Abstract
Introduction
Malignant mesothelioma (MM) is an aggressive cancer that primarily arises from the pleura (MPM) or peritoneum (MPeM), mostly due to asbestos exposure. This study reviewed the Dutch population-based incidence, treatment and survival since the national ban on asbestos in 1993.
Materials and methods
Patients with MPM or MPeM diagnosed from 1993 to 2018 were selected from the Dutch cancer registry. Annual percentage change (APC) was calculated for (age-specific and sex-specific) revised European standardised incidence rates (RESR). Treatment pattern and Kaplan-Meier overall survival analyses were performed.
Results
In total, 12 168 patients were included in the study. For male patients younger than 80 years, the MM incidence significantly decreased in the last decade (APC ranging between −9.4% and −1.8%, p<0.01). Among both male and female patients aged over 80 years, the incidence significantly increased during the entire study period (APC 3.3% and 4.6%, respectively, p<0.01). From 2003 onwards, the use of systemic chemotherapy increased especially for MPM (from 9.3% to 39.4%). Overall, 62.2% of patients received no antitumour treatment. The most common reasons for not undergoing antitumour treatment were patient preference (42%) and performance status (25.6%). The median overall survival improved from 7.3 (1993–2003) to 8.9 (2004–2011) and 9.3 months from 2012 to 2018 (p<0.001).
Conclusion
The peak of MM incidence was reached around 2010 in the Netherlands, and currently the incidence is declining in most age groups. The use of systemic chemotherapy increased from 2003, which likely resulted in improved overall survival over time. The majority of patients do not receive treatment though and prognosis is still poor.
Keywords: mesothelioma, clinical epidemiology, asbestos induced lung disease
Key messages.
What is the key question?
How did the 1993 national ban on asbestos affect malignant mesothelioma incidence and how did therapeutic advances affect mesothelioma prognosis in the Netherlands?
What is the bottom line?
Malignant mesothelioma incidence has peaked about 10 years earlier than predicted after the Dutch national ban on asbestos.
While treatment advances have led to somewhat better survival, prognosis is still dismal.
Why read on?
These findings show that asbestos regulation leads to a decreasing mesothelioma incidence sooner than earlier predicted, thereby supporting the notion that malignant mesothelioma cases can, for the most part, be effectively prevented.
The treatment patterns and survival outcomes we observed in over 12 000 patients with mesothelioma highlight the persistent need for better mesothelioma therapeutics.
Introduction
Malignant mesothelioma (MM) is a highly lethal tumour that primarily arises from the pleura and, to a lesser extent, from the peritoneum. In sporadic cases, it originates from the pericardium or tunica vaginalis testis.1 Malignant pleural mesothelioma (MPM) represents over 90% of all MM cases. MPM prognosis is very poor, with a median overall survival (OS) of approximately 1 year when treated with chemotherapy.2 Recently, combination checkpoint inhibition therapy, consisting of nivolumab (anti-programmed death-ligand 1 (PD-1)) and ipilimumab (anti-cytotoxic T lymphocyte-associated protein 4 (CTLA4)), has been shown to increase OS in patients with MPM compared with standard first-line chemotherapy (median OS 18.1 months vs 14.1 months).3 As a result, the US Food and Drug Administration (FDA) approved the combination of nivolumab and ipilimumab as first-line treatment for unresectable MPM.4 For malignant peritoneal mesothelioma (MPeM), the median OS is about 6 months.5 For a long time, treatment options were identical to those for MPM. Since 2009, several studies have demonstrated that long-term survival can be achieved in selected patients with MPeM treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).6–8
The main risk factor for MPM development is exposure to asbestos.9 10 This correlation is less prominent in MPeM. The definitive relation between asbestos exposure and MM was proven in 1960 by Wagner et al.11 Despite this discovery, production of asbestos peaked worldwide between 1970 and 1980, with a production of more than 4 000 000 metric tons per year. The use of asbestos in the Netherlands also peaked in these years. Its use declined in the following decade due to new regulations, but the ban on asbestos in the Netherlands was finally realised in 1993. Countries such as Russia, Kazakhstan and China are still mass producers of asbestos to this day and others have only recently banned its use. 12 13 Predicting the future incidence of MM is difficult due to the considerable variation in latency time (ie, the time between asbestos exposure and MM diagnosis) that has been reported, varying between 10 years and over 50 years.12 13 In the Netherlands, a peak in MM mortality was expected around 2020.14 15 The current population-based study reviews the national MM incidence 25 years after the nationwide asbestos ban. This could aid others in predicting MM incidence after implementation of asbestos regulations. Concurrently, it aims to identify patterns in incidence, MM characteristics, treatment and survival for both MPM and MPeM.
Methods
Data collection
Patients diagnosed with MM between 1993 and 2018 were identified in the Netherlands Cancer Registry (NCR) by searching for cases with ICD-O codes 8000–8005, 9050–9053 and 9990 located in the pleura (C38.4) or peritoneum (C48.2) (International Classification of Diseases for Oncology, Third Edition, First Revision). Incidence rates were available from 1989 to 2018. Data were extracted after the approval of the NCR Monitoring Committee and the NCR Scientific Committee on Pulmonary Oncology. Data were handled in accordance with the latest European privacy regulations (General Data Protection Regulation, European Union 2016/679). Data on all patients diagnosed with cancer in the Netherlands are collected by the NCR. De Boer et al 5 previously described their methods. In short, the NCR identifies patients with cancer by using the Dutch Pathological Anatomical National Automated Archive and the National Registry of Hospital Discharge Diagnoses. Specialised personnel collect information on diagnosis, stage of the disease and treatment from medical records. Information on vital status is updated annually through the National Municipal Personal Records Database. Vital status was updated to 1 February 2020. Data were extracted and provided to the investigators by trained personnel who were not part of the study team.
The tumour stage was registered for all patients. For MPM, the tumour, node, metastases (TNM) classification current at the moment of diagnosis was used.16 For MPeM, the Extent of Disease (EOD) coding, according to the Surveillance, Epidemiology, and End Results (SEER), was used.17 The EOD coding stratifies the tumour stage into local, regional and distant progression. It is used by the NCR when no prevailing staging system is available. The location of metastases was specified from 2008 onwards. The cause of death was unavailable due to privacy regulations.
Incidence analyses
Incidence rates from 1989 until 2018 were analysed. Age-specific and sex-specific rates were calculated using the revised European standard rate (RESR) (Eurostat 2013, International Standard Serial Number (ISSN) 1977–0375). The SEER ‘Joinpoint Regression Program’ (V.4.8.0.1, April 2020) (IMS; under contract for the National Cancer Institute, Bethesda, Maryland, USA) was used to identify trends in incidence.18 This software fits the simplest regression model to incidence rates, thereby identifying trend-breaks or the so-called ‘joinpoints’. The number of joinpoints is determined by the use of the Monte Carlo permutation test. Annual percentage changes (APCs) were calculated by the software by fitting a log-linear regression model to the data, using the natural logarithm of the incidence rates with the year of diagnosis as the independent variable. APCs were calculated over the segment between two joinpoints or over the entire period when the number of joinpoints was zero.
Treatment pattern analyses
Treatment was stratified into four main categories for trend analyses: systemic chemotherapy, surgery, radiotherapy and best supportive care. Surgery included several procedures, such as debulking, CRS, decortication and extrapleural pneumonectomy. Other treatment categories were targeted therapy and immunotherapy, including the angiogenesis inhibitor bevacizumab, tyrosine kinase inhibitors (not further specified in the data), and checkpoint inhibitors comprising the anti-PD(L)-1 and anti-CTLA4 agents nivolumab, pembrolizumab and ipilimumab. Treatment strategies were reviewed per year for MPM and per 5 years for MPeM. From 2015 onwards, the reason for not undergoing antitumour therapy was recorded.
Survival analyses
To define survival trends, data were stratified into three time periods. The first period included cases diagnosed from 1993 until 2003, the second period included cases from 2004 until 2011, and the third period comprised cases diagnosed from 2012 up to 2018. The year 2003 was deliberately chosen as Vogelzang et al 2 published the results of their phase III trial on combination chemotherapy with cisplatin and pemetrexed in this year. The following years were divided into two equal periods to assess if survival had improved since. To compare survival between patients with MPM diagnosed at different stages of disease, only data from 2008 to 2018 were used, as the new and improved seventh edition of the TNM staging manual was published in 2007. Stage of disease was not compared for MPeM as there is no widely used staging system. Kaplan-Meier OS curves were also drafted for different treatment modalities (ie, chemotherapy, surgery and ‘best supportive care’) to illustrate survival for these different groups, but were deliberately not compared statistically as these outcomes are heavily influenced by (selection) bias.
Statistical analyses
Continuous variables are shown as median with IQR and were compared with the independent samples median test. Categorical variables are given as numbers with percentages and were compared with the χ2 test. Survival analyses were performed by use of the Kaplan-Meier method. Survival between groups was compared with the log-rank method in case of proportional hazards or generalised Wilcoxon in case of non-proportional hazards. Two-sided p values smaller than 0.05 were considered statistically significant. Statistical analyses were performed with ‘Statistical Package for Social Sciences’ (SPSS) V.25.0.0.1 and R V.4.0.2 (http://www.r-project.org). Incidence rates were calculated with SAS V.9.4.
Results
Patient and tumour characteristics
There were 12 168 patients with MM identified in the NCR between 1993 and 2018. This comprised 11 539 (94.8%) cases of MPM and 629 (5.2%) cases of MPeM. A comprehensive overview of patient and tumour characteristics at the time of diagnosis is provided in table 1. Generally, patients with MPeM were younger (median age 69 (61–76) years vs 71 (64–77) years, p=0.004). The MPM group comprised more male patients (87.4% vs 72.3%, p<0.001). Furthermore, there were more cases with epithelioid subtype among patients with MPeM (88% vs 76.2%, p<0.001). An attempt to detect trends in the occurrence of histological subtypes over time failed due to the lack of pathological registration data. It did reveal though that subtypes have been increasingly specified by pathologists over time, from 46.7% of cases in the first 10 years of the study period to 83% of cases in the last 10 years (online supplemental figure 1). Different staging systems were used for both tumour types and could therefore not be compared between MPM and MPeM. Also, an attempt to determine trends for stage of disease at diagnosis failed due to the large number of cases with unknown stage. This analysis revealed that the use of staging has slightly increased during the study period, but still for about one-third of patients no stage is recorded (online supplemental figure 2).
Table 1.
Patient and tumour characteristics
| MPM (n=11 539) |
MPeM (n=629) |
Total (N=12 168) |
P value | |
| Age, median (IQR) | 71 (64–77) | 69 (61–76) | 71 (64–77) | 0.004 |
| Male, n (%) | 10 085 (87.4) | 458 (72.3) | 10 543 (86.6) | <0.001 |
| Histology, n (%)* | ||||
| Epithelioid | 6061 (76.2) | 368 (88) | 6429 (76.8) | <0.001* |
| Sarcomatoid | 1149 (14.5) | 18 (4.3) | 1167 (13.9) | |
| Biphasic | 739 (9.3) | 32 (7.7) | 771 (9.2) | |
| Unknown | 3590 | 211 | 3801 | |
| Side, n (%)* | ||||
| Left | 4499 (40.4) | – | – | |
| Right | 6631 (59.6) | – | – | |
| Unknown | 409 | – | – | |
| Stage (TNM), n (%)* | ||||
| I | 2840 (37.2) | – | – | |
| II | 918 (12) | – | – | |
| III | 1971 (25.8) | – | – | |
| IV | 1897 (24.9) | – | – | |
| Unknown | 3913 | – | – | |
| Stage (EOD), n (%)* | ||||
| 1. In situ | – | 0 | – | |
| 2. Local disease | – | 190 (40.8) | – | |
| 3. Contiguous/invasive | – | 123 (26.4) | – | |
| 4. Regional lymph nodes | – | 22 (4.7) | – | |
| 5. Regional progression | – | 12 (2.6) | – | |
| 6. Distant progression | – | 119 (25.5) | – | |
| Unknown | – | 163 | – | |
| Metastases, n (%)† | 610 (10.6) | 54 (18.6) | 664 (11) | <0.001 |
| Multiple metastatic sites | 149 (2.6) | 11 (3.8) | 160 (2.6) | 0.021 |
| Most common metastatic sites, n (%)† | ||||
| Lung | 163 (2.8) | 7 (2.4) | 170 (2.8) | |
| Lymph node | 159 (2.8) | 11 (3.8) | 170 (2.8) | |
| Bone | 135 (2.3) | – | 135 (2.2) | |
| Liver | 73 (1.3) | 13 (4.5) | 86 (1.4) | |
| Peritoneum | 79 (1.4) | 5 (1.7) | 84 (1.4) | |
| Soft tissue | 59 (1) | 3 (1) | 62 (1) | |
| Adrenal glands | 52 (0.9) | 1 (0.3) | 53 (0.9) | |
| Pleura | 12 (0.2) | 15 (5.2) | 27 (0.4) | |
| Cutaneous | 12 (0.2) | – | 12 (0.2) | |
| Brain | 15 (0.3) | – | 15 (0.2) | |
*Percentage and p value based on known cases.
†Metastases were registered nationwide from 2008 onwards; percentages based on 6038 cases (MPM n=5757, MPeM n=291).
EOD, Extent of Disease classification; MPeM, malignant peritoneal mesothelioma; MPM, malignant pleural mesothelioma; TNM, tumour, node, metastases classification.
thoraxjnl-2021-217709supp001.pdf (820.8KB, pdf)
thoraxjnl-2021-217709supp002.pdf (823.7KB, pdf)
Incidence
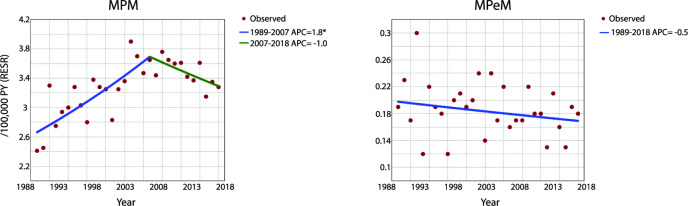
During the study period, the MM incidence for both sexes combined ranged between 2.6 and 4.1 cases per 100 000 person-years (RESR) (supplementary table A). The incidence significantly increased at 1.6% annually (95% CI 1 to 2.1) up to 2010, after which a non-significant decrease, or plateau, was observed of −1.7% annually (95% CI −3.9 to 0.6).
thoraxjnl-2021-217709supp003.pdf (204.2KB, pdf)
For MPM, the incidence was increasing with a rate of 1.8% per year (95% CI 1.1 to 2.5) from 1989 to 2007, thereby ranging between 2.4 and 3.5 cases per 100 000 person-years (RESR). From 2007 onwards, a non-significant decrease was observed (APC=−1%, 95% CI −2.5 to 0.4), with rates ranging between 3.3 and 3.8 cases per 100 000 person-years (RESR) (figure 1).
Figure 1.
Malignant pleural and peritoneal mesothelioma incidence per 100 000 PY (RESR) between 1989 and 2018 in the Netherlands. *Indicates that the APC is significantly different from 0 at the alpha level of 0.05. APC, annual percentage change; MPeM, malignant peritoneal mesothelioma; MPM, malignant pleural mesothelioma; PY, person-years; RESR, revised European standard rate.
For MPeM, about 0.15–0.25 cases per 100 000 person-years (RESR) were reported annually during the entire study period (figure 1); no significant trend was observed (APC=−0.5, 95% CI −1.4 to 0.4). Male incidence ranged between 0.2 and 0.5 cases per 100 000 person-years (RESR), whereas female incidence ranged between 0 and 0.2 cases per 100 000 person-years (RESR).
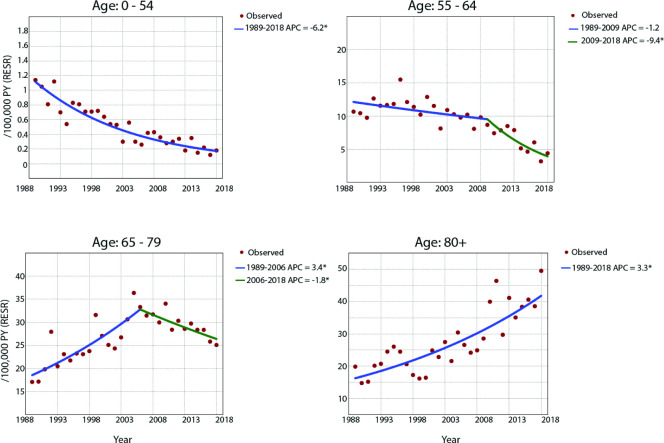
Analyses of sex-specific and age-specific incidence rates were performed for both MPM and MPeM combined, as separate age-specific rates were not available for MPeM only. For male patients, significantly decreasing MM incidence rates were observed for all age groups except for patients older than 80 years, for whom the incidence increased during the entire study period, with an average APC of 3.3% (95% CI 2.5 to 4.1) (figure 2). For male patients between 0 and 54 years, there was an average decrease of 6.2% per year between 1989 and 2018 (95% CI −7.2 to −5.2). For male patients between 55 and 64 years, significantly declining incidence was observed between 2009 and 2018 (APC=−9.4%, 95% CI −13.1 to −5.4). For male patients aged 65–79, there was a significant increase between 1989 and 2006 (APC=3.4%, 95% CI 2.3 to 4.5), after which a significant decrease was observed between 2006 and 2018 (APC=−1.8%, 95% CI −3.5 to −0.1). For female patients younger than 65 years, no significant trends in MM incidence were observed. For female patients aged 65 years or older, a significant increase in incidence was observed over the entire study period (APC for 65–79 years=1.8%, 95% CI 0.9 to 2.6; APC for 80 and older=4.6%, 95% CI 2.2 to 6.9) (supplementary table B and C).
Figure 2.
Age-specific incidence rates for male patients with malignant mesothelioma (both pleural and peritoneal mesothelioma) per 100 000 PY (RESR). *Indicates that the APC is significantly different from 0 at the alpha level of 0.05. APC, annual percentage change; PY, person-years; RESR, revised European standard rate.
Treatment
A comprehensive overview of treatment modalities is provided in table 2. From 1993 until 2018, the majority of patients with MM (62.1%) received ‘best supportive care’. From 2015, the reason for not undergoing antitumour treatment was registered for 898 patients. For most cases, this was due to patient preference (42%) or due to performance status (25.6%). Chemotherapy was given to 28.1% of patients with MM, radiotherapy was given in 8.8% of patients, and 3.4% of patients received a type of surgery. No information was registered whether a macroscopically complete resection was the intended goal of the surgery or whether this was achieved. In total, 4.2% of patients with MM were treated with a multimodality approach. Targeted therapy was used in 74 cases (0.6%) and immunotherapy in 55 (0.5%).
Table 2.
Treatment
| MPM (n=11 539) | MPeM (n=629) | Total (N=12 168) | |
| All, n (%)* | |||
| Chemotherapy | 3273 (28.4) | 142 (22.6) | 3415 (28.1) |
| Surgery | 343 (3.0) | 65 (10.3) | 408 (3.4) |
| Radiotherapy | 1072 (9.3) | 4 (0.6) | 1076 (8.8) |
| Immunotherapy | 52 (0.5) | 3 (0.5) | 55 (0.5) |
| Targeted therapy | 72 (0.6) | 2 (0.3) | 74 (0.6) |
| Best supportive care | 7132 (61.8) | 423 (67.2) | 7555 (62.1) |
| Unknown | 144 (1.2) | 7 (1.1) | 151 (1.2) |
| Multimodal treatment, n (%) | |||
| Chemotherapy and surgery | 92 (0.8) | 10 (1.6) | 102 (0.8) |
| Chemotherapy and radiotherapy | 252 (2.2) | 1 (0.2) | 253 (2.1) |
| Chemotherapy and targeted therapy | 44 (0.4) | 1 (0.1) | 45 (0.4) |
| Chemotherapy and immunotherapy | 11 (0.1) | – | 11 (0.1) |
| Surgery and radiotherapy | 47 (0.4) | 2 (0.3) | 49 (0.4) |
| Surgery and chemotherapy and radiotherapy | 40 (0.3) | – | 40 (0.3) |
| Other | 9 (0.1) | 3 (0.5) | 12 (0.1) |
| Total | 495 (4.3) | 17 (2.7) | 512 (4.2) |
| Reason for no treatment (ie, BSC), n (%)† | |||
| Comorbidity | 18 (2.1) | 3 (5.9) | 21 (2.3) |
| Performance status | 214 (25.3) | 16 (31.4) | 230 (25.6) |
| Age | 32 (3.8) | 1 (2) | 33 (3.7) |
| Patient preference | 363 (42.9) | 14 (27.5) | 377 (42) |
| Disease too extensive | 55 (6.5) | 8 (15.7) | 63 (7) |
| Deceased before start of treatment | 30 (3.5) | 0 (0) | 30 (3.3) |
| Other/unknown | 135 (15.9) | 9 (17.6) | 144 (16) |
*Total percentage >100% due to 4.2% of patients receiving multimodal treatment.
†Registered for 898 patients (847 pleural, 51 peritoneal); percentages based on registered cases.
BSC, best supportive care; MPeM, malignant peritoneal mesothelioma; MPM, malignant pleural mesothelioma.
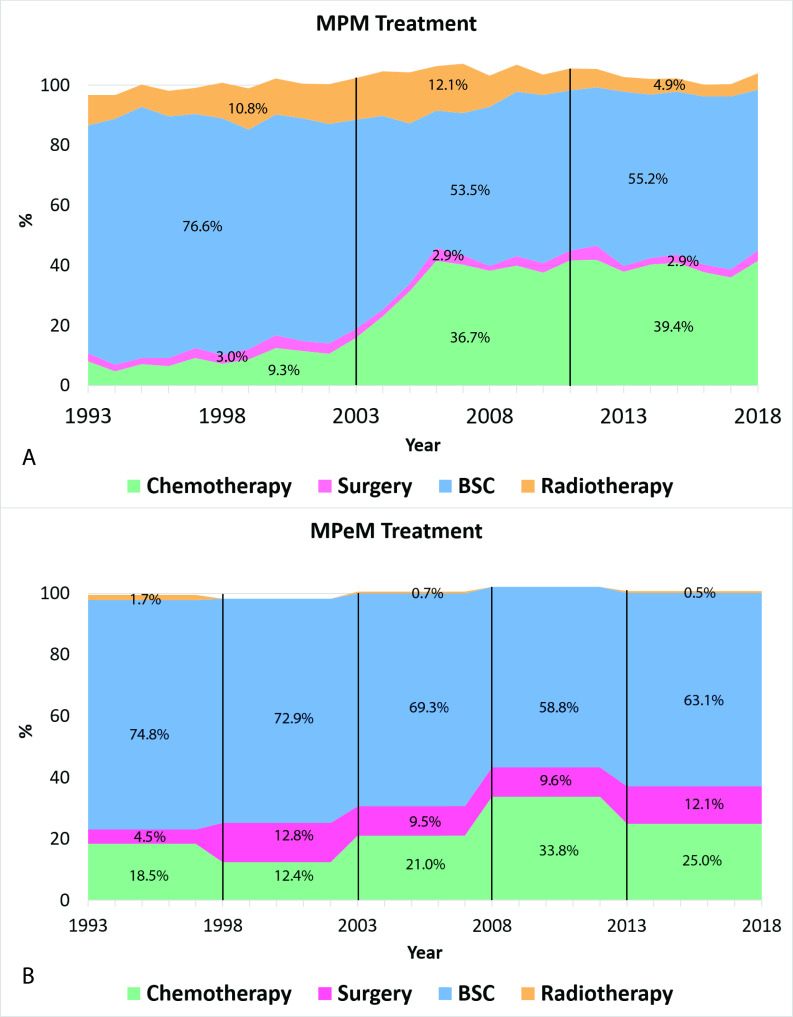
Patients with MPM were treated more frequently with chemotherapy (28.4% vs 22.6%) and radiotherapy (9.3% vs 0.6%) than patients with MPeM. On the other hand, surgery was more often applied in patients with MPeM (10%) compared with MPM (3%). Figure 3A shows an increase in the use of systemic chemotherapy between 2002 and 2006 for MPM from approximately 10% to 40% yearly. After 2006, the use of chemotherapy for the treatment of MPM remained stable. Radiotherapy was used in about 10%–15% of cases up to 2008, after which its use decreased to 4%–5% of cases yearly. As can be seen in figure 3B, patients with MPeM were most commonly treated with best supportive care. Its use ranges from about 75% between 1993 and 1997 to about 65% from 2013 to 2018. The use of chemotherapy increased from around 2003 onwards and peaked between 2008 and 2012, after which its use decreased again. The role of surgery remained limited during the study period and varied between 4% and 13% per 5 years.
Figure 3.
Treatment patterns for malignant pleural mesothelioma (MPM) per year (A) from 1993 to 2018. Treatment patterns per 5 years for malignant peritoneal mesothelioma (MPeM) from 1993 to 2018 (B). Stacked areas do not add up to 100% due to patients receiving multimodal treatment or patients receiving other treatment. BSC, best supportive care.
Survival
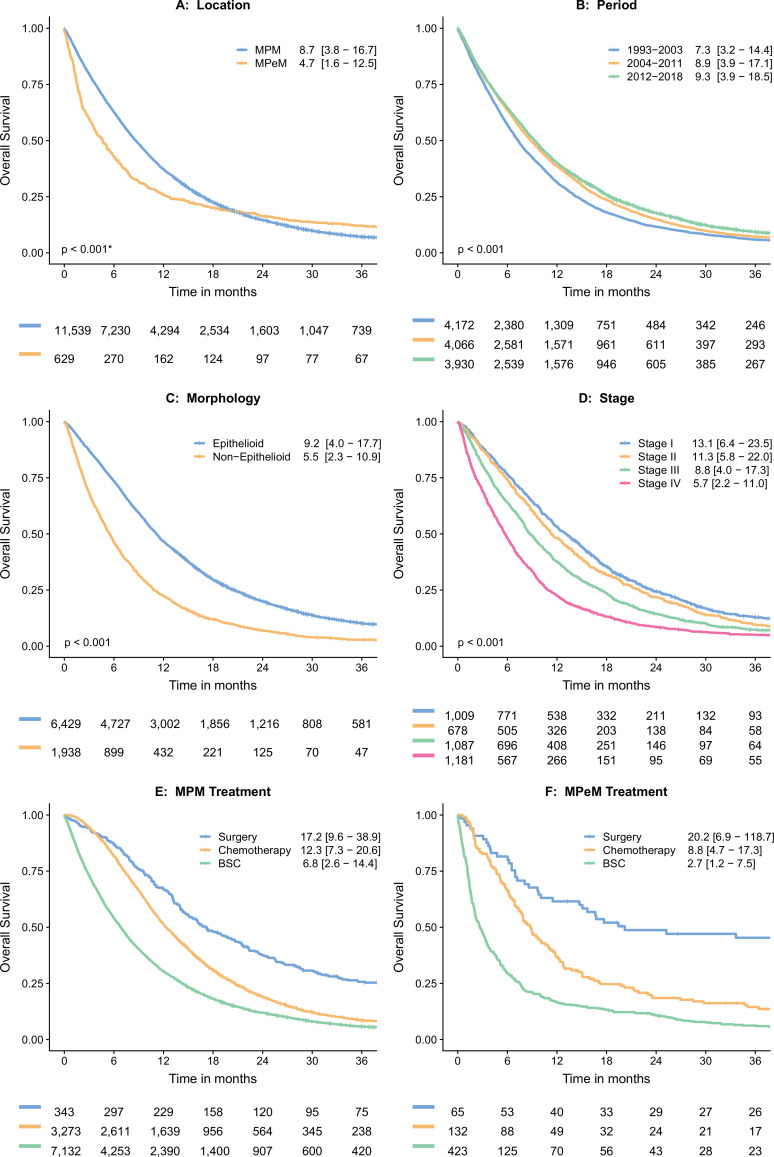
The median OS for the entire cohort was 8.4 months (IQR: 3.6–16.6 months). Kaplan-Meier curves with the median OS for different subgroups are provided in figure 4. Patients with MPeM had worse median survival compared with MPM (4.7 months vs 8.7 months, p<0.001), and also when treated with systemic chemotherapy (8.9 months vs 12.7 months, p<0.001; data not shown). Survival of the entire cohort significantly improved from about 2003, likely due to the introduction of combination chemotherapy, together with the increased use of this treatment. Between 2012 and 2018, the median OS for all patients was 9.3 months compared with 7.3 months between 1993 and 2003. Patients with non-epithelioid morphology (ie, biphasic and sarcomatoid histology) had a significantly worse prognosis (5.5 months vs 9.2 months, p<0.001), and also when treated with systemic chemotherapy (8.9 months vs 13.6 months; data not shown). For MPM, the OS significantly differed for different stages of disease (data used from 2008 to 2018). Patients diagnosed with stage I pleural mesothelioma had a median survival of 13.1 months (IQR 6.4–23.5) compared with 5.7 months (IQR 2.2–11.0) for patients with stage IV disease. Figure 4E, F illustrates the survival for chemotherapy, surgery and ‘best supportive care’ for MPM and MPeM, respectively. These outcomes were not statistically compared because they are subjected to selection bias.
Figure 4.
Kaplan-Meier actuarial overall survival curves with median overall survival (IQR) in months and numbers at risk. *Breslow (generalised Wilcoxon) p value, calculated for non-proportional hazards. (A) Overall survival (OS) compared per location (MPM vs. MPeM), (B) OS compared per time period. (C) OS compared per morphological subtype. (D) OS compared per stage for pleural mesothelioma (MPM), only data from 2008 to 2018 were used as the seventh edition of the TNM staging manual was published in 2007. (E) Showing OS for different treatment modalities in MPM. (F) Showing OS for different treatment modalities in MPeM. BSC, best supportive care; MPeM, malignant peritoneal mesothelioma; MPM, malignant pleural mesothelioma; TNM, tumour, node, metastases.
Discussion
This study shows that MM incidence among most age groups is currently declining in the Netherlands. The peak of MM incidence appears to have been passed around 2010, and currently there is a strong trend towards declining incidence for the whole population. Historically, the Netherlands has been among countries with relatively high MM incidence rates.9 In recent decades, the rapid increase in MM incidence has been monitored with some apprehension.14 19 20 Earlier studies have tried to predict the impact and mortality of mesothelioma in the Netherlands.14 15 Now, for the first time, with updated incidence numbers, this study observed decreasing MM incidence among most age groups in the Netherlands. Similar results have earlier been reported for Sweden, the USA and Australia.21–23 For other countries with high MM incidence that have banned the use of asbestos, such as the UK, these outcomes can further aid in predicting future MM incidence.
These findings are remarkable, as it was predicted that incidence would peak in the Netherlands around 2020.14 However, this peak has already been reached, approximately around 2010. This indicates that MM incidence is not only associated with the complete ban on asbestos but also with measures that were implemented before 1993. The thesis of J Stumphius24 (an occupational physician at a Dutch shipyard) that was published in 1969 unmistakably related asbestos exposure to mesothelioma in the Netherlands. This thesis led to additional research, which finally resulted in governmental regulation in 1978, resulting in a decrease of approximately 75% in the amount of asbestos that was processed in the Netherlands in the 1980s compared with the 1970s. As the processing and use of asbestos plummeted from 1980 onwards and the latency time for mesothelioma is 30 years on average, the decline of incidence from 2010 onwards could have been expected. This also explains why we observed that incidence among patients younger than 55 years was already declining before the complete ban on asbestos in 1993.
The age-specific incidence rates that were analysed in this study indicate that the MPM incidence among most age groups is currently declining, with the exception of male patients aged over 80 years and female patients aged over 65 years. These observations were to be expected considering the latency time between asbestos exposure and MM development.13 25 26 Patients who are currently older than 80 years have likely been heavily (occupationally) exposed to asbestos in the past. However, the current decline in incidence among all other male age groups indicates that the number of new MM cases will further diminish in the near future and the peak incidence lies behind us. The group of patients that have been heavily exposed to asbestos will become smaller each year due to the ban on asbestos in 1993 and other earlier measures. The 80+ age group has relatively high incidence rates, but the group size is decreasing each year. Therefore, the weight of this age group on the total incidence rates diminishes. Combined with declining incidence rates among all other age groups, this causes the current decline of the total MM incidence.
Age-specific incidence rates were unavailable for MPeM due to the small number of patients. For the general MPeM population, however, the incidence rate remained more or less stable over time. This could imply that the link between asbestos exposure and MPeM is less prevailing than it is in MPM.27 This also suggests that there are other causes for MPeM development that are more dominant, such as previous radiation therapy or germline mutations.27 28 The proportion of MPeM in this cohort was 5.2%. This is lower than expected based on literature, where rates between 10% and 30% have been reported.27 29 It has been observed though that MPeM is often misdiagnosed, which can explain the small proportion of observed cases in this cohort.27
With regard to treatment, between 2002 and 2006, there was an evident increase in the use of systemic chemotherapy, especially for MPM. In 2003, Vogelzang et al showed that platinum-pemetrexed treatment increased the median OS by 3 months, compared with cisplatin alone in MPM.2 The increased treatment rate, together with the fact that combination treatment prolongs survival, likely resulted in improved survival at a population level from 2003 onwards.
The majority of patients, however, did not undergo antitumour treatment. For patients with MPM who did not receive antitumour treatment, over 40% were due to patient preference. Perhaps, patients are reluctant to receive toxic therapy, especially when the benefit is limited. Similar patterns were seen in Belgium and England.30 For patients with MPeM, it was more often due to poor performance status (31.4%) or extensive disease (15.7%). Patients with MPeM are known to be often diagnosed at an advanced stage of the disease, likely due to the rareness of their condition and non-specific presentation.31 32
The use of radiotherapy for MPM was common but decreased around 2009. The updated 2008 European Society for Medical Oncology (ESMO) guideline can explain this. It stated that the use of radiotherapy should be limited to local palliative use and pain control because of the potentially severe side effects, while its benefit in local disease control is controversial.33 In 2016 and 2019, the lack of benefit from prophylactic tract irradiation was confirmed in randomised trials.34 35 These results might lead to a further decrease in its use.
In the current cohort, the use and thus the impact of immunotherapy and targeted therapy were minimal, with less than 1% of patients treated with either modality. This is due to the fact that checkpoint inhibition therapy is not yet registered as a treatment option and there have been no major advances regarding targeted therapy.36 37 Recently, the results of the phase III CheckMate743 study showed that combination checkpoint inhibition therapy for patients with MPM in first line increased OS by 4 months compared with standard first-line chemotherapy.3 For the non-epithelioid subtype, the survival benefit was even greater. For this group, the estimated survival at 2 years was 38% in the immunotherapy group compared with 8% in the chemotherapy group. As a result, the FDA has approved the combination of nivolumab plus ipilimumab for the first-line treatment of MM.4 Monotherapy for MM should not be written off entirely though as there are selected patients who might benefit from its use.38 Nonetheless, novel treatment options are still urgently required to improve survival in patients. An overview of new developments is given by Yap et al.39 Within our group, we are working on dendritic cell therapy both in pleural and peritoneal mesothelioma.40 41
The role of surgery for MM remains controversial. Its use was minimal in the current cohort, with only 3% of patients with MPM and about 10% of patients with MPeM receiving surgical treatment. Especially for patients with MPeM, this could be a missed opportunity. Several series have been published on the use of CRS-HIPEC for MPeM.6–8 42 A large series of Yan et al,6 for example, observed a median OS of 53 months with 3-year and 5-year survival rates of 60% and 47%, respectively. This survival can partly be explained by patient selection. However, studies on the use of systemic chemotherapy are also subjected to patient selection but rarely show long-term survivors.43–45
Strengths and limitations
The strengths of our study are the number of patients included and the length of time in which the data were collected. There are also some limitations that need to be discussed. As with most population-based registries, the overarching theme of the limitations lies in the details of the data. For example, the stage of disease for MPM was noted according to the TNM stage that was used at the time of diagnosis. As there were different TNM stages used between 1993 and 2018, it was hard to compare stage of disease at the time of diagnosis throughout time. Moreover, for about one-third of patients there was no stage recorded. This is likely due to the minimal use of surgery for MPM in the Netherlands. Therefore the pathological TNM stage (pTNM) is mostly lacking and the clinical TNM stage (cTNM) has no consequences for treatment as systemic chemotherapy is considered the gold standard. Patient selection for systemic chemotherapy is mostly based on performance status and patient preference, rather than stage of disease. Also, details regarding treatment were often missing. With regard to survival analyses, the cause of death is not registered by the NCR due to privacy regulations. Thus cancer-specific survival was unavailable. Nonetheless, given the very poor prognosis of MM, it is very unlikely that this affected our outcomes, even for patients at advanced age. Although these limitations complicated indepth additional analysis on survival between different stages and treatment modalities, they did not influence the main conclusion of our data on the decreasing incidence in the Netherlands from 2010 onwards nor did they influence the trends in treatment throughout time.
Conclusion
This study shows that MM incidence has reached a peak in the Netherlands around 2010, following the national ban on asbestos in 1993. In most age groups the MM incidence is currently declining, with the exception of male patients aged over 80 years and female patients aged over 65 years. The number of patients receiving treatment has increased since 2003, although most patients did not receive any antitumour therapy. In this period, survival improved slightly, but the prognosis is still poor. Recent advances in therapy might change this perception.
Footnotes
JPvK and RAB contributed equally.
RC and EVEM contributed equally.
Contributors: All authors were involved in constructing the study concept and design. JPvK, RAB, MJA, RC and EVEM were involved in data acquisition. Statistical analyses were performed by JPvK, RAB and MJA. All authors were involved in the interpretation of outcomes. JPvK and RAB drafted and edited the manuscript. All authors were involved in the manuscript review. EVEM acts as guarantor and corresponding author for the current work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JAB reports reimbursements for his institution (Netherlands Cancer Institute) outside the submitted work from Roche, AstraZeneca and Boehringer Ingelheim. JA reports grants from Amphera, grants from Roche, ownership interest (including patents) from Amphera, and advisory roles for Amphera, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, MSD and Roche, outside the submitted work. PB reports financial support to his institution (Netherlands Cancer Institute) for studies by Bristol Myers Squibb and Merck, and advisory roles for Bristol Myers Squibb, Merck and BeiGene. RC reports personal speakers fees from Roche, Pfizer and Bristol Myers Squibb, and personal advisory fees from MSD and Roche, outside the submitted work.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Mezei G, Chang ET, Mowat FS, et al. Epidemiology of mesothelioma of the pericardium and tunica vaginalis testis. Ann Epidemiol 2017;27:348–59. 10.1016/j.annepidem.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 2. Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636–44. 10.1200/JCO.2003.11.136 [DOI] [PubMed] [Google Scholar]
- 3. Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375–86. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 4. Wright K. Fda Approves nivolumab plus ipilimumab for previously untreated unresectable malignant pleural mesothelioma. Oncology 2020;34:502–3. 10.46883/ONC.2020.3411.0502 [DOI] [PubMed] [Google Scholar]
- 5. de Boer NL, van Kooten JP, Damhuis RAM, et al. Malignant peritoneal mesothelioma: patterns of care and survival in the Netherlands: a population-based study. Ann Surg Oncol 2019;26:4222–8. 10.1245/s10434-019-07803-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237–42. 10.1200/JCO.2009.23.9640 [DOI] [PubMed] [Google Scholar]
- 7. Sugarbaker PH. Update on the management of malignant peritoneal mesothelioma. Transl Lung Cancer Res 2018;7:599–608. 10.21037/tlcr.2018.08.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Helm JH, Miura JT, Glenn JA, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol 2015;22:1686–93. 10.1245/s10434-014-3978-x [DOI] [PubMed] [Google Scholar]
- 9. Bianchi C, Bianchi T. Malignant mesothelioma: global incidence and relationship with asbestos. Ind Health 2007;45:379–87. 10.2486/indhealth.45.379 [DOI] [PubMed] [Google Scholar]
- 10. Stayner L, Welch LS, Lemen R. The worldwide pandemic of asbestos-related diseases. Annu Rev Public Health 2013;34:205–16. 10.1146/annurev-publhealth-031811-124704 [DOI] [PubMed] [Google Scholar]
- 11. Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med 1960;17:260–71. 10.1136/oem.17.4.260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frank AL, Joshi TK. The global spread of asbestos. Ann Glob Health 2014;80:257–62. 10.1016/j.aogh.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 13. Alpert N, van Gerwen M, Taioli E. Epidemiology of mesothelioma in the 21st century in Europe and the United States, 40 years after restricted/banned asbestos use. Transl Lung Cancer Res 2020;9:S28–38. 10.21037/tlcr.2019.11.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peto J, Decarli A, La Vecchia C, et al. The European mesothelioma epidemic. Br J Cancer 1999;79:666–72. 10.1038/sj.bjc.6690105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segura O, Burdorf A, Looman C. Update of predictions of mortality from pleural mesothelioma in the Netherlands. Occup Environ Med 2003;60:50–5. 10.1136/oem.60.1.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berzenji L, Van Schil PE, Carp L. The eighth TNM classification for malignant pleural mesothelioma. Transl Lung Cancer Res 2018;7:543–9. 10.21037/tlcr.2018.07.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NIH NCI, surveillance, epidemiology, and end results (seer) program. Available: https://seer.cancer.gov/tools/codingmanuals/historical.html
- 18. Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335–51. [DOI] [PubMed] [Google Scholar]
- 19. Peto J, Hodgson JT, Matthews FE, et al. Continuing increase in mesothelioma mortality in Britain. Lancet 1995;345:535–9. 10.1016/S0140-6736(95)90462-X [DOI] [PubMed] [Google Scholar]
- 20. Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol 1997;145:211–8. 10.1093/oxfordjournals.aje.a009093 [DOI] [PubMed] [Google Scholar]
- 21. Hemminki K, Li X. Time trends and occupational risk factors for pleural mesothelioma in Sweden. J Occup Environ Med 2003;45:456–61. 10.1097/01.jom.0000058341.05741.7e [DOI] [PubMed] [Google Scholar]
- 22. Delgermaa V, Takahashi K, Park E-K, et al. Global mesothelioma deaths reported to the world Health organization between 1994 and 2008. Bull World Health Organ 2011;89:716–24. 10.2471/BLT.11.086678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leigh J, Driscoll T. Malignant mesothelioma in Australia, 1945-2002. Int J Occup Environ Health 2003;9:206–17. 10.1179/oeh.2003.9.3.206 [DOI] [PubMed] [Google Scholar]
- 24. Stumphius J, Meyer PB. Asbestos bodies and mesothelioma. Ann Occup Hyg 1968;11:283–93. 10.1093/annhyg/11.4.283 [DOI] [PubMed] [Google Scholar]
- 25. Marinaccio A, Binazzi A, Cauzillo G, et al. Analysis of latency time and its determinants in asbestos related malignant mesothelioma cases of the Italian register. Eur J Cancer 2007;43:2722–8. 10.1016/j.ejca.2007.09.018 [DOI] [PubMed] [Google Scholar]
- 26. van der Bij S, Koffijberg H, Burgers JA, et al. Prognosis and prognostic factors of patients with mesothelioma: a population-based study. Br J Cancer 2012;107:161–4. 10.1038/bjc.2012.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Oncol 2007;18:985–90. 10.1093/annonc/mdl345 [DOI] [PubMed] [Google Scholar]
- 28. Attanoos RL, Churg A, Galateau-Salle F, et al. Malignant mesothelioma and its non-asbestos causes. Arch Pathol Lab Med 2018;142:753–60. 10.5858/arpa.2017-0365-RA [DOI] [PubMed] [Google Scholar]
- 29. Teta MJ, Mink PJ, Lau E, et al. Us mesothelioma patterns 1973-2002: indicators of change and insights into background rates. Eur J Cancer Prev 2008;17:525–34. 10.1097/CEJ.0b013e3282f0c0a2 [DOI] [PubMed] [Google Scholar]
- 30. Damhuis RA, Khakwani A, De Schutter H, et al. Treatment patterns and survival analysis in 9014 patients with malignant pleural mesothelioma from Belgium, the Netherlands and England. Lung Cancer 2015;89:212–7. 10.1016/j.lungcan.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 31. Acherman YIZ, Welch LS, Bromley CM, et al. Clinical presentation of peritoneal mesothelioma. Tumori 2003;89:269–73. 10.1177/030089160308900307 [DOI] [PubMed] [Google Scholar]
- 32. Kaya H, Sezgı C, Tanrıkulu AC, et al. Prognostic factors influencing survival in 35 patients with malignant peritoneal mesothelioma. Neoplasma 2014;61:433–8. 10.4149/neo_2014_053 [DOI] [PubMed] [Google Scholar]
- 33. Stahel RA, Weder W, Felip E, et al. Malignant pleural mesothelioma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2008;19 Suppl 2:ii43–4. 10.1093/annonc/mdn083 [DOI] [PubMed] [Google Scholar]
- 34. Clive AO, Taylor H, Dobson L, et al. Prophylactic radiotherapy for the prevention of procedure-tract metastases after surgical and large-bore pleural procedures in malignant pleural mesothelioma (smart): a multicentre, open-label, phase 3, randomised controlled trial. Lancet Oncol 2016;17:1094–104. 10.1016/S1470-2045(16)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bayman N, Appel W, Ashcroft L, et al. Prophylactic irradiation of tracts in patients with malignant pleural mesothelioma: an open-label, multicenter, phase III randomized trial. J Clin Oncol 2019;37:1200–8. 10.1200/JCO.18.01678 [DOI] [PubMed] [Google Scholar]
- 36. Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the mesothelioma Avastin cisplatin pemetrexed study (maps): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405–14. 10.1016/S0140-6736(15)01238-6 [DOI] [PubMed] [Google Scholar]
- 37. Brosseau S, Assoun S, Naltet C, et al. A review of bevacizumab in the treatment of malignant pleural mesothelioma. Future Oncol 2017;13:2537–46. 10.2217/fon-2017-0307 [DOI] [PubMed] [Google Scholar]
- 38. Lievense LA, Sterman DH, Cornelissen R, et al. Checkpoint blockade in lung cancer and mesothelioma. Am J Respir Crit Care Med 2017;196:274–82. 10.1164/rccm.201608-1755CI [DOI] [PubMed] [Google Scholar]
- 39. Yap TA, Aerts JG, Popat S, et al. Novel insights into mesothelioma biology and implications for therapy. Nat Rev Cancer 2017;17:475–88. 10.1038/nrc.2017.42 [DOI] [PubMed] [Google Scholar]
- 40. Belderbos RA, Baas P, Berardi R, et al. A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: dendritic cell immunotherapy for mesothelioma (DENIM) trial. Transl Lung Cancer Res 2019;8:280–5. 10.21037/tlcr.2019.05.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Boer NL, van Kooten JP, Burger JWA, et al. Adjuvant dendritic cell based immunotherapy (DCBI) after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal mesothelioma, a phase II single centre open-label clinical trial: rationale and design of the MESOPEC trial. BMJ Open 2019;9:e026779. 10.1136/bmjopen-2018-026779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kusamura S, Torres Mesa PA, Cabras A, et al. The role of Ki-67 and Pre-cytoreduction parameters in selecting diffuse malignant peritoneal mesothelioma (DmpM) patients for cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2016;23:1468–73. 10.1245/s10434-015-4962-9 [DOI] [PubMed] [Google Scholar]
- 43. Jänne PA, Wozniak AJ, Belani CP, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer 2005;7:40–6. 10.3816/CLC.2005.n.020 [DOI] [PubMed] [Google Scholar]
- 44. Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International expanded access program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211–8. 10.1016/j.lungcan.2008.08.013 [DOI] [PubMed] [Google Scholar]
- 45. Fujimoto E, Kijima T, Kuribayashi K, et al. First-Line chemotherapy with pemetrexed plus cisplatin for malignant peritoneal mesothelioma. Expert Rev Anticancer Ther 2017;17:865–72. 10.1080/14737140.2017.1340157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2021-217709supp001.pdf (820.8KB, pdf)
thoraxjnl-2021-217709supp002.pdf (823.7KB, pdf)
thoraxjnl-2021-217709supp003.pdf (204.2KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.