign non-IgE-mediated delayed immune response to allergenic foods such as a cow or soymilk protein. It usually presents with a bloody mucoid stool in a well-appearing healthy infant aged one to four weeks. It occurs mainly in exclusively breastfed infants (60%) and resolves when the mother eliminates CMP and soy proteins from their diet[45]. As skin allergy tests and IgE are negative in infants with FPIAP, the diagnosis is usually made by exclusion and confirmed with an elimination/rechallenge test with CMP or soymilk protein[46]. The symptoms usually disappear within 1-3 d of the elimination of the offending CMP or soy protein from the diet of breastfeeding mothers. However, it may take a longer time to resolve the symptoms. With a dairy-eliminated diet, the mother should be supported with a daily calcium intake of at least 800 mg and multivitamins as needed. Bottle-feeding babies may benefit from extensively hydrolyzed formulas or sometimes amino acid-based formulas. FPIAP is benign, and most cases resolve by the first birthday[47].
CMPA in adults
CMPA is not as common in adults as in children, but when present, it usually has a severe and protracted course. The symptoms can be elicited by traces of milk as small as 0.3 mg of CMPs[40]. Adults with CMPA have allergies to the same major allergenic milk proteins (casein and whey). However, they usually display robust immune responses, as illustrated by powerful SPTs and high IgE reactivity[48]. Even dermal or respiratory exposure to CMP can induce a severe form of CMPA, including anaphylaxis. Repeated exposure to CMP by food handling or inhalation of dairy products could induce cutaneous sensitization in adult patients with a personal history of atopy[49,50]. Hansen et al[51] showed that only 1%-3% of children with CMPA would continue to have CMPA as an adult, usually severe and life-threatening. Stöger and Wüthrich[52] showed that CMPA was more common in females (92%); 39% of them developed CMPA during or shortly after pregnancy. Casein was the predominant sensitizing allergen in 71%, while whey protein sensitization (alpha-lactalbumin and beta-lactoglobulin) was rare. He et al[53] demonstrated that A1 β-casein is the responsible component of casein that can induce CMPA, whereas A2 β-casein alleviates the acute gastrointestinal symptoms in Chinese adult patients with CMPA. The presence of other autoimmune diseases could increase the risk of CMPA. Kristjánsson et al[54] showed that 50% of adult patients with coeliac disease developed a mucosal inflammatory response similar to that of gluten with rectal CMP. Casein was the main allergic protein. About one-quarter of patients with irritable bowel syndrome (IBS) have food hypersensitivity, including CMPA[55].
Different factors can affect the prevalence of CMPA in adults, such as ethnic origin and geographical area. A study by Domínguez-García et al[56] on young adult students (18- to 25-year-olds) in a Mexican university showed that the prevalence of CMPA among them was 1/400 compared to 1/10 for lactose intolerance. A risk factor for developing CMPA in adulthood is the excessive intake of dairy products[57]. Sousa et al[58] reported a 24-year-old man who developed CMPA after excessive intake of hydrolyzed casein and whey CMPs for bodybuilding for two years. CMPA was confirmed by positive IgE against cow’s milk α-lactalbumin, β-lactoglobulin, and casein extracts, suggesting that excessive CM intake may induce CMPA. On the other hand, adult patients with CMPA have lower IgG levels than controls[58]. The respiratory tract and skin are the main organs affected in adulthood CMPA in adults, while gastrointestinal and cardiovascular manifestations are less frequent than in childhood CMPA[59]. Due to the higher rate of lactose intolerance compared to CMPA in adults, some cases of CMPA are wrongly diagnosed as lactose intolerance due to the common symptoms. Lactose intolerance can be excluded by the negative hydrogen breath test for lactose, the positive cow’s milk challenge and SPTs, and high IgE levels against CMP[60]. Therefore, cases with refractory lactose intolerance should be investigated for CMPA, as IgE-mediated sensitivity to CMP is a common comorbidity in patients with refractory lactose intolerance not responding to a lactose-free diet[61]. Although most CMPAs reported in adults are IgE-mediated, non-IgE-mediated CMPAs may also occur. For example, FPIES, which are non-IgE-mediated CMPAs, can also be observed in older children and adults[62,63]. IgG-mediated CMPAs were also reported by Anthoni et al[12]. They reported a significant association of high IgG levels with self-reported milk-provoked gastrointestinal symptoms, especially constipation, in the adult population. However, the serum IgG levels decrease with the increasing age of the affected patients[10].
Despite adulthood CMPA prevalence being about one-quarter of childhood CMPA, the adulthood type is more severe and more liable to complications and even death. CMPA is rarely implicated in the worsening of coexisting atopic dermatitis during adolescence and adulthood[64]. Recurrent acute pancreatitis was reported as a rare complication of IgE-mediated CMPA. de Diego Lorenzo et al[65] reported recurrent episodes of acute pancreatitis in a 23-year-old patient with characteristic abdominal pain and high serum pancreatic enzyme levels, confirmed by the presence of swelling and edema of the pancreas on the sonogram. The episodes were induced by milk consumption and associated with diarrhea and signs of generalized urticaria, such as conjunctival injection, facial erythema, and generalized pruritus. The blood showed eosinophilia and high serum levels of CMP-specific IgE and anti-beta-lactoglobulin IgE[66].
In addition, young adults with CMPA in infancy are at an increased risk of failing to reach their growth potential and height. Therefore, they are candidates for proper growth and nutritional monitoring and need appropriate dietary intervention[65]. They are more prone to reduced bone mass density and developing early osteoporosis. This effect could be reversed by milk desensitization, adequate calcium supplements, and optimal nutritional rules for these patients. Eliminating dairy products in treating adult patients with CMPA may increase the risk of gout and hyperuricemia, as milk consumption protects against gout[67].
LACTOSE INTOLERANCE
Lactose is a disaccharide composed of galactose linked to glucose that can be hydrolyzed in the small intestine brush border membrane by β-galactosidase (lactase enzyme). After infancy, lactase activity progressively decreases due to a gradual decrease in lactase synthesis ability. Therefore, adults do not tolerate large amounts of lactose[68]. Lactose intolerance is a common condition of food intolerance characterized by the development of many symptoms following the consumption of foods containing lactose, the primary milk sugar, due to absolute or relative deficiency of the lactase enzyme in the mucosal brush border of the small intestine. As a result of inadequate lactose digestion, the lactose reaches the colon undigested, where the gut microbiota ferments it, causing nonspecific symptoms such as abdominal pain, bloating, flatulence, and mushy to watery diarrhea. The symptoms usually develop within 30 min to a few hours after lactose ingestion. The severity of the symptoms correlates with the deficiency of the lactase enzyme. Therefore, nausea and vomiting may occur after consuming large amounts of lactose-containing foods such as dairy products[69-71].
There are four main types of lactose intolerance; developmental lactose intolerance, which occurs in premature babies as lactase enzyme production starts after 34 wk of gestation; congenital lactose intolerance, which is inherited from lactase deficiency due to a defect in the gene responsible for lactase synthesis; primary lactose intolerance, which results from the normal aging process and is the most common cause of lactose intolerance; and secondary lactose intolerance which results from damage of the brush border of the intestinal mucosa due to infection, inflammation, or trauma and improves with treatment of the cause[72]. Lactose intolerance can be isolated or part of a broader intolerance to variable saccharides, including monosaccharides, disaccharides, oligosaccharides, and polyols. It is crucial to determine whether lactose intolerance is isolated or compounded during the treatment to ensure successful therapy of a lactose-free diet[73].
The manifestations of lactose intolerance depend on many factors, in addition to the degree of lactase deficiency. These factors include the dose of ingested lactose; the osmolality of the food; the dietary fat content; gut motility and gastric emptying time; gut microbiota and its ability to ferment lactose; small intestinal bacterial overgrowth; water absorptive capacity of the colon; and the pain threshold due to sensitivity of the intestine to the generated gases and other fermented substrates due to lactose fermentation[73]. For example, a patient with lactose intolerance may tolerate up to 12 g of lactose (equivalent to a glass of milk); an amount between 12 and 18 g can be tolerated when mixed with other types of food; while an amount between 18 and 50 g starts to produce symptoms of lactose intolerance and the symptoms increase with increased the amount. Lactose over 50 g causes significant symptoms in most patients. However, the relation between the amount of ingested lactose and the severity of the symptoms needs more valid evidence[70]. These symptoms include abdominal distension, bloating, colic, abdominal pain, increased borborygmi, flatus, and osmotic diarrhea induced by lactose in dairy products. Nonspecific symptoms of lactose intolerance may include headaches, muscle pain, chronic fatigue, depression, and concentration problems[74].
Diagnosis of lactose intolerance
Diagnosis of lactose intolerance depends on self-reported symptoms, dietary challenges, and investigative testing, including physiological, genetic, and endoscopic testing. Physiologic testing depends on the evaluation of lactase activity by different methods. It is also essential to rule out secondary causes. When lactose intolerance is assumed, a trial of a lactose-free diet should be conducted for 2-4 wk with the elimination of all lactose sources, including hidden lactose sources. Then, lactose is reintroduced to the diet. If symptoms recover during the 2-4 wk period and reappear with lactose reintroduction, a lactose intolerance diagnosis can be made[75]. Indirect evidence of lactose malabsorption due to lactase deficiency includes measuring stool pH and reducing substances. Fecal pH of less than 6.0 may suggest lactose intolerance. However, this test is not recommended in infants less than two years of age due to the high rate of false negative results[76]. Fecal-reducing substances are another indirect tool to diagnose lactose (or other carbohydrates) maldigestion and malabsorption. Positive results may suggest an absence of the related enzyme[77]. However, false negative results could occur if the patient has not recently ingested lactose.
The lactose hydrogen breath test: The lactose hydrogen breath test is commonly done in suspected lactose-intolerant patients. The test depends on the principle that lactase deficiency causes lactose indigestion, which undergoes gut microbiota fermentation and subsequent hydrogen gas production. The patient ingests 25 to 50 g of lactose, and then the hydrogen gas is checked every 15 min for 3-6 h. The increasing hydrogen concentration in the breath by more than 20 ppm (parts per million) over baseline after lactose ingestion indicates hypolactasia. However, the test needs a long duration (3-6 h) with a risk of a false negative in 10% of cases[78]. A false negative test may be related to the presence of non-hydrogen-producing fermenting bacteria. In this situation, methane-producing bacteria may cause methane gas production in about one-third of the adult population and may have additional health consequences worse than excess hydrogen levels. Therefore, combined measuring of hydrogen and methane significantly improves the diagnosis of malabsorption syndromes, including lactose intolerance and small intestinal bacterial overgrowth, compared with a single hydrogen breath test[79].
The lactose tolerance test: The lactose tolerance test examines the ability to digest lactose to its components by checking the glucose level after administering 50 g of lactose orally. The blood glucose levels are checked before and after 30, 60, and 120 min of lactose intake. The absence of increased blood glucose levels after oral lactose intake indicates the inability of the body to digest lactose and hence possible lactase deficiency. However, this test is affected by other factors, such as gastric emptying time and mechanisms of glucose metabolism, and has lower sensitivity and specificity, with false positive and negative results in 20% of patients. Therefore, it is rarely performed. However, it can detect patients with lactose intolerance and a negative hydrogen breath test due to a lack of hydrogen gas-producing bacteria. Patients with diabetes mellitus are not candidates for this test as their blood sugar will increase even in the presence of lactose intolerance[80,81].
The gaxilose test: The gaxilose test is considered the new gold standard for lactose intolerance diagnosis. It uses gaxilose, a synthetic disaccharide formed of -O-β-D-galactopyranosyl-D-xylose that can be metabolized with lactase enzyme into galactose and xylose due to its structural similarity to lactose. Xylose is absorbed by the enterocyte, reaches the blood, and is then excreted in the urine. Therefore, the blood and urine levels of xylose will reflect the activity of the lactase enzyme available to metabolize gaxilose. However, this recent test needs more studies to confirm its efficacy, safety, sensitivity, and specificity[82-84].
Genetic tests: Genetic tests apply real-time polymerase chain reaction or sequencing of DNA extracted from buccal mucosa or venous blood to detect the genetic type of lactose intolerance. Determining the lactase enzyme activity on intestinal biopsies is done for other reasons to detect a primary or secondary cause of lactose intolerance. The patchy activity of lactase should be considered during the biopsy. Therefore, more than a single biopsy may be needed to achieve optimal test accuracy. The genetic test is a good predictor of lactase persistence or non-persistence in specific populations[85-87].
Lactose intolerance comorbidities
Lactose intolerance should not be considered an isolated disorder as it may trigger many other diseases. There are significant correlations between lactose intolerance and the prevalence of osteoporosis, mental status changes, and the existence of other food intolerances[74]. Infant colic is a common problem. Subclinical lactose intolerance could be an underlying pathophysiologic mechanism[88]. About one-third of patients with IBS have lactose intolerance as a part of their malabsorption syndrome. Therefore, a trial of a lactose-free diet is a common practice in managing IBS. Consequently, a hydrogen breath test is recommended in newly diagnosed patients with IBS to identify those who would benefit from a lactose-free diet[89]. At the same time, patients with inflammatory bowel disease (IBD) have a 2.7-fold increased risk of lactose intolerance, indicating the need to screen patients with IBD for lactose intolerance to avoid overlapping or confusing symptoms[90].
CMPA could be a comorbidity of lactose intolerance or the underlying cause as the CMP-immune mediated inflammation destroys the brush border of the intestinal mucosa that contains lactase enzyme, resulting in lactase deficiency and lactose intolerance. In addition, CMPA is commonly mistaken for lactose intolerance as both have common symptoms. Therefore, CMPA should be considered in lactose-free diet-refractory lactose intolerance. However, there are critical differences between cow’s milk allergy and lactose intolerance which could limit misunderstandings in diagnosing these two conditions[60,91]. Grundmann et al[92] showed that 21.4% of patients with chronic pruritus had lactase deficiency, and 38.3% had an excellent anti-pruritic effect after four weeks of a lactose-free diet. Therefore, lactase deficiency could be an independent underlying cause of chronic pruritus. Hence, lactase deficiency screening is a reasonable diagnostic step in investigating chronic pruritus. A lactose-free diet should be tried if lactose intolerance is confirmed in patients with chronic pruritus[92]. Patients with systemic sclerosis have a 44% higher prevalence of lactose intolerance than those in control. Lactose intolerance occurs as a part of the malabsorption syndrome that results from gut inflammation as a feature of systemic inflammation associated with systemic sclerosis[93]. Other saccharides malabsorption, such as fructose malabsorption, is also common in patients with systemic sclerosis[94]. On the other hand, a cross-sectional study over ten years showed that patients with lactose intolerance might have a reduced risk of gastric and colon cancer[95].
Management of lactose intolerance
Management of lactose intolerance is sometimes tricky. First, we should confirm the diagnosis, detect secondary causes, and determine the amount of lactose the patient can tolerate. This step is crucial in the management, as complete lactose restriction is not advised. Usually, 12-15 g of daily lactose can be tolerated by most adult patients and up to 5 g by most children, especially when mixed with foods. We start with a lactose-restricted diet, then gradually reintroduce milk and milk products according to the person’s tolerance to improve the symptoms and induce tolerance[96]. Mixing lactose with other foods causes slow lactose release in the small intestine and better tolerance. Some lactose-containing foods can be more easily tolerated than others. Yogurt is better tolerated as it contains partially hydrolyzed lactose. On the same track, high-fat-containing dairy products cause delayed gastric emptying and slow lactose release, while skimmed milk can produce severe symptoms due to low fat and high lactose content[71,97].
Consistent and continuous gradual administration of lactose often improves the number and effectiveness of colonic bacteria metabolizing lactose, generating fewer symptoms[98]. Probiotics containing lactose-fermenting bacteria such as Streptococcus thermophilus, L. reuteri, L. rhamnosus, L. acidophilus, L. bulgaricus, and Bifidobacterium longum promote lactose digestion and help to improve gastrointestinal symptoms of lactose intolerance[99].
Therefore, lactose-free milk is usually unnecessary except when a large daily amount of milk is needed, such as during infancy or in severe cases when small doses produce marked symptoms of lactose intolerance. Milk alternatives such as coconut, almond, rice, or oat milk should not be used as the primary nutritional milk source for children below the age of five years. These types of milk should also be fortified with vitamin D. Soy milk, or any plant-based milk, is not recommended for infants below the age of one year. However, soy milk might be considered in infants older than six months if they cannot tolerate satisfactory amounts of cows’ milk formula[100,101]. Children with lactose intolerance using lactose-free milk or plant-based formula are more likely to develop osteoporosis and decreased bone density due to a deficiency of calcium, vitamin D, riboflavin, and protein. They should be supplemented with adequate calcium and vitamin D intake to ensure optimal peak bone mass in childhood and adolescence. Adequate vitamin D and calcium intake can be achieved by increasing consumption of calcium-rich non-dairy foods such as fish, dark green leafy vegetables, tofu, seeds, and nuts, as well as getting enough sunlight exposure through daily walks and other outdoor activities[102,103].
If symptoms of lactose intolerance are still present despite adequate nutritional management, lactase enzyme supplements can be tried as an adjunct and not a replacement for dietary management. Enzyme replacement therapy may not completely alleviate symptoms, and it is hard to calculate the effective dose[96]. However, a recent study showed that oral lactase enzyme supplementation significantly lessened the clinical symptoms and diminished hydrogen breath excretion in subjects with lactose intolerance[104]. Breastfeeding mothers of infants with lactose intolerance do not need to have a low or free-lactose diet, as the lactose content of the breast milk has no relation to the lactose intake by the mother. On the other side, formula-fed infants benefit from a lactose-free formula with a trial of lactose-containing reintroduction food over 2-4 wk[105].
COW’S MILK-RELATED FUNCTIONAL GASTROINTESTINAL DISORDERS
Functional gastrointestinal disorders (FGIDs) are frequent disorders in infants, children, and adults, characterized by persistent and recurring gastrointestinal symptoms (e.g., dysphagia, abdominal pain, dyspepsia, bloating, constipation, or diarrhea) in the absence of clear underlying pathological conditions. However, diet is an essential factor in the pathogenesis and management of FGIDs[106]. Functional dyspepsia is characterized by recurrent symptoms and signs of indigestion without apparent cause. The link between cow’s milk and functional dyspepsia is not well established. Unfortunately, intolerance of dairy products is usually not one of the differential diagnoses of functional dyspepsia. In their study, Wortmann et al[107] showed that adult-type lactose intolerance was present in 44.7% of patients with functional dyspepsia. Mishkin et al[108] showed that the prevalence of lactose intolerance in patients with functional dyspepsia is affected by ethnic group and age, with a decreased prevalence between 25-55 years and an increase after 55 years.
Persistent regurgitation is a common nonspecific symptom of CMPA in infants. It usually presents with excessive crying, irritability, pain, respiratory symptoms, and feeding or sleep disturbance. CMPA was documented in up to 50% of infants with persistent GER, which could be just an association or CMPA-induced GER. The likelihood of CMPA increases in the presence of atopy and multiorgan symptoms such as failure to thrive, diarrhea, rectal bleeding, or atopic dermatitis[32,109,110]. A negative SPT does not rule out CMPA as it is IgE-and non-IgE-mediated. At the same time, clinical improvement with a cow’s milk-free diet is not solid proof of immune system involvement. Therefore, esophageal pH impedance, oral food challenge, and endoscopy are recommended to reach a correct clinical diagnosis and classification, but they are not always possible in all infants[111]. We should consider using 2-4 wk of a protein hydrolysate or amino acid-based formula in a formula-fed infant or eliminating cow’s milk in the maternal diet in breastfed infants[112]. However, there is not enough evidence to avoid drinking milk for GER. Patients with GER who tolerate cow’s milk may continue to consume it. Avoidance of milk consumption should be avoided only if symptoms of GER increase with milk consumption[113]. Due to its acid-neutralizing properties, milk has long been used to treat the symptoms of peptic ulcers and GERD. However, the high calcium and protein content significantly increased acid production by 30% for every 250 mL of milk. In addition, patients with peptic ulcers are more vulnerable to the effects of milk on the gastric parietal cells[114]. As confirmed by an endoscopic study[115], patients with peptic ulcers who avoided a milk-based diet had better ulcer cicatrization results than those who consumed milk. However, patients with a peptic ulcer can consume a moderate amount of dairy products according to their tolerance to benefit from their high nutritional content[116].
Infant colic is a functional disorder characterized by full-force crying for a minimum of three hours per day for a minimum of three days per week in infants younger than five months. It is a worldwide disorder affecting many infants and families. The precise mechanisms are not well known. While it does not indicate the presence of disease, it occasionally represents an underlying severe disorder in a small percentage of infants who may require a medical assessment[117]. Cow’s milk may precipitate infant colic through CMPA and lactose intolerance. Both conditions can induce colic through gut inflammation (as indicated by fecal calprotectin) and gut dysmotility and dysbiosis, with fewer Bifidobacilli inducing abnormal peristalsis and colicky pain[118]. Colic may arise as a delayed reaction within a few hours to days of CMP consumption. Infant colic may be one of the many symptoms of CMPA. A cohort of 100 infants with colic showed a positive challenge-proven CMPA in 44% of the infants during a cow’s-milk challenge[119]. Moravej et al[120] showed that the SPT for CMP was positive in 2.6% of infants with colic who responded well to cow’s milk elimination from the mothers’ diet. Many infants with colic have transient lactose intolerance, causing excessive gas production. Lactase enzyme activity may be low in many infants during the first weeks of life and may take a few weeks to improve. Pretreatment of feeds with lactase can improve colic[121]. For breastfeeding colicky infants, a trial of dairy product exclusion by the mother for 2-4 wk could produce symptomatic improvement. For bottle-fed infants, a partially hydrolyzed formula with Galacto-Oligosaccharides/Fructo-Oligosaccharides and added β-palmitate might be beneficial in cases where CMPA is not suspected. The mother can also use a formula containing prebiotics and/or ferments or a lactose-reduced formula[122].
Functional constipation is a common disorder in children, negatively impacting their quality of life. Simeone et al[123] showed that 17.3% of children with functional constipation had evidence of CMPA and improved with a CMP elimination diet. The European Society for Paediatric Gastroenterology, Hepatology and Nutrition-North American Society for Paediatric Gastroenterology, Hepatology and Nutrition advised that a CMP-free diet be tried only in cases of laxative-resistant constipation and only after consulting an expert. However, a literature review by Sopo et al[124] showed that 28% to 78% of children with functional constipation benefited from a CMP-free diet. The review results give solid scientific evidence for a causal relationship between functional constipation and CMPA. Therefore, they recommended a two-to-four-week restricted diet as a first-line diagnostic and therapeutic strategy in children with functional constipation[124]. The same result was also found in the literature review by Gelsomino et al[125]. They propose that a CMP-free diet should be considered a first-line treatment for functional constipation, at least in preschool children and children with a previous diagnosis of CMPA or a personal or family history of atopy.
IBS is one of the most common FGIDs with poorly understood mechanisms that affect a significant proportion of the population with a strong negative impact on the quality of life[126]. The lack of well-understood underlying pathophysiologic mechanisms makes choosing effective treatment strategies difficult. Clinicians commonly recommend a lactose-free diet to manage this syndrome[118]. Approximately one-third of patients with IBS have some degree of lactose intolerance, which could be present with diarrhea, gas, and bloating. Milk lactose irritates the already vulnerable intestines of people with IBS. However, a systematic review by Vaiopoulou et al[127] did not find convincing evidence to indicate an objective relationship between IBS and any recognized malabsorption syndrome involving lactose intolerance.
On the other hand, a significant portion of people with IBS and self-reported lactose intolerance and negative hydrogen breath testing were proved to be due to underlying immune-mediated reactions to CMP. Carroccio et al[55] in a study with retrospective and prospective phases, showed that a percentage of self-reported milk intolerance in patients with IBS was not related to lactose intolerance; instead, they clinically reacted when subjected to whole cow’s milk, indicating CMPA. Patients with IBS and lactose intolerance had more severe symptoms and a higher degree of fatigue, anxiety, and depression[128,129]. Therefore, we should consider that a significant minority of patients with IBS could benefit from a dairy elimination diet, especially in the presence of evidence of CMPA. More studies are necessary to identify the complex pathogenic mechanisms of FGIDs and to improve their management[130].
COW’S MILK AND IBDS
The relationship between cow’s milk and IBD is cause and effect. There is much evidence of an increased prevalence of ulcerative colitis in children with a previous history of CMPA. Knoflach et al[131] found high IgG and IgM against CMP in patients with IBD than in controls, with a good correlation between disease activity and the levels of IgG and IgA against certain CMPs. In addition, Virta et al[132] found in two separate studies that a past history of CMPA increases the prevalence of pediatric IBD. In contrast, asthma increases the likelihood of Crohn’s disease[132,133].
On the other hand, lactose malabsorption prevalence is significantly more common in patients with Crohn’s disease affecting the small intestine than in patients with Crohn’s disease affecting the colon or patients with ulcerative colitis. In IBD affecting the colon, lactose malabsorption prevalence is affected by other factors, such as ethnic risk determined by genetic factors. The degree of lactose malabsorption in Crohn’s disease of the small intestine is also affected by bacterial overgrowth and small intestine transit time, in addition to lactase enzyme activity[132].
Avoiding dairy products is common dogmatic advice given by many physicians to patients with IBD. However, Strisciuglio et al[134] showed that dietary CMP elimination has no substantial role in managing ulcerative colitis in non-sensitized children. Due to dairy product restrictions in patients with IBD (either due to unnecessary fear of the presence of CMPA or due to the occurrence of secondary lactose intolerance), there is an increased risk of inadequate intake of calcium, an essential element to prevent the decrease in bone mineral density, which consequently increases the risk of osteoporosis. Therefore, proper dietary management is crucial to prevent osteoporosis through education and adequate dietary management with low/free-lactose milk, fermented milk, plant-based milk supplemented with calcium and vitamin D, calcium-rich foods, and calcium supplements[135]. The exclusion of certain types of foods should be based on solid science. Indiscriminate exclusion of certain foods increases the risk of nutritional deficiencies. Lim et al[136] showed that the mean daily calcium, vitamin A, and zinc intake were significantly decreased in the food exclusion group. Milk was the most common restricted food, followed by dairy products, raw fish, deep-spicy foods, and ramen. Therefore, the magnitude of osteoporosis will be further increased by dairy product restriction, in addition to the effects of IBD itself[137].
Fermented milk using specific lactic acid bacteria could help avoid dairy product restrictions. Fermented milk with lactic acid-producing bacteria contains exopolysaccharides, peptides, and short-chain fatty acids that help to modulate intestinal lumen pH, help recovery of intestine mucosa, modulate the gut microbiota, and alleviate the inflammatory response by modifying the innate and adaptive immune system. As a result, bioactive compounds derived from fermented milk can alleviate the negative symptoms of IBD[138]. Consequently, the disease activity can be significantly reduced by the oral administration of specific probiotics containing B. subtilis JNFE0126. Zhang et al[139] showed that B. subtilis-containing fermented milk could decrease the intestinal mucosa inflammatory response, induce intestinal stem cell proliferation, and promote mucosal barrier reconstruction. B. subtilis-containing fermented milk helps to rebalance the gut mucosa through the enrichment of Lactobacillus, Bacillus, and Alistipes and decrease Escherichia and Bacteroides abundance.
COW’S MILK-RELATED GASTROINTESTINAL CANCER
Despite data from the geographic distribution of colon cancer showing increased milk consumption[140], a meta-analysis found that milk and whole milk products are associated with a lower risk of colorectal cancer[141]. The protective effects of milk and whole dairy products are related to the high calcium content of milk. Calcium is the primary anti-carcinogen, especially in doses equal to or higher than 1200 mg/d. Therefore, calcium supplementation is indicated in patients with a contraindication to milk intake[142]. Vitamin D is another milk ingredient that protects against colon cancer. However, Baron et al[143] showed that daily vitamin D3 (1000 IU), calcium (1200 mg), or supplementation with both after surgical removal of colorectal adenomas did not show significant risk reduction of recurrent colorectal adenomas over a follow-up period of three to five years.
MILK AND GASTROINTESTINAL INFECTIONS
As milk is a part of everyday human food from birth onwards, it may be a source of microbial infection in humans, causing many diseases. Milk is rich in sugars, lipids, and proteins, which are ideal media for the growth of a broad spectrum of organisms. Diseases produced by milk-borne organisms include Mycobacterium avium, Mycobacterium bovis, Salmonella species, brucellosis, streptococcal infections, “summer diarrhea”, Yersinia enterocolitica, diphtheria, Escherichia coli (E. coli), Campylobacter jejuni, Citrobacter species, Shiga toxin-producing E. coli, Pseudomonas aeruginosa, Proteus mirabilis, and Klebsiella species[144]. Campylobacter species and Salmonella species are the most common identified etiological agents, while other zoonotic infections, particularly yersiniosis and listeriosis, are increasingly reported. Most infections were due to improperly treated cows’ milk or dairy products and increasingly polluted “heat-treated” milk products. Milk pasteurization decreased infectious diseases and their high infant mortality rates by only 50%, even with concurrent medical and dairy hygiene advances, particularly in the less developed world[145]. Most of these infections presented with manifestations of gastroenteritis, food poisoning, and hepatosplenomegaly, in addition to systemic manifestations such as fever, muscle aches, severe headache, meningitis, sepsis, pneumonia, and renal failure. Raw milk consumption, a common practice by milk producers on their farms, is a significant risk factor for milk-transmitted infection, despite the other health benefits of drinking fresh milk over pasteurized milk. In addition, raw milk consumption increases the risk of horizontal gene transfer of antimicrobial resistance genes where the bovine strains may meet the human microbiota and change them into resistant pathogenic strains, a fundamental reason for increasing the prevalence of antimicrobial resistance[146].
CONCLUSION
Cow’s milk induces various gastrointestinal and systemic manifestations from birth to the elderly with various underlying mechanisms. Cow’s milk allergy is a common disorder, especially at pediatric age, with different presentations according to the site of major implication. However, when it is present in adulthood, it is usually severe. Eliminating the offending CMP from breastfeeding mothers’ diets and using extensively hydrolyzed or amino acid-based formulas are the main lines of treatment in infancy and childhood. Avoiding dairy and dairy products is also conducted for adults with CMPA. Different types of lactose intolerance can occur with different presentations and prevalence according to age and ethnicity. It can be isolated or be a part of a broader intolerance to various saccharides. CMPA and lactose intolerance, in addition to milk-induced gut microbiota dysbiosis, are commonly associated with various FGIDs such as gastroesophageal reflux, peptic ulcers, infant colic, IBS, and constipation. Cow’s milk consumption may be implicated in the pathogenesis of some cancers and the prevention of others. In addition, cow’s milk is a significant source of many zoonotic infections that could affect human health. It may also play a role in the development of antimicrobial resistance. This special issue will cover these various topics in more detail. Physicians and patients should be well oriented with milk-related disorders to avoid unnecessary nutritional mismanagement.
ACKNOWLEDGEMENTS
We thank the anonymous referees for their valuable suggestions.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Faculty of Medicine, Tanta University, Egypt; University Medical Center, King Abdulla Medical City. Arabian Gulf University.
Peer-review started: August 14, 2022
First decision: August 29, 2022
Article in press: October 14, 2022
Specialty type: Pediatrics
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Li J, China; Margiotta G, Italy; Wen XL, China S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
Contributor Information
Mohammed Al-Beltagi, Department of Pediatrics, Faculty of Medicine, Tanta University, Tanta 31511, Algharbia, Egypt; Department of Pediatrics, University Medical Center, Arabian Gulf University, Manama 26671, Bahrain. mbelrem@hotmail.com.
Nermin Kamal Saeed, Department of Pathology, Microbiology Section, Salmaniya Medical Complex, Manama 26671, Bahrain; Department of Pathology, Microbiology Section, Royal College of Surgeons in Ireland - Bahrain, Busaiteen 15503, Muharraq, Bahrain.
Adel Salah Bediwy, Department of Chest Diseases, Faculty of Medicine, Tanta University, Tanta 31527, Algharbia, Egypt; Department of Chest Diseases, University Medical Center, Arabian Gulf University, Manama 26671, Bahrain.
Reem Elbeltagi, Department of Medicine, The Royal College of Surgeons in Ireland - Bahrain, Busiateen 15503, Muharraq, Bahrain.
References
- 1.Zeder MA. Domestication and early agriculture in the Mediterranean Basin: Origins, diffusion, and impact. Proc Natl Acad Sci U S A. 2008;105:11597–11604. doi: 10.1073/pnas.0801317105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corsello A, Pugliese D, Gasbarrini A, Armuzzi A. Diet and Nutrients in Gastrointestinal Chronic Diseases. Nutrients. 2020;12 doi: 10.3390/nu12092693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aslam H, Marx W, Rocks T, Loughman A, Chandrasekaran V, Ruusunen A, Dawson SL, West M, Mullarkey E, Pasco JA, Jacka FN. The effects of dairy and dairy derivatives on the gut microbiota: a systematic literature review. Gut Microbes. 2020;12:1799533. doi: 10.1080/19490976.2020.1799533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannetti A, Toschi Vespasiani G, Ricci G, Miniaci A, di Palmo E, Pession A. Cow's Milk Protein Allergy as a Model of Food Allergies. Nutrients. 2021;13 doi: 10.3390/nu13051525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shokouhi Shoormasti R, Fazlollahi MR, Barzegar S, Teymourpour P, Yazdanyar Z, Lebaschi Z, Nourizadeh M, Tazesh B, Movahedi M, Kashani H, Pourpak Z, Moin M. The Most Common Cow's Milk Allergenic Proteins with Respect to Allergic Symptoms in Iranian Patients. Iran J Allergy Asthma Immunol. 2016;15:161–165. [PubMed] [Google Scholar]
- 6.Sletten GB, Halvorsen R, Egaas E, Halstensen TS. Changes in humoral responses to beta-lactoglobulin in tolerant patients suggest a particular role for IgG4 in delayed, non-IgE-mediated cow's milk allergy. Pediatr Allergy Immunol. 2006;17:435–443. doi: 10.1111/j.1399-3038.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 7.Mastrorilli C, Santoro A, Caffarelli C. Primary Prevention of Allergic Diseases: The Role of Early Exposure to Cow's Milk Formula. Front Pediatr. 2020;8:420. doi: 10.3389/fped.2020.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landi N, Ragucci S, Di Maro A. Amino Acid Composition of Milk from Cow, Sheep and Goat Raised in Ailano and Valle Agricola, Two Localities of 'Alto Casertano' (Campania Region) Foods. 2021;10 doi: 10.3390/foods10102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martelli A, De Chiara A, Corvo M, Restani P, Fiocchi A. Beef allergy in children with cow's milk allergy; cow's milk allergy in children with beef allergy. Ann Allergy Asthma Immunol. 2002;89:38–43. doi: 10.1016/s1081-1206(10)62121-7. [DOI] [PubMed] [Google Scholar]
- 10.Restani P, Gaiaschi A, Plebani A, Beretta B, Cavagni G, Fiocchi A, Poiesi C, Velonà T, Ugazio AG, Galli CL. Cross-reactivity between milk proteins from different animal species. Clin Exp Allergy. 1999;29:997–1004. doi: 10.1046/j.1365-2222.1999.00563.x. [DOI] [PubMed] [Google Scholar]
- 11.Candreva AM, Smaldini PL, Curciarello R, Cauerhff A, Fossati CA, Docena GH, Petruccelli S. Cross-reactivity between the soybean protein p34 and bovine caseins. Allergy Asthma Immunol Res. 2015;7:60–68. doi: 10.4168/aair.2015.7.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anthoni S, Savilahti E, Rautelin H, Kolho KL. Milk protein IgG and IgA: the association with milk-induced gastrointestinal symptoms in adults. World J Gastroenterol. 2009;15:4915–4918. doi: 10.3748/wjg.15.4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anthoni S, Elg P, Haahtela T, Kolho KL. Should milk-specific IgE antibodies be measured in adults in primary care? Scand J Prim Health Care. 2008;26:197–202. doi: 10.1080/02813430802117442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–1113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Martorell A, Plaza AM, Boné J, Nevot S, García Ara MC, Echeverria L, Alonso E, Garde J, Vila B, Alvaro M, Tauler E, Hernando V, Fernández M. Cow's milk protein allergy. A multi-centre study: clinical and epidemiological aspects. Allergol Immunopathol (Madr) 2006;34:46–53. doi: 10.1157/13086746. [DOI] [PubMed] [Google Scholar]
- 16.Sakai K, Yoshino K, Satter MA, Ota F, Nii Y, Fukuta K, Ueda N, Shimizu Y, Yamamoto S. Effects of pH variation and NaCl on in vitro digestibility of cow's milk proteins in commercially available infant formulas. J Nutr Sci Vitaminol (Tokyo) 2000;46:325–328. doi: 10.3177/jnsv.46.325. [DOI] [PubMed] [Google Scholar]
- 17.Perezabad L, López-Abente J, Alonso-Lebrero E, Seoane E, Pion M, Correa-Rocha R. The establishment of cow's milk protein allergy in infants is related with a deficit of regulatory T cells (Treg) and vitamin D. Pediatr Res. 2017;81:722–730. doi: 10.1038/pr.2017.12. [DOI] [PubMed] [Google Scholar]
- 18.Vandenplas Y, Al-Hussaini B, Al-Mannaei K, Al-Sunaid A, Helmi Ayesh W, El-Degeir M, El-Kabbany N, Haddad J, Hashmi A, Kreishan F, Tawfik E. Prevention of Allergic Sensitization and Treatment of Cow's Milk Protein Allergy in Early Life: The Middle-East Step-Down Consensus. Nutrients. 2019;11 doi: 10.3390/nu11071444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil F, Amezqueta A, Martinez D, Aznal E, Etayo V, Durá T, Sánchez-Valverde F. Association between Caesarean Delivery and Isolated Doses of Formula Feeding in Cow Milk Allergy. Int Arch Allergy Immunol. 2017;173:147–152. doi: 10.1159/000477725. [DOI] [PubMed] [Google Scholar]
- 20.Molloy J, Allen K, Collier F, Tang ML, Ward AC, Vuillermin P. The potential link between gut microbiota and IgE-mediated food allergy in early life. Int J Environ Res Public Health. 2013;10:7235–7256. doi: 10.3390/ijerph10127235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Zheng S, Yang X, Huazeng B, Cheng Q. Influences of non-IgE-mediated cow's milk protein allergy-associated gut microbial dysbiosis on regulatory T cell-mediated intestinal immune tolerance and homeostasis. Microb Pathog. 2021;158:105020. doi: 10.1016/j.micpath.2021.105020. [DOI] [PubMed] [Google Scholar]
- 22.Tan W, Zhou Z, Li W, Lu H, Qiu Z. Lactobacillus rhamnosus GG for Cow's Milk Allergy in Children: A Systematic Review and Meta-Analysis. Front Pediatr. 2021;9:727127. doi: 10.3389/fped.2021.727127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward CM, Geng L, Jyonouchi H. Fetal sensitization to cow's milk protein and wheat: cow's milk protein and wheat-specific TNF-alpha production by umbilical cord blood cells and subsequent decline of TNF-alpha production by peripheral blood mononuclear cells following dietary intervention. Pediatr Allergy Immunol. 2007;18:276–280. doi: 10.1111/j.1399-3038.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 24.Høst A. Frequency of cow's milk allergy in childhood. Ann Allergy Asthma Immunol. 2002;89:33–37. doi: 10.1016/s1081-1206(10)62120-5. [DOI] [PubMed] [Google Scholar]
- 25.Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, Dean T. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–1124. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 26.Saarinen KM, Pelkonen AS, Mäkelä MJ, Savilahti E. Clinical course and prognosis of cow's milk allergy are dependent on milk-specific IgE status. J Allergy Clin Immunol. 2005;116:869–875. doi: 10.1016/j.jaci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Vanto T, Helppilä S, Juntunen-Backman K, Kalimo K, Klemola T, Korpela R, Koskinen P. Prediction of the development of tolerance to milk in children with cow's milk hypersensitivity. J Pediatr. 2004;144:218–222. doi: 10.1016/j.jpeds.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 28.Kvenshagen B, Halvorsen R, Jacobsen M. Adverse reactions to milk in infants. Acta Paediatr. 2008;97:196–200. doi: 10.1111/j.1651-2227.2007.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacob S, Bonito Vitor A. [Cow's milk protein intolerance imitating septic shock in a young infant] An Pediatr (Engl Ed) 2019;90:52–53. doi: 10.1016/j.anpedi.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Walsh J, Meyer R, Shah N, Quekett J, Fox AT. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: understanding the underlying mechanisms and presentations. Br J Gen Pract. 2016;66:e609–e611. doi: 10.3399/bjgp16X686521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yimyaem P, Chongsrisawat V, Vivatvakin B, Wisedopas N. Gastrointestinal manifestations of cow's milk protein allergy during the first year of life. J Med Assoc Thai. 2003;86:116–123. [PubMed] [Google Scholar]
- 32.Salvatore S, Agosti M, Baldassarre ME, D'Auria E, Pensabene L, Nosetti L, Vandenplas Y. Cow's Milk Allergy or Gastroesophageal Reflux Disease-Can We Solve the Dilemma in Infants? Nutrients. 2021;13 doi: 10.3390/nu13020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teoh T, Mill C, Chan E, Zimmer P, Avinashi V. Liberalized Versus Strict Cow's Milk Elimination for the Treatment of Children with Eosinophilic Esophagitis. J Can Assoc Gastroenterol. 2019;2:81–85. doi: 10.1093/jcag/gwy030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vinit C, Dieme A, Courbage S, Dehaine C, Dufeu CM, Jacquemot S, Lajus M, Montigny L, Payen E, Yang DD, Dupont C. Eosinophilic esophagitis: Pathophysiology, diagnosis, and management. Arch Pediatr. 2019;26:182–190. doi: 10.1016/j.arcped.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 35.Machado RS, Kawakami E, Goshima S, Patrício FR, Fagundes Neto U. [Hemorrhagic gastritis due to cow's milk allergy: report of two cases] J Pediatr (Rio J) 2003;79:363–368. [PubMed] [Google Scholar]
- 36.Alabsi HS, Reschak GL, Fustino NJ, Beltroy EP, Sramek JE, Alabsi SY. Neonatal eosinophilic gastroenteritis: possible in utero sensitization to cow's milk protein. Neonatal Netw. 2013;32:316–322. doi: 10.1891/0730-0832.32.5.316. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Homma T, Kurokawa M, Matsukura S, Adachi M, Wakabayashi K, Nozu F, Tazaki T, Kimura T, Matsuura T, Fukuda M, Shiozawa E, Takimoto M. Eosinophilic gastroenteritis due to cow's milk allergy presenting with acute pancreatitis. Int Arch Allergy Immunol. 2012;158 Suppl 1:75–82. doi: 10.1159/000337782. [DOI] [PubMed] [Google Scholar]
- 38.Lyngbaek S, Adamsen S, Aru A, Bergenfeldt M. Recurrent acute pancreatitis due to eosinophilic gastroenteritis. Case report and literature review. JOP. 2006;7:211–217. [PubMed] [Google Scholar]
- 39.Feketea G, Popp A, Ionescu DM, Berghea EC. Case Report: Food Protein-Induced Protein Losing Enteropathy (FPIPLE) in Infancy. Front Nutr. 2022;9:810409. doi: 10.3389/fnut.2022.810409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umar SB, DiBaise JK. Protein-losing enteropathy: case illustrations and clinical review. Am J Gastroenterol. 2010;105:43–9; quiz 50. doi: 10.1038/ajg.2009.561. [DOI] [PubMed] [Google Scholar]
- 41.Feuille E, Nowak-Węgrzyn A. Food Protein-Induced Enterocolitis Syndrome, Allergic Proctocolitis, and Enteropathy. Curr Allergy Asthma Rep. 2015;15:50. doi: 10.1007/s11882-015-0546-9. [DOI] [PubMed] [Google Scholar]
- 42.Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome and allergic proctocolitis. Allergy Asthma Proc. 2015;36:172–184. doi: 10.2500/aap.2015.36.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Järvinen KM, Nowak-Węgrzyn A. Food protein-induced enterocolitis syndrome (FPIES): current management strategies and review of the literature. J Allergy Clin Immunol Pract. 2013;1:317–322. doi: 10.1016/j.jaip.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Bulsa K, Standowicz M, Baryła-Pankiewicz E, Czaja-Bulsa G. Chronic Milk-Dependent Food Protein-Induced Enterocolitis Syndrome in Children from West Pomerania Region. Nutrients. 2021;13 doi: 10.3390/nu13114137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassilopoulou E, Feketea G, Konstantinou GN, Zekakos Xypolias D, Valianatou M, Petrodimopoulou M, Vourga V, Tasios I, Papadopoulos NG. Food Protein-Induced Allergic Proctocolitis: The Effect of Maternal Diet During Pregnancy and Breastfeeding in a Mediterranean Population. Front Nutr. 2022;9:843437. doi: 10.3389/fnut.2022.843437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martin VM, Virkud YV, Seay H, Hickey A, Ndahayo R, Rosow R, Southwick C, Elkort M, Gupta B, Kramer E, Pronchick T, Reuter S, Keet C, Su KW, Shreffler WG, Yuan Q. Prospective Assessment of Pediatrician-Diagnosed Food Protein-Induced Allergic Proctocolitis by Gross or Occult Blood. J Allergy Clin Immunol Pract. 2020;8:1692–1699.e1. doi: 10.1016/j.jaip.2019.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, Staiano A, Dupont C. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007;92:902–908. doi: 10.1136/adc.2006.110999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam HY, van Hoffen E, Michelsen A, Guikers K, van der Tas CH, Bruijnzeel-Koomen CA, Knulst AC. Cow's milk allergy in adults is rare but severe: both casein and whey proteins are involved. Clin Exp Allergy. 2008;38:995–1002. doi: 10.1111/j.1365-2222.2008.02968.x. [DOI] [PubMed] [Google Scholar]
- 49.Morfín-Maciel BM, Castillo-Morfín BM. [Anaphylaxis caused by dermal exposure with cow's milk in an adult with food allergy. A case report] Rev Alerg Mex. 2020;67:73–78. doi: 10.29262/ram.v67i1.669. [DOI] [PubMed] [Google Scholar]
- 50.Quirantes Sierra B, Lara Jiménez A, Skodova M. Sensitization to cow's milk protein in a dairy worker. Occup Med (Lond) 2017;67:579–580. doi: 10.1093/occmed/kqx101. [DOI] [PubMed] [Google Scholar]
- 51.Hansen MM, Nissen SP, Halken S, Høst A. The natural course of cow's milk allergy and the development of atopic diseases into adulthood. Pediatr Allergy Immunol. 2021;32:727–733. doi: 10.1111/pai.13440. [DOI] [PubMed] [Google Scholar]
- 52.Stöger P, Wüthrich B. Type I allergy to cow milk proteins in adults. A retrospective study of 34 adult milk- and cheese-allergic patients. Int Arch Allergy Immunol. 1993;102:399–407. doi: 10.1159/000236589. [DOI] [PubMed] [Google Scholar]
- 53.He M, Sun J, Jiang ZQ, Yang YX. Effects of cow's milk beta-casein variants on symptoms of milk intolerance in Chinese adults: a multicentre, randomised controlled study. Nutr J. 2017;16:72. doi: 10.1186/s12937-017-0275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristjánsson G, Venge P, Hällgren R. Mucosal reactivity to cow's milk protein in coeliac disease. Clin Exp Immunol. 2007;147:449–455. doi: 10.1111/j.1365-2249.2007.03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carroccio A, Brusca I, Mansueto P, Soresi M, D'Alcamo A, Ambrosiano G, Pepe I, Iacono G, Lospalluti ML, La Chiusa SM, Di Fede G. Fecal assays detect hypersensitivity to cow's milk protein and gluten in adults with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2011;9:965–971.e3. doi: 10.1016/j.cgh.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Domínguez-García V, Flores-Merino MV, Morales-Romero J, Bedolla-Pulido A, Mariscal-Castro J, Bedolla-Barajas M. [Allergy to cow's milk protein, or lactose intolerance: a cross-sectional study in university students] Rev Alerg Mex. 2019;66:394–402. doi: 10.29262/ram.v66i4.640. [DOI] [PubMed] [Google Scholar]
- 57.Carroccio A, Soresi M, Mantia B, Fayer F, La Blasca F, Seidita A, D'Alcamo A, Florena AM, Tinè C, Garlisi C, Mansueto P. Whole Cow's Milk but Not Lactose Can Induce Symptoms inPatients with Self-Reported Milk Intolerance: Evidence of Cow's Milk Sensitivity in Adults. Nutrients. 2021;13 doi: 10.3390/nu13113833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sousa MJCS, Reis Ferreira AL, Moreira da Silva JP. Bodybuilding protein supplements and cow's milk allergy in adult. Eur Ann Allergy Clin Immunol. 2018;50:42–44. doi: 10.23822/EurAnnACI.1764-1489.28. [DOI] [PubMed] [Google Scholar]
- 59.Tedner SG, Asarnoj A, Thulin H, Westman M, Konradsen JR, Nilsson C. Food allergy and hypersensitivity reactions in children and adults-A review. J Intern Med. 2022;291:283–302. doi: 10.1111/joim.13422. [DOI] [PubMed] [Google Scholar]
- 60.Olivier CE, Lorena SL, Pavan CR, dos Santos RA, dos Santos Lima RP, Pinto DG, da Silva MD, de Lima Zollner R. Is it just lactose intolerance? Allergy Asthma Proc. 2012;33:432–436. doi: 10.2500/aap.2012.33.3584. [DOI] [PubMed] [Google Scholar]
- 61.Leonard SA, Nowak-Węgrzyn A. Food Protein-Induced Enterocolitis Syndrome. Pediatr Clin North Am. 2015;62:1463–1477. doi: 10.1016/j.pcl.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 62.Du YJ, Nowak-Węgrzyn A, Vadas P. FPIES in adults. Ann Allergy Asthma Immunol. 2018;121:736–738. doi: 10.1016/j.anai.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 63.Celakovská J, Ettlerová K, Ettler K, Vanecková J, Bukac J. Evaluation of cow's milk allergy in a large group of adolescent and adult patients with atopic dermatitis. Acta Medica (Hradec Kralove) 2012;55:125–129. doi: 10.14712/18059694.2015.49. [DOI] [PubMed] [Google Scholar]
- 64.Sinai T, Goldberg MR, Nachshon L, Amitzur-Levy R, Yichie T, Katz Y, Monsonego-Ornan E, Elizur A. Reduced Final Height and Inadequate Nutritional Intake in Cow's Milk-Allergic Young Adults. J Allergy Clin Immunol Pract. 2019;7:509–515. doi: 10.1016/j.jaip.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 65.de Diego Lorenzo A, Robles Fornieles J, Herrero López T, Cos Arregui E. Acute pancreatitis associated with milk allergy. Int J Pancreatol. 1992;12:319–321. doi: 10.1007/BF02924372. [DOI] [PubMed] [Google Scholar]
- 66.Nachshon L, Goldberg MR, Schwartz N, Sinai T, Amitzur-Levy R, Elizur A, Eisenberg E, Katz Y. Decreased bone mineral density in young adult IgE-mediated cow's milk-allergic patients. J Allergy Clin Immunol. 2014;134:1108–1113.e3. doi: 10.1016/j.jaci.2014.06.026. [DOI] [PubMed] [Google Scholar]
- 67.Min KB, Min JY. Increased risk for hyperuricemia in adults sensitized to cow milk allergen. Clin Rheumatol. 2017;36:1407–1412. doi: 10.1007/s10067-016-3457-9. [DOI] [PubMed] [Google Scholar]
- 68.Kaskous S. Cow's milk consumption and risk of disease. Emir J Food Agric. 2021;33:1–11. [Google Scholar]
- 69.Vesa TH, Marteau P, Korpela R. Lactose intolerance. J Am Coll Nutr. 2000;19:165S–175S. doi: 10.1080/07315724.2000.10718086. [DOI] [PubMed] [Google Scholar]
- 70.Mattar R, de Campos Mazo DF, Carrilho FJ. Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 2012;5:113–121. doi: 10.2147/CEG.S32368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Szilagyi A, Ishayek N. Lactose Intolerance, Dairy Avoidance, and Treatment Options. Nutrients. 2018;10 doi: 10.3390/nu10121994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amiri M, Diekmann L, von Köckritz-Blickwede M, Naim HY. The Diverse Forms of Lactose Intolerance and the Putative Linkage to Several Cancers. Nutrients. 2015;7:7209–7230. doi: 10.3390/nu7095332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Y, Misselwitz B, Dai N, Fox M. Lactose Intolerance in Adults: Biological Mechanism and Dietary Management. Nutrients. 2015;7:8020–8035. doi: 10.3390/nu7095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schiffner R, Kostev K, Gothe H. Do patients with lactose intolerance exhibit more frequent comorbidities than patients without lactose intolerance? Ann Gastroenterol. 2016;29:174–179. doi: 10.20524/aog.2016.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rocco A, Compare D, Sgamato C, Martino A, De Simone L, Coccoli P, Melone ML, Nardone G. Blinded Oral Challenges with Lactose and Placebo Accurately Diagnose Lactose Intolerance: A Real-Life Study. Nutrients. 2021;13 doi: 10.3390/nu13051653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su HM, Jiang Y, Hu YL, Yang H, Dong TJ. [Lactose intolerance in neonates with non-infectious diarrhea] Zhongguo Dang Dai Er Ke Za Zhi. 2016;18:306–310. doi: 10.7499/j.issn.1008-8830.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caballero B, Solomons NW, Torún B. Fecal reducing substances and breath hydrogen excretion as indicators of carbohydrate malabsorption. J Pediatr Gastroenterol Nutr. 1983;2:487–490. doi: 10.1097/00005176-198302030-00016. [DOI] [PubMed] [Google Scholar]
- 78.Yang JF, Fox M, Chu H, Zheng X, Long YQ, Pohl D, Fried M, Dai N. Four-sample lactose hydrogen breath test for diagnosis of lactose malabsorption in irritable bowel syndrome patients with diarrhea. World J Gastroenterol. 2015;21:7563–7570. doi: 10.3748/wjg.v21.i24.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Lacy Costello BP, Ledochowski M, Ratcliffe NM. The importance of methane breath testing: a review. J Breath Res. 2013;7:024001. doi: 10.1088/1752-7155/7/2/024001. [DOI] [PubMed] [Google Scholar]
- 80.Domínguez-Jiménez JL, Fernández-Suárez A, Ruiz-Tajuelos S, Puente-Gutiérrez JJ, Cerezo-Ruiz A. Lactose tolerance test shortened to 30 minutes: An exploratory study of its feasibility and impact. Rev Esp Enferm Dig. 2014;106:381–385. [PubMed] [Google Scholar]
- 81.Ghoshal UC, Kumar S, Chourasia D, Misra A. Lactose hydrogen breath test versus lactose tolerance test in the tropics: does positive lactose tolerance test reflect more severe lactose malabsorption? Trop Gastroenterol. 2009;30:86–90. [PubMed] [Google Scholar]
- 82.Hermida C, Corrales G, Martínez-Costa OH, Fernández-Mayoralas A, Aragón JJ. Noninvasive evaluation of intestinal lactase with 4-galactosylxylose: comparison with 3- and 2-galactosylxylose and optimization of the method in rats. Clin Chem. 2006;52:270–277. doi: 10.1373/clinchem.2005.058446. [DOI] [PubMed] [Google Scholar]
- 83.Monsalve-Hernando C, Crespo L, Ferreiro B, Martín V, Aldeguer X, Opio V, Fernández-Gil PL, Gaspar MJ, Romero E, Lara C, Santander C, Torrealba L, Savescu T, Hermida C. Phase IV noninferiority controlled randomized trial to evaluate the impact on diagnostic thinking and patient management and the test-retest reproducibility of the Gaxilose test for hypolactasia diagnosis. Medicine (Baltimore) 2018;97:e13136. doi: 10.1097/MD.0000000000013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aragón JJ, Hermida C, Martínez-Costa OH, Sánchez V, Martín I, Sánchez JJ, Codoceo R, Cano JM, Cano A, Crespo L, Torres Y, García FJ, Fernández-Mayoralas A, Solera J, Martínez P. Noninvasive diagnosis of hypolactasia with 4-Galactosylxylose (Gaxilose): a multicentre, open-label, phase IIB-III nonrandomized trial. J Clin Gastroenterol. 2014;48:29–36. doi: 10.1097/MCG.0b013e318297fb10. [DOI] [PubMed] [Google Scholar]
- 85.Catanzaro R, Sciuto M, Marotta F. Lactose Intolerance-Old and New Knowledge on Pathophysiological Mechanisms, Diagnosis, and Treatment. SN Compr Clin Med. 2021;3:499–509. [Google Scholar]
- 86.Santonocito C, Scapaticci M, Guarino D, Annicchiarico EB, Lisci R, Penitente R, Gasbarrini A, Zuppi C, Capoluongo E. Lactose intolerance genetic testing: is it useful as routine screening? Clin Chim Acta. 2015;439:14–17. doi: 10.1016/j.cca.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 87.Buzás GM. [Lactose intolerance: past and present. Part 1] Orv Hetil. 2015;156:1532–1539. doi: 10.1556/650.2015.30261. [DOI] [PubMed] [Google Scholar]
- 88.Gordon M, Biagioli E, Sorrenti M, Lingua C, Moja L, Banks SS, Ceratto S, Savino F. Dietary modifications for infantile colic. Cochrane Database Syst Rev. 2018;10:CD011029. doi: 10.1002/14651858.CD011029.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cancarevic I, Rehman M, Iskander B, Lalani S, Malik BH. Is There a Correlation Between Irritable Bowel Syndrome and Lactose Intolerance? Cureus. 2020;12:e6710. doi: 10.7759/cureus.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Asfari MM, Sarmini MT, Kendrick K, Hudgi A, Uy P, Sridhar S, Sifuentes H. Association between Inflammatory Bowel Disease and Lactose Intolerance: Fact or Fiction. Korean J Gastroenterol. 2020;76:185–190. doi: 10.4166/kjg.2020.76.4.185. [DOI] [PubMed] [Google Scholar]
- 91.Di Costanzo M, Berni Canani R. Lactose Intolerance: Common Misunderstandings. Ann Nutr Metab. 2018;73 Suppl 4:30–37. doi: 10.1159/000493669. [DOI] [PubMed] [Google Scholar]
- 92.Grundmann SA, Stratmann E, Brehler R, Luger TA, Ständer S. Lactase deficiency: a potential novel aetiological factor in chronic pruritus of unknown origin. Acta Derm Venereol. 2011;91:698–703. doi: 10.2340/00015555-1150. [DOI] [PubMed] [Google Scholar]
- 93.Marie I, Leroi AM, Gourcerol G, Levesque H, Menard JF, Ducrotte P. Lactose malabsorption in systemic sclerosis. Aliment Pharmacol Ther. 2016;44:1123–1133. doi: 10.1111/apt.13810. [DOI] [PubMed] [Google Scholar]
- 94.Marie I, Leroi AM, Gourcerol G, Levesque H, Ménard JF, Ducrotte P. Fructose Malabsorption in Systemic Sclerosis. Medicine (Baltimore) 2015;94:e1601. doi: 10.1097/MD.0000000000001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asfari MM, Hamid O, Sarmini MT, Kendrick K, Pappoppula LP, Sifuentes H, Sridhar S. The Association of Lactose Intolerance With Colon and Gastric Cancers: Friend or Foe? Cureus. 2022;14:e24713. doi: 10.7759/cureus.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rozenberg S, Body JJ, Bruyère O, Bergmann P, Brandi ML, Cooper C, Devogelaer JP, Gielen E, Goemaere S, Kaufman JM, Rizzoli R, Reginster JY. Effects of Dairy Products Consumption on Health: Benefits and Beliefs--A Commentary from the Belgian Bone Club and the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. Calcif Tissue Int. 2016;98:1–17. doi: 10.1007/s00223-015-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 2013;1:151–159. doi: 10.1177/2050640613484463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bayless TM, Brown E, Paige DM. Lactase Non-persistence and Lactose Intolerance. Curr Gastroenterol Rep. 2017;19:23. doi: 10.1007/s11894-017-0558-9. [DOI] [PubMed] [Google Scholar]
- 99.Leis R, de Castro MJ, de Lamas C, Picáns R, Couce ML. Effects of Prebiotic and Probiotic Supplementation on Lactase Deficiency and Lactose Intolerance: A Systematic Review of Controlled Trials. Nutrients. 2020;12 doi: 10.3390/nu12051487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mäkinen OE, Wanhalinna V, Zannini E, Arendt EK. Foods for Special Dietary Needs: Non-dairy Plant-based Milk Substitutes and Fermented Dairy-type Products. Crit Rev Food Sci Nutr. 2016;56:339–349. doi: 10.1080/10408398.2012.761950. [DOI] [PubMed] [Google Scholar]
- 101.Concerns for the use of soy-based formulas in infant nutrition. Paediatr Child Health. 2009;14:109–118. [PMC free article] [PubMed] [Google Scholar]
- 102.Katoch GK, Nain N, Kaur S, Rasane P. Lactose Intolerance and Its Dietary Management: An Update. J Am Nutr Assoc. 2022;41:424–434. doi: 10.1080/07315724.2021.1891587. [DOI] [PubMed] [Google Scholar]
- 103.Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK. The Determinants of Peak Bone Mass. J Pediatr. 2017;180:261–269. doi: 10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 104.Baijal R, Tandon RK. Effect of lactase on symptoms and hydrogen breath levels in lactose intolerance: A crossover placebo-controlled study. JGH Open. 2021;5:143–148. doi: 10.1002/jgh3.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heine RG, AlRefaee F, Bachina P, De Leon JC, Geng L, Gong S, Madrazo JA, Ngamphaiboon J, Ong C, Rogacion JM. Lactose intolerance and gastrointestinal cow's milk allergy in infants and children - common misconceptions revisited. World Allergy Organ J. 2017;10:41. doi: 10.1186/s40413-017-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fikree A, Byrne P. Management of functional gastrointestinal disorders. Clin Med (Lond) 2021;21:44–52. doi: 10.7861/clinmed.2020-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wortmann AC, Simon D, Mazzoleni LE, Sander GB, Francesconi CFM, Nabinger DD, Grott CS, Rech TF, Mazzoleni F, Lunge VR, Bona LR, Milbradt TC, Silveira TRD. The association between adult-type hypolactasia and symptoms of functional dyspepsia. Genet Mol Biol. 2018;41:92–97. doi: 10.1590/1678-4685-GMB-2017-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mishkin D, Sablauskas L, Yalovsky M, Mishkin S. Fructose and sorbitol malabsorption in ambulatory patients with functional dyspepsia: comparison with lactose maldigestion/malabsorption. Dig Dis Sci. 1997;42:2591–2598. doi: 10.1023/a:1018841402133. [DOI] [PubMed] [Google Scholar]
- 109.Salvatore S, Vandenplas Y. Gastroesophageal reflux and cow milk allergy: is there a link? Pediatrics. 2002;110:972–984. doi: 10.1542/peds.110.5.972. [DOI] [PubMed] [Google Scholar]
- 110.Iacono G, Carroccio A, Cavataio F, Montalto G, Kazmierska I, Lorello D, Soresi M, Notarbartolo A. Gastroesophageal reflux and cow's milk allergy in infants: a prospective study. J Allergy Clin Immunol. 1996;97:822–827. doi: 10.1016/s0091-6749(96)80160-6. [DOI] [PubMed] [Google Scholar]
- 111.Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, Gupta S, Langendam M, Staiano A, Thapar N, Tipnis N, Tabbers M. Pediatric Gastroesophageal Reflux Clinical Practice Guidelines: Joint Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2018;66:516–554. doi: 10.1097/MPG.0000000000001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin CR, Ling PR, Blackburn GL. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients. 2016;8 doi: 10.3390/nu8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Uscanga-Domínguez LF, Orozco-García IJ, Vázquez-Frias R, Aceves-Tavares GR, Albrecht-Junnghans RE, Amieva-Balmori M, Bazaldua-Merino LA, Bernal-Reyes R, Camacho-de León ME, Campos-Gutiérrez JA, Carmona-Sánchez RI, Castro-Marín LV, Coss-Adame E, Cuevas-Estrada AJ, Escobedo-Martínez JA, González-Franco LR, Huerta-Iga FM, Lozano-Lozano R, Martínez-Vázquez SE, Milke García MP, Nogueira-de Rojas JR, Padilla-González M, Pérez Y López N, Silva-Campechano F, Treviño-Mejía MC, Velázquez-Alva MC. Technical position on milk and its derivatives in adult health and disease from the Asociación Mexicana de Gastroenterología and the Asociación Mexicana de Gerontología y Geriatría. Rev Gastroenterol Mex (Engl Ed) 2019;84:357–371. doi: 10.1016/j.rgmx.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 114.Colemont L. Gastro-duodenal ulcers. Who? Acta Gastroenterol Belg. 1998;61:303–304. [PubMed] [Google Scholar]
- 115.Kumar N, Kumar A, Broor SL, Vij JO, Anand BS. Effect of milk on patients with duodenal ulcers. Br Med J (Clin Res Ed) 1986;293:666. doi: 10.1136/bmj.293.6548.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kulshreshtha M, Srivastava G, Singh MP. Pathophysiological status and nutritional therapy of peptic ulcer: An Update. Environ Dis. 2017;2:76–86. [Google Scholar]
- 117.Ong TG, Gordon M, Banks SS, Thomas MR, Akobeng AK. Probiotics to prevent infantile colic. Cochrane Database Syst Rev. 2019;3:CD012473. doi: 10.1002/14651858.CD012473.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rhoads JM, Collins J, Fatheree NY, Hashmi SS, Taylor CM, Luo M, Hoang TK, Gleason WA, Van Arsdall MR, Navarro F, Liu Y. Infant Colic Represents Gut Inflammation and Dysbiosis. J Pediatr. 2018;203:55–61.e3. doi: 10.1016/j.jpeds.2018.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bishop JM, Hill DJ, Hosking CS. Natural history of cow milk allergy: clinical outcome. J Pediatr. 1990;116:862–867. doi: 10.1016/s0022-3476(05)80641-9. [DOI] [PubMed] [Google Scholar]
- 120.Moravej H, Imanieh MH, Kashef S, Handjani F, Eghterdari F. Predictive value of the cow's milk skin prick test in infantile colic. Ann Saudi Med. 2010;30:468–470. doi: 10.4103/0256-4947.72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vandenplas Y. Lactose intolerance. Asia Pac J Clin Nutr. 2015;24 Suppl 1:S9–13. doi: 10.6133/apjcn.2015.24.s1.02. [DOI] [PubMed] [Google Scholar]
- 122.Vandenplas Y, Alturaiki MA, Al-Qabandi W, AlRefaee F, Bassil Z, Eid B, El Beleidy A, Almehaidib AI, Mouawad P, Sokhn M. Middle East Consensus Statement on the Diagnosis and Management of Functional Gastrointestinal Disorders in <12 Months Old Infants. Pediatr Gastroenterol Hepatol Nutr. 2016;19:153–161. doi: 10.5223/pghn.2016.19.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Simeone D, Miele E, Boccia G, Marino A, Troncone R, Staiano A. Prevalence of atopy in children with chronic constipation. Arch Dis Child. 2008;93:1044–1047. doi: 10.1136/adc.2007.133512. [DOI] [PubMed] [Google Scholar]
- 124.Sopo SM, Arena R, Scala G. Functional constipation and cow's-milk allergy. J Pediatr Gastroenterol Nutr. 2014;59:e34. doi: 10.1097/MPG.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 125.Gelsomino M, Vescovo ED, Bersani G, Sopo SM. Functional constipation related to cow's milk allergy in children: A management proposal. Allergol Immunopathol (Madr) 2021;49:17–20. doi: 10.15586/aei.v49i3.72. [DOI] [PubMed] [Google Scholar]
- 126.Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759–6773. doi: 10.3748/wjg.v20.i22.6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vaiopoulou A, Karamanolis G, Psaltopoulou T, Karatzias G, Gazouli M. Molecular basis of the irritable bowel syndrome. World J Gastroenterol. 2014;20:376–383. doi: 10.3748/wjg.v20.i2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rana SV, Malik A. Breath tests and irritable bowel syndrome. World J Gastroenterol. 2014;20:7587–7601. doi: 10.3748/wjg.v20.i24.7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dainese R, Casellas F, Mariné-Barjoan E, Vivinus-Nébot M, Schneider SM, Hébuterne X, Piche T. Perception of lactose intolerance in irritable bowel syndrome patients. Eur J Gastroenterol Hepatol. 2014;26:1167–1175. doi: 10.1097/MEG.0000000000000089. [DOI] [PubMed] [Google Scholar]
- 130.Caffarelli C, Di Mauro D, Garrubba M, Mastrorilli C. Allergy in Children with Functional Constipation and Irritable Bowel Syndrome. Iran J Pediat. 2016 [Google Scholar]
- 131.Knoflach P, Park BH, Cunningham R, Weiser MM, Albini B. Serum antibodies to cow's milk proteins in ulcerative colitis and Crohn's disease. Gastroenterology. 1987;92:479–485. doi: 10.1016/0016-5085(87)90145-4. [DOI] [PubMed] [Google Scholar]
- 132.Virta LJ, Ashorn M, Kolho KL. Cow's milk allergy, asthma, and pediatric IBD. J Pediatr Gastroenterol Nutr. 2013;56:649–651. doi: 10.1097/MPG.0b013e318285e9d8. [DOI] [PubMed] [Google Scholar]
- 133.Mishkin S. Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. Am J Clin Nutr. 1997;65:564–567. doi: 10.1093/ajcn/65.2.564. [DOI] [PubMed] [Google Scholar]
- 134.Strisciuglio C, Giannetti E, Martinelli M, Sciorio E, Staiano A, Miele E. Does cow's milk protein elimination diet have a role on induction and maintenance of remission in children with ulcerative colitis? Acta Paediatr. 2013;102:e273–e278. doi: 10.1111/apa.12215. [DOI] [PubMed] [Google Scholar]
- 135.Ratajczak AE, Rychter AM, Zawada A, Dobrowolska A, Krela-Kaźmierczak I. Lactose intolerance in patients with inflammatory bowel diseases and dietary management in prevention of osteoporosis. Nutrition. 2021;82:111043. doi: 10.1016/j.nut.2020.111043. [DOI] [PubMed] [Google Scholar]
- 136.Lim HS, Kim SK, Hong SJ. Food Elimination Diet and Nutritional Deficiency in Patients with Inflammatory Bowel Disease. Clin Nutr Res. 2018;7:48–55. doi: 10.7762/cnr.2018.7.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krela-Kaźmierczak I, Michalak M, Szymczak-Tomczak A, Czarnywojtek A, Wawrzyniak A, Łykowska-Szuber L, Stawczyk-Eder K, Dobrowolska A, Eder P. Milk and dairy product consumption in patients with inflammatory bowel disease: Helpful or harmful to bone mineral density? Nutrition. 2020;79-80:110830. doi: 10.1016/j.nut.2020.110830. [DOI] [PubMed] [Google Scholar]
- 138.Sigala-Robles R, Santiago-López L, Hernández-Mendoza A, Vallejo-Cordoba B, Mata-Haro V, Wall-Medrano A, González-Córdova AF. Peptides, Exopolysaccharides, and Short-Chain Fatty Acids from Fermented Milk and Perspectives on Inflammatory Bowel Diseases. Dig Dis Sci. 2022 doi: 10.1007/s10620-022-07382-2. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X, Tong Y, Lyu X, Wang J, Wang Y, Yang R. Prevention and Alleviation of Dextran Sulfate Sodium Salt-Induced Inflammatory Bowel Disease in Mice With Bacillus subtilis-Fermented Milk via Inhibition of the Inflammatory Responses and Regulation of the Intestinal Flora. Front Microbiol. 2020;11:622354. doi: 10.3389/fmicb.2020.622354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shrier I, Szilagyi A, Correa JA. Impact of lactose containing foods and the genetics of lactase on diseases: an analytical review of population data. Nutr Cancer. 2008;60:292–300. doi: 10.1080/01635580701745301. [DOI] [PubMed] [Google Scholar]
- 141.Aune D, Lau R, Chan DSM, Vieira R, Greenwood DC, Kampman E, Norat T. Dairy products and colorectal cancer risk: a systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 142.Wallace K, Baron JA, Cole BF, Sandler RS, Karagas MR, Beach MA, Haile RW, Burke CA, Pearson LH, Mandel JS, Rothstein R, Snover DC. Effect of calcium supplementation on the risk of large bowel polyps. J Natl Cancer Inst. 2004;96:921–925. doi: 10.1093/jnci/djh165. [DOI] [PubMed] [Google Scholar]
- 143.Baron JA, Barry EL, Mott LA, Rees JR, Sandler RS, Snover DC, Bostick RM, Ivanova A, Cole BF, Ahnen DJ, Beck GJ, Bresalier RS, Burke CA, Church TR, Cruz-Correa M, Figueiredo JC, Goodman M, Kim AS, Robertson DJ, Rothstein R, Shaukat A, Seabrook ME, Summers RW. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med. 2015;373:1519–1530. doi: 10.1056/NEJMoa1500409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dhanashekar R, Akkinepalli S, Nellutla A. Milk-borne infections. An analysis of their potential effect on the milk industry. Germs. 2012;2:101–109. doi: 10.11599/germs.2012.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharp JC. Infections associated with milk and dairy products in Europe and North America, 1980-85. Bull World Health Organ. 1987;65:397–406. [PMC free article] [PubMed] [Google Scholar]
- 146.Tóth AG, Csabai I, Krikó E, Tőzsér D, Maróti G, Patai ÁV, Makrai L, Szita G, Solymosi N. Antimicrobial resistance genes in raw milk for human consumption. Sci Rep. 2020;10:7464. doi: 10.1038/s41598-020-63675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]